Summary
Various modeling and experimental studies have analyzed the reactions, interconnections, and motifs in two-component systems, with an eye towards understanding their physiological implications and the differences between alternative designs. Examples where recent progress has been made include aspects of autoregulation, signal integration in branched pathways, cross-talk suppression, and cross-regulation via connector proteins.
Introduction
Studies of two-component systems have revealed a diversity of designs that control the flow of information within and between circuits. Despite the variety and increasing complexity of these systems, some aspects are amenable to simple modeling. This type of analysis, when closely coupled with experiments, bolsters one's intuition, gives insights into the functioning of these circuits, and provides a starting point for analyzing the larger networks within which two-component systems are embedded. This review will cover some of the simple design features that are commonly found in two-component systems and that have been explored through modeling and experiment. The emphasis will not be on the methods for modeling regulatory circuits but rather on the conclusions reached as a result of modeling.
Phosphorylation and dephosphorylation
Single-step phosphotransfer
The pathways of phosphorylation and dephosphorylation are at the heart of two-component signaling. In some circuits, the histidine kinase plays a role only in response regulator phosphorylation. The best-studied example is the histidine kinase CheA in chemotaxis circuits, where response regulator dephosphorylation is controlled by a separate phosphatase (see [1] for a recent review). In many two-component systems, however, the histidine kinase participates in the phosphorylation and dephosphorylation of its partner response regulator. For these bifunctional histidine kinases, the input stimulus controls the ratio of kinase to phosphatase activity, by modulating one or both of these reactions, and thereby setting the response regulator phosphorylation level [2]. A simple kinetic model of this cycle of phosphorylation and dephosphorylation predicts that the steady state output of the circuit—the level of phosphorylated response regulator—is robust or insensitive to fluctuations in the concentrations of histidine kinase and response regulator proteins [3]. The analysis assumed that the histidine kinase is in low abundance relative to the response regulator, which is consistent with measurements for at least three two-component systems in E. coli (EnvZ/OmpR, KdpD/KdpE, PhoQ/PhoP) [4-6]. The concentration robustness predicted by the model has been observed experimentally for the E. coli. EnvZ/OmpR [3], PhoQ/PhoP [6], and CpxA/CpxR [7] systems. The model also predicts that for sufficiently high expression of the histidine kinase, a significant amount of response regulator will be complexed with the histidine kinase, with a concomitant decrease in response regulator phosphorylation. These predictions are consistent with experiments overexpressing the histidine kinase EnvZ, which show a decrease in OmpR-regulated transcription [3] and, in cells expressing an OmpR-GFP fusion, an increase in fluorescence at the cell periphery [8], consistent with OmpR-GFP binding to the transmembrane histidine kinase.
A related model was recently analyzed, which predicts a stronger form of robustness with respect to the regulatory proteins [9]. As with the model in [3], the response regulator is assumed to be dephosphorylated by the histidine kinase. However an additional assumption is that the major pathway for histidine kinase dephosphorylation is through phosphotransfer to the response regulator; the contributions from histidine kinase auto-dephosphorylation or reverse phosphotransfer to ADP are assumed to be sufficiently small to be negligible. If phosphoryl groups are removed from the histidine kinase only through phosphotransfer to the response regulator then at steady state, the rate of histidine kinase autophosphorylation must equal the rate of response regulator dephosphorylation by the histidine kinase. The concentration of histidine kinase cancels from the two sides of the equation for this rate balance. The end result is that the output (the concentration of phosphorylated response regulator) becomes strictly independent of the concentration of histidine kinase. The output is also independent of the total amount of response regulator down to a threshold value, below which all of the response regulator is phosphorylated.
Another recent modeling study considered the behavior of systems where a dead-end complex can form between the unphosphorylated histidine kinase and response regulator [10]. Under certain conditions this system can show bistability, in which the output has two different stable steady states (corresponding to two different levels of phosphorylated response regulator) for the same input. This would translate to bimodal cell populations and switch-like responses to increasing stimulus (e.g. [11]). The bistability requires not only the dead end complex, but also that the dominant mode of response regulator dephosphorylation is histidine kinase-independent [10]. Therefore, these predictions are not in conflict with observations of uniform, rather than bimodal, cell populations for several two-component systems with bifunctional histidine kinases [6,12].
