Abstract
Charcot-Marie-Tooth disease type 2D, a hereditary axonal neuropathy, is caused by mutations in glycyl-tRNA synthetase (GARS). The mutations are distributed throughout the protein in multiple functional domains. In biochemical and cell culture experiments, some mutant forms of GARS have been indistinguishable from wild-type protein, suggesting that these in vitro tests may not adequately assess the aberrant activity responsible for axonal degeneration. Recently, mouse and fly models have offered new insight into the disease mechanism. There are still gaps in our understanding of how mutations in a ubiquitously expressed component of the translation machinery result in axonal neuropathy. Here we review recent reports, weigh the evidence for and against possible mechanisms, and suggest areas of focus for future work.
Keywords: glycyl-tRNA synthetase, tRNA synthetase, Charcot-Marie-Tooth Disease, protein translation, neurodegeneration
Charcot-Marie-Tooth disease (CMT), one of the most common inherited neurological disorders, includes a genetically diverse group of peripheral neuropathies characterized by progressive weakness and atrophy in the hands and feet 1. Pathophysiological studies categorize CMT as type I (demyelinating) or type 2 (axonal) 2. There is overlap between axonal forms of CMT and a similar group of clinical syndromes classified as distal hereditary motor neuropathy (dHMN) or distal spinal muscular atrophy (dSMA), which are distinguished from CMT2 only by the absence of sensory loss 3. CMT type 2D often causes more weakness in the distal upper extremities, a finding that is unusual among the CMTs, which usually present with length-dependent axonal degeneration affecting the feet more than the hands 3.
In 2003, the glycyl-tRNA synthetase gene GARS was found to be mutated in CMT2D 4. GARS is responsible for covalently linking glycine with corresponding tRNAs in an ATP-dependent reaction. Charged tRNAgly is then used in protein synthesis in the ribosome. GARS is thus ubiquitously expressed and absolutely necessary for protein translation in all cells. Since the CMT2D gene discovery, the challenge has been to determine how missense mutations in this critical and widely expressed protein which causes selective degeneration of axons in peripheral nerves, a common conundrum with hereditary neurodegenerative diseases linked to ubiquitous proteins and presenting selective neuronal vulnerability. Here we review work done to determine a mechanism for axonal degeneration in CMT2D and critically assess the likely mechanisms and suggest avenues for future investigation.
Genetics of CMT2D
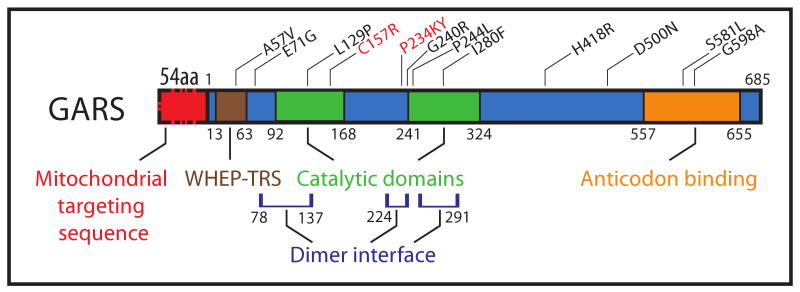
GARS is one of thirty seven tRNA synthetase genes. It encodes both cytosolic and mitochondrial isoforms of the protein, which differ by a 54 amino acid N-terminal mitochondrial targeting sequence (Figure 1). In the protein region common to both isoforms, there are four functional domains: a WHEP-TRS domain that is highly conserved across tRNA synthetases (amino acids 13-63), two domains that form the catalytic core (amino acids 92-168 and 241-324) and an anticodon binding domain (amino acids 557-655). To date, 11 mutations in GARS have been reported (Table 1). There are varying degrees of genetic evidence to indicate that the mutations are pathogenic. The mutations E71G, L129P, and G240R, which segregate in pedigrees with lod scores over 5, are the most tightly linked to the disease 4-7, while the L129P mutation is incompletely penetrant. Variants identified by sequencing GARS in small families and isolated patients with peripheral neuropathy, including A57V, P244L, I280F, D500N, G526R, S581L, G598A and H418R (which also has reduced penetrance), are less clearly implicated 4, 8-12.
Figure 1.
The GARS transcript produces two protein products, a cytosolic isoform and a mitochondrial isoform that has a 54 amino acid mitochondrial targeting sequence. The remaining 685 amino acids encoded by the transcript are shared by both isoforms. The mutations that have been identified in GARS (black, human; red, mouse) are distributed across the entire protein in all of the functional domains. Many of the mutations that have been identified lie in one of the three regions that form the dimer interface.
