Abstract
Convection-enhanced delivery (CED) of GDNF and NTN was employed to determine the tissue clearance of these factors from the rat striatum and the response of the dopaminergic system to a single infusion. Two doses of GDNF (15 and 3 μg) and NTN (10 μg and 2 μg) were infused into the rat striatum. Animals were euthanized 3, 7, 14, 21, and 28 days post-infusion. Brains were processed for ELISA, HPLC, and immunohistochemistry (IHC). Both doses of the infused GDNF resulted in a sharp increase in striatal GDNF levels followed by a rapid decrease between day 3 and 7. Interestingly, IHC revealed GDNF in the septum and the base of the brain 14 days after GDNF administration. Dopamine (DA) turnover was significantly increased in a dose-dependent manner for more than 7 days after a single GDNF infusion. NTN persisted in the brain for at least two weeks longer than GDNF. It also had more persistent effects on DA turnover, probably due to its precipitation in the brain at neutral pH after infusion. Our data suggest that daily or continuous dosing may not be necessary for delivering growth factors into the CNS.
Keywords: glial cell line-derived factor (GDNF), neurturin (NTN), convection-enhanced delivery (CED), rat, brain
Introduction
One of the most recent revolutions in modern neurology is the concept of the regenerating brain. New discoveries have proven that the adult human brain has the potential to generate new neurons (Curtis et al., 2007; Eriksson et al., 1998). Control of neuroplasticity could be valuable in devising new strategies for treatment of central nervous system (CNS) disorders, for example, neurodegenerative diseases (Parkinson's, Alzheimer's, amyotrophic lateral sclerosis, etc). The use of growth factors with neurotrophic capacity has opened a new era of exploration of restorative therapies. Over the past few years, glial cell-derived neurotrophic factor (GDNF) has attracted much attention as a potential way to prevent ongoing neurodegeneration and to restore degenerating dopaminergic (DA) neurons in parkinsonian patients. GDNF was originally characterized in 1993 as a growth factor that promoted the survival of ventral midbrain DA neurons (Lin et al., 1993). Subsequently, it was found to have similar effects on other types of neurons within both the central and peripheral nervous systems (CNS and PNS). Its pleiotropic roles have been also confirmed in proliferation, migration, and differentiation of neuronal cells (Airaksinen et al., 1999; Gianino et al., 2003; Henderson et al., 1994; Natarajan et al., 2002; Taraviras et al., 1999). In general, GDNF plays a crucial role in the development and function of the nervous system. All these beneficial functions encouraged the initiation of clinical trials in Parkinson's disease (PD) (Gill et al., 2003; Lang et al., 2006; Slevin et al., 2005). However, clinical results after intra-cerebral infusions of GDNF are mixed, with small Phase 1 studies reporting promising clinical improvements (Gill et al., 2003; Slevin et al., 2005) but the corresponding placebo-controlled Phase 2 study (Lang et al., 2006) reporting negative results. Differences in outcome of the earlier and later studies may be related to the use of a relatively less efficient delivery system in the Phase 2 study that failed to achieve consistent and effective delivery of GDNF in the putamen.
In this context, there is even less information concerning a GDNF homolog, neurturin (NTN). Discovered in 1996 by Johnson and Milbrandt's group, it paralleled many of the biological activities of GDNF (Kotzbauer et al., 1996). Like GDNF, it promoted the survival and proliferation of developing rat enteric neuronal precursors, resulting in an increased number of neurons and glia (Heuckeroth et al., 1998). It also promoted significant parasympathetic (TH-negative) neurite outgrowth (Stewart et al., 2008). Horger and colleagues found that NTN was expressed in the nigrostriatal system, and that it exerted potent effects on survival and function of midbrain DA neurons (Horger et al., 1998). Direct injection of NTN into the substantia nigra protected mature DA neurons from cell death induced by 6-hydroxy-dopamine (6-OHDA). Moreover, administration of NTN into the striatum of intact adult animals induced behavioral and biochemical changes associated with functional upregulation of nigral DA neurons. These results suggested the possibility that GDNF and NTN may exert redundant trophic effects on the nigrostriatal system. In this light, recombinant NTN may be yet another good candidate for treatment of PD.
In contrast to our knowledge of cerebroventricular (ICV) and nigral infusions (Lapchak et al., 1997), little is known about the tissue clearance of GDNF and NTN proteins infused into brain parenchyma, including distribution and effects on dopamine metabolism. To address some of these important issues, we infused single doses of recombinant GDNF (15 μg and 3 μg) and NTN (10 μg and 2 μg) protein into the rat striatum and evaluated their pharmacokinetic properties in the brain. We found that both GDNF and NTN displayed strikingly extended tissue clearance and correspondingly enhanced duration of effect on the nigro-striatal dopaminergic system in naïve rats. These data might support the concept that intermittent, rather than continuous, delivery of NTN or GDNF into the striatum could be a more reasonable approach for the treatment of PD.
Methods
Experiments were performed with 170 male Sprague-Dawley rats (Charles River Laboratories; Wilmington, MA) each weighing 200-250 g. All procedures were approved by, and in accordance with, the regulations of the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Pharmacokinetics of GDNF and NTN protein infused into the rat brain
To examine the tissue distribution and clearance of infused GDNF, and subsequent response of the dopaminergic system within the rat brain, we infused two doses of GDNF (15 and 3 μg) into the rat striatum (total volume of 10 μl for 20 min; the infusion rate was 0.5 μl/min) by convection-enhanced delivery (CED) as previously described (Hadaczek et al. 2006). After 3, 7, 14, 21, and 28 days, we euthanized animals at the appropriate time. Brains were processed for ELISA (n=10), HPLC (n=10), and immunohistochemical staining against GDNF (n=2). Similar conditions (10 μl for 20 min; infusion rate: 0.5 μl/min) were used for NTN protein with the exception of lower dosage (10 μg and 2 μg) due problematic solubility. After NTN infusion, brains were processed only for HPLC (n=10) and immunohistochemical staining against NTN (n=2 for each time point). Details of the study design are described in Fig. 1.
Fig. 1.
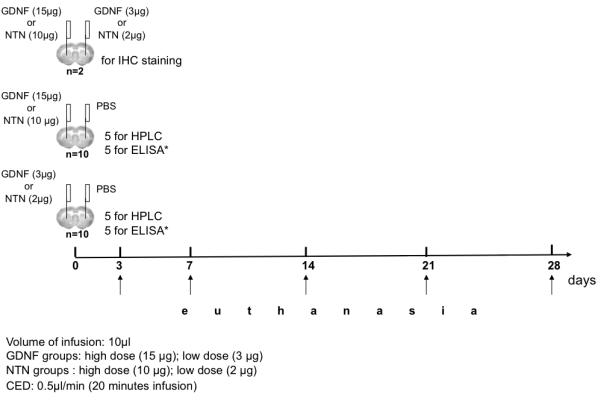
Pharmacokinetics of GDNF and NTN infused into the rat brain – study design. The scheme shows our experimental design for the striatal infusion of GDNF and NTN proteins. Two different doses of GDNF and NTN were used (15 μg and 3 μg for GDNF; 10 μg and 2 μg for NTN). Rats were euthanized at different time points to measure the tissue levels of GDNF and dopamine turnover as a response to a single GDNF and NTN infusion. The brains were processed for ELISA (only for the rats infused with GDNF), HPLC, and IHC staining (see details in Material and Methods).
Stereotactic surgery and growth factors
The rats were anesthetized with 2% v/v isoflurane (Baxter; Deerfield, IL) in 3 L/min oxygen, and were placed in a small-animal stereotactic frame (David Kopf Instruments; Tujunga, CA). A