Phosphorelays
The two-component systems considered above involve a single phosphotransfer step. Phosphorelays, on the other hand, employ multiple phosphotransfers: following histidine kinase autophosphorylation, the phosphoryl group is passed to an aspartate residue on a receiver domain, then to a histidine on an HPt domain, and finally to an aspartate on the receiver domain of a response regulator. The various phosphorylated intermediates can be separate proteins, as in the sporulation phosphorelay of Bacillus subtilis [13], or they can be fused in “hybrid” proteins. For many of these systems, response regulator dephosphorylation proceeds by reverse phosphotransfer [14-16]. The general properties of phosphorelay architectures are relatively unexplored and it remains a mystery why some signaling systems make use of single-step phosphotransfers and others phosphorelays. One possibility is that the intermediate steps in phosphorelays provide additional points of control. This is the case for the sporulation phosphorelay [13]. Several phosphatases act at various points to provide additional inputs and multiple levels of regulation to control the switch between sporulation and alternative cell fates [17-22]. However, among the many other well-studied phosphorelays, there are few reports of additional regulators acting at points along the phosphotransfer pathway. It may be that the additional regulators simply have not yet been uncovered in these systems. It is also possible that phosphorelays have other important properties. One modeling study considered hybrid kinases that contain the first three sites of phosphorylation of the phosphorelay [23]. By scanning through ranges of parameter sets it was argued that these systems are likely to show activation kinetics that is a sigmoidal function of time, and to filter noise in the input stimulus. These predictions have yet to be explored experimentally.
Autoregulation
It is not uncommon to find that a phosphorylated response regulator activates transcription of its own gene and also the gene encoding its partner histidine kinase. This autogenous control is intuitively associated with positive feedback. The nature of this feedback, however, depends on the effects of histidine kinase and response regulator expression level on response regulator phosphorylation. For systems with a bifunctional histidine kinase, an application of the modeling described above suggested that the positive feedback associated with autoregulation can be strongly dependent on input stimulus [6]. Indeed, such behavior was observed for the PhoQ/PhoP system in E. coli, where the steady state response was unaffected by autoregulation over a wide range of input stimulus levels (magnesium concentrations). However when cells were grown in sufficiently high stimulus conditions (growth-limiting levels of magnesium), autoregulation amplified the output. In contrast, a strain with a mutated PhoQ that lacks phosphatase activity, and therefore functions as a monofunctional protein, showed strong amplification from autoregulation and considerable cell-to-cell variability, irrespective of the stimulus conditions [6].
The effects of autoregulation on steady-state behavior were also explored in the Bordetella bronchiseptica BvgS/BvgA system [24], which involves a phosphorelay through the hybrid sensor kinase BvgS. It was found that autoregulation modulates the sensitivity of the system to applied stimulus. The properties of the phosphorelay that give rise to this shift in sensitivity have not yet been established.
Positive autoregulation may also have significant effects on the kinetics of activation and inactivation of two-component systems. A study of the E. coli PhoR/PhoB system demonstrated that autoregulation produces a memory or “learning” behavior in the activation kinetics [25], a result of the stability of the histidine kinase and response regulator proteins. This is likely to be a relatively short-term memory, with a decay time on the order of the cell generation time. The temporal control of Bvg-regulated gene expression is also affected by autoregulation [24]. In this case, autoregulation produces a gradual increase in BvgA phosphorylation, which may be important for sustained expression of genes that are transcribed only under intermediate levels of activation. A similar role for autoregulation in controlling the temporal progression of gene expression has been proposed for the B. subtilis sporulation phosphorelay [26].
Autoregulation can also lead to a transient overshoot or surge in response regulator phosphorylation, which subsequently relaxes to a lower steady-state level [27]. The requirement of autoregulation for this behavior was shown explicitly for the Salmonella PhoQ/PhoP system. Similar surges have also been observed for several other autoregulated two-component systems [5,27-29]. A detailed kinetic model of the KdpD/KdpE system did not predict this overshoot [5]. This discrepancy between modeling and experiment suggests it may be necessary to include additional reaction steps or possibly additional regulators in the model to understand the origin of the overshoot.
