Abstract
To successfully interact with their environment, developing organisms need to correctly process sensory information and generate motor output appropriate to their size and structure. Patterned sensory experience has long been known to induce various forms of developmental plasticity which ultimately shape mature neural circuits. Yet these same types of plasticity also allow developing organisms to respond appropriately to the external world by dynamically adapting neural circuit function to ongoing changes in brain circuitry and sensory input. Recent work on the visual system of frogs and fish has provided an unprecedented view into how visual experience dynamically affects circuit function at many levels, ranging from gene expression to network function, ultimately leading to system-wide functional adaptations.
Patterned visual activity and the developing visual system: an overview
The survival of an organism depends on its ability to successfully interact with the environment, which for a young animal typically begins before its nervous system has completed development. The immature nervous system, though considerably different from the adult brain, is obviously capable of providing essential functions for the animal to thrive 1. However, as development proceeds and the organism grows, the nervous system undergoes profound activity-dependent and independent changes in both its neural circuit architecture and the electrical properties of individual neurons within those circuits. This type of developmental plasticity has traditionally been invoked to explain how the adult brain comes to be sculpted from the immature circuit over development. In the short-term, however, plasticity also mediates important adaptive changes that help developing organisms to respond appropriately to the environment in the face of their own ongoing changes.
The vertebrate visual system provides one of the best-studied examples of the role of sensory experience in circuit development 2. Starting with seminal discoveries by Hubel and Wiesel 3, a long history of increasingly sophisticated experiments have implicated spatiotemporal patterns of neural activity, arising either spontaneously or through visual stimulation in the refinement of several fundamental aspects of mammalian visual system organization, including proper retinotopy, segregation of monocular inputs and stimulus selectivity 4-9. Visual activity and patterned spontaneous activity are believed to guide this process through the induction of various forms of use-dependent plasticity —such as long-term potentiation and depression and synaptic scaling— which, over development, gradually shape the functional organization of the visual system 10-12. However, it is also becoming evident that the visual system can rapidly adapt to changes in the visual environment to maintain stable function 13-15. Some of the same plasticity mechanisms implicated in development are also important for these functional adaptations, although some novel ones may be also involved.
Here we review several recent studies describing the role of patterned retinal activity on the functional properties of the visual systems of developing frogs and fish, mainly in Xenopus tadpoles and zebrafish embryos. These studies have helped elucidate general principles that probably extend to a variety of sensory systems across vertebrate species. External fertilization and development makes fish and frogs particularly amenable to early surgical and genetic manipulations, including transplantation, dye labeling and transfection of single cells. This has led to their increasing popularity for in vivo imaging and electrophysiological studies during embryo-nic development. The main visual center in these animals is the optic tectum, which receives direct input from the retina. The output of the tectum is directly related with the activation of visually-guided behaviors 16-18, and thus it is an ideal preparation in which to study the functional development of neural circuits. Much is known about the development of the retinotectal projection, which is organized topographically such that neighboring cells in the retina project to neighboring sites in the tectum 19. Initial development of topography in this projection depends on an array of molecular cues, but its precise refinement is believed to require neural activity 20, 21 (see Textbox 1).
Box 1. Patterned retinal activity and the wiring of the developing visual system in frogs and fish.
Visual experience participates in the structural refinement of the retinotectal projection through activity-dependent mechanisms. Is activity permissive or instructive for map plasticity? An anatomical screen for retinotectal projection defects in zebrafish 91, 92 has revealed several lines in which defects in neural activity or synaptic transmission are associated with abnormal development of the retinotectal projection 24, 93. However these experiments did not distinguish between permissive and instructive roles of patterned activity.
A key aspect of instructive plasticity is that spatiotemporally patterned activity in the inputs provides the information necessary to direct the stabilization or elimination of specific connections. This may be achieved by Hebbian mechanisms in which cells with similar firing patterns should consolidate their connections while cells with mismatched firing patterns disconnect from one another. In mammals much of this patterned activity is generated spontaneously in the form of retinal waves before eye opening takes place. In frogs and fish the retina is already able to transmit visual information by the time RGC axons first innervate the tectum 94, permitting experiments that examine an instructive role of activity. When inputs across the entire retina are artificially correlated during development by strobe-rearing goldfish, largely eliminating instructive cues from retinal firing patterns, RGC axon arbors and receptive fields fail to refine normally 95. Further evidence for an instructive role of neural activity comes from experiments demonstrating that pharmacological blockade of NMDA-type glutamate receptors in developing zebrafish drives an expansion of the RGC terminal arbor coverage area, suggesting that correlation detection through NMDA receptors rather than the overall activity level may be required for axonal refinement 96. Experiments in frogs have also shown that the retinotopic precision of axonal terminations is degraded if NMDARs are blocked in the mature tectum 97, consistent with the idea that the same mechanisms mediating map refinement probably continue to maintain the map long after it is initially established.
The formation of eye-specific bands in the tecta of fish and frogs experimentally manipulated to have binocular innervation of the same tectal lobe constitutes the strongest evidence for an instructive role of neural activity in circuit development. In these experiments, inputs from the two eyes segregate into a unique alternating ocular dominance pattern across the tectum that compromises between the constraints imposed by topographic molecular cues and activity-dependent mechanisms 98-100. This segregation, which is dependent on neural activity in RGCs 101, 102 and activation of tectal NMDA receptors 103, involves a process of stochastic extension of new branches along axons and their selective elimination from territories dominated by inputs from the other eye 104. Taken together these studies are consistent with a model where correlated activity activates postsynaptic NMDARs, resulting in stabilization of the co-active inputs via Hebbian modifications, while inputs that are not co-active are selectively eliminated.
On the other hand, more recent studies also support the existence of non-Hebbian forms of competition. Selectively silencing individual RGCs (by transfecting them early in development with an inwardly-rectifying leak K+ channel subunit) results in relatively smaller and less branched terminal arbors of those neurons 105. This effect is blocked if the entire eye is silenced with TTX, showing that competition among axons is necessary to cause retraction of the less active input. In a converse experiment, single RGCs were implanted in a zebrafish lakritz mutant lacking endogenous RGCs. In this experiment the axon from the single RGC grew into an appropriate tectal location, but its terminal arbor was expanded as it had no neighboring axons to compete with 106. These experiments reveal the participation of diverse forms of competition in the refinement of the retinotectal projection.