Table 1. The genetics of GARS variants identified in patients.
| Mutation | Geographical Origins | Age of Onset | Disease Phenotype | Penetrance | Genetic Evidence | Reference |
|---|---|---|---|---|---|---|
| A57V | Ghana | 12 years | NA | Found in a screen of 33 patients | Rohkamm et al., 2007 | |
| E71G | Mongolia | 18 years (average) |
CMT2D/dSMA-V | Complete | Segregation in a large pedigree Lod score of 6.07 |
Antonellis et al., 2003 Sambuughin et al., 1998 |
| L129P | Bulgaria | 17 years (average) |
dSMA-V | Incomplete | Segregation in a large pedigree Lod score of 5.99 |
Antonellis et al., 2003 Christodoulou et al., 1995 |
| G240R | North America | 23 years (average) |
CMT2D | Complete | Segregation in large pedigree Lod score of 6.89 |
Antonellis et al., 2003 Ionasescu et al., 1996 |
| P244L | Japan | Adolescence | CMT2D | NA | Found in a screen of 89 patients absent from 100 healthy controls | Abe et al., 2006 |
| I280F | UK | 11 to 18 years | CMT2 | Complete | Segregation in two generations of a small pedigree | James et al., 2006 |
| H418R | UK/Australia | 26 years (average) |
dSMA-V | Incomplete | Segregation in a large family. 13 individuals carry the mutation | Sivakumar et al., 2005 |
| D500N | Italy | 6 years to >53 years | CMT2D/dSMA-V | Incomplete | Segregation in a 3 generation pedigree with 5 affected individuals | Del Bo et al., 2006 |
| G526R | France | 20 years (average) |
dSMA-V | Complete | Segregation in a two generations of a small pedigree | Antonellis et al. 2003 |
| S581L | UK | 4 years | Lower-limb predominant CMT2 | Complete | 4 generation pedigree | James et al. 2006 |
| G598A | UK | 6 months | severe HMN | NA | de novo | James et al. 2006 |
Cell culture studies of GARS function and localization
In determining the functional consequences of GARS mutations, the initial focus was on the ability of the mutant enzyme to charge tRNAgly. tRNA charging can be assayed in two ways: (1) aminoacylation as measured by quantitative capture of tritiated glycine, and (2) radioactively labeled inorganic phosphate release as a proxy for charging activity. These approaches were used to evaluate the activity of mutant GARS compared to wild-type enzyme. Of the seven mutations studied in aminoacylation assays with tritiated glycine, E71G, P234KY, D500N, and S581L were all active, while L129P, G240R, and G526R were inactive 13. The inactivity of G526R was confirmed with the phosphate release assay 14. These assays suggest that there is no direct correlation between disease pathogenesis and the in vitro charging capacity of the mutant proteins. Moving forward, it may be useful to determine whether these mutant enzymes can charge tRNAs in neurons and specifically in the axon.
Recent structural studies of GARS indicated that a majority of disease causing mutations lie on the dimer interface of the protein 14, 15. GARS normally forms a homodimer, and the effects of GARS mutations on dimerization have been examined by co-immunoprecipitation of endogenous mouse Gars with V5-tagged human GARS 13. In this study, L129P and G240R had weaker dimer associations, P234KY and H418R were similar to wild-type, and D500N, G526R and S581L had stronger dimer interactions 13. Disease-associated mutations thus have a range of effects on dimer interactions. More investigation is required to examine whether and how these effects on dimer interactions are relevant to the pathogenesis.
A number of studies have used cell-culture models to study how GARS mutations lead to CMT2D. Antonellis et al. found punctate structures by immunohistochemistry in normal human tissue sections and neuroblastoma cells, but they were unable to identify other proteins that colocalize with these GARS granules. Notably, the antibody stained granules did not colocalize with survival motor neuron (SMN, a nucleolar RNA binding protein), alpha-tubulin or oxidative phosphorylation complex IV, a marker of mitochondria 16. As they are observed in spinal cord, root, and nerve sections of human autopsy tissue, these puncta indicate that GARS is transported into peripheral axons, where it has a distinctive distribution 16. The GARS antibody also stains the nucleolus, indicating a possible role in RNA processing in this organelle 16. The key to confirming the relevance of GARS granules to the pathogenesis of axonal degeneration is to determine whether there are different distributions in CMT2D patients and animal models and to identify binding partners that colocalize with GARS in neurons. Other studies have used recombinant GARS with fluorescent tags to determine its subcellular distribution in transfected SH-SY5Y cells. The mitochondrial isoform of GARS tagged with eGFP on the C-terminus forms puncta in the cell, which interestingly do not form when eGFP-tagged GARS contains L129P, G240R, and H418R mutations 16. It is, however, unclear whether these GARS-eGFP granules are analogous to endogenous GARS puncta. It is also unexpected that these wild-type GARS-eGFP puncta (seen with overexpression of the mitochondrial isoform) do not colocalize with mitochondria.
Another study compared the distribution of transfected epitope-tagged GARS to the distribution of endogenous GARS in the projections of N2a cells. It was found that wild-type transfected GARS had the same distribution as endogenous, but transfected mutant GARS failed to localize to sprouting neurites13.
Research into the subcellular localization is important partly because studies of tRNA synthetases in cell-free systems have shed little light on the CMT2D disease mechanism. The identification of GARS mutations in peripheral nerve disease has naturally shifted the focus of investigation to understanding how the wild type and mutant proteins function in a cellular context. The negative biochemical results have also raised the possibility that GARS may have a unique function in neurons, which is disrupted in the disease. Other tRNA synthetases have been shown to have functions other than charging, and this possibility is worth exploring with GARS in cell culture and animal models.