Negative autoregulation, in which the response regulator represses it's own expression, appears to be less common, at least among two-component systems that have been studied to date. However, a few examples have been reported. In the CovS/CovR system, found in Streptococcus pyogenes, phosphorylated CovR represses transcription of the covRcovS operon [30]. Modeling of this system suggests that autorepression may give rise to oscillatory behavior [31]. Examples of mixed positive and negative autoregulation have also been observed in some systems. For example transcription of the gene encoding the response regulator CtrA in Caulobacter crescentus is both activated and repressed by phosphorylated CtrA. The resulting feedback loops are part of a complex phosphorelay network that functions as an oscillator to control the Caulobacter cell cycle [32,33]. In atleast a few cases a response regulator will repress its own expression, irrespective of its phosphorylation status. Two examples are the response regulators TorR [34] and LuxO [35]. The effects of these phosphorylation-independent negative feedback loops on the regulatory circuits have not yet been established.
It is also important to note that in circuits where auxiliary proteins interact with the histidine kinase or response regulator to modulate signal transduction (discussed below), autoregulation may affect the system by controlling the relative concentrations of the interacting proteins.
Signal Integration and Distribution Among Phosphotransfer Pathways
Branched Pathways
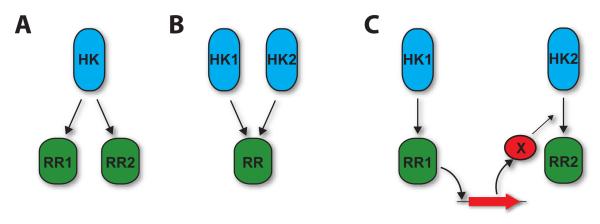
In some two-component systems, the phosphorylation pathway is branched, with more than one source or target of phosphotransfer [36], Fig. 1A,B. Such branched pathways can either bemany-to-one, in which multiple phosphodonors phosphorylate a single protein, or “one-to-many” in which the phosphodonor phosphorylates multiple targets. A well-studied example of “one-to-many” branched regulation is found in bacterial chemotaxis, where the histidine kinase CheA phosphorylates the two response regulators CheY and CheB [1].
Fig. 1.
Three different architectures that extend two-component systems beyond simple linear pathways
A) One-to-many branched pathway. One histidine kinase phosphorylates two response regulators.
B) Many-to-one branched pathway. Two histidine kinases phosphorylate the same response regulator.
C) Connector-mediated pathway. The HK1/RR1 two-component system activates expression of a connector protein X, which modulates the activity of the HK2/RR2 system. X may act on the histidine kinase or response regulator and may increase or decrease RR2 phosphorylation.
An interesting example of “many-to-one” regulation is found in the quorum sensing network of Vibrio harveyi, where three different phosphorelays converge on the same HPt-domain containing protein LuxU [16]. The three sensor kinases, LuxN, LuxQ, and CqsS, detect three distinct autoinducer signals AI-1, AI-2, and CAI-1, respectively. The signal integrating properties of the LuxN and LuxQ branches have been studied in detail. Remarkably, it was found that the output of this circuit is very well fit by a simple linear combination of the contributions from LuxN and LuxQ [37]. This is surprising since, given the combined kinase and phosphatase activities of the two converging pathways, one would have expected some form of nonlinear integration of the two inputs. From a simple model of this system, it was shown that this linearity can be neatly explained if the phosphatase activity of LuxN and LuxQ are constitutive and the input signalsonly modulate the kinase activities of these receptors [37]. The convergence of the AI-1, AI-2, and CAI-1 signaling branches suggests the autoinducers will interfere with each other and limit the ability of the circuit to separately detect individual concentrations of the three different signals. A recent study, which used information theory to explore the limitations imposed by this interference, found that the optimal condition for the circuit to acquire information about the concentrations of both AI-1 and AI-2 is obtained when the kinase rates of the active LuxN and LuxQ receptors are comparable [38] – a condition that was observed to be the case experimentally [37]. It was also shown that modifications of the signaling circuit, through feedback or modulation of autoinducer production, can improve the information transmission [38]. The basic limitation arising from interference of multiple input signals may account for the use of alternative pathway integration architectures, discussed below, rather than convergent phosphorylation pathways in many systems.