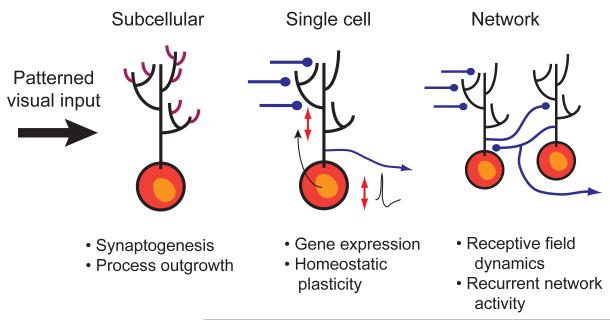
While there is ample evidence that patterned visual activity is important for establishing the fine structure of the central visual pathways, there is an increasing body of evidence suggesting that patterned activity is also important for fine-tuning the functional properties of the developing visual system. During the developmental time period in which the retinotectal map is being established and refined, both Xenopus tadpoles and zebrafish embryos are able to see and behave appropriately in response to visual stimuli 22-24Thus, the visual system must be able to continuously adapt its response properties to respond appropriately to the visual environment, even while the neural circuitry responsible for visual processing is rapidly changing. Patterned visual activity plays an important role in instructing these adaptive changes which occur at many levels; these range from synaptogenesis and process outgrowth, regulation of gene expression, homeostatic adaptations of dynamic range, receptive field properties and temporal response characteristics of the tectal network (Figure 1). Each of these levels will be reviewed in turn.
Figure 1. Rapid functional modulation of the retinotectal circuit by visual experience.
Although neural activity is known to be critical for the gradual sculpting of neural circuits over development, there is ample evidence that patterned activity also induces rapid functional adaptations which may allow for the optimization of neural circuit function in response to a changing environment and neuronal architecture. In this article we review recent studies from the visual system of frogs and fish that show that these adaptations can occur at many levels of function in the optic tectum, the principal visual area in the brains of these species. (A) On the subcellular scale visual activity can induce rapid changes in optic tectal neurons which involve synaptogenesis and process outgrowth. This provides a substrate for rapid experience-dpendent plasticity. (b) At the single cell level visual experience triggers gene expression which can have cell-wide effects, particularly in altering dendritic structure. It can also allow homoestatic adaptations in synaptic transmission and intrinsic excitability which can normalize the input-output properties of tectal neurons. (C) At the level of neural circuits, visual experience can modify receptive field properties of tectal neurons as well as the temporal activation pattern of recurrent circuits within the tectum.
Patterned Activity Leads to Dynamic Synaptogenesis and Process Outgrowth
A remarkable amount of neural circuit remodeling occurs late in development — for instance, synapse density in the mammalian visual cortex is known to peak shortly after birth, but is subsequently reduced by intensive synaptic pruning 25, 26. The ability to at least crudely process sensory information with circuits that are still in the process of undergoing developmental change is a common challenge across species. Fish and amphibians, for example, exhibit extensive neurogenesis well into maturity, and this leads to the continual expansion and distortion of topographically organized neural maps. The retina, which adds new neurons at the margin nearest the lens, expands radially, whereas the target of RGC axons, the optic tectum, expands by adding new cells linearly along its periventricular proliferative zone. One consequence of this ongoing neurogenesis is the need for all RGC axonal inputs to the tectum to shift their termination zones gradually over time as the structures expand in order to maintain a relatively evenly-spaced retinotopic map 27, 28. To maintain optimal function, the synaptic contacts formed between the eye and the brain are thus obliged to dissolve and reform at new sites throughout the life of the animal.
Early in vivo imaging studies in both zebrafish and Xenopus systems revealed the RGC axon terminal and the tectal dendritic arbor to be remarkably dynamic structures, even in animals actively relying on visual function to guide their behaviors 29, 30. Subsequent experiments revealed that the motility of axons and dendrites was indeed not independent of visual function, but instead directly modulated by visually driven synaptic activity 31-33. The use of targeted expression of GFP-tagged synaptic proteins in live imaging, including GFP-synaptobrevin and synaptophysin-GFP to reveal clusters of presynaptic vesicles in axons 34-36, and PSD95-GFP to visualize the postsynaptic density in dendrites 37, 38, lent support for the idea that synapses were being rapidly assembled and disassembled within the functioning, but immature, retinotectal projection.
An influential way of thinking about the developmental interplay between synaptogenesis and projection stability is the “Synaptotropic Model”, first put forth by Vaughn 37, 39. This model proposes that stabilizing interactions at synaptic contacts could modulate the intrinsic tendency of cells to branch and form new synapses in such a way as to bias connectivity. Indeed considerable evidence in the retinotectal system supports the notion that synapse maturation, as measured by increased accumulation of presynaptic vesicles at presynaptic sites 35, 40 or by addition of AMPA receptors to postsynaptic synapses 41, can both confer additional stability onto axonal and dendritic arbors at these sites, but also enhance local branching of these processes34, 36, 37, resulting in activity-regulated, local control of arbor elaboration through synaptic modulation.
Control of Gene Expression by Visual Experience
Plasticity induced by visual experience and synaptic activation does not only impact activated synapses. Neural activity is one of the major regulators of nuclear transcription in neurons, largely through the activation of voltage-gated calcium channels and calcium-dependent transcription factors. Several such transcriptional regulators have been shown to directly modulate synaptic or structural properties of developing neurons 42-45.