Animal Models of GARS-Linked Axonopathy
Animal models can help to explain the neuronal specificity of the effects of GARS mutations. In addition to the reports of human mutations described above, mutations have been identified in mice and flies. As biochemical and cell culture studies point to possible pathogenic mechanisms, these animal models play an important role in the confirmation and extension of the findings.
Mouse Models of CMT2D
The two mouse models that have been reported with Gars mutations have motor and sensory deficits of different severity. Phenotypic features and the results of informative crosses are summarized in Table 2. The first CMT2D model, Nmf249, is a spontaneous mutation identified at the Jackson Laboratory in a line with a dominantly inherited neurological phenotype and decreased lifespan. The defect was mapped to a 1.9 Mb critical region containing Gars, the mouse ortholog of GARS. The causal mutation, a CC → AAATA replacement, results in an in-frame change of a proline to a lysine and tyrosine at residue 278 in the mouse Gars gene, equivalent to P234KY in the human protein (the annotation of the mouse protein starts at the beginning of the mitochondrial targeting sequence, while the human protein does not include those residues in the count) 17. The affected mice have sensory and motor deficits and survive for about 6-8 weeks. Marked loss of integrity of the neuromuscular junctions (NMJ) is evident at post-natal day 36. Motor and sensory nerves in affected mice showed decreased evoked potential amplitude and decreased nerve conduction velocity with histological and ultrastructural evidence of a decrease in large nerve fibers with normal myelin thickness around the remaining fibers 17. These observations indicate a severe, dominantly inherited motor and sensory neuropathy.
Table 2. Mouse phenotypes with Gars alleles.
| Gars Genotype | Life Expectancy | Strain Differences | Phenotype | Reference |
|---|---|---|---|---|
|
GarsNmf249/+ or GarsP278KY/+ |
6-8 weeks | Outcross to CAST/EiJ extended lifespan but did not alter onset or severity of motor phenotype | Sensory and motor deficits Abnormal NMJ morphology Impaired nerve impulse transmission Reduced NCVs Loss of large diameter peripheral axons |
Seburn et al. 2006 |
| GarsNmf249/Nmf249 | Embryonic lethal | NA | NA | Seburn et al. 2006 |
| GarsXM256/+ | Normal lifespan | NA | Reduced GARS RNA levels Normal NMJ morphology Normal NCVs |
Seburn et al. 2006 |
| GarsXM256/XM256 | Embryonic lethal | NA | NA | Seburn et al. 2006 |
| GarsNmf249/XM256 | Embryonic lethal | NA | NA | Seburn et al. 2006 |
| GarsC201R/+ | Normal lifespan | Motor phenotype is more severe on B6 background than on C3H | Decreased grip strength Poor skilled motor function Increased total GARS protein at p15 Reduction in large diameter axons in sciatic nerve |
Achilli et al. 2009 |
| GarsC201R/C201R | 17 days (maximum) |
Maximum life expectancy 15 days on C3H background | Reduced weight and viability Impaired limb movement |
Achilli et al. 2009 |
| GarsC201R/XM256 | Embryonic lethal | NA | NA | Achilli et al. 2009 |
Total Gars mRNA levels were equivalent to those found in wild-type mice, and in in vitro aminoacylation assays the P234KY mutant GARS protein was fully active. To investigate this further, a hypomorphic allele was examined. This XM256 allele contains an insertion in the second intron of the Gars gene and results in a 50% reduction in Gars mRNA levels 17. These heterozygous mice have a normal lifespan and no NMJ pathology 17. These results indicate that a simple loss of GARS function is not responsible for the disease.
Another Gars allele was recently identified in an ENU mutagenesis screen for neurological phenotypes at the MRC Mammalian Genetics Unit, Harwell UK 18. Two littermates were found with deficits in rotarod and grip strength testing. Positional cloning and candidate gene sequencing showed a missense mutation in Gars, which results in a cysteine to arginine change at position 201 (C201R), equivalent to C157R in the human protein. The phenotype of the GarsC201R/+ mouse is considerably less severe than the GarsNmf249/+ mouse. Heterozygotes have a normal lifespan with a 50% decrease in grip strength detectable in one month old mice; homozygotes are viable, albeit with a severe deficit. Most of the characterization was performed on the C3H background, which has a less severe pathology. No abnormal histology was observed in spinal cord sections of affected animals, but a 50% decrease in large diameter motor axons was seen in the sciatic nerve in 17-month-old GarsC201R/+ mice. Brain lysates from GarsC201R/+ mice and from GarsC201R/C201R mice were evaluated for aminoacylation. While no significant difference was seen in the charging activity of lysates from heterozygotes, the lysates from homozygotes had a 60% reduction in enzyme activity. GARS protein levels were elevated in both heterozygotes and homozygotes at P15, perhaps indicating compensatory upregulation; in adult heterozygotes no difference in protein levels was observed in comparison to wild-type mice 18.