Cross-talk
In principle, one might imagine cross-phosphorylation between otherwise distinct signal transduction pathways, or cross-talk, could be an effective means to integrate signals to control multiple outputs. However few examples of bona-fide cross-talk have been reported in the literature (reviewed in [36]). Such cross-talk is ultimately controlled by the specificity of interactions ([36,39]), but the design features of many two-component systems also play a role in suppressing phosphorylation from non-partner histidine kinases or alternative phosphodonors. In particular, bifunctional histidine kinases can buffer against cross-phosphorylation [7,40,41]. In this case, a high flux of phosphorylation and dephosphorylation will render the system insensitive to weak sources of phosphorylation from other histidine kinases or alternative phosphodonors. A second mechanism that limits cross-talk from a histidine kinase is mediated by the partner response regulator. This cross-talk suppression likely results from the partner response regulator out competing non-partner response regulators for interaction with the histidine kinase [7,42].
Cross-regulation via Auxiliary Proteins
Despite the relatively rare appearance of cross-talk, two-component systems do not always function independently. Indeed, there are many examples of one two-component system regulating the activity of another [19,43-48]. In these cases, cross-regulation is achieved by controlling the expression of an auxiliary protein that modulates the activity of a histidine kinase or response regulator of a second system Fig. 1C,(see [49] for a recent review). Such proteins, which effectively connect two signaling pathways, have been named “connectors” [50]. Some connectors also act in feedback loops [19,48,49,51] and are part of a growing list of auxiliary proteins that mediate positive and negative feedback in two-component systems by acting on histidine kinases or response regulators [52-56].
The protein PmrD, which connects the PhoQ/PhoP and PmrB/PmrA path ways in Salmonella has been studied in some detail. Transcription of pmrD is activated by the PhoQ/PhoP system. PmrD binds to the phosphorylated form of the response regulator PmrA, preventing dephosphorylation by the histidine kinase PmrB [57]. Using a combination of modeling and experimental analysis, this architecture for indirect regulation of a gene by PhoQ/PhoP via PmrD and PmrA was compared with alternative scenarios in which regulation is directly controlled by PhoP or in which both forms of regulation were present (a feedforward connector loop) [50,58]. The PmrD connector-mediated circuit resulted in higher levels of activation upon PhoQ stimulation, and a more sustained level of target gene transcription following a decrease in PhoQ stimulus, when compared with the direct pathway. This latter property can be understood as arising from the persistence of PmrD protein in the cell after pmrD transcription has stopped. Interestingly, for the regulation of the gene pbgP by PhoQ/PhoP and PmrB/PmrA, the three different architectures (connector-mediated, direct pathway, and feedforward connector loop) are found in three different species (Salmonella enterica, Yersinia pestis, Klebsiella pneumoniae) [58].
Concluding Remarks
Studies of phosphotransfer-based signaling circuits continue to reveal new layers of complexity for these systems. Indeed, there are fewer and fewer examples of signal transduction pathways that really are made up of only “two components”. To provide some level of organization and understanding for this subject, there is a growing need to characterize the properties of different circuit architectures and, wherever possible, the general principles that govern their function, through modeling and experiment. Some examples of open questions and future research directions are listed in Box 1. Most of the properties of two-component signaling circuits covered above were derived from considerations of one or a few systems and therefore the conclusions may not readily generalizable in all cases. However, one of the benefits of a tight interplay between theory and experiment is that identifying the exceptions, deviations, and violations will be even more informative and help to formulate a circuit science of bacterial cell signaling.
Box 1.
There are numerous open questions concerning the design and organization of two-component signaling systems. Some questions and future research directions that follow from the results reviewed here include:
What are the functional differences between simple single-step two-component systems and the various phosphorelays?
What are the limitations imposed by noise and interference for different circuit architectures? Are there specific designs that effectively proofread or increase fidelity?
What are the general principles that distinguish branched phosphotransfer pathways, various connectors, or other mechanisms for integrating signals and that may account for the use of one architecture over another?
Progress in addressing these and many other questions will likely depend on the development of new methods to follow histidine kinase and response regulator activity in vivo.
Future research directions will also likely include an increasing focus on the interactions between two-component systems and other regulatory networks in the cell.
