Abstract
The purpose of this experiment was to test in the rat the hypotheses that activation of the brain reward system would attenuate the effects of intracranial nociceptive stimulation and would potentiate the antinociceptive effects of morphine. In this experiment pain (nociception) was generated by electrical stimulation of a brain pain pathway, the mesencephalic reticular formation (MRF) of the rat. Reward pathway stimulation was to the medial forebrain bundle at the level of the lateral hypothalamus (MFB-LH). Current thresholds for escape from MRF stimulation were determined using a modification of the psychophysical methods of limits. MRF stimulation was delivered concurrently with different intensities of non-contingent MFB-LH stimulation. The effects of morphine and saline were determined under all stimulation conditions. Contrary to expectation MFB-LH stimulation significantly lowered MRF stimulation escape thresholds. Morphine administration elevated MRF thresholds in the absence of MFB-LH stimulation. However, this effect was blocked by concurrent MFB-LH stimulation. These findings, which mimic the effects of the opiate antagonist naloxone, i.e., potentiating of pain and antagonism of morphine’s analgesic effects, suggest the presence of an endogenous opiate receptor antagonist.
Keywords: Analgesia Intracranial Pain Reward
Introduction
There have been several experiments in which the analgesic action of direct activation of the brain reward pathways by electrical stimulation of the lateral hypothalamus has been assessed. The non-contingent stimulation of the lateral hypothalamus produces attenuation of the aversive effects of peripheral stimuli delivered in tail-flick and foot withdrawal (Cox and Valenstein, 1965; Dafny et al., 1996). Cox and Valenstein pointed out that there were clinical reports that stimulation of brain areas that were putatively rewarding modified aversive states. In their experiment they found that rats’ choice of a chamber in which they received rewarding brain stimulation was not altered by simultaneous foot shock. They concluded that hypothalamic stimulation, a reward site, “…attenuates the aversive properties of foot shock.” The analgesic effects of rewarding stimulation were most clearly shown in a study in which lateral hypothalamic stimulation self-administered by animals was found to attenuate tonic pain (Lopez and Cox, 1992). Although these studies suggest that lateral hypothalamic stimulation can have an antinociceptive effect on the response to peripheral aversive stimuli none of these experiments specifically measured nociceptive thresholds or the effects or morphine on this activation of the reward systems effect on nociception.
Although the above mentioned experiments suggest that stimulation a reward pathway would attenuate nociception in the rat some experiments found that lateral hypothalamic stimulation appeared to enhance the aversive effects of stimulation of either the tegmentum (Olds and Olds, 1962) or the nucleus gigantocellularis reticularis (NGC) (Keen and Casey, 1970). Other investigators have reported the opposite result for paired LH-NGC stimulation (Carr and Coons, 1982). In these experiments, as in other investigations of the antinociceptive effects of lateral hypothalamic stimulation, nociceptive thresholds were not measured nor were the effects of morphine on this system determined.
The specific hypothesis of this investigation was that activation of the brain reward pathway would attenuate the nociception resulting from direct stimulation of an ascending pain pathway as well as potentiating the analgesic effect of morphine on the stimulation of the pain pathway. We have previously used classical psychophysical procedures to determine the threshold for escape from the aversive stimulation of the mesencephalic reticular formation (MRF) in the study of nociception and analgesia in the rat (Wheeling et al., 1981; Unterwald et al., 1987; Izenwasser and Kornetsky, 1989; Sasson and Kornetsky, 1983; Sasson et al., 1986; Hubner and Kornetsky, 1972; Crosby et al., 2005). The advantage of the technique over the commonly used reflexive techniques is that an actual threshold can be determined and defined in terms of intensity of stimulation, e.g., μAmps, as opposed to reflexive techniques, i.e., the “tail-flick.” method in which the intensity of stimulation is defined in terms of latency of response to a fixed stimulus intensity, e.g., the flicking of the rats tail to escape from the burning effect of a focused beam of light. Also, the psychophysical method of determining threshold measures behavior controlled at supraspinal levels which is not the case for the tail-flick reflexive approach to the measurement of nociception. In the present experiment, the effects of non-contingent MFB-LH stimulation on thresholds for escape from MRF stimulation were examined both in the presence of either morphine or saline.