Both mutant alleles have been crossed to the hypomorphic allele GarsXM256. In both cases, no compound heterozygous offspring were produced, confirming by non-complementation that Gars mutations cause the motor phenotypes in the GarsC201R/+ and GarsNMF249/+ mice 18. Why these GarsC201R/XM256 and GarsNMF249/XM256 mice do not survive is unclear, given the retained enzymatic activity in the heterozygous mutant mice, but it may indicate that both Gars P278KY and C201R require the presence of some wild-type enzyme for functionality. Alternatively, the toxicity of the mutant protein may be mitigated by the presence of wild-type enzyme, or the mutant proteins lack some other, unknown activity.
The histopathology and electrophysiology done on these mice show that both are good models for the disease phenotype. While the milder GarsC201R/+ mouse phenotype is more consistent with the disease seen in humans, with late onset, grip strength deficit, and no reduction in lifespan, the observations from the GarsNmf249/+ mouse provide an indication of the effects of a more rapidly progressive pathology, potentially facilitating short-duration interventional studies.
Although the findings in the mice argue against a simple loss of function mechanism for the GARS mutations, there is still some ambiguity because of the lack of viability of the compound heterozygotes and because of the reduced enzymatic activity in the GarsC201R homozygote. If the mutations did not cause a loss of aminoacylation function, then deleting the other copy of Gars should not result in lethality. In fact, one could argue that the phenotype should not have been worse because the compound heterozygotes have the same dose of mutant protein as the heterozygous mutants. The differences in severity of both models on different backgrounds show that the GARS phenotype is easily modified. This is consistent with the range of onset and incomplete penetrance seen in patients. Important questions could be answered with transgenic mice overexpressing GARS. If robust overexpression of a mutant transgene replicates the phenotype, then we could conclude that axons degenerate because of a dominant negative or toxic gain of function in GARS. Similarly, if robust expression of wild-type protein is able to rescue the mutant phenotypes, then we could conclude that a loss of GARS function, perhaps a non-canonical function, is the cause of disease.
Drosophila Models of GARS deficiency
The aats-gly gene in Drosophila has 60 percent homology with human GARS, and 10 of the mutations identified in humans are missense changes in amino acids that are conserved in fly. In 2007 the Luo group identified a fly mutation in a random mutagenesis screen for genes necessary for dendrite and axon formation and arborization in olfactory projection neurons. The screen was performed with the Mosaic Analysis with a Repressible Cell Marker (MARCM) technique, which allows individual cells deficient in a protein to be studied on a heterozygous background 19.
The Drosophila mutation that was identified caused a missense change equivalent to P98L in the human protein 20. Three additional experiments supported the conclusion that the homozygous mutations probably cause a loss of translation as deletion of aats-gly, two other tRNA synthetases, and the locus Df(3L)mito, which contains two genes involved in the mitochondrial translation machinery all replicated the phenotype 20. It is particularly noteworthy that removal of the mitochondrial translation machinery resembled the phenotype in severity and penetrance. It is also interesting that the neuronal cell bodies appeared healthy in all of these mutants, indicating that axons and dendrites have a greater sensitivity to reduced protein translation 20. This study also attempted to connect the fly phenotype to the human disease through a series of rescue experiments. It was demonstrated that the wild-type human GARS transgene fully rescued the phenotype, while the human mutants GARSE71G and GARSL129P did not 20.
The Drosophila work underscores the importance of GARS, and tRNA synthetases in general, in neurite growth and arborization. Other non-neuronal tissues were not examined to determine the effects of deficiency in GARS, so it is unclear if neurons are particularly vulnerable to loss of housekeeping function. The most remarkable observation from this study was that human GARSE71G was unable to rescue the arborization defect. This is the only assay in which GARSE71G is distinguishable from wild type. This finding questions the sensitivity or relevance of other functional assays that have been reported. In subcellular localization, in vitro aminoacylation activity, and yeast rescue, GARSE71G (which is strongly implicated genetically) behaves like wild-type, but in Drosophila olfactory axons, GARSE71G is not equivalent to wild-type GARS.
Of particular interest is that in the Chihara et al. study, overexpression of several GARS and aats-gly wild-type and mutant transgenes did not cause any overt phenotype or problems with projection neuron arborization, suggesting that the mutant protein does not have dominant negative or toxic effects in Drosophila. Overexpression was not, however, the focus of this study. It is difficult to evaluate the relevance of this invertebrate model that has recessive inheritance and where effects are only viewed in individual neurons, when there are mouse models that point to toxicity rather than simple loss of function of the mutant protein.
Diseases Caused by Mutations in other tRNA Synthetase Genes
GARS was the first tRNA synthetase gene to be linked to a disease. Since then mutations in 4 of the 36 other tRNA synthetase genes have been linked to neurological phenotypes in humans or mice, indicating that tRNA synthetases play a particularly important role in nervous system development and maintenance.