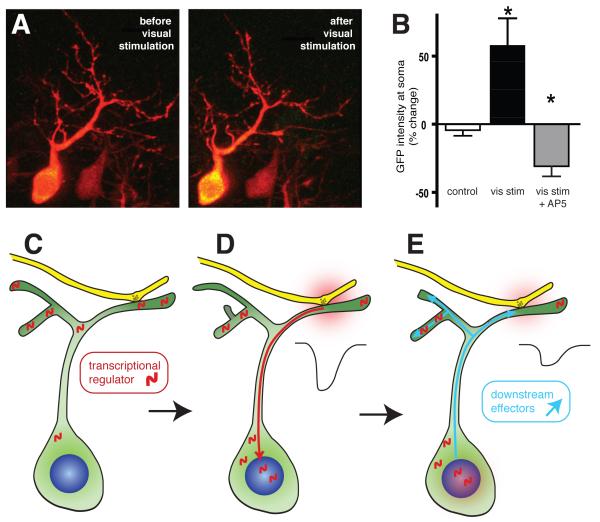
The calcineurin-activated transcriptional regulator Nuclear Factor in Activated T-Cells (NFAT) is implicated in the regulation of both activity-dependent structural remodeling of dendrites and in the regulation of synaptic maturation in the developing Xenopus visual system 46. Low-frequency patterned visual stimulation drives the translocation of NFAT from dendrites into the nucleus of tectal neurons where it engages a transcriptional program that limits synaptic maturation and reduces neural activity-dependent dendritic branch extension (Figure 2). Importantly, inhibition of NFAT in tectal neurons not only enhances the cell-wide rates of synapse maturation and branch formation, but also modulates how the cell responds to activity changes at synaptic inputs, effectively reprogramming the cell with a new set of plasticity rules based on transcription driven by the cell’s prior neural activity. Thus, neurons can respond differently to the same activity patterns depending on their recent transcriptional history.
Figure 2. Activity-dependent regulation of gene transcription by NFAT alters dendritic and synaptic development.
In Xenopus tadpoles, visual stimulation causes the transcriptional regulator NFAT to translocate toward the nucleus where it drives changes in the expression levels of a number of plasticity-associated gene products. (A) Expression of NFAT tagged with EGFP (green) in tectal neurons, co-expressing cell-filling tdTomato to visualize dendritic morphology, reveals an increase in NFAT-GFP fluorescence in the cell soma and nucleus with a concommitant decrease in dendritic intensity following 40min of continuous visual stimulation. (B) The nuclear translocation of NFAT-GFP in response to visual stimulation requires synaptic activation of NMDA receptors. Blocking NMDA receptors prevents this translocation, even causing a decrease in nuclear NFAT-GFP levels, suggesting that basal synaptic transmission may be sufficient to drive an intermediate level of NFAT activation. (C-E) These data suggest that dendritic stores of NFAT, perhaps associated with synapses, translocate to the nucleus in response to NMDAR activation. At the nucleus NFAT regulates expression levels of gene products that control both dendritic branching and synaptogenesis or synapse maturation. Blocking NFAT activation increases both branching and mEPSC frequency. Data adapted from Schwartz et al., 2009.
Numerous screens for activity-regulated genes have resulted in long lists of candidate plasticity genes that increase expression within minutes of neuronal activation. Many of these turn out to play critical roles in development and plasticity. The ease of live imaging and patch clamp physiology in the Xenopus retinotectal system has permitted several striking in vivo demonstrations of how activity-regulated genes impact neural circuit development and function. For example, CPG15, also known as neuritin, was originally identified as part of a subtraction screen for cDNAs differentially expressed by neuronal activation of rat dentate gyrus 47. Over-expression of CPG15 in the tadpole optic tectum caused a dramatic increase in the elaboration of tectal neuron dendrites and presynaptic axonal arbors 48, 49. These changes were accompanied by enhanced synaptic maturation revealed by the recruitment of AMPA receptors to retinotectal synapses. Another plasticity-associated gene rapidly up-regulated by visual stimulation in the developing optic tectum is the short form of Homer (Homer 1a) 50. In contrast to the increase in synaptic efficacy at AMPARs driven by CPG15, the visual stimulation-induced increase in Homer 1a expression was found to prevent the increase in AMPAR-mediated transmission in tectal neurons that normally occurs when mGluR1 receptors are activated.
Brain derived neurotrophic factor (BDNF) is implicated in activity-dependent developmental plasticity and is robustly regulated at the level of transcription 51-53, translation 54, and release 55. A series of experiments by Cohen-Cory and colleagues using in vivo time lapse imaging in developing Xenopus tadpoles has demonstrated that BDNF in the optic tectum, signaling through TrkB receptors on RGC axons, positively regulates the density of GFP-synaptobrevin clusters, a marker of synapses used for live imaging, along the axon terminals 34, 56. At the same time, BDNF rapidly stabilizes RGC axon branches, thereby resulting in more complex arbors with more potential synaptic contacts 57, 58. Effects on the density of postsynaptic sites were also observed by imaging PSD95-GFP, however these appeared to be subsequent to the presynaptic changes 38. Consistent with these results, application of exogenous BDNF to the optic tectum results in a rapid, presynaptically-mediated increase in the EPSC evoked in tectal neurons by RGC stimulation 59. Dendritic trafficking of BDNF mRNA for local protein synthesis is thought to be important for BDNF-dependent synaptic plasticity in the hippocampus 54 although this has not yet been demonstrated in the retinotectal system. However, proper function of the RNA binding protein CPEB in tectal neurons has been shown to be critical for the development of efficient synaptic transmission in Xenopus 60. Thus, CPG15, Homer and BDNF exemplify gene products whose expression is regulated by and in turn regulates the response of the circuit to sensory stimuli. It is likely that this feedback property of activity-regulated genes constitutes a fundamental mechanism for the important homeostatic control of neuronal excitability.
Homeostatic Changes in Functional Response Properties
In order for neurons to maximize the dynamic range of their firing rates, they must be able to adapt their spike output to account for changing levels of synaptic input 61, 62. Neurons can achieve this homeostatic adaptation by at least two different strategies. In the first, neurons can adjust the strength of all of their synaptic inputs equally, so as to normalize the overall level of synaptic activity while maintaining the relative differences between different synaptic weights. This process is known as synaptic scaling 63. In the second strategy, neurons can adjust their own intrinsic excitability, to regulate the amount of spike output in relation to the total level of synaptic input they receive 64. Much of the initial data describing these phenomena was derived from in vitro experiments in which the overall levels of neuronal activity were manipulated either pharmacologically or genetically, and resulting changes in synaptic strength and intrinsic excitability were measured after several days 63, 65, 66. However, more recently, these phenomena have also been measured in vivo in a variety of systems and different time scales 15, 67-70.
