Abstract
Primary hepatocytes are commonly used as liver surrogates in toxicology and tissue engineering fields, therefore, maintenance of functional hepatocytes in vitro is an important topic of investigation. This paper sought to characterize heparin-based hydrogel as a three-dimensional scaffold for hepatocyte culture. The primary rat hepatocytes were mixed with a prepolymer solution comprised of thiolated heparin and acrylated poly(ethylene glycol) (PEG). Raising the temperature from 25° to 37°C initiated Michael addition reaction between the thiol and acrylated moieties and resulted in formation of hydrogel with entrapped cells. Analysis of liver-specific products, albumin and urea, revealed that the heparin hydrogel was non-cytotoxic to cells and, in fact, promoted hepatic function. Hepatocytes entrapped in the heparin-based hydrogel maintained high levels of albumin and urea synthesis after three weeks in culture. Because heparin is known to bind growth factors, we incorporated hepatocyte growth factor (HGF) – an important liver signaling molecule - into the hydrogel. HGF release from heparin hydrogel matrix was analyzed using enzyme linked immunoassay (ELISA) and was shown to occur in a controlled manner with only 40% of GF molecules released after 30 days in culture. Importantly, hepatocytes cultured within HGF-containing hydrogels exhibited significantly higher levels of albumin and urea synthesis compared to cells cultured in the hydrogel alone. Overall, heparin-based hydrogel showed to be a promising matrix for encapsulation and maintenance of difficult-to-culture primary hepatocytes. In the future, we envision employing heparin-based hyrogels as matrices for in vitro differentiation of hepatocytes or stem cells and as vehicles for transplantation of these cells.
1. Introduction
Hepatocytes are used widely as liver surrogates in drug screening/toxicology, liver biology, and tissue engineering fields [1–3]. Upon excision from the liver and cultivation in vitro primary hepatocytes de-differentiate rapidly, losing hallmark liver functions related to protein synthesis and detoxification. However, hepatic phenotype may be rescued by recapitulating, in the Petri dish, components of the in vivo microenvironment [3, 4]. Various approaches for rescuing hepatocyte function in vitro include coating culture substrates with extracellular matrix proteins (ECM) [5, 6], entrapment within biomimetic gels (collagen- or Matrigel-based) [4, 7, 8] and co-cultivation with other cell types [9–11].
Three-dimensional matrices have been shown to improve polarity and organotypic structure formation in hepatocytes cultured in vitro [7, 12] and may also serve as vehicles for cell transplantation [6, 13]. A number of matrices comprised of natural and synthetic biomaterials have been utilized for entrapment of hepatocytes [14–16]. The approaches utilizing natural biomaterials include culturing hepatocytes in a collagen gel sandwich or encapsulating these cells within Matrigel. More recently, PEG-based synthetic hydrogels, bearing cell-adhesive peptides, have been employed by Bhatia and co-workers for encapsulation and patterning of primary hepatocytes [17, 18]. Both categories of biomaterials, natural and synthetic, have advantages and disadvantages. Natural polymers provide an excellent microenvironment for cultivation of functional hepatocytes; however, these biomaterials are difficult to manipulate (e.g. control chemical/mechanical properties) and may not be suitable for cell transplantation due to difficulties with fabrication of delivery vehicles and possibility of immune response. On the other hand, synthetic biomaterials such as PEG hydrogels may be easily manipulated to create materials of desired chemical and mechanical properties. While suitable for entrapment and cultivation of robust mesenchymal or endothelial cells [19–22], synthetic hydrogels are less optimal for maintenance of difficult-to-culture epithelial cells such as primary hepatocytes [17, 18].
Recently, we developed a “hybrid” natural/synthetic hydrogel formed by a Michael-type addition reaction between thiolated heparin and diacrylated poly(ethylene glycol) (PEG) [23]. Heparin is a natural biomaterial that is abundant in the liver [24, 25] and is known to sequester a number of different ECM proteins and GFs via heparin binding domains [26, 27]. Leveraging affinity of heparin for GF molecules, we previously demonstrated controlled release of human growth hormone (hGF) and vascular endothelial growth factor (VEGF) from heparin-based hydrogels [28, 29]. In addition to excellent bioactivity afforded by heparin, the mechanical properties of the hydrogel may be easily controlled by changing the length or functionality of synthetic PEG molecules [23]. Furthermore, heparin-based hydrogel may be fabricated into miniature constructs suitable for cell delivery/transplantation [30] and was found to cause little or no inflammation and hemolysis in vivo [28].
As a step towards development of heparin hydrogel scaffolds for hepatocyte or stem cell transplantation, the present study sought to characterize cultivation of primary rat hepatocytes in this gel and to test the effects of incorporating hepatocyte growth factor (HGF) [31–33] - a potent liver morphogen – into the hydrogel. Future applications of heparin hydrogel as a microenvironment niche for hepatocyte or stem cell differentiation and as a vehicle for cell transplantation are envisioned.
2. Materials and Methods
2.1. Materials
Heparin (sodium salt, from porcine intestinal mucosa, MW 12 kDa) was purchased from Cellsus Ins. (Cincinnati, IA, USA). Poly(ethylene glycol) diacrylate (PEG-DA, MW 3.4 and 6 kDa, 98 % degree of substitution) and tetra-functional poly(ethylene glycol) sulfhydryl (PEG-SH4, MW 10 kDa) were purchased from SunBio Inc. (Anyang, Korea). Ethanol, epidermal growth factor (EGF), collagenase type IV, bovine serum albumin (BSA), and hepatocyte growth factor (HGF) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glucagon and recombinant human insulin were obtained from Eli Lilly (Indianapolis, IN, USA), and hydrocortisone sodium succinate was obtained from Pfizer Inc. (Ann Arbor, MI, USA). Concentrated phosphate-buffered saline (10X PBS) was purchased from Lonza (Walkersville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM), sodium pyruvate, nonessential amino acids, fetal bovine serum (FBS), penicillin/streptomycin, and Live/Dead viability/cytotoxicity kit were purchased from Invitrogen (Carlsbad, CA, USA). Cell Proliferation reagent WST-1 was purchased from Roche Ltd, (Basel, Switzerland). Rat albumin ELISA kit was obtained from Bethyl Laboratories (Montgomery, TX, USA) and urea analysis kit was purchased from Stan Bio Laboratory (Boerne, TX, USA). Human HGF antibody and Goat IgG secondary antibody - H&L (HRP) were purchased from Abcam Inc. (Cambridge, MA, USA). Goat anti-rat IgG Texas Red was purchased from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA, USA). Formalin was purchased from Fisher (Pittsburgh, PA, USA). Mounting medium with DAPI was purchased from Vector Laboratories, Inc. (Burlingame, CA, USA).