Two recessive diseases of the central nervous system are associated with mutations in mitochondrial tRNA synthetases: a leukoencephalopathy caused by mutations in the mitochondrial aspartyl-tRNA synthetase gene (DARS2)21, and an infantile encephalopathy most severely affecting the cerebellum caused by mutations in the mitochondrial arginyl-tRNA synthetase gene (RARS2) 22. In mice, mutations in the editing domain of alanyl-tRNA synthetase (Aars) cause Purkinje cell degeneration, ataxia, and hair follicle dystrophy (sticky) 23. This study reported that the sticky Aars mutant mischarges tRNAala with serine, and hypothesized that this leads to accumulation of misfolded proteins due to a resulting decrease in the fidelity of protein translation 23.
Two missense mutations and a four amino acid deletion in the tyrosyl-tRNA synthetase gene (YARS) have been identified in dominantly inherited intermediate CMT (DI-CMT) 24. Of the diseases that have been connected to tRNA synthetase genes, this disease is most similar to GARS-linked CMT2D in inheritance pattern and phenotype. The differences in the disease manifestations are that DI-CMT has both axonal and demyelinating features and lower limb predominance. It was initially demonstrated that neither of the YARS missense mutants release pyrophosphate as quickly24. More recent data suggest that one of them, E196K, is fully active in an aminoacylation assay, in yeast experiments, and in Drosophila genetic complementation assays (these results are more consistent with the observations with GARS)25.
A Drosophila model of DI-CMT has also been generated through transgenic overexpression of mutant YARS and aats-tyr, the fly ortholog. While wild-type overexpression did not result in an abnormal phenotype, overexpression of transgenes with disease causing mutations produced expression-level-dependent phenotypes. High levels of expression of some of the mutant transgenes in all tissues caused lethality, while lower levels of ubiquitous expression and neuronal expression of mutant transgenes resulted in motor performance defects as measured by negative geotaxis, jumping, and flying25. This work nicely demonstrates that there is some toxicity of the mutant protein in neurons and that this toxicity is conserved in invertebrates. This is the first report of toxicity of a mutant tRNA synthetase and should guide future work on GARS.
The similarities between the results of the functional assays that have been performed seem to increase the likelihood that the mechanism of YARS and GARS-linked CMT is the same. With this in mind, it will be interesting to see how research in each area will advance our understanding of both diseases. The dosage-dependent effects of mutant YARS overexpression suggest that perhaps expression levels of mutant GARS were simply not high enough to generate a phenotype in the experiments reported by Chihara et al[HK1].
Possible Mechanisms for GARS Axonopathy
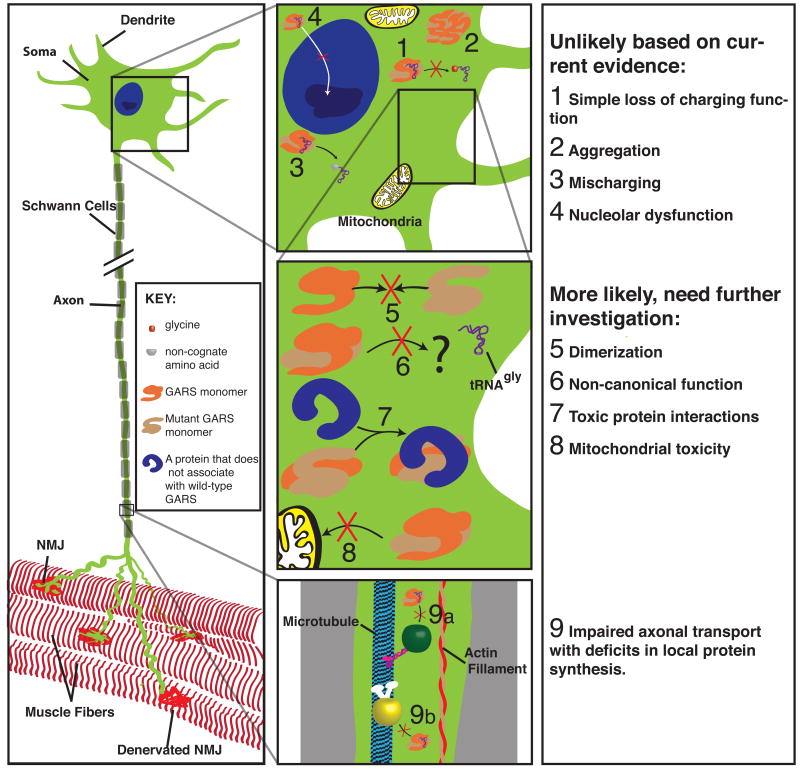
There are at least nine possible mechanisms for axonal degeneration with GARS mutations. None are mutually exclusive; some have more support than others, and some are unlikely but difficult to rule out completely (Figure 2).
Figure 2.
Possible mechanisms by which mutations in GARS could be leading to axon degeneration in these uniquely shaped cells. Some of these are unlikely or have been ruled out by functional analysis ((1) Loss of charging function, (2) Aggregation, (3) Mischarging), others require further investigation ((4) Nucleolar dysfunction, (5) Dimerization, (6) Non-canonical functions, (7) novel interactions resulting from mutations, (8) Mitochondrial toxicity or dysfunction, (9) Impaired axonal transport that lead to deficits in local translation).