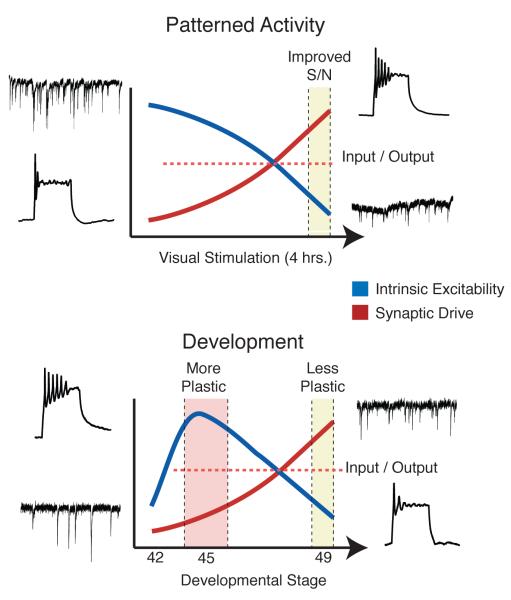
In Xenopus tadpoles, both processes have been described and are known to be modulated by visual activity both in short and long time scales (Figure 3). Developing tectal neurons have a large proportion of AMPA-type glutamate receptors (AMPAR) lacking the GluR2 subunit; this renders them permeable to Ca++ and sensitive to modulation by polyamines (PAs)71. PAs are positively-charged molecules which bind to Ca++-permeable AMPAR in a voltage-dependent manner, resulting in inward rectification and a reduction in synaptic currents even at the resting membrane potential of the cell 72. The synthesis of PAs is regulated by neural activity, primarily through changes in expression of ornithine decarboxylase, the rate limiting enzyme of the PA synthesis pathway 73. This was demonstrated in a series of experiments in which Xenopus tadpoles were exposed to a four-hour period of enhanced, patterned visual stimulation. Exposure to patterned visual activity resulted in an increase in PA synthesis, which in turn resulted in an overall reduction in AMPAR-mediated synaptic transmission 71. Furthermore, the same manipulation also caused an increase in the intrinsic excitability of tectal neurons, expressed as an increase in voltage-gated Na+ current, and this increase required the PA-mediated decrease in synaptic transmission, but it was not mediated directly by PAs 74. As a result, these homeostatic adaptations allowed tectal cells to reduce their activation by background neural activity, but enhance their responses to visually-evoked events, resulting in an enhanced signal-to-noise ratio.
Figure 3. Homeostatic regulation of synaptic transmission and intrinsic excitability in Xenopus tadpole tectal neurons.
Tectal cells are known to adjust their intrinsic excitability in order to maintain a broad dynamic range in response to changes in levels of synaptic input. Both short periods of patterned visual input as well as overall activity levels during development can trigger these changes, which are expressed as changes in voltage-gated Na+ currents. In (A), freely-swimming tadpoles were exposed to 4 hours of enhanced, patterned visual stimulation. This resulted in an overall decrease in spontaneous excitatory synaptic transmission caused by enhanced blockade of Ca++-permeable AMPA receptors by polyamines. As a consequence of this decrease, the amplitude of voltage-gated Na+ currents was enhanced, increasing the intrinsic excitability of tectal cells. This combination of synaptic and intrinsic changes allows tectal cells to filter out noisy background stimuli, while enhancing stimulus sensitivity to more salient visual stimuli. In (B), the relationship between background spontaneous excitatory synaptic input and voltage-gated Na+ currents was measured over development. Between developmental stages 45 and 49, tectal neurons undergo a period of rapid growth and synaptic maturation. Starting from stage 45 tectal neurons show enhanced excitability and low levels of background excitatory synaptic input. By stage 49, the amount of synaptic input dramatically increases. This results in a homeostatic downregulation of voltage-gated Na+ currents, resulting in decreased intrinsic excitability. Both of these example illustrate how tectal neurons can dynamically adapt their intrinsic properties in response to changes in overall levels of synaptic drive. Figure based on findings from Aizenman et al. 2002, 2003 and Pratt et al. 2007.
A similar relationship between synaptic transmission and intrinsic excitability is observed during development68. Between developmental stages 45 and 49, during which the retinotectal map is being established and refined, there is an inverse relation between synaptic transmission and intrinsic excitability in tectal neurons. During this time, the net amount of excitatory synaptic drive received by tectal neurons increases significantly. Concurrently, the intrinsic excitability of tectal cells decreases, and this decrease is mediated by changes in voltage-gated Na+ currents. The result is that the input-output properties of the cell remain stable; when synaptic drive is weak, cells are most excitable, whereas when synaptic drive is strong, cells are less excitable. Genetically decreasing excitatory synaptic transmission during development prevents the observed decrease in intrinsic excitability, suggesting that the mechanisms are linked. Thus, tectal neurons are able to homeostatically maintain a wide dynamic range by altering their intrinsic excitability through the adjustment of voltage gated Na+ currents. This can occur gradually over development, or more rapidly in order to adapt to changing input characteristics.
Receptive field properties are dynamically regulated
Sperry’s chemoaffinity hypothesis posits that guidance cues with orderly distributions in the optic tectum guide retinal axons to their proper synaptic partners along the dorsoventral and mediolateral axes75. While the fundamental tenets of this model are supported by molecular biological evidence, any complete description must also incorporate the nuances of activity-dependent developmental map refinement and receptive field (RF) plasticity 20, 76. During normal development in frogs and fish the tectum is much smaller than its adult size. As a result during tectal development there is a massive topographic sorting out of axons that are initially extensively overlapping in the smaller immature tectum 30, 77. As the tectum grows, these terminals occupy a smaller percentage of the tectum, leading to connection refinement that inevitably contributes to RF refinement. However more recent experimental evidence reveals that activity-dependent synaptic plasticity mechanisms also have a role to play in sculpting RFs.
Taking advantage of the transparency of zebrafish larvae, Niell and co-workers carried out systematic mapping of RF properties in developing optic tecta bulk-loaded with the cell permeant calcium indicator Oregon Green BAPTA 1-AM, permitting dozens of tectal neurons to be characterized simultaneously for their responses to a range of visual stimuli78. They observed that from the earliest stages at which the lens forms a sharp image on the retina (70 hours post-fertilization) cells exhibited stimulus selectivity that closely resembled those in mature fish including direction selectivity, responsiveness to moving spots and spots flashed in the RF. However a modest increase in selectivity for all of these features was reported with age, most notably a more reliable response evoked by small spots within the RF, indicative of improved visual acuity.
In agreement with these findings, whole-cell recordings of visually evoked synaptic potentials, made in Xenopus tectal neurons at different stages of development similarly revealed an incremental improvement in visual acuity in older animals 22, 79. Interestingly dark rearing had little impact on the development of these properties in zebrafish, although visual acuity was slightly reduced, suggesting that they may either develop as part of an intrinsic program or that spontaneous activity is able to instruct the development of the circuit. In support of a role for activity, disrupting normal sensory activity in Xenopus tadpoles either by rearing under conditions of intensive visual stimulation or by pharmacologically disrupting normal synaptic transmission through NMDARs or GABARs did abrogate the improvement in visual acuity normally observed. Furthermore, blumenkohl mutant zebrafish, which have defects in release of neuro-transmitter, exhibit both larger RGC terminal arbors, expanded RFs and reduced visual acuity compared with wildtype fish 24.
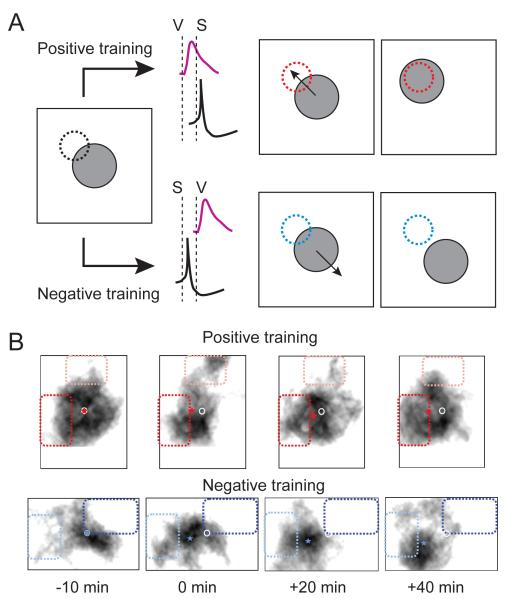
The strongest evidence to support a role for visual experience in the development of RF properties comes from experiments in which specific patterns of retinotectal activation or visual stimulation are applied to drive predictable changes in the RF. Retinotectal synapses in Xenopus tadpoles exhibit spike timing-dependent long-term potentiation and depression 80. By carefully timing the firing of an action potential in a tectal neuron with respect to the arrival of visually evoked EPSPs in a conditioning stimulus designed to induce either potentiation or depression, Vislay-Meltzer and colleagues were able to effectively remodel the shape of receptive fields of tectal neurons in tadpoles (Figure 4)81. In addition to simple properties like visual field location, conditioning stimuli have also been used to modulate more complex properties like the direction selectivity of tectal neurons, which involves both retinotectal inputs and intrinsic tectal circuitry 23. Training by repeated presentation of directional stimuli modifies the responses of tectal neurons such that cells shift their preference to fire more vigorously when presented with bars sweeping in the trained direction 82, 83.
Figure 4. Patterned visual activity can dynamically alter the spatial properties of tectal neuron receptive fields.
While visual activity-induced synaptic plasticity has been long believed to play a role in refinement of retinotopic maps, this same type of plasticity can also rapidly alter the functional response properties of visual neurons, including retinotopy. In this example Engert and colleagues use a spike-timing dependent plasticity (STDP) protocol to alter the spatial location of tectal cell receptive fields in Xenopus tadpoles. (A) Training protocol for inducing (STDP) of receptive field location. After rapidly mapping the receptive field of a tectal neuron from which whole-cell recordings are being performed, either a positive or a negative training protocol was administered. In the positive training, an area of visual space on the edge of the receptive field was activated to induce a visual stimulus. In the diagram the receptive field is indicated by the gray circle and the training area by the dotted circle. A few milliseconds after the visual input activated the tectal cell, the cell was briefly depolarized to generate an action potential. Based on a STDP rule where the visual input (V) occurs before the spike (S), this would result in potentiation of the visual inputs on the edge of the receptive field, and would eventually cause a shift of the receptive field center towards the training area. In the negative training protocol, the neuron is made to spike a few milliseconds before the visual input arrives (S before V). This would result in depression of the visual input and a shift in the receptive field center away from the training area. (B) Representative data showing the results of positive and negative conditioning. The black blob is the map of the visual receptive field. The darker dotted areas represent the training areas. The white circle is the original receptive field center and the star represents the receptive field center during and after conditioning. Notice the shift of the receptive field center towards or away from the positively or negatively trained areas, respectively. Data adapted from Vislay-Meltzer, 2006.
Do these experience-dependent modifications of RF properties reflect the same mechanisms that ultimately form mature RFs, or are they a means for rapid but transient modulation of RFs? Indeed most of the phenomena described above turn out to be disappointingly labile. They persist as long as tectal cells are maintained in a quiescent state, but once spontaneous firing resumes cells tend to return to their previous states. Importantly, stable changes are produced when conditioning epochs are properly spaced in time rather than presented as a single massed training 84. The implication for the functional processing of visual inputs is that the neural processing landscape is far more dynamic than generally considered. Certain patterns of activity may indeed underlie the long-lasting modifications of tectal RFs that underlie map refinement but there are also many, more transient modulations of neuronal response properties that reflect recent sensory experience.
Temporal Network Properties Can be Sculpted by Visual Inputs
Patterned visual activity not only affects retinotectal inputs, but can also shape the response properties of local intratectal circuitry. Intratectal circuits represent a large proportion of synaptic inputs received by tectal neurons 85. These local circuits are important for coordinating the activity of the tectal network output and are important for generating precise visual avoidance behavior and may also be a substrate for rapid modulation of RF properties 22. Over development, changes are observed in the temporal dynamics of local network activity within the Xenopus tadpole tectum, such that recurrent network activity —initially triggered by incoming retinal input—becomes more temporally coherent and less variable, increasing the precision of tectal cell spiking 68.
How does this refinement of local circuits occur? One possibility is that temporal properties of recurrent tectal circuits are sculpted by incoming retinal input, perhaps via a timing-dependent synaptic plasticity rule. If so, then if a visual stimulus is presented in which a specific time interval is repeated, a trace of this interval should be detectable in the pattern of recurrent activity evoked by single stimuli. In a series of in vivo experiments, Pratt et al. tested this hypothesis by presenting freely-swimming tadpoles with pairs of light flashes with an inter-stimulus interval (ISI) of either 150 msec or 400 msec over a period of 4 hrs 85. RGCs are known to fire bursts in response to a single visual stimulus 86, however by imposing a specific ISI during training, this interval would be overrepresented in the input frequencies received by tectal neurons. Thus, visual input in tadpoles trained with a short interval is predicted to elicit recurrent activity at a shorter latency than in those trained with the longer interval. This prediction was borne out — after 4 hours of training, tadpoles trained with the short ISI exhibited recurrent network activity in response to single optic nerve shocks which lasted significantly shorter than tadpoles trained with the longer ISI. A similar finding was obtained in vitro in which the temporal pattern of recurrent activity evoked by direct optic nerve stimulation could be conditioned to organize itself temporally around a precisely controlled time interval. This suggests that this type of network plasticity is expressed within the tectal circuitry, and can be explained by a mechanism involving spike timing-dependent plasticity of recurrent intratectal connections. These data suggest that temporal sculpting of local excitatory tectal circuits allows inappropriately timed recurrent inputs to weaken, and appropriately timed inputs to potentiate, resulting in a gradual increase in temporal coherence and in increased precision of network driven tectal spiking. Moreover these changes can occur within a few hours, modulating the network dynamics to adapt to changing temporal input patterns.
Entraining of network activity by repetition of temporally-spaced stimuli had also been previously described in the fish, reptile, amphibian and mammalian retina 87-89. In a phenomenon known as an ‘omitted stimulus potential’, repetitive presentation of a given stimulus results in a response timed to match the stimulus interval in trials in which the actual stimulus was absent. A similar phenomenon was recently described by Sumbre et al. in larval zebrafish optic tectum 90. In this study, the authors used Ca++-sensitive dyes to image activity of tectal cells in response to repetitive visual stimuli. They found that after presenting a series of evenly-spaced stimuli, a subset of tectal neurons remained entrained after the end of the stimulus train, and the timing of their post-stimulus activation matched the ISI of the stimulus. Interestingly, the authors used much longer ISIs (ranging from 4 to 10 sec) than used in the Xenopus study described above, suggesting that a very different, yet still unknown, neural mechanism may be used to entrain the network activity.
Both of these studies show that temporally-organized activity originating from the retina can allow the intratectal circuitry to self-organize, possibly optimizing the temporal response properties of the tectal network. While the functional consequences of this temporal adaptation remain poorly understood, it is known that properly timed tectal activity is required for generating normal visual avoidance behavior22, and therefore this process may be important for fine tuning of sensorimotor integration in these species.
Conclusions
Neural processing involves the precise synthesis of circuit wiring, signaling between elements and the well-timed integration performed by each element in the circuit. Given the enormous challenges in constructing a functional brain circuit, it has been tempting to consider developmental plasticity as a process that is solely dedicated to the task of building a mature brain. However, this oversimplification ignores the remarkable degree of dynamism observed at the molecular, structural, and functional levels in developing neurons and nervous systems ( Box 2). The experiments described above offer the insight that the developing circuit is a not merely an unrefined version of its future self, but also a sublime reflection of its past history, its prior experience. The developing organism is not merely a miniature version of the adult, but has specific sensory processing requirements appropriate to its size and structure. As the organism develops and grows, its brain must continually adapt and refine itself while simultaneously functioning as required to process and respond appropriately to ongoing sensory input.
Box 2. Outstanding questions.
A number of outstanding questions regarding short-term developmental plasticity, covering the gamut from mechanism to behavior remain unresolved. Here we highlight several of the key topics that should be the subject of future investigations:
Dynamic synaptogenesis and outgrowth
◦ Is the dynamic behavior of axons and dendrites observed during the initial formation of the map subject to regulation by the same mechanisms that maintain the map in older animals as the brain grows?
◦ The axonal and dendritic arbors, as well as the pre- and postsynaptic structures, have thus far only rarely been imaged together in the central nervous system. What is the prevalence of transient hemisynapses (in which only pre- or postsynaptic elements are present), and how does the association of pre- with postsynaptic structure contribute to relative stability of the arbors and synapses? What are the trans-synaptic signaling pathways that contribute to structural plasticity?
◦ How many cell types are there in the retinotectal circuit? Do they each respond differently to sensory stimulation? Enhancer trap experiments will be useful for better characterizing the range of cell types.
Regulation of gene expression by experience
◦ The list of activity-regulated genes that participate in circuit plasticity is far from complete. Even for BDNF for which much is known, the detailed signaling cascades by which it exerts its effects on synapse formation, plasticity, and function are still poorly understood. More work will be required to reveal of all the players involved and how they function.
◦ Synaptic activity appears to regulate subcellular trafficking of some mRNAs for local synthesis. What are consequences of local protein synthesis in circuit function and plasticity during development?
◦ How do microRNAs modulate the signaling underlying such events?
Homeostasis in response properties
◦ What are the molecular sensors and transcriptional regulators that read neuronal activity levels to drive homeostatic adaptation like synaptic scaling and changes in intrinsic excitability?
Receptive field plasticity
◦ Do the spike-timing-dependent mechanisms that acutely to modify RF structure in immature animals also contribute to long-term refinement of RFs seen over development?
◦ What is the range of changes in local tectal circuitry that contributes to RF plasticity, including tectotectal excitatory and inhibitory inputs?
Temporal properties of networks
◦ The zebrafish visual circuit can entrain to stimuli with ISI of many seconds. Are there specific pacemaker cells or circuits that participate in this process or is this an emergent property of the network?
◦ How does spatial and temporal RF plasticity contribute to the complex behavior of the organism?
◦ What kinds of reinforcement signals from higher brain structures might guide RF spatiotemporal plasticity in a behaviorally relevant manner?
Acknowledgements
CDA is supported by the National Science Foundation, National Institutes of Health and The Whitehall Foundation; additional funding was provided by a generous gift of the Klingenstein Fund. ESR holds a Canada Research Chair and is supported by grants from the Canadian Institutes for Health Research, the EJLB Foundation, and NARSAD.
Glossary
- Receptive field
In the case of the visual system, the receptive field of a neuron is the area of visual space in which a visual stimulus will activate the neuron.
- Retinotopic map
An orderly projection between the retina and a central visual area (in this case the optic tectum) in which near-neighbor relationships are maintained, such that nearby cells in the retina project to nearby cells in the tectum. This maintains the relative topographical organization of visual space in the different visual areas.
- Spike-timing dependent plasticity
A type of long-term synaptic plasticity in which the polarity of the change in synapse strength (either potentiation or depression) depends on the relative timing between the synaptic input and the spiking of the postsynaptic cell.
- Synaptic scaling
A type of adaptive synaptic plasticity in which the synaptic strengths of all synapses in a neuron are up or down regulated in response to long-term decreases or increases in global levels of neural activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruthazer ES. You’re perfect, now change--redefining the role of developmental plasticity. Neuron. 2005;45:825–828. doi: 10.1016/j.neuron.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 3.Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.Cang J, et al. Development of Precise Maps in Visual Cortex Requires Patterned Spontaneous Activity in the Retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Huberman AD, et al. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52:247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, et al. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin T, et al. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- 9.Penn AA, et al. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- 10.Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond, B, Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cline HT. Dendritic arbor development and synaptogenesis. Current Opinion in Neurobiology. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko M, et al. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardin JA, et al. Cellular mechanisms underlying stimulus-dependent gain modulation in primary visual cortex neurons in vivo. Neuron. 2008;59:150–160. doi: 10.1016/j.neuron.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragoi V, et al. Foci of orientation plasticity in visual cortex. Nature. 2001;411:80–86. doi: 10.1038/35075070. [DOI] [PubMed] [Google Scholar]
- 15.Maffei A, et al. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 16.Gahtan E, et al. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J Neurosci. 2005;25:9294–9303. doi: 10.1523/JNEUROSCI.2678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewert JP. Neural correlates of key stimulus and releasing mechanism: a case study and two concepts. Trends Neurosci. 1997;20:332–339. doi: 10.1016/s0166-2236(96)01042-9. [DOI] [PubMed] [Google Scholar]
- 18.Pratt K, Aizenman C. Multisensory integration in mesencephalic trigeminal neurons in Xenopus tadpoles. Journal of Neurophysiology. 2009 doi: 10.1152/jn.91317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemke G, Reber M. RETINOTECTAL MAPPING: New Insights from Molecular Genetics. Annu. Rev. Cell. Dev. Biol. 2005;21:551–580. doi: 10.1146/annurev.cellbio.20.022403.093702. [DOI] [PubMed] [Google Scholar]
- 20.Ruthazer ES, Cline HT. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J Neurobiol. 2004;59:134–146. doi: 10.1002/neu.10344. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin T, O’Leary DDM. Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- 22.Dong W, et al. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. Journal of Neurophysiology. 2009;101:803–815. doi: 10.1152/jn.90848.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramdya P, Engert F. Emergence of binocular functional properties in a monocular neural circuit. Nat Neurosci. 2008 doi: 10.1038/nn.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smear MC, et al. Vesicular glutamate transport at a central synapse limits the acuity of visual perception in zebrafish. Neuron. 2007;53:65–77. doi: 10.1016/j.neuron.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. I. Qualitative analysis. J Neurocytol. 1983;12:599–616. doi: 10.1007/BF01181526. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Easter SS, Stuermer CA. An evaluation of the hypothesis of shifting terminals in goldfish optic tectum. J Neurosci. 1984;4:1052–1063. doi: 10.1523/JNEUROSCI.04-04-01052.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reh TA, Constantine-Paton M. Retinal ganglion cell terminals change their projection sites during larval development of Rana pipiens. J Neurosci. 1984;4:442–457. doi: 10.1523/JNEUROSCI.04-02-00442.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaethner RJ, Stuermer CA. Dynamics of terminal arbor formation and target approach of retinotectal axons in living zebrafish embryos: a time-lapse study of single axons. J Neurosci. 1992;12:3257–3271. doi: 10.1523/JNEUROSCI.12-08-03257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Rourke NA, Fraser SE. Dynamic changes in optic fiber terminal arbors lead to retinotopic map formation: an in vivo confocal microscopic study. Neuron. 1990;5:159–171. doi: 10.1016/0896-6273(90)90306-z. [DOI] [PubMed] [Google Scholar]
- 31.Sin WC, et al. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 32.Witte S, et al. In vivo observations of timecourse and distribution of morphological dynamics in Xenopus retinotectal axon arbors. J Neurobiol. 1996;31:219–234. doi: 10.1002/(SICI)1097-4695(199610)31:2<219::AID-NEU7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 33.Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsina B, et al. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 35.Ruthazer ES, et al. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niell CM, et al. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez AL, et al. BDNF increases synapse density in dendrites of developing tectal neurons in vivo. Development. 2006;133:2477–2486. doi: 10.1242/dev.02409. [DOI] [PubMed] [Google Scholar]
- 39.Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 40.Hu B, et al. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- 41.Haas K, et al. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA. 2006;103:12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aizawa H, et al. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- 43.Ince-Dunn G, et al. Regulation of thalamocortical patterning and synaptic maturation by NeuroD2. Neuron. 2006;49:683–695. doi: 10.1016/j.neuron.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Ramanan N, et al. SRF mediates activity-induced gene expression and synaptic plasticity but not neuronal viability. Nat Neurosci. 2005;8:759–767. doi: 10.1038/nn1462. [DOI] [PubMed] [Google Scholar]
- 45.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz N, et al. Neural activity regulates synaptic properties and dendritic structure in vivo through calcineurin/NFAT signaling. Neuron. 2009;62:655–669. doi: 10.1016/j.neuron.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Nedivi E, et al. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 48.Nedivi E, et al. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science. 1998;281:1863–1866. doi: 10.1126/science.281.5384.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantallops I, et al. Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat Neurosci. 2000;3:1004–1011. doi: 10.1038/79823. [DOI] [PubMed] [Google Scholar]