Acknowledgements
The research from the Goulian lab that was covered in this review was supported by grants from the National Science Foundation and National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirby JR. Chemotaxis-like regulatory systems: unique roles in diverse bacteria. Annu Rev Microbiol. 2009;63:45–59. doi: 10.1146/annurev.micro.091208.073221. [DOI] [PubMed] [Google Scholar]
- 2.Russo FD, Silhavy TJ. The essential tension: opposed reactions in bacterial two-component regulatory systems. Trends Microbiol. 1993;1:306–310. doi: 10.1016/0966-842x(93)90007-e. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor E, Goulian M. Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc Natl Acad Sci U S A. 2003;100:691–696. doi: 10.1073/pnas.0234782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai SJ, Inouye M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem. 2002;277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- 5.Kremling A, Heermann R, Centler F, Jung K, Gilles ED. Analysis of two-component signal transduction by mathematical modeling using the KdpD/KdpE system of Escherichia coli. Biosystems. 2004;78:23–37. doi: 10.1016/j.biosystems.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6*.Miyashiro T, Goulian M. High stimulus unmasks positive feedback in an autoregulated bacterial signaling circuit. Proc Natl Acad Sci U S A. 2008;105:17457–17462. doi: 10.1073/pnas.0807278105. This article considers the effects of positive autoregulation in a system with a bifunctional histidine kinase and demonstrates that amplification from positive feedback is strongly stimulus dependent. The results are likely to be applicable to many other examples of positive autoregulation in two-component signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Siryaporn A, Goulian M. Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Mol Microbiol. 2008;70:494–506. doi: 10.1111/j.1365-2958.2008.06426.x. This article, along with reference 41, describe mechanisms of cross-talk suppression between the EnvZ/OmpR and CpxA/CpxR two-component systems. The mechanisms depend on the cycle of phosphorylation and dephosphorylation by bifunctional histidine kinases in these systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batchelor E, Goulian M. Imaging OmpR localization in Escherichia coli. Mol Microbiol. 2006;59:1767–1778. doi: 10.1111/j.1365-2958.2006.05048.x. [DOI] [PubMed] [Google Scholar]
- 9.Shinar G, Milo R, Martinez MR, Alon U. Input output robustness in simple bacterial signaling systems. Proc Natl Acad Sci U S A. 2007;104:19931–19935. doi: 10.1073/pnas.0706792104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Igoshin OA, Alves R, Savageau MA. Hysteretic and graded responses in bacterial two-component signal transduction. Mol Microbiol. 2008;68:1196–1215. doi: 10.1111/j.1365-2958.2008.06221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veening JW, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol. 2008;62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor E, Silhavy TJ, Goulian M. Continuous control in bacterial regulatory circuits. J Bacteriol. 2004;186:7618–7625. doi: 10.1128/JB.186.22.7618-7625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoch JA. Control of Cellular Development in Sporulating Bacteria by the Phosphorelay Two-Component Signal Transduction System. In: Hoch JA, Silhavy TJ, editors. Two-component Signal Transduction. ASM Press; 1995. pp. 129–144. [Google Scholar]
- 14.Georgellis D, Kwon O, De Wulf P, Lin EC. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem. 1998;273:32864–32869. doi: 10.1074/jbc.273.49.32864. [DOI] [PubMed] [Google Scholar]
- 15.Ansaldi M, Jourlin-Castelli C, Lepelletier M, Theraulaz L, Mejean V. Rapid dephosphorylation of the TorR response regulator by the TorS unorthodox sensor in Escherichia coli. J Bacteriol. 2001;183:2691–2695. doi: 10.1128/JB.183.8.2691-2695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perego M, Glaser P, Hoch JA. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol. 1996;19:1151–1157. doi: 10.1111/j.1365-2958.1996.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 18.Perego M. A new family of aspartyl phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol Microbiol. 2001;42:133–143. doi: 10.1046/j.1365-2958.2001.02611.x. [DOI] [PubMed] [Google Scholar]
- 19.Perego M, Hoch JA. Two-Component Systems, Phosphorelays, and Regulation of their Activities by Phosphatases. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and its Closest Relatives: from Genes to Cells. American Society for Microbiology Press; 2002. pp. 473–481. [Google Scholar]
- 20.Veening JW, Hamoen LW, Kuipers OP. Phosphatases modulate the bistable sporulation gene expression pattern in Bacillus subtilis. Mol Microbiol. 2005;56:1481–1494. doi: 10.1111/j.1365-2958.2005.04659.x. [DOI] [PubMed] [Google Scholar]