2.2. Isolation and culture of primary hepatocytes
Hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Boston, MA, USA) weighing 125–200 g, using a two-step collagenase perfusion procedure as described previously [34]. Typically, 100–200 million hepatocytes were obtained with viability > 90% as determined by trypan blue exclusion. Primary hepatocytes were maintained in DMEM supplemented with 10% FBS, 200 U/ml penicillin, 200 μg/ml streptomycin, 7.5 μg/ml hydrocortisone, 20 ng/ml EGF, 14 ng/ml glucagon, and 0.5 U/ml insulin at 37 °C in a humidified 5 % CO2 atmosphere. To induce the spheroid formation of hepatocytes, cells were cultured in hanging drops [35]. Briefly, 20 μL drops containing ~500 cells were placed onto lids of 150 mm dishes, which were inverted over dishes containing 10 ml of PBS. After 2 days, hepatocytes aggregated into spheroids and were collected for encapsulation in hydrogels.
2.3. Preparation of hydrogels and encapsulation of hepatocytes
Heparin-based hydrogels were prepared by Michael-type addition reaction between thiolated heparin (Hep-SH) and diacrylated poly (ethylene glycol) (PEG-DA), as previously reported by us [23]. Heparin-based hydrogels were made by dissolving 40 % thiolated Hep-SH and 6 kDa PEG-DA (1:1 molar ratio of thiol group and acrylate group) in hepatocyte culture medium to make 5, 10, 15, or 20 wt % of gel precursor solutions which were sterilized by filtration. In all cases gelation was completed within 10 min at 37 °C and pH 7.4. More detailed information on the gelation kinetics was reported previously [23].
In order to compare heparin-based hydrogel to another gel of similar physical and mechanical properties but lacking in bioactivity, we prepared PEG hydrogels by Michael addition reaction of tetra-functional poly (ethylene glycol) sulfhydryl (PEG-SH4, 10 kDa) and 3.4 kDa PEG-DA (1:1 molar ratio of thiol group and acrylate group). These PEG precursors were used at the same concentration (10 wt %) as bioactive gesl and were shown by us previously to result in hydrogels with similar mesh size and mechanical properties of the heparin-based hydrogel [29]. HGF-contained hydrogels were prepared by adding HGF solution to both heparin-based and PEG gel precursor solution during gelation, resulting in 1 μg/ml final concentration of HGF.
The hepatocytes – either in the form of single cells or spheroids – were encapsulated into the hydrogel by adding cells to the precursor solution during gelation. The gel precursor solution (50 μL) containing hepatocytes (2 × 106 cells/ml) was poured into a 96 well plate with an ultralow attachment surface (Corning, NY, USA), and incubated for 30 min at 37 °C in a humidified 5 % CO2 atmosphere. These conditions caused formation of the gel layer with encapsulated hepatocytes at the bottom of the microtiter plate. In the next step, the gel layer was immersed in 200 μL/per well of hepatocyte culture medium (described in detail in Section 2.2) and then maintained in a standard cell culture condition up to 20 days. The same procedures were followed for formation of heparin-based and PEG hydrogels. The medium was collected daily and stored at -20 °C for albumin and urea analysis.
2.4. Viability and morphology of encapsulated hepatocytes
The viability of hepatocytes in the hydrogel was determined by double-staining procedure using Live/Dead viability/cytotoxicity kit. Stained cells were imaged by a confocal microscope (Zeiss LSM 5 Pascal, NJ, USA). Cell viability was determined by counting the number of cells that appeared dead (stained red) or live (green), summing the number of cells, and expressing the viability as percent of viable cells. In addition, cell viability was measured by WST-1 assay as discussed below. The changes in morphology of single hepatocytes and spheroids, as well as spheroid diameter were observed daily by optical microscopy (Zeiss Axiovert 40, Carl Zeiss, NJ, USA).
2.5. Proliferation and function of encapsulated hepatocytes
The proliferation of hepatocytes in hydrogel was characterized by WST-1 assay. At each measurement point, WST-1 was added for 4 h, and the colorimetric absorbance of the produced formazan at 450 nm was measured using a microplate reader (Thermo max microplate reader, Molecular Devices, Sunnyvale, CA, USA). Hydrogels containing no cells were used as control. For analysis of hepatic function, culture medium was collected every 24 h and was analyzed using a urea standard kit from StanBio and rat albumin ELISA kit from Bethyl Laboratories. Absorbance was measured with a microplate reader using a 492 nm filter for albumin content and a 570 nm filter for urea content. The sample size for these experiments was n =4.
2.6. In vitro HGF release from the hydrogels
HGF release profiles from heparin-based and PEG hydrogels were compared. In these experiments 10 wt % gel was prepared as described above with 1 μg/ml final concentration of HGF. After gelation, hepatocyte culture media (200 μL) was added as a release buffer and samples were kept at 37 °C in humidified 5 % CO2 incubator to provide the same condition to cell culture. The release medium was replaced with fresh one every day and collected samples were immediately frozen at −20 °C prior to analysis. The HGF released at different time points was analyzed with human HGF ELISA kit from Abcam Inc. using the human HGF antibody (diluted 1:2,000 in blocking solution) and Goat IgG secondary antibody - H&L (HRP) (diluted 1:5,000 in blocking solution). Absorbance was measured using a microplate reader with a 492 nm filter. The sample size was n =3.
2.7. Immunofluorescence staining
For detecting intracellular albumin, hydrogels were sectioned using cryo-sectioning procedure with 1:250 diluted anti-rat serum albumin antibody and 1:100 diluted anti-sheep IgG conjugated with Texas Red. Finally, samples were counterstained with DAPI to determine the location of nuclei. In addition, heparin hydrogel without cells was used as a negative control. Stained cells were visualized and imaged using a confocal microscope.
2.8. Statistical analysis
Data were statistically evaluated by Student’s t-test. The minimum level of significance was set at p < 0.05.