- 50.Van Keuren-Jensen K, Cline HT. Visual experience regulates metabotropic glutamate receptor-mediated plasticity of AMPA receptor synaptic transmission by homer1a induction. J Neurosci. 2006;26:7575–7580. doi: 10.1523/JNEUROSCI.5083-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson SL, et al. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 52.Hong EJ, et al. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 54.An JJ, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshak S, et al. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 58.Hu B. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- 59.Du J-L, Poo M.-m. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature. 2004;429:878–883. doi: 10.1038/nature02618. [DOI] [PubMed] [Google Scholar]
- 60.Bestman JE, Cline HT. The RNA binding protein CPEB regulates dendrite morphogenesis and neuronal circuit assembly in vivo. Proc Natl Acad Sci USA. 2008;105:20494–20499. doi: 10.1073/pnas.0806296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stemmler M, Koch C. How voltage-dependent conductances can adapt to maximize the information encoded by neuronal firing rate. Nat Neurosci. 1999;2:521–527. doi: 10.1038/9173. [DOI] [PubMed] [Google Scholar]
- 62.Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- 63.Turrigiano GG, et al. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 64.Desai NS, et al. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- 65.Burrone J, et al. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 66.Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J Neurosci. 1998;18:2212–2225. doi: 10.1523/JNEUROSCI.18-06-02212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandrasekaran AR, et al. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007;27:1746–1755. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tyler WJ, et al. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. J Neurosci. 2007;27:9427–9438. doi: 10.1523/JNEUROSCI.0664-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilhelm JC, et al. Compensatory changes in cellular excitability, not synaptic scaling, contribute to homeostatic recovery of embryonic network activity. Proc Natl Acad Sci U S A. 2009;106:6760–6765. doi: 10.1073/pnas.0813058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aizenman CD, et al. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- 72.Rozov A, et al. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol. 1998;511:361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 74.Aizenman CD, et al. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- 75.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fraser SE, Perkel DH. Competitive and positional cues in the patterning of nerve connections. J. Neurobiol. 1990;21:51–72. doi: 10.1002/neu.480210105. [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi DS, Murphey RK. Map formation in the developing Xenopus retinotectal system: an examination of ganglion cell terminal arborizations. J Neurosci. 1985;5:3228–3245. doi: 10.1523/JNEUROSCI.05-12-03228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Niell C, Smith S. Functional Imaging Reveals Rapid Development of Visual Response Properties in the Zebrafish Tectum. Neuron. 2005;45:941–951. doi: 10.1016/j.neuron.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 79.Tao HW, Poo M.-m. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 80.Zhang LI, et al. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- 81.Vislay-Meltzer RL, et al. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron. 2006;50:101–114. doi: 10.1016/j.neuron.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 82.Engert F, et al. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature. 2002;419:470–475. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- 83.Mu Y, Poo M.-m. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron. 2006;50:115–125. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Q, et al. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–1957. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]
- 85.Pratt KG, et al. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci. 2008;11:467–475. doi: 10.1038/nn2076. [DOI] [PubMed] [Google Scholar]
- 86.Chung SH, et al. The structural and functional development of the retina in larval Xenopus. Journal of embryology and experimental morphology. 1975;33:915–940. [PubMed] [Google Scholar]
- 87.Bullock TH, et al. Event-related potentials in the retina and optic tectum of fish. J Neurophysiol. 1990;64:903–914. doi: 10.1152/jn.1990.64.3.903. [DOI] [PubMed] [Google Scholar]
- 88.Prechtl JC, Bullock TH. Event-related potentials to omitted visual stimuli in a reptile. Electroencephalogr Clin Neurophysiol. 1994;91:54–66. doi: 10.1016/0013-4694(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz G, et al. Detection and prediction of periodic patterns by the retina. Nat Neurosci. 2007;10:552–554. doi: 10.1038/nn1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sumbre G, et al. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456:102–106. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baier H, et al. Genetic dissection of the retinotectal projection. Development. 1996;123:415–425. doi: 10.1242/dev.123.1.415. [DOI] [PubMed] [Google Scholar]
- 92.Karlstrom RO, et al. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- 93.Gnuegge L, et al. Analysis of the activity-deprived zebrafish mutant macho reveals an essential requirement of neuronal activity for the development of a fine-grained visuotopic map. J Neurosci. 2001;21:3542–3548. doi: 10.1523/JNEUROSCI.21-10-03542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang LI, et al. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt JT, Buzzard M. Activity-driven sharpening of the retinotectal projection in goldfish: development under stroboscopic illumination prevents sharpening. J Neurobiol. 1993;24:384–399. doi: 10.1002/neu.480240310. [DOI] [PubMed] [Google Scholar]
- 96.Schmidt JT, et al. MK801 increases retinotectal arbor size in developing zebrafish without affecting kinetics of branch elimination and addition. J Neurobiol. 2000;42:303–314. [PubMed] [Google Scholar]
- 97.Cline HT, Constantine-Paton M. NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron. 1989;3:413–426. doi: 10.1016/0896-6273(89)90201-8. [DOI] [PubMed] [Google Scholar]
- 98.Straznicky C, Glastonbury J. Anomalous ipsilateral optic fibre projection in Xenopus induced by larval tectal ablation. Journal of embryology and experimental morphology. 1979;50:111–122. [PubMed] [Google Scholar]
- 99.Law MI, Constantine-Paton M. Anatomy and physiology of experimentally produced striped tecta. J Neurosci. 1981;1:741–759. doi: 10.1523/JNEUROSCI.01-07-00741.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ide CF, et al. Eye dominance columns from an isogenic double-nasal frog eye. Science. 1983;221:293–295. doi: 10.1126/science.6857287. [DOI] [PubMed] [Google Scholar]
- 101.Reh TA, Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985;5:1132–1143. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boss VC, Schmidt JT. Activity and the formation of ocular dominance patches in dually innervated tectum of goldfish. J Neurosci. 1984;4:2891–2905. doi: 10.1523/JNEUROSCI.04-12-02891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cline HT, Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruthazer ES, et al. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- 105.Hua JY, et al. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 106.Gosse NJ, et al. Retinotopic order in the absence of axon competition. Nature. 2008;452:892–895. doi: 10.1038/nature06816. [DOI] [PMC free article] [PubMed] [Google Scholar]