Methods
In five adult F344 rats two bipolar electrodes (0.125 mm in diameter and insulated except at the tips), were contralaterally implanted with one in the mesencephalic reticular formation (MRF) (AP−7, ML +2.5, DV −7), a pain pathway, and the other at a 12° angle into the medial forebrain bundle at the level of the lateral hypothalamus (MFB-LH) (AP −4, ML +3.2, DV −8.7), a reward pathway. At the completion of the experiment each rat was killed with an injection of pentobarbital (150 mg/kg) and perfused with 60 ml of saline followed by 60 ml of 10% formalin. Brains were removed and stored in 10% formalin until sectioned with a vibratome. The sections (100 μm) were placed on glass slides and stained with cresyl-violet and examined under a light microscope to confirm electrode placement verified by the Paxinos and Watson Rat Brain Atlas (1986).
The threshold for escape from MRF nociceptive stimulation was determined in a chamber (23 cm × 23 cm × 40 cm) with a wheel manipulandum (10 cm wide and a diameter of 5 cm) in one wall of the chamber. Fastened to one endplate of the wheel were four equally spaced cams. Rotation of the manipulandum caused the cam to close a microswitch, which resulted in the termination of the nociceptive stimulus. A modification of the classic psychophysical method of limits was used to determine the escape (nociceptive) threshold. Stimuli were presented in alternating ascending and descending intensities with a step size of 3.0 μAmps. An ascending series was initiated at a previously determined subthreshold intensity. Three trials were given in succession at each stimulus intensity level. Two or three escapes were scored as a plus while less than two were scored as a minus. An ascending series was conducted until plus scores were obtained in two successive intensity levels. A descending series was then imitated at one intensity step lower and current intensities continued to decrease until two successive minus scores were observed. The threshold for a particular ascending or descending series was defined as the midpoint between those intensities that delimited the transition from plus to minus scores. A session was comprised of four stimulation series, two ascending and two descending, comprised a session. A session threshold was based on the mean of the four series thresholds.
Once the rat learned that it could terminate the nociceptive stimulus the threshold was determined on 10 spaced saline treatment days and the mean saline treatment threshold ± the standard deviation in μAmps was determined in each animal. All treatments, saline or morphine sulfate (0.25, 5.0. 10.0 mg/kg) were administered subcutaneously (s.c.). The obtained drug treatment threshold for each experimental animal was converted to a z-score based on the mean and standard deviation threshold of the respective animal’s 10 saline treatment days.
Prior to the obtaining the threshold for escape, the threshold for rewarding brain stimulation delivered to the medial forebrain bundle at the level of the lateral hypothalamus (MFB-LH) was first determined in each rat using the rate independent psychophysical method (Esposito and Kornetsky, 1977; Kornetsky and Bain, 1992). This was conducted in a distinctly different chamber (32 cm × 25 cm × 33 cm) from that used to determine the MRF stimulation threshold with one retractable lever located 6.5 cm above the stainless steel grid floor. Different manipulanda were used for the two procedures so that the rat would immediately be able to discriminate the respective functions of the two manipulanda. After the reward threshold levels were ascertained for each rat then the threshold for MRF nociceptive stimulation was determined with the simultaneous delivery of the threshold intensity of rewarding brain stimulation delivered to the MFB-LH of the respective rat. The specific hypothesis was that the nociceptive MRF threshold would be significantly raised in the presence of rewarding MFB-LH stimulation and that MFB-LH stimulation would enhance the threshold elevating effects of morphine.
All MRF nociceptive thresholds levels were transformed to a z-score based on the respective mean and standard deviation (SD) of the individual animal’s 10 saline treatment days, e.g., z-score=2 indicates the treatment mean effect is 2SDs from the saline control treatment. Results were analyzed using paired t-tests for within subject comparisons.
Results
The anatomical locations of the tips of the electrodes are shown in the reconstruction in Figure 1. MFB-LH and MRF placements were confirmed in all of the animals.
Fig. 1.
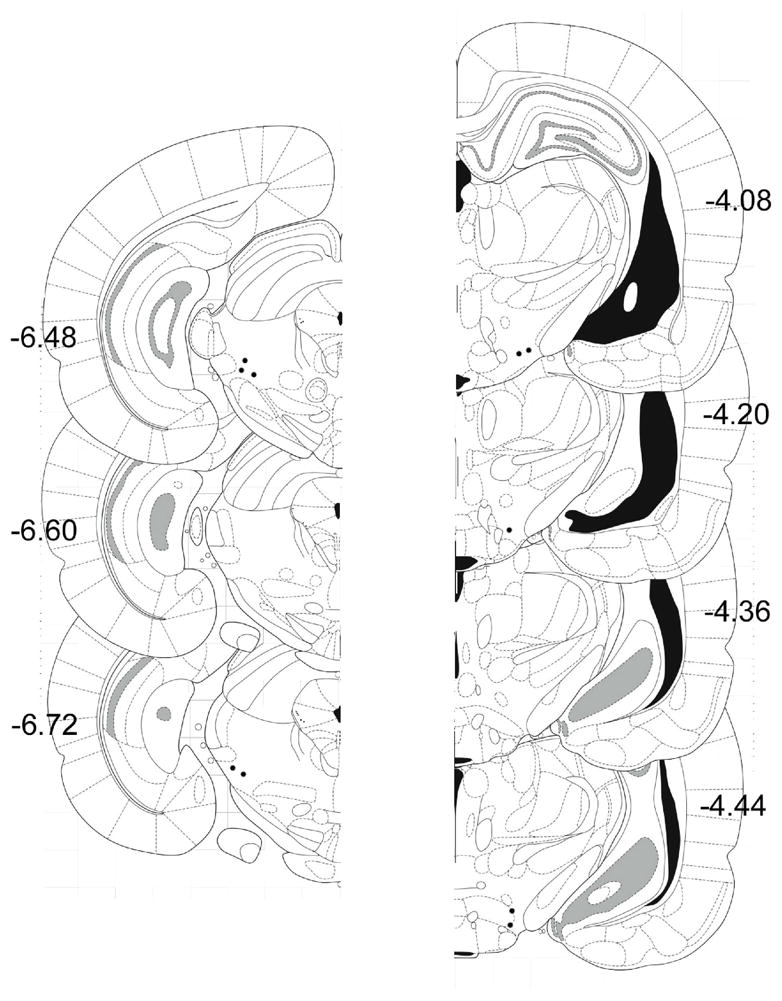
Coronal sections showing electrode placements for MRF on left. MFB-LH on right (N=5). Sections shown are adapted from the atlas of Paxinos and Watson (2005).
The mean (± SD) of the means for the 5 individual rat’s baseline nociceptive (MRF) stimulation threshold was 38±4 μAmps. The mean threshold for rewarding intracranial stimulation to the MFB-LH was 59±8 μAmps.
In the first rat tested in which the simultaneous stimulation of the MRF at threshold intensity and MFB-LH at threshold took place the animal responded in a manner only seen in response to nociceptive stimulus intensities that were well above a threshold level. Consequently, the MFB-LH stimulus intensity level was immediately reduced to a level of 5 or 10 μAmps, and only these current intensities were delivered thereafter when MFB stimulation was administered concurrently with MRF stimulation. At the 5 to 10 μAmp intensity levels MFB-LH stimulation by itself had no consequence; the stimulation was neither rewarding nor aversive. However, as illustrated in
Figure 2, when these low intensity levels of MFB-LH stimulation were simultaneously delivered with stimulation to the MRF, the nociception threshold was significantly lowered. This potentiating of a nociceptive stimulation is characteristic of the effects of the opioid antagonist naloxone (Sasson and Kornetsky, 1983). In order to determine the extent that the combined MFB-LH and MRF stimulation mimic the effects of naloxone, the effects of morphine on the nociceptive MRF threshold was determined in the presence of 5 μAmps of MFB-LH stimulation. Figure 3 illustrates the threshold raising effect of morphine alone on the nociceptive threshold for MRF stimulation and the threshold for morphine in combination with low intensity MFB-LH stimulation. As illustrated, subcutaneous administration of 5 or 10 mg/kg of morphine monotonically raised the threshold for escape from nociceptive MRF stimulation. However, in the presence of 5 μAmps of MFB-LH stimulation, the analgesic effect of both 5 and 10 mg/kg of morphine was completely antagonized. Not only were these mean effects statistically significant, each of the animals exhibited effects similar to the mean effects illustrated in the figures.
Fig 2.
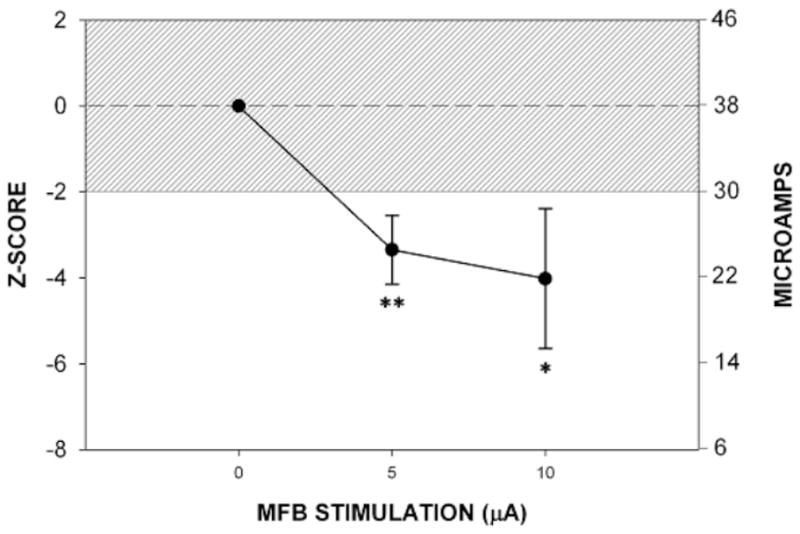
Mean ± SE effects of low intensity level MFB stimulation on the nociceptive threshold.
*p< 0.03
**p<0.01
Fig 3.
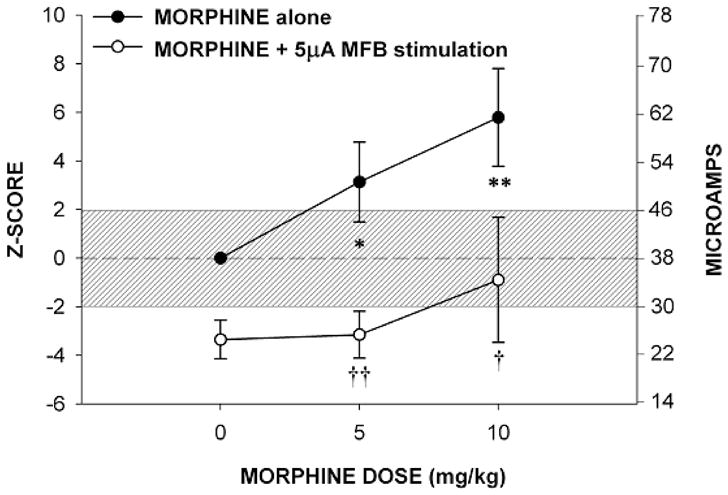
Mean ± SE analgesic effects of morphine alone and saline treatment
Between respective dose of morphine alone and saline treatment.
*p< 0.07
**p<0.02
Between morphine alone and respective dose of morphine plus 5 μA of MFB stimulation.
†p<0.06
††p<0.01
Discussion
Non-contingent low current stimulation of the MFB-LH lowered the threshold at which rats would work to escape stimulation delivered to the MRF. MFB-LH stimulation also blocked morphine-induced elevations of nociceptive MRF escape thresholds. While the stimulation delivered to the MFB-LH was non-contingent, prior to combined MRF and MFB-LH stimulation the electrodes in MFB-LH placement sites were found to support responding for brain stimulation reward, demonstrating a clear connection to reward pathways. The effects of MFB-LH stimulation on MRF escape responding in the present experiment were similar to those observed following the administration of the opioid antagonist naloxone, a significant lowering the nociceptive threshold and a reversal of morphine-induced threshold elevation (Sasson and Kornetsky, 1983).
The results of the present investigation are not consistent with the hypotheses that activation of a reward pathway would result in antinociceptive effects and the enhancement of morphine-induced antinociception. The results of two older studies are also not consistent with hypothesis that the activation of reward pathways results in antinociceptive effects. Olds and Olds (1962) found that responding for escape from aversive dorsal tegmental stimulation was enhanced by continuous stimulation of areas that support self-stimulation. In an experiment with greater similarly to the one described here, stimulation delivered to the nucleus gigantocellulariis (NGC) at subthreshold levels became aversive by the non-contingent stimulation of hypothalamic sites that support self-stimulation (Keene and Casey, 1970). Since the NGC is part of the medullary reticular formation this finding along with those of the present study suggest that the stimulation of hypothalamic reward areas could enhance the effects of aversive stimuli delivered throughout the extent of the recticular formation.
Carr and Coons (1982) report that rates of escape responding from NGC stimulation are attenuated when paired with MFB-LH stimulation. Also, after the pairing of NGC and rewarding LH stimulation rats will not escape from a compartment in which NGC is administered to a second compartment in which it is not delivered (Diotte et al., 2000). These findings seem contrary to the idea that combining LH stimulation with reticular formation stimulation results in an increase in the strength of the nociceptive stimulation. It should be noted, however, that LH and NGC stimulation in the Carr and Coons and the Diotte studies were delivered sequentially, e.g. LH stimulation was delivered first, followed by NSC stimulation with an interval of several milliseconds separating the stimulation of the different sites. Thus, the behavioral effects of paired LH-reticular formation stimulation appear to be dependent on the timing of the delivery of the different stimuli.
In the present study concurrent stimulation of the MFB-LH and the MRF blocked the effects of systemically administered morphine. This raises questions as to precisely which areas of the brain are involved in mediating this effect. There is evidence that points to the MRF as a possible site of interaction between morphine and the combined MFB-LH/MRF stimulation. The microiontophoretic application of either morphine or the synthetic opioid peptide met-enkephalinamide into the MRF significantly increases tail flick latency (Haigler and Mittleman, 1978; Haigler and Spring, 1978). Morphine infusion into the MRF also attenuates the response to the hemostat pinch test. These results suggest that the MRF plays a significant role in the pathways in the mediation of the nociceptive stimuli evoked by noxious stimuli applied to peripheral sites such as the paw and the tail. Pressure delivered to the paws of rats and paw pinch evokes firing in MRF neurons (Haigler, 1978; Hosford and Haigler, 1980). Evoked firing is inhibited in this area by the microinjection of either morphine or the opioid peptide, methionine-enkephalin, into this area, suggesting that a possible mechanism of opioid analgesic action within the MRF results from a decrease of cell firing produced by noxious stimuli. Putative neurotransmitters that may be involved in the transmission of nociceptive stimuli within the MRF include acetylcholine, norepinephrine, neurotensin, and substance P (Haigler and Spring, 1981). The ability of either acetylcholine or norepinephrine to increase neuronal firing within the MRF is attenuated by the local application of morphine (Haigler and O’Neill, 1983.
Anatomical studies have shown that MRF sends ascending projections via the MFB to the lateral hypothalamus (Jones and Yang, 1985). The existence of functional connections between the lateral hypothalamus and MRF is supported by the finding that low frequency stimulation of one area results in altered neuronal firing in the other area (Barone et al., 1981). One possible explanation for the increase in sensitivity to aversive MRF stimulation produced by the simultaneous delivery of MFB and MRF is that the currents from these two sources simply sum. This summed stimulation may over ride the inhibitory effects of morphine within the MRF leading to the release of neurotransmitters that act to facilitate the transmission of the nociceptive signal. However, the intensity of the currents delivered concurrently to the MRF and MFB-LH required to block the analgesic effects of morphine in this study were extremely low, and it is not clear that the simple sum of these would be sufficient to significantly attenuate the antinociceptive effects of morphine.
Another possible explanation for the escape threshold lowering effects of the addition of MFB to MRF stimulation is that the net effect of this combined stimulation is the facilitation of release of excitatory neurotransmitters. This might occur locally within the MRF. Glutamatergic fibers, however, project from the MRF to the periaqueductal gray (Beitz et al., 1989). The periaqueductal gray is another midbrain structure that modulates the transmission of nociceptive stimuli, and excitatory input from the MRF might modulate the activity of this structure.
A third explanation for the observation that combined MRF-LH/MRF stimulation lowers the threshold for escape and blocks the effects of morphine is that this stimulation leads to an increase activity of some anti-opioid substance. Several such substances have been identified. They include neuropeptide FF (Wang et al., 1999; Wei et al., 1998); Yang et al., 2008), interleukin-1 (Shavit et al., 2005), cholecystokinin (Faris et al., 1983; Li and Han, 1989), nociceptin (Heinricher et al., 1997; Rossi et al., 1998), and dopamine (Izenwasser and Kornetsky, 1989; King et al., 2001). Whether combined MRF/MFB low intensity stimulation leads to the release of any of these substances within the MRF or other brain areas remains to be determined. Nociceptin and putative nociceptin receptor binding sites have been shown to be present in the MRF, and could therefore act as an anti-opioid substance within this area (Letchworth et al., 2000; Neal et al., 1999).
The anti-opioid substances such as cholecystokinin, nociceptin, and neuorpeptide FF do not act as competitive antagonists that bind directly to opioid receptors. The finding that combining of MFB-LH rewarding stimulation with MRF nociceptive stimulation produces effects that resemble those of naloxone in MRF stimulation escape experiments, potentiating nociception and the blocking of the nociceptive threshold raising effects of morphine, suggest the presence of a yet to be identified endogenous opioid receptor antagonist. This antagonist would not only block the effects of morphine, but might also act to antagonize the actions of endogenous opioid peptides.
Fields et al., (2006) in a review of central nervous system mechanisms of pain modulation gives evidence that an endogenous opioid-mediated pain modulator system exists. If an endogenous opioid receptor antagonist does exist, it is reasonable to assume it serves some function. Pain itself has survival value. It signals things that need to be avoided. In the presence of pain there is a release of the endorphins (Bodner et al. 1980). This probably attenuates the painful stimulus; however, if the attenuation of the pain is sufficient such that the animal does not try to escape from the pain source it is unlikely that it would survive. Thus, a function of an endogenous opioid receptor antagonist would be to modulate the endogenous opioids and an appropriate name for the substance that fits with the name naloxone would be “endoxone.”
Acknowledgments
This work was supported by NIDA grant R21 DA25586 and NIDA Research Scientist Award KO5 DA000099 to Conan Kornetsky
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barone FC, Wayner MJ, Zarco de Coronado I, Tsai WH. Mesencephalic reticular formation stimulation effects on hypothalamic neuronal activity. Brain Res Bull. 1981;7:419–25. doi: 10.1016/0361-9230(81)90040-x. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. Possible origin of glutamatergic projections to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain Res Bull. 1989;23:25–35. doi: 10.1016/0361-9230(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Bodner R, Kelley DD, Brutus M, Glusman M. Stress induced analgesia: neural and hormonal determinants. Neurscience Biobehav Rev. 1980;4:87–100. doi: 10.1016/0149-7634(80)90028-7. [DOI] [PubMed] [Google Scholar]
- Carr KD, Coons EE. Rats self-administer nonrewarding brain stimulation to ameliorate aversion. Science 1982. 1982;215:1516–7. doi: 10.1126/science.7063859. [DOI] [PubMed] [Google Scholar]
- Cox VC, Valenstein ES. Attenuation of aversive properties of peripheral shock by hypothalamic stimulation. Science . 1965;149:323–325. doi: 10.1126/science.149.3681.323. [DOI] [PubMed] [Google Scholar]
- Crosby S, Knapp CM, Kornetsky C. Nociceptive threshold and analgesic response to morphine in aged and young adult rats as determined by thermal radiation and intracerebral electrical stimulation. Pharm Biochem Behav. 2005;84:148–157. doi: 10.1016/j.pbb.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Dafny N, Donog WQ, Prieto-Gomez C, Reyes-Vazquez C, Stanford J, Qiao JT. Lateral hypothalamus: site involved in pain modulation. Neuroscience. 1996;70:449–60. doi: 10.1016/0306-4522(95)00358-4. [DOI] [PubMed] [Google Scholar]
- Diotte M, Miquelez M, Miliaressis E, Bielajew C. Interactions between rewarding lateral hypothalamic and aversive nucleus reticularis giigantocellularis stimulation. Behavior and Brain Res. 2000;116:149–156. doi: 10.1016/s0166-4328(00)00268-0. [DOI] [PubMed] [Google Scholar]
- Esposito R, Kornetsky C. Morphine lowering of self-stimulation thresholds: lack of tolerance with long term administration. Science. 1977;195:189–191. doi: 10.1126/science.831268. [DOI] [PubMed] [Google Scholar]
- Faris PL, Komisaruk BR, Watkins LR. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983;219:310–312. doi: 10.1126/science.6294831. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Heinricher MM. Central nervous systems mechanisms of pain modulation. Chapter 7. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. Elsevier; Phildadlphia: 2006. [Google Scholar]
- Haigler HJ. Morphine: ability to block neuronal activity evoked by a nociceptive stimulus. Life Sci. 1976;19:841–57. doi: 10.1016/0024-3205(76)90312-x. [DOI] [PubMed] [Google Scholar]
- Haigler HJ, Mittleman RS. Analgesia produced by direct injection of morphine into the mesencephalic reticular formation. Brain Res Bull. 1978;3:655–62. doi: 10.1016/0361-9230(78)90013-8. [DOI] [PubMed] [Google Scholar]
- Haigler HJ, O’Neill TP. Interactions of morphine with putative neurotransmitters in the mesencephalic reticular formation. Life Sci. 1983;32:759–69. doi: 10.1016/0024-3205(83)90310-7. [DOI] [PubMed] [Google Scholar]
- Haigler HJ, Spring DD. A comparison of the analgesic and behavioral effects of [D-Ala2] Met-enkepha-linamide and morphine in the mesencephalic reticular formation of rats. Life Sci. 1978;23:1229–39. doi: 10.1016/0024-3205(78)90500-3. [DOI] [PubMed] [Google Scholar]
- Haigler HJ, Spring DD. Putative nociceptive neurotransmitters in the mesencephalic reticular formation. Life Sci. 1981;29:33–43. doi: 10.1016/0024-3205(81)90112-0. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Grandy DK. Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J Neurophysiol. 1997;78:3351–3358. doi: 10.1152/jn.1997.78.6.3351. [DOI] [PubMed] [Google Scholar]
- Hosford DA, Haigler HJ. Morphine and methionine-enkephalin: different effects on spontaneous and evoked neuronal firing in the mesencephalic reticular formation of the rat. J Pharmacol Exp Ther. 1980;213:355–63. [PubMed] [Google Scholar]
- Hubner CB, Kornetsky C. Heroin, 6-acetylmorphine and morphine effects on threshold for rewarding and aversive brain stimulation. J Pharmacol Exp Therap. 1992;260:562–567. [PubMed] [Google Scholar]
- Izenwasser S, Kornetsky C. Potentiation of morphine analgesia by D-amphetamine is mediated by norepinephrine and not dopamine. Pharmacol Biochem Behav. 1989;32:983–984. [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;1;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Keene JJ, Casey KL. Excitatory connection from lateral hypothalamic self-stimulation sites to escape sites in medullary reticular formation. Exp Neurol. 1970;28:155–66. doi: 10.1016/0014-4886(70)90170-6. [DOI] [PubMed] [Google Scholar]
- King MA, Bradshaw S, Chang AH, Pintar JE, Pasternak GW. Potentiation of opioid analgesia in dopamine2 receptor knock-out mice: evidence for a tonically active anti-opioid system. J Neurosci. 2001;21:7788–92. doi: 10.1523/JNEUROSCI.21-19-07788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Bain G. Brain stimulation reward: a model for the study of the rewarding effects of abused substances. NIDA Monograph . 1992;124:73–93. [PubMed] [Google Scholar]
- Letchworth SR, Mathis JP, Rossi GC, Bodnar RJ, Pasternak GW. Autoradiographic localization of (125)I[Tyr(14)]orphanin FQ/nociceptin and (125)I[Tyr(10)]orphanin FQ/nociceptin(1–11) binding sites in rat brain. J Comp Neurol. 2000;423:319–29. [PubMed] [Google Scholar]
- Li Y, Han JS. Cholecystokinin-octapeptide antagonizes morphine analgesia in periaqueductal gray of the rat. Brain Res. 1989;480:105–110. doi: 10.1016/0006-8993(89)91572-2. [DOI] [PubMed] [Google Scholar]
- Lopez R, Cox VC. Analgesia for tonic pain by self-administered lateral hypothalamic stimulation. Neuroreport. 1992;3:311–314. doi: 10.1097/00001756-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–47. [PubMed] [Google Scholar]
- Olds ME, Olds J. Approach-escape interactions in rat brain. Am J Physiol . 1962;203:803–810. doi: 10.1152/ajplegacy.1962.203.5.803. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. San Diego, CA: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Burlington, MA: Elsevier Academic Press; 2005. [Google Scholar]
- Rossi GC, Perlmutter M, Leventhal L, Talatti A, Pasternak GW. Orphanin FQ/nociception analgesia in the rat. Brain Res. 1998;792:327–330. doi: 10.1016/s0006-8993(97)01490-x. [DOI] [PubMed] [Google Scholar]
- Sasson S, Kornetsky C. Naloxone lowers brain-stimulation escape threshold. Pharm Biochem Behav. 1983;18:231–233. doi: 10.1016/0091-3057(83)90368-4. [DOI] [PubMed] [Google Scholar]
- Sasson S, Unterwald EM, Kornetsky C. Potentiation of morphine analgesia by amphetamine. Psychopharmacol. 1986;90:163–165. doi: 10.1007/BF00181233. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Sasson S, Kornetsky C. Evaluation of the supraspinal analgesic activity and abuse liability of ethylketocyclazocine. Eur J Pharmacol. 1987;133:275–81. doi: 10.1016/0014-2999(87)90023-9. [DOI] [PubMed] [Google Scholar]
- Wang JL, Zhu CB, Cao XD, Wu GC. Distinct effect of intracerebroventricular and intrathecal injections of nociceptin/orphanin FQ in the rat formalin test. Regul Pept. 1999;79:159–163. doi: 10.1016/s0167-0115(98)00161-x. [DOI] [PubMed] [Google Scholar]
- Wei H, Panula P, Pertovaara A. A differential modulation of allodynia, hyperalgesia and nociception by neuropeptide FF in the periaqueductal gray of neuropathic rats: interactions with morphine and naloxone. Neuroscience. 1998;86:311–319. doi: 10.1016/s0306-4522(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Wheeling HS, Sasson S, Kornetsky C. Tolerance to the effects of morphine on escape from reticular formation stimulation. Subst Alcohol Action Misuse . 1981;2:107–114. [PubMed] [Google Scholar]
- Yang HY, Tao T, Iadarola MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides. 2008;42:1–18. doi: 10.1016/j.npep.2007.06.004. [DOI] [PubMed] [Google Scholar]