3. Results and discussion
In the present study, heparin-based hydrogel (referred to as heparin hydrogel for simplicity) was employed as a three-dimensional matrix for cultivation of primary rat hepatocytes in the form of single cells or spheroids and in the presence or absence of HGF (see Figure 1). Over the course of three weeks, hepatocyte spheroids entrapped in heparin hydrogels exhibited high levels of albumin and urea synthesis with hepatic function increasing over time whereas hepatocytes entrapped as single cells showed gradual loss of hepatic function. Importantly, inclusion of HGF into the hydrogel reversed the loss of function in single hepatocytes and enhanced hepatic phenotype of hepatocyte spheroids. Overall, heparin hydrogel was found to be an excellent biomaterial for maintenance of functional primary hepatocytes and may be used in the future as a matrix for differentiation of hepatocytes or stem cells in vitro and as a scaffold for transplantation of these cells.
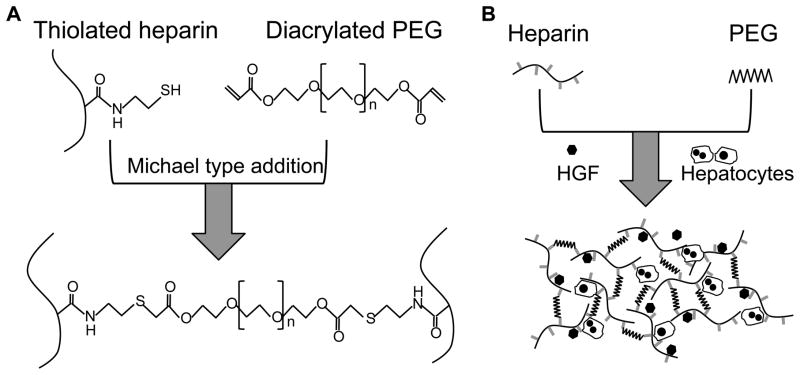
Figure 1.
Diagrams describing gel chemistry and cell cultivation experiments. (A) Heparin gel was formed by Michael addition reaction of thiolated heparin and acrylated poly(ethylene glycol) (PEG). (B) Primary rat hepatocytes were mixed with liquid gel precursors at room temperature and became encapsulated in the gel upon increasing the temperature to 37°C. Hepatocyte growth factor (HGF) was added into the gel precursor solution along with cells.
3.1. Viability and morphology of primary hepatocytes inside of the hydrogels
To examine the compatibility of primary hepatocytes with heparin hydrogel, the viability of encapsulated cells was monitored using live/dead staining and WST-1 assay. As shown with live/dead staining in Figure 2(A–F), the viability of single cells or hepatocyte spheroids exceeded 70% one day after encapsulation whereas viability for both types of hepatocyte cultures was ~20% in PEG hydrogels. A more quantitative analysis of viability, carried out using WST-1 assay (Figure 2G), confirmed superiority of heparin hydrogel in maintaining viable hepatocytes immediately after encapsulation. As seen from Figure 2B, viability of single hepatocytes and spheroids was 71 ± 3% and 86 ± 5% respectively in heparin hydrogels and only ~20 % for both types of hepatocytes entrapped in PEG gel. In the case of hepatocyte spheroid cultures, the spheroid diameter was observed to be similar (236 ± 14 μm) in heparin and PEG hydrogels immediately after encapsulation. However, cell spheroids in PEG gel became deformed after 5 days in culture and disintegrated after two weeks in culture whereas spheroids within the heparin gel remained compact and intact throughout the three week culture period. Beyond measuring cell viability one day after entrapment, we used WST-1 assays to characterize cell-carrying hydrogels throughout the three week culture period and did not observe drop off in viability (Figure 2G). Presence of live hepatocytes is corroborated by liver function analysis data presented in the following sections of this manuscript. The results presented in Figure 2 are significant as they demonstrate hepatocyte viability in heparin hydrogel to be much higher than in PEG hydrogel. Given similar polymerization conditions and physical/mechanical properties of both gel types, the enhanced survival of hepatocytes should be attributed to the presence of bioactive groups in the heparin gel. Our findings of low hepatocyte viability in PEG gel are consistent with a recent report by Underhill et al. who noted poor survival of hepatocytes in PEG gels lacking cell adhesive domains [17].
Figure 2.
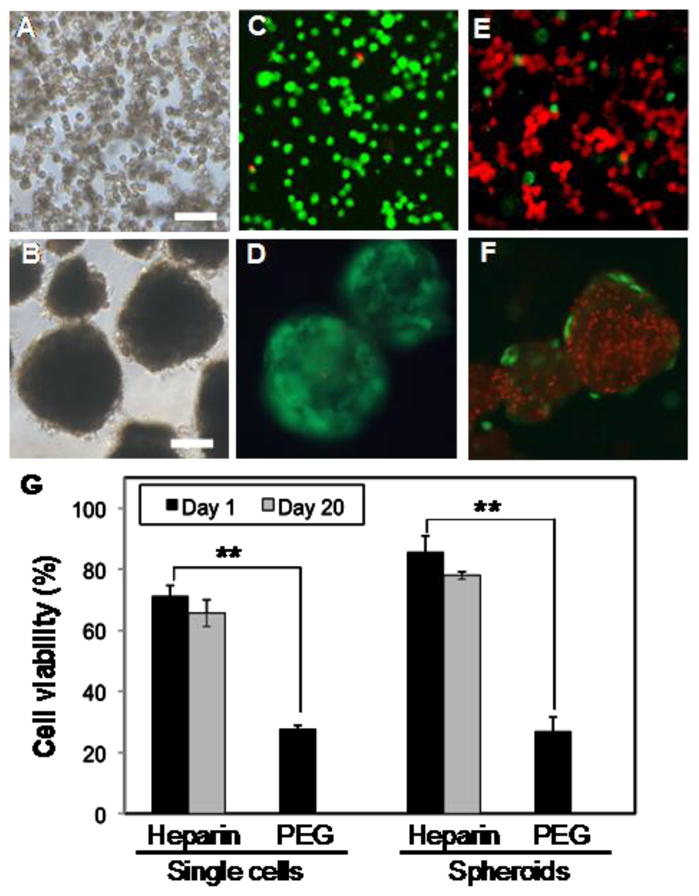
Characterizing viability of primary rat hepatocytes entrapped in heparin and PEG hydrogel. (A–B) Representative brightfield images of hepatocytes in the form of single cells (A) and spheroids (B) entrapped in a gel. Scale bars are 200 μm. (C–D) Live/dead staining of hepatocytes entrapped in heparin hydrogel as single cells (C) and spheroids (D) for one day. Viability exceeded 70%. (E–F) Live/dead staining of hepatocytes entrapped in PEG hydrogel as single cells (E) and spheroids (F) for one day. In both cases viability was ~20%. (G) Confirming hepatocyte viability with WST-1 assay. In both cases of the single cells and spheroids, higher viability was observed for hepatocytes entrapped in heparin hydrogels compared to PEG hydrogels. (**) p<0.001 (n=4).