Loss of charging function
Some of the reported mutations cause loss of function as assayed by in vitro aminoacylation or yeast viability. These results are consistent with a disease mechanism of haploinsufficiency in charging function. However, other mutations that segregate with the disease do not alter enzyme activity in these assays, making it unlikely that the disease is due to a simple loss of this function. The finding that the mouse that is heterozygous for the hypomorphic allele (XM256/+) has no phenotype also presents strong evidence that the disease is not caused by a simple loss of function. There is, however, evidence from mouse models that a loss of function may contribute to the pathogenesis of the disease. The lethality observed in compound heterozygous mice (GarsNmf249/XM256, GarsC201R/XM256) suggests that despite the activity of both the C157R and P234KY mutant enzymes in vitro, neither can restore viability to a mouse deficient in wild-type Gars. Finally, the E71G mutant protein was unable to fully rescue the arborization defects in Aats-gly-deficient olfactory projection neurons in Drosophila, suggesting that this mutation may also cause some loss of function that is not detectable with other assays. While toxic-gain or dominant-negative mechanisms may be more likely, the loss of function seen with these mutations may still be a part of the pathogenesis.
Aggregation
The GARS mutations in CMT2D resemble SOD1 missense mutations in familial amyotrophic lateral sclerosis (ALS), as in being distributed across different functional domains in the protein, and in the fact that the mutations do not have a consistent effect on the known function of the protein. As with other mutant proteins in neurodegenerative disease, mutant SOD1 forms aggregates. While there is still debate about whether mutant protein aggregates are a cause of toxicity or a result of a protective mechanism to sequester toxic protein, the aggregates generally correlate with toxicity 26. Aggregates of mutant GARS have not been observed in patient cells or in cell culture systems overexpressing mutant GARS 13, 16. As this work has also used multiple antibodies, it is unlikely that antibody sites are masked in aggregates of mutant protein. Thus it is unlikely that aggregates are formed in cells expressing mutant GARS, and aggregation is probably not part of the pathogenesis of CMT due to GARS mutations. This does not, however, rule out the possibility that GARS is toxic to the motor neuron through aberrant protein interactions.
Mischarging
Unlike AARS, GARS does not have a known editing domain. It is worth testing whether mutations that cause CMT decrease the fidelity of tRNA charging, but with current data it is unlikely that this is the mechanism of toxicity. AARS mutations cause the sticky phenotype only when bred to homozygosity. While the recessive inheritance suggests a loss of function (unlike GARS), the fact that mutations in this member of the tRNA synthetase family cause neurodegeneration in mice may be relevant. It will be interesting to see whether a link between these two diseases can be found and whether there are any human diseases caused by mutations in tRNA synthetase editing domains.
Nucleolar dysfunction
The function of GARS in the nucleolus is unknown. Antonellis et al. showed that GARS colocalizes with nucleolar antigen in SH-SY5Y cells, but the reason for its presence there is unclear16. It is difficult to know whether mutants have impaired nuclear import, because recombinant GARS does not appear to be concentrated in the nucleolus. It is also possible that nucleolar staining is an artifact of the antibody. Methionyl-tRNA synthetase (MARS) has also been found in the nucleolus and has been shown to regulate rRNA synthesis in proliferative cells. MARS is, however, absent from the nucleolus in quiescent cells 27. If GARS has a similar function in the nucleolus, it seems unlikely that this function plays an important role in a disease manifested primarily in terminally differentiated motor and sensory neurons.
Dimerization
There are a number of ways that aberrant dimer formation could lead to GARS dysfunction or GARS toxicity in neurons. Dimerization may not be an independent mechanism, but rather a potential link between basic GARS biochemistry and the other pathogenic mechanisms listed here. For example GARS monomers may not be distributed to nerve terminals as well as dimers. Similarly, it may be that an accumulation of monomers is toxic to the cell.
Non-canonical functions
Essential housekeeping proteins commonly acquire additional functions through evolution, and various non-canonical functions have been ascribed to tRNA synthetases (as reviewed in 28, 29). These range from anti-angiogenic properties observed with YARS and WARS, to regulation of transcription seen with KARS. Yeast GARS1 has been shown to participate in the formation of mRNA 3′-ends. It could be that while the canonical charging function may be preserved with some mutations, all of the mutations alter a function of GARS that has not yet been identified.
Disease-associated mutations lead to new, toxic, protein-protein or protein-RNA interactions
If mutant GARS is toxic to motor neurons, it may be because of a protein-protein or protein-RNA interaction that is detrimental to axon health. The expression profile of this interacting protein could help explain the motor and sensory specificity of the phenotype. The neuronal specificity of GARS mutation effects in CMT2D could be due to the cell-specific expression of a key interacting factor, as has been proposed, for example, in Huntington's disease30.
Mitochondrial toxicity or dysfunction
Mitochondrial defects have been reported in various neurodegenerative diseases, including axonal CMT. CMT2A is caused by mutations in mitofusin 2 (MFN2) 31, a membrane protein that is responsible for the maintenance of mitochondrial morphology. Mutations in ganglioside-induced differentiation associated protein 1 (GDAP1) likely lead to CMT2K and CMT4A by a similar mechanism of altered mitochondrial structure 32, 33. GARS is not a membrane protein, and it is unlikely to have a similar function in mitochondrial architecture, but it is a mitochondrial protein, and the pathogenesis of the disease could be linked to mitochondrial toxicity or altered function in this organelle.