- 21.Smits WK, Bongiorni C, Veening JW, Hamoen LW, Kuipers OP, Perego M. Temporal separation of distinct differentiation pathways by a dual specificity Rap-Phr system in Bacillus subtilis. Mol Microbiol. 2007;65:103–120. doi: 10.1111/j.1365-2958.2007.05776.x. [DOI] [PubMed] [Google Scholar]
- 22.Bischofs IB, Hug JA, Liu AW, Wolf DM, Arkin AP. Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay. Proc Natl Acad Sci U S A. 2009;106:6459–6464. doi: 10.1073/pnas.0810878106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JR, Cho KH. The multi-step phosphorelay mechanism of unorthodox two-component systems in E. coli realizes ultrasensitivity to stimuli while maintaining robustness to noises. Comput Biol Chem. 2006;30:438–444. doi: 10.1016/j.compbiolchem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Williams CL, Cotter PA. Autoregulation is essential for precise temporal and steady-state regulation by the Bordetella BvgAS phosphorelay. J Bacteriol. 2007;189:1974–1982. doi: 10.1128/JB.01684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffer SM, Westerhoff HV, Hellingwerf KJ, Postma PW, Tommassen J. Autoamplification of a two-component regulatory system results in “learning” behavior. J Bacteriol. 2001;183:4914–4917. doi: 10.1128/JB.183.16.4914-4917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita M, Losick R. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 2005;19:2236–2244. doi: 10.1101/gad.1335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin D, Lee EJ, Huang H, Groisman EA. A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science. 2006;314:1607–1609. doi: 10.1126/science.1134930. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external copper. Mol Microbiol. 2005;56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 29.Hutchings MI, Hong HJ, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol Microbiol. 2006;59:923–935. doi: 10.1111/j.1365-2958.2005.04953.x. [DOI] [PubMed] [Google Scholar]
- 30.Gusa AA, Scott JR. The CovR response regulator of group A streptococcus (GAS) acts directly to repress its own promoter. Mol Microbiol. 2005;56:1195–1207. doi: 10.1111/j.1365-2958.2005.04623.x. [DOI] [PubMed] [Google Scholar]
- 31.Mitrophanov AY, Churchward G, Borodovsky M. Control of Streptococcus pyogenes virulence: modeling of the CovR/S signal transduction system. J Theor Biol. 2007;246:113–128. doi: 10.1016/j.jtbi.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtzendorff J, Hung D, Brende P, Reisenauer A, Viollier PH, McAdams HH, Shapiro L. Oscillating global regulators control the genetic circuit driving a bacterial cell cycle. Science. 2004;304:983–987. doi: 10.1126/science.1095191. [DOI] [PubMed] [Google Scholar]
- 33.Biondi EG, Reisinger SJ, Skerker JM, Arif M, Perchuk BS, Ryan KR, Laub MT. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444:899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- 34.Ansaldi M, Simon G, Lepelletier M, Mejean V. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J Bacteriol. 2000;182:961–966. doi: 10.1128/jb.182.4.961-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svenningsen SL, Tu KC, Bassler BL. Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. EMBO J. 2009;28:429–439. doi: 10.1038/emboj.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- 37*.Long T, Tu KC, Wang Y, Mehta P, Ong NP, Bassler BL, Wingreen NS. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 2009;7:e68. doi: 10.1371/journal.pbio.1000068. This article considers the integration of multiple signals by the branched quorum sensing circuit in V. harveyi. A simple and elegant quantitative analysis is used to conclude that the system combines the AI-1 and AI-2 autoinducers signals additively. The analysis also indicates that that the AI signals control the kinase and not the phosphatase activities of the sensor kinases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Mehta P, Goyal S, Long T, Bassler BL, Wingreen NS. Information processing and signal integration in bacterial quorum sensing. Mol Syst Biol. 2009;5:325. doi: 10.1038/msb.2009.79. This article takes a relatively unexplored approach to studying information processing in bacterial signaling circuits. Using the V. harveyi quorum sensing circuit as a model, information theory is applied to the problem of distinguishing multiple input signals in many-to-one branched pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szurmant H, Hoch JA. Interaction fidelity in two-component signaling. Current Opinion In Microbiology. 2010;13 doi: 10.1016/j.mib.2010.01.007. ??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves R, Savageau MA. Comparative analysis of prototype two-component systems with either bifunctional or monofunctional sensors: differences in molecular structure and physiological function. Mol Microbiol. 2003;48:25–51. doi: 10.1046/j.1365-2958.2003.03344.x. [DOI] [PubMed] [Google Scholar]