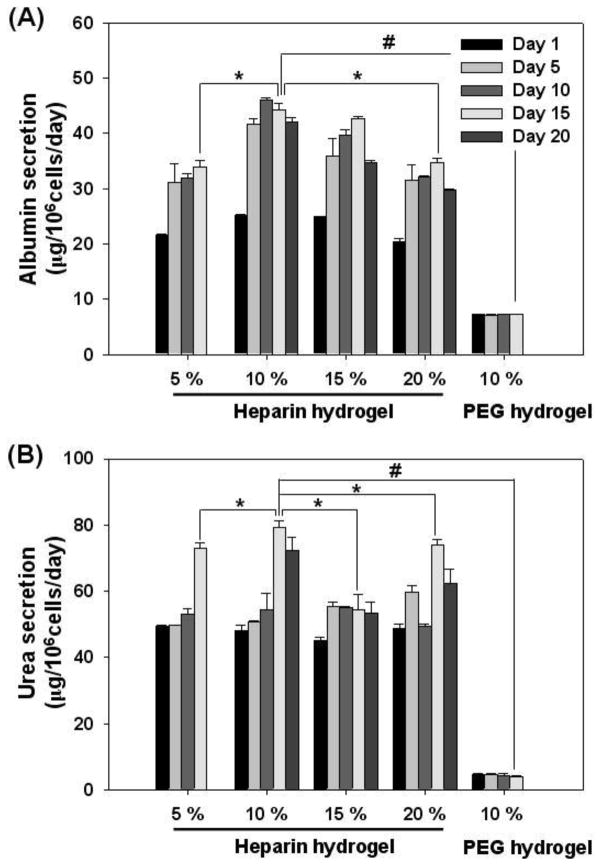
3.2. Effects of the gel concentration on function of hepatocytes
The elasticity or stiffness of the matrix is an important cue for regulating cellular survival, proliferation, and differentiation [5, 14, 36–38]. For instance, while hepatocytes cultured on soft collagen gels maintain differentiated phenotype, hepatocytes cultured on stiff collagen monolayer spread-out, proliferate, and dedifferentiate [39–42]. In the case of gel formation, prepolymer concentration in aqueous medium is a key parameter determining mechanical properties of the resultant hydrogel [23, 43]. Therefore, we prepared heparin hydrogels from precursor solutions of concentrations varying from 5% to 20% and evaluated function of hepatocytes within these hydrogels. In the case of single cell encapsulation, we did not observe significant differences in hepatic phenotype expression as a function of hydrogel concentration (data not shown). However, hepatocyte spheroids did exhibit differences in hepatic function (Figure 3A,B), with spheroids encapsulated inside 10 wt % heparin hydrogel showing higher levels of albumin and urea synthesis. In our previous studies, we determined elastic moduli for 5 and 10 wt % heparin gel to be 680 ± 40 and 2,300 ± 100 Pa respectively in a fully hydrated state [23, 44]. Given that the elastic modulus of the healthy liver was reported to be ~1,500 Pa [43], 10 wt% heparin hydrogel offered a reasonable match to mechanical properties of native tissue. It is therefore not surprising that spheroid encapsulation in 10 wt% heparin gel resulted in highest levels of hepatic phenotype expression. It is also possible that 10 wt% heparin gels had optimal porosity conducive to retention of secreted ECM molecules. This gel concentration was used in all subsequent hepatocyte encapsulation and characterization experiments.
Figure 3.
The effects of heparin gel concentration on function of hepatocyte spheroids. Albumin (A) and urea (B) production of hepatocyte spheroids suggested that 10 wt % heparin hydrogel was optimal. (*) p<0.05 and (#) p<0.0001 (n=4).
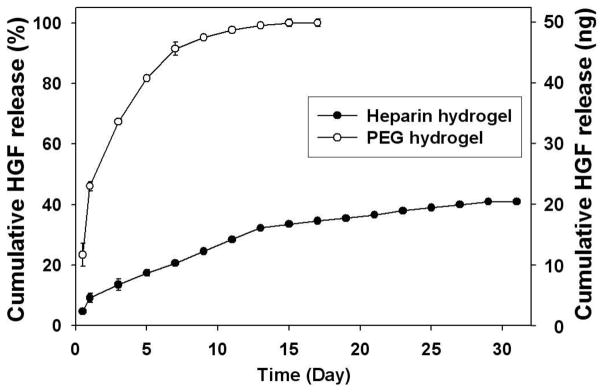
3.3. Controlled release of HGF from heparin hydrogels
GFs provide cues that guide tissue development and regeneration. In particular, HGF has been determined to be a key signaling molecule contributing to embryonic development and regeneration of the liver [32]. Traditionally, GF molecules are added to the cell culture media in soluble form. A number of alternative approaches for delivering GF signals to cells include controlled release from biomaterial scaffolds and solid-phase presentation on culture substrates [15, 29, 45–47]. Previously, our laboratory demonstrated that primary rat hepatocytes cultured on HGF-carrying ECM protein arrays were stimulated from the bottom-up and retained hepatic phenotype after 10 days of cultivation [31]. We also showed that heparin hydrogel could be used for binding (via heparin binding domains) and controlled release of VEGF [28] and hGH [29] due to their specific affinities to heparin. In the liver, heparin modulates binding of HGF to its cell-surface receptor (c-met) and also contributes to HGF-mediated stimulation of in vitro hepatocyte cultures. In addition, hepatic clearance of HGF has been reported to decrease in the presence of heparin suggesting stabilizing effect of HGF-heparin interactions [48]. Given the relevance of heparin-HGF interaction in the liver, we examined incorporation of HGF into heparin hydrogels and the effects of HGF on hepatic phenotype expression.