Impaired axonal transport
Peripheral motor axons are particularly long, highly terminally branched, and support nerve terminals that are much larger than elsewhere in the nervous system. With the distance and volume of proteins to travel along the narrow axon, active transport is critical to the health of the axon and its terminals. Impaired transport has been shown to cause motor neuron degeneration: kinesin mutations cause motor deficits in Drosophila 34, and mutations in dynactin p150 cause motor neuron phenotypes in humans, mice and flies 35-37.
There is evidence of local protein translation in axons 38, and it is likely that the peripheral motor axon in particular is translationally active. GARS is likely among the cargo that is transported in both directions along microtubules between the soma and the nerve terminal. It is possible that axons degenerate because mutant GARS protein is not included in anterograde or retrograde transport cargo, and the axon is unable to clear the mutant protein.
BOX 1: Outstanding questions
How do the variable effects on charging function and dimer formation relate to disease pathogenesis?
Which GARS isoform (cytosolic or mitochondrial) is most relevant to disease?
Is GARS transported down the axon?
Does GARS have non-canonical functions? Are any of these functions unique to neurons?
Do mutations in GARS cause it to associate with new RNAs or proteins?
Do post-translational modifications of GARS impact its localization and binding partners.
Will transgenic overexpression of mutant GARS model the disease?
Will transgenic overxpression of wild-type GARS mitigate the pathology?
Do YARS and GARS mutations cause CMT by the same mechanism?
Concluding Thoughts
Many mechanisms may contribute to the disease manifestations, but all the mutations likely share the same pathophysiology. A functional assay for GARS mutations that correlates with pathogenicity is needed. A specific focus must be placed on mutations that segregate with disease. Studying mutations with weak genetic evidence could confuse the search for a common mechanism. It is worthwhile to focus on mutations such as E71G, L129P, C157R, P234KY, and G240R that are most likely pathogenic because they segregate with disease in large human and mouse pedigrees. Of the mutations with the strongest supporting evidence, E71G continues to be an outlier. An assay that is able to identify a functional consequence of this mutation would likely point to the critical mechanism.
At this juncture, the most likely candidates for a unifying mechanism are impaired axonal transport, mitochondrial dysfunction, new protein associations that lead to toxicity and loss or gain of a non-canonical activity. Amino-acid mischarging, abnormal dimerization, aggregation, and a simple loss of charging function are unlikely to be primarily responsible for the pathogenesis of the disease.
A special focus should be placed on determining if the mutant protein is damaging to cells and tissues through a toxicity or dominant negative effect, and if overexpression of wild-type protein is protective. These experiments would help categorize the mechanism as either a loss-of-function or purely dominant effect. This determination will allow us to develop and test therapeutic measures. There is still much to be learned about normal and abnormal protein-protein and protein-RNA interactions of GARS and about its activity and toxicity in cells and especially neurons. As work in cell culture helps direct the search for a CMT2D mechanism, these observations need to be confirmed in vivo in animal models and ultimately in patients.
Acknowledgments
The authors would like to thank Anthony Antonellis, Isabella Palazzolo, Deborah Kwon, Michael Mooney, Katherine Gribble, and Barrington Burnett for helpful comments on the manuscript. We are grateful for support from the NINDS intramural program and the Marshall Commission (to W.W.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jani-Acsadi A, et al. Charcot-Marie-Tooth neuropathies: diagnosis and management. Semin Neurol. 2008;28:185–194. doi: 10.1055/s-2008-1062264. [DOI] [PubMed] [Google Scholar]
- 2.Bienfait H, et al. Phenotype of Charcot-Marie-Tooth disease Type 2. Neurology. 2007;68:1658–1667. doi: 10.1212/01.wnl.0000263479.97552.94. [DOI] [PubMed] [Google Scholar]
- 3.Barisic N, et al. Charcot-Marie-Tooth disease: a clinico-genetic confrontation. Ann Hum Genet. 2008;72:416–441. doi: 10.1111/j.1469-1809.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 4.Antonellis A, et al. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet. 2003;72:1293–1299. doi: 10.1086/375039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sambuughin N, et al. Autosomal dominant distal spinal muscular atrophy type V (dSMA-V) and Charcot-Marie-Tooth disease …. J Neurol Sci. 1998 doi: 10.1016/s0022-510x(98)00264-0. [DOI] [PubMed] [Google Scholar]
- 6.Ionasescu V, et al. Autosomal dominant Charcot-Marie-Tooth axonal neuropathy mapped on chromosome 7p (CMT2D) Human Molecular Genetics. 1996 doi: 10.1093/hmg/5.9.1373. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulou K, et al. Mapping of a distal form of spinal muscular atrophy with upper limb predominance to chromosome 7p. Human Molecular Genetics. 1995 doi: 10.1093/hmg/4.9.1629. [DOI] [PubMed] [Google Scholar]