- 41*.Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA. Kinetic buffering of cross talk between bacterial two-component sensors. J Mol Biol. 2009;390:380–393. doi: 10.1016/j.jmb.2009.05.007. This article, along with reference 7, describe mechanisms of cross-talk suppression between the EnvZ/OmpR and CpxA/CpxR two-component systems. The mechanisms depend on the cycle of phosphorylation and dephosphorylation by bifunctional histidine kinases in these systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva JC, Haldimann A, Prahalad MK, Walsh CT, Wanner BL. In vivo characterization of the type A and B vancomycin-resistant enterococci (VRE) VanRS two-component systems in Escherichia coli: a nonpathogenic model for studying the VRE signal transduction pathways. Proc Natl Acad Sci U S A. 1998;95:11951–11956. doi: 10.1073/pnas.95.20.11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kox LF, Wosten MM, Groisman EA. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 2000;19:1861–1872. doi: 10.1093/emboj/19.8.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eguchi Y, Utsumi R. A novel mechanism for connecting bacterial two-component signal-transduction systems. Trends Biochem Sci. 2005;30:70–72. doi: 10.1016/j.tibs.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Tu X, Latifi T, Bougdour A, Gottesman S, Groisman EA. The PhoP/PhoQ two-component system stabilizes the alternative sigma factor RpoS in Salmonella enterica. Proc Natl Acad Sci U S A. 2006;103:13503–13508. doi: 10.1073/pnas.0606026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eguchi Y, Itou J, Yamane M, Demizu R, Yamato F, Okada A, Mori H, Kato A, Utsumi R. B1500, a small membrane protein, connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli. Proc Natl Acad Sci U S A. 2007;104:18712–18717. doi: 10.1073/pnas.0705768104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tschowri N, Busse S, Hengge R. The BLUF-EAL protein YcgF acts as a direct anti-repressor in a blue-light response of Escherichia coli. Genes Dev. 2009;23:522–534. doi: 10.1101/gad.499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerken H, Charlson ES, Cicirelli EM, Kenney LJ, Misra R. MzrA: a novel modulator of the EnvZ/OmpR two-component regulon. Mol Microbiol. 2009;72:1408–1422. doi: 10.1111/j.1365-2958.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitrophanov AY, Groisman EA. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008;22:2601–2611. doi: 10.1101/gad.1700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato A, Mitrophanov AY, Groisman EA. A connector of two-component regulatory systems promotes signal amplification and persistence of expression. Proc Natl Acad Sci U S A. 2007;104:12063–12068. doi: 10.1073/pnas.0704462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato A, Latifi T, Groisman EA. Closing the loop: the PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc Natl Acad Sci U S A. 2003;100:4706–4711. doi: 10.1073/pnas.0836837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garnerone AM, Cabanes D, Foussard M, Boistard P, Batut J. Inhibition of the FixL sensor kinase by the FixT protein in Sinorhizobium meliloti. J Biol Chem. 1999;274:32500–32506. doi: 10.1074/jbc.274.45.32500. [DOI] [PubMed] [Google Scholar]
- 53.Raivio TL, Popkin DL, Silhavy TJ. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 1999;181:5263–5272. doi: 10.1128/jb.181.17.5263-5272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gon S, Jourlin-Castelli C, Theraulaz L, Mejean V. An unsuspected autoregulatory pathway involving apocytochrome TorC and sensor TorS in Escherichia coli. Proc Natl Acad Sci U S A. 2001;98:11615–11620. doi: 10.1073/pnas.211330598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan S, Junker A, Helmann JD, Mascher T. Regulation of LiaRS-dependent gene expression in Bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system. J Bacteriol. 2006;188:5153–5166. doi: 10.1128/JB.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lippa AM, Goulian M. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009;5:e1000788. doi: 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato A, Groisman EA. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 2004;18:2302–2313. doi: 10.1101/gad.1230804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Mitrophanov AY, Jewett MW, Hadley TJ, Groisman EA. Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet. 2008;4:e1000233. doi: 10.1371/journal.pgen.1000233. This article describes a “feedforward connector loop” circuit architecture, which connects a pair of two-component systems. Klebsiella pneumoniae has this circuitry, whereas several closely related bacteria are missing different components of this design. The implications of this circuit are explored through modeling and it is suggested that the feedforward loop structure provides an evolutionary intermediate between direct and indirect transcriptional control. [DOI] [PMC free article] [PubMed] [Google Scholar]