HGF was incorporated into heparin and PEG hydrogels of similar mesh size [29] and its release was analyzed using ELISA. The hydrogels were incubated in media under cell culture conditions. As shown in Figure 4, while release of HGF from both types of hydrogels showed an initial burst, this burst was significantly lower from heparin hydrogels than PEG gel (10 and 50% for heparin and PEG gel respectively). The presence of heparin binding domains on HGF as well as its net positive charge was expected to promote the sustained release of these molecules from negatively charged heparin hydrogels. Indeed, as seen from Figure 4, HGF in heparin hydrogel showed a much slower release profile compared to PEG hydrogels. Only 40% of HGF was released from heparin hydrogel after four weeks whereas 50% of HGF was released within two days in culture and more than 90% of HGF was released in a week. These results suggest that heparin hydrogel can serve as a reservoir for sequestration and controlled release of HGF. Importantly, association of HGF with heparin occurs via secondary bond formation and does not involve chemical modification steps that may compromise activity of this morphogen.
Figure 4.
In vitro release profile of HGF release from heparin (●) and PEG (○) hydrogels. HGF was mixed at 1 μg/ml concentration with gel precursor solution. More than 60% of HGF was retained in heparin hydrogels after 30 days while PEG hydrogels completely released HGF in 15 days (n=3).
3.4. Culture of primary hepatocytes inside heparin hydrogel in the absence or presence of HGF
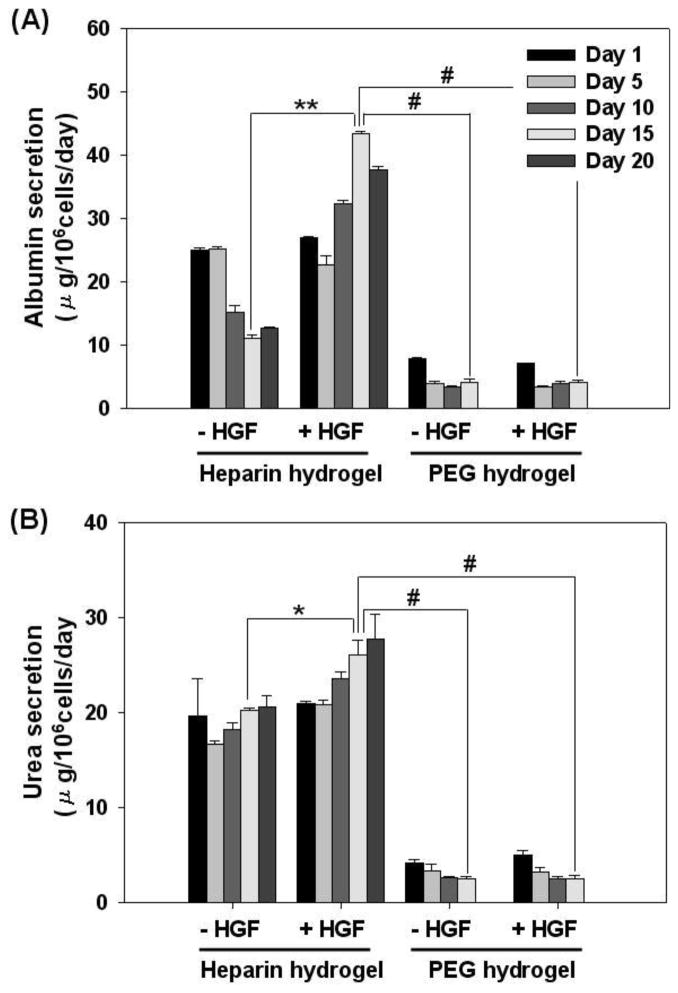
3.4.1. Function of hepatocytes encapsulated in hydrogel as a single cell suspension
Primary hepatocytes are difficult-to-culture cells that lose hepatic function within days of isolation unless specific microenvironment cues are provided in the Petri dish. A number of approaches including cultivation of hepatocytes on ECM-coated substrates, within collagen or Matrgel matrices and in co-culture with stromal cells have been shown to rescue and maintain hepatic phenotype [2, 4, 7, 49, 50]. In the present work, we sought to characterize phenotype maintenance of hepatocytes encapsulated in heparing hydrogel as single cells and spheroids. Furthermore, given the physiological relevance of heparin in sequestering HGF and modulating its activity in the liver, we hypothesized that inclusion of HGF into heparin hydrogels will have synergistic effect on expression of hepatic phenotype. As a control, hepatocytes were also cultured within PEG hydrogel of similar mesh size and modulus of elasticity in order to delineate contributions to hepatic phenotype of mechanical/physical properties and bioactivity of the gel.
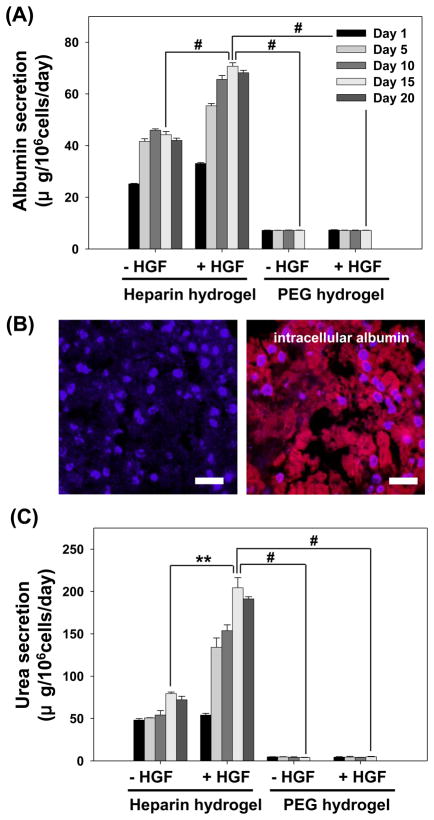
Phenotype of encapsulated hepatocytes was analyzed by monitoring hallmark liver products, albumin and urea, secreted by hepatocytes [4, 18]. As shown in Figure 5A, albumin production of hepatocytes encapsulated in heparin hydrogel as single cells started to decline after day 5 and stabilized by day 15 in culture. The albumin secretion levels ranged from 25 to 10 μg/106 cells/day at early and late time points respectively. Importantly, inclusion of HGF into heparin hydrogel had a profound effect in enhancing albumin synthesis of hepatocytes. The albumin secretion level initially decreased at day 5, but then rose, reaching the maximum at day 15. The albumin levels of 25 – 50 μg/106 cells/day produced by hepatocytes in HGF containing heparin gel was ~ 2 fold higher than the albumin synthesis within heparin gels lacking HGF. In comparison, albumin production by hepatocytes encapsulated in PEG gel was 3 to 5 fold lower compared to heparin gel and was unaffected by the addition of HGF. This lack of change in hepatic function due to incorporation of HGF into PEG gel is in stark contrast to significant upregulation of hepatic albumin synthesis in HGF-containing heparin gel. This suggests that physical incorporation of HGF into the gel matrix is insufficient and that hepatic activity of this morphogen is enhanced by interactions with heparin. This observation is in agreement with reports of GF bioactivity being enhanced by interactions with ECM components [48, 51] as well as our previous work demonstrating enhanced hepatic phenotype in cells cultured on HGF/ECM protein substrates [31].
Figure 5.
The function of primary hepatocytes encapsulated in heparin and PEG hydrogels as single cell with or without HGF. The levels of hepatic production of albumin (A) and urea (B) were significantly higher in heparin hydrogel compared to PEG hydrogel. The addition of HGF to heparin hydrogel further enhanced albumin and urea synthesis of hepatocyte. In contrast, incorporation of HGF into PEG hydrogel had no significant effect on hepatic function. (*) p<0.05, (**) p<0.001, and (#) p<0.0001 (n=4).
Hepatic urea synthesis is an important part of liver metabolism. In vitro, urea synthesis is commonly used to gauge phenotype expression of primary hepatocytes. As seen from Figure 5B, hepatocytes encapsulated within heparin hydrogels synthesized 4 to 6 fold higher levels of urea compared to cells encapsulated in PEG gel. Incorporation of HGF into cell-carrying gels was again observed to upregulate urea production in hepatocytes within heparin hydrogel but had no effect on hepatocytes inside PEG gel.
It should be noted that in addition to hepatic function analysis shown in Figure 5 we monitored hepatic viability using WST-1 assay and found it to be unchanged over the course of three weeks (see Figure 2G). This is expected given the limited proliferation capacity of hepatocytes in vitro. Therefore, changes in albumin and urea synthesis in response to HGF stimulation may be attributed to upregulation of hepatic function.
3.4.2. Function of hepatocyte spheroids cultured within hydrogel
Formation of cell spheroids is thought to better mimic in vivo tissue architecture and has been reported to increase survival and enhance tissue-specific function of multiple cell types including primary hepatocytes [27, 52–57]. In the present study, spheroids (236 ± 14 μm diameter) comprised of primary rat hepatocytes were encapsulated in heparin and PEG hydrogels of comparable mechanical/physical properties and were analyzed for hepatic phenotype expression. As shown in Figure 6, hepatocyte spheroids were highly functional inside heparin hydrogel over the course of three weeks. Unlike single hepatocyte cultures described in the previous section, albumin synthesis in hepatocyte spheroids residing within heparin hydrogel increased by 60 – 70 % by day 5 and stabilized at 45 μg/106 cells/day for the remainder of culture period (Figure 6A). The level of albumin synthesis on per cell basis was 4 fold higher in spheroids than in single cells cultured within heparin hydrogels. Inclusion of HGF into heparin hydrogel upregulated albumin production of the hepatocyte spheroids in comparison to spheroids cultured without HGF. The albumin secretion was 33 μg/106 cells/day at day 1 then reached ~70 μg/106 cells/day by day 10 and was stable until the end of experiment at day 20. Spheroid-carrying heparin hydrogels were sectioned and immunofluorescently stained to investigate distribution of intracellular albumin within the spheroid. As shown in Figure 6B, albumin signal was uniform throughout the spheroid suggesting that hepatocytes in the center of the spheroid did not experience nutrient depravation. The urea synthesis of hepatocyte spheroids followed a similar trend to that of albumin (Figure 6C). The urea production increased to 80 μg/106 cells/day by day 15 and was stable thereafter. Presence of HGF in heparin gel enhanced urea synthesis in hepatocyte spheroids by ~ 2.5 fold. At the same time hepatocyte spheroids in PEG gel produced 10 to 40 fold less of urea and were unaffected by inclusion of HGF into the gel.
Figure 6.
The function of hepatocyte spheroids encapsulated in heparin and PEG hydrogels with or without HGF. The albumin (A,B) and urea (C) synthesis of hepatocyte spheroids was maintained at a high level after 20 days inside heparin hydrogel and was enhanced even further by incorporating HGF into the gel. In contrast, hepatocyte spheroids in PEG gel had much lower albumin and urea production and were unaffected by addition of HGF. (**) p<0.001 and (#) p<0.0001 (n=4). (B) Immunofluorescence staining for albumin in hepatocyte spheroids after 14 days of cultivation in heparin hydrogel. Hepatocytes in heparin hydrogels showed strong staining for albumin (red). Staining of cell nucleus with DAPI (blue) was used to demonstrate localization of albumin in the cytoplasm. All scale bars are 50 μm.
The results presented in Figures 5 and 6 demonstrate that primary hepatocytes encapsulated in heparin hydrogels were highly functional after 20 days in culture. The levels of urea and albumin production of hepatocyte spheroids entrapped in heparin hydrogels were comparable to results reported for hepatocytes cultivated in a collagen gel sandwich - one of the better known methods for long term maintenance of functional hepatocytes.[34, 58] Our data also compare well with hepatocytes cultured in other natural[12, 59–61] and synthetic hydrogels [62]. A particularly exciting feature of our hydrogel is high natural affinity of heparin for a large number GFs and ECM proteins possessing heparin binding domains.[63–65] This obviates the need for covalent attachment of cell-adhesive or cell–stimulatory molecules and opens a range of possibilities for engineering the microenvironment inside the gel. As a step towards designing hepatocellular environment, we incorporated HGF into the gel and demonstrated significantly higher levels of hepatic phenotype expression in GF-containing heparin hydrogels. Although HGF is a mitogen, we did not observe an increase in numbers of single cells or the size of spheroids; therefore, increase in albumin and urea synthesis was attributed to enhanced phenotype expression of existing cells. Ishii et al. reported a similar increase in albumin synthesis of HGF-stimulated hepatocytes and suggested transcriptional activation as the cause of this upregulation [48]. Interestingly, incorporation of HGF in PEG gel of similar physical properties did not affect function of entrapped hepatocytes, suggesting that binding to heparin was important for hepatic activity of HGF. Once again, this observation is in agreement with previous reports of heparin extending/enhancing activity HGF and slowing down hepatic clearance of this morphogen [48].
4. Conclusions
The present paper investigated the use of heparin hydrogel as a matrix for encapsulation and cultivation of primary rat hepatocytes. Analysis of hallmark liver products, albumin and urea, revealed that the heparin hydrogel provided an excellent environment for long-term cultivation of functional primary hepatocytes. Hepatocyte spheroids cultured in the heparin gel were found to be significantly more functional compared to hepatocytes entrapped as single cell suspension. Importantly, heparin hydrogel was shown to serve as a reservoir for sequestration and controlled release of HGF – an important liver morphogen. Inclusion of HGF into heparin hydrogel upregulated albumin and urea production in both single cell and hepatocyte spheroid cultures by 50 to 400% at day 20 in culture. In comparison, incorporation of HGF into PEG hydrogels of comparable mesh size and mechanical properties had no effect on function of encapsulated hepatocytes. While the exact mechanism of HGF-induced hepatic phenotype induction in the hydrogel is unclear, we hypothesize that binding to heparin may protect HGF against proteolytic degradation and may improve bioactivity of this morphogen. Overall, heparin hydrogel has proven to be a promising matrix for cultivation of hepatocytes. Importantly, a large number of GFs possess heparin binding domains and can therefore be incorporated into the hydrogel. We therefore envision future experiments aimed at designing GF-containing heparin hydrogels for directing differentiation of adult hepatocytes, hepatic progenitors or embryonic stem cells.
Acknowledgments
This work was partially supported by the World Class University (WCU) program at GIST through a grant provided by the Ministry of Education, Science and Technology (MEST) of Korea (Project No. R31-20008-000-10026-0) and by the Research Center for Biomolecular Nanotechnology, GIST. This study was also supported in part by NIH grant (R21EB006519) awarded to AR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13(7):880–5. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 2.Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, Yarmush ML. Hepatic tissue engineering for adjunct and temporary liver support: Critical technologies. Liver Transplant. 2004;10(11):1331–42. doi: 10.1002/lt.20229. [DOI] [PubMed] [Google Scholar]
- 3.Allen JW, Bhatia SN. Engineering liver therapies for the future. Tissue Eng. 2002;8(5):725–37. doi: 10.1089/10763270260424097. [DOI] [PubMed] [Google Scholar]
- 4.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuartion. FASEB J. 1996:101471–84. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 5.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes - Control by extracellular-matrix. J Cell Physiol. 1992;151(3):497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 6.Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, et al. Replacement of liver-function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233(4769):1190–2. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 7.Moghe PV, Berthiaume F, Ezzell RM, Toner M, Tompkins RG, Yarmush ML. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials. 1996;17(3):373–85. doi: 10.1016/0142-9612(96)85576-1. [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, et al. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29(1):90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka S, Nakamura T, Ichihara A. Stimulation of growth of primary cultured adult-rat hepatocytes without growth-factors by coculture with nonparenchymal liver-cells. Exp Cell Res. 1987;172(1):228–42. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J Biomed Mater Res. 1997;34(2):189–99. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Jones C, Zern MA, Revzin A. Analysis of local tissue-specific gene expression in cellular micropatterns. Anal Chem. 2006;78(24):8305–12. doi: 10.1021/ac0613333. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Pt A. 2008;14(2):227–36. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 13.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perezatayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediat Surg. 1988;23(1):3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 14.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat Med. 1996;2(7):824–6. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 15.Griffith LG, Naughton G. Tissue engineering - Current challenges and expanding opportunities. Science. 2002;295(5557):1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann PM, Heimrath S, Kim BS, Mooney DJ. Highly porous polymer matrices as a three-dimensional culture system for hepatocytes. Cell Transplant. 1997;6(5):463–8. doi: 10.1177/096368979700600505. [DOI] [PubMed] [Google Scholar]
- 17.Underhill GH, Chen AA, Albrecht DR, Bhatia SN. Assessment of hepatocellular function within PEG hydrogels. Biomaterials. 2007;28(2):256–70. doi: 10.1016/j.biomaterials.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21(3):790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 19.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30):5991–8. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Patel PN, Gobin AS, West JL, Patrick CW. Poly(ethylene glycol) hydrogel system supports preadipocyte viability, adhesion, and proliferation. Tissue Eng. 2005;11(9–10):1498–505. doi: 10.1089/ten.2005.11.1498. [DOI] [PubMed] [Google Scholar]
- 21.Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30(26):4318–24. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67A(4):1430–6. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 23.Tae G, Kim YJ, Choi WI, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8(6):1979–86. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 24.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997:27660–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 25.Kato S, Ishii T, Hara H, Sugiura N, Kimata K, Akamatsu N. Hepatocyte Growth-Factor Immobilized onto Culture Substrates through Heparin and Matrigel Enhances DNA-Synthesis in Primary Rat Hepatocytes. Exp Cell Res. 1994;211(1):53–8. doi: 10.1006/excr.1994.1058. [DOI] [PubMed] [Google Scholar]
- 26.Dudas J, Ramadori G, Knittel T, Neubauer K, Raddatz D, Egedy K, et al. Effect of heparin and liver heparan sulphate on interaction of HepG2-derived transcription factors and their cis-acting elements: altered potential of hepatocellular carcinoma heparan sulphate. Biochem J. 2000:350245–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9–10):1172–84. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 28.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomat Sci-Polym E. 2006;17(1–2):187–97. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 29.Choi WI, Kim M, Tae G, Kim YH. Sustained release of human growth hormone from heparin-based hydrogel. Biomacromolecules. 2008;9(6):1698–704. doi: 10.1021/bm701391b. [DOI] [PubMed] [Google Scholar]
- 30.Kim M, Lee JY, Shah SS, Tae G, Revzin A. On-cue detachment of hydrogels and cells from optically transparent electrodes. Chem Commun. 2009;(39):5865–7. doi: 10.1039/b909169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, et al. Cultivating liver cells on printed arrays of hepatocyte growth factor. Biomaterials. 2009;30(22):3733–41. doi: 10.1016/j.biomaterials.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119(4):591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 33.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth-factor - from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129(5):1177–80. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn JCY, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989:3174–9. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 35.Del Duca D, Werbowetski T, Del Maestro RF. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol. 2004;67(3):295–303. doi: 10.1023/b:neon.0000024220.07063.70. [DOI] [PubMed] [Google Scholar]
- 36.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47(4):1394–400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 37.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 39.Fassett J, Tobolt D, Hansen LK. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2006:17345–56. doi: 10.1091/mbc.E05-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen LK, Wilhelm J, Fassett JT. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr Top Dev Biol. 2006:72205–36. doi: 10.1016/S0070-2153(05)72004-4. [DOI] [PubMed] [Google Scholar]
- 41.Semler EJ, Moghe PV. Engineering hepatocyte functional fate through growth factor dynamics: The role of cell morphologic priming. Biotechnol Bioeng. 2001;75(5):510–20. doi: 10.1002/bit.10113. [DOI] [PubMed] [Google Scholar]
- 42.Ng S, Wu YN, Zhou Y, Toh YE, Ho ZZ, Chia SM, et al. Optimization of 3-D hepatocyte culture by controlling the physical and chemical properties of the extra-cellular matrices. Biomaterials. 2005;26(16):3153–63. doi: 10.1016/j.biomaterials.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Sharma N, Chippada U, Jiang X, Schloss R, Yarmush ML, et al. Functional modulation of ES-derived hepatocyte lineage cells via substrate compliance alteration. Anna Biomed Eng. 2008;36(5):865–76. doi: 10.1007/s10439-008-9458-3. [DOI] [PubMed] [Google Scholar]
- 44.Kim M, Shin Y, Hong B, Kim YJ, Chun JS, Tae G, et al. In vitro chondrocyte culture in a heparin-based hydrogel for cartilage regneration. Tissue Eng Pt C-Meth. 2009 doi: 10.1089/ten.TEC.2008.0548. [DOI] [PubMed] [Google Scholar]
- 45.Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, et al. Functional immobilization of signaling proteins enables control of stem cell fate. Nature Methods. 2008;5(7):645–50. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- 46.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–34. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Kitajima T, Terai H, Ito Y. A fusion protein of hepatocyte growth factor for immobilization to collagen. Biomaterials. 2007;28(11):1989–97. doi: 10.1016/j.biomaterials.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Ishii T, Sato M, Sudo K, Suzuki M, Nakai H, Hishida T, et al. Hepatocyte growth-factor stimulates liver-regeneration and elevates blood protein level in normal and partially hepatectomized rats. J Biochem. 1995;117(5):1105–12. doi: 10.1093/oxfordjournals.jbchem.a124814. [DOI] [PubMed] [Google Scholar]
- 49.Bhandari RN, Riccalton LA, Lewis AL, Fry JR, Hammond AH, Tendler SJ, et al. Liver tissue engineering: a role for co-culture systems in modifying hepatocyte function and viability. Tissue Eng. 2001;7(3):345–57. doi: 10.1089/10763270152044206. [DOI] [PubMed] [Google Scholar]
- 50.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 51.Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor-beta binds collagen IV of basement membrane matrix: Implications for development. Dev Biol. 1991:143303–8. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Absi SF, Hansen LK, Hu W-S. Three-dimensional co-culture of hepatocytes and stellate cells. Cytotechnology. 2004;45(3):125–40. doi: 10.1007/s10616-004-7996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat-liver cells - histotypic reorganization, biomatrix deposition, and maintenance of functional activities. Journal of Cell Biology. 1985;101(3):914–23. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu FJ, Friend JR, Hsiao CC, Zilliox MJ, Ko WJ, Cerra FB, et al. Efficient assembly of rat hepatocyte spheroids for tissue engineering applications. Biotechnology and Bioengineering. 1996;50(4):404–15. doi: 10.1002/(SICI)1097-0290(19960520)50:4<404::AID-BIT7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, et al. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49(2):578–86. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park KH, Na K, Kim SW, Jung SY, Park KH, Chung HM. Phenotype of hepatocyte spheroids behavior within thermo-sensitive Poly(NiPAAm-co-PEG-g-GRGDS) hydrogel as a cell delivery vehicle. Biotechnology Letters. 2005;27(15):1081–6. doi: 10.1007/s10529-005-8453-0. [DOI] [PubMed] [Google Scholar]
- 57.Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnology and Bioengineering. 2002;78(3):257–69. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 58.Dunn JCY, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991:7237–45. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 59.Seo SJ, Choi YJ, Akaike T, Higuchi A, Cho CS. Alginate/galactosylated chitosan/heparin scaffold as a new synthetic extracellular matrix for hepatocytes. Tissue Engineering. 2006;12(1):33–44. doi: 10.1089/ten.2006.12.33. [DOI] [PubMed] [Google Scholar]
- 60.Seo SJ, Akaike T, Choi YJ, Shirakawa M, Kang IK, Cho CS. Alginate microcapsules prepared with xyloglucan as a synthetic extracellular matrix for hepatocyte attachment. Biomaterials. 2005;26(17):3607–15. doi: 10.1016/j.biomaterials.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 61.Seo SJ, Kim IY, Choi YJ, Akaike T, Cho CS. Enhanced liver functions of hepatocytes cocultured with NIH 3T3 in the alginate/galactosylated chitosan scaffold. Biomaterials. 2006;27(8):1487–95. doi: 10.1016/j.biomaterials.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007:21790–81. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 63.Vlodavsky I, Miao HQ, Medalion B, Danagher P, Ron D. Invovlement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer and Metastasis Reviews. 1996;14(2):177–86. doi: 10.1007/BF00437470. [DOI] [PubMed] [Google Scholar]
- 64.Bashkin P, Doctrow S, Kagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin like molecules. Biochemistry. 1989;28(4):1737–43. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- 65.Ashikari-Hada S, Habuch H, Kariya Y, Itoh N, Reddi AH, Kimata K. Characterization of growth factor-binding structures in heparin/haparan sulfacte. J Bio Chem. 2004;279(13):12346–54. doi: 10.1074/jbc.M313523200. [DOI] [PubMed] [Google Scholar]