- 8.Rohkamm B, et al. Further evidence for genetic heterogeneity of distal HMN type V, CMT2 with predominant hand involvement and Silver syndrome. J Neurol Sci. 2007;263:100–106. doi: 10.1016/j.jns.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James P, et al. Severe childhood SMA and axonal CMT due to anticodon binding domain mutations in the GARS gene. Neurology. 2006;67:1710–1712. doi: 10.1212/01.wnl.0000242619.52335.bc. [DOI] [PubMed] [Google Scholar]
- 10.Sivakumar K, et al. Phenotypic spectrum of disorders associated with glycyl-tRNA synthetase mutations. Brain. 2005;128:2304–2314. doi: 10.1093/brain/awh590. [DOI] [PubMed] [Google Scholar]
- 11.Del Bo R, et al. Coexistence of CMT-2D and distal SMA-V phenotypes in an Italian family with a GARS gene mutation. Neurology. 2006;66:752–754. doi: 10.1212/01.wnl.0000201275.18875.ac. [DOI] [PubMed] [Google Scholar]
- 12.Abe A, Hayasaka K. The GARS gene is rarely mutated in Japanese patients with Charcot-Marie-Tooth neuropathy. J Hum Genet. 2009;54:310–312. doi: 10.1038/jhg.2009.25. [DOI] [PubMed] [Google Scholar]
- 13.Nangle L, et al. Charcot-Marie-Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc Natl Acad Sci USA. 2007;104:11239–11244. doi: 10.1073/pnas.0705055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie W, et al. Long-range structural effects of a Charcot-Marie-Tooth disease-causing mutation in human glycyl-tRNA synthetase. Proc Natl Acad Sci U S A. 2007;104:9976–9981. doi: 10.1073/pnas.0703908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cader MZ, et al. Crystal structure of human wildtype and S581L-mutant glycyl-tRNA synthetase, an enzyme underlying distal spinal muscular atrophy. FEBS Lett. 2007;581:2959–2964. doi: 10.1016/j.febslet.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Antonellis A, et al. Functional Analyses of Glycyl-tRNA Synthetase Mutations Suggest a Key Role for tRNA-Charging Enzymes in Peripheral Axons. J Neurosci. 2006;26:10397–10406. doi: 10.1523/JNEUROSCI.1671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seburn KL, et al. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot-Marie-Tooth 2D mouse model. Neuron. 2006;51:715–726. doi: 10.1016/j.neuron.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Achilli F, et al. An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy. Dis Model Mech. 2009 doi: 10.1242/dmm.002527. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JS, Luo L. A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat Protoc. 2006;1:2583–2589. doi: 10.1038/nprot.2006.320. [DOI] [PubMed] [Google Scholar]
- 20.Chihara T, et al. Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat Neurosci. 2007;10:828–837. doi: 10.1038/nn1910. [DOI] [PubMed] [Google Scholar]
- 21.Scheper G, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat Genet. 2007;39:534–539. doi: 10.1038/ng2013. [DOI] [PubMed] [Google Scholar]
- 22.Edvardson S, et al. Deleterious mutation in the mitochondrial arginyl-transfer RNA synthetase gene is associated with pontocerebellar hypoplasia. Am J Hum Genet. 2007;81:857–862. doi: 10.1086/521227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 24.Jordanova A, et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet. 2006;38:197–202. doi: 10.1038/ng1727. [DOI] [PubMed] [Google Scholar]
- 25.Storkebaum E, et al. Dominant mutations in the tyrosyl-tRNA synthetase gene recapitulate in Drosophila features of human Charcot-Marie-Tooth neuropathy. Proc Natl Acad Sci U S A. 2009;106:11782–11787. doi: 10.1073/pnas.0905339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown RHJ. SOD1 aggregates in ALS: Cause, correlate or consequence? Nat Med. 1998;4:1362–1364. doi: 10.1038/3945. [DOI] [PubMed] [Google Scholar]
- 27.Ko YG, et al. Nucleolar localization of human methionyl-tRNA synthetase and its role in ribosomal RNA synthesis. J Cell Biol. 2000;149:567–574. doi: 10.1083/jcb.149.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SG, et al. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci U S A. 2008;105:11043–11049. doi: 10.1073/pnas.0802862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonellis A, Green ED. The role of aminoacyl-tRNA synthetases in genetic diseases. Ann Rev Genomics Hum Genet. 2008;9:87–107. doi: 10.1146/annurev.genom.9.081307.164204. [DOI] [PubMed] [Google Scholar]
- 30.Subramaniam S, et al. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Züchner S, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 32.Baxter RV, et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet. 2002;30:21–22. doi: 10.1038/ng796. [DOI] [PubMed] [Google Scholar]
- 33.Cuesta A, et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 34.Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puls I, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 36.Teuling E, et al. A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Hum Molec Genet. 2008;17:2849–2862. doi: 10.1093/hmg/ddn182. [DOI] [PubMed] [Google Scholar]
- 37.Eaton BA, et al. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 38.Brittis PA, et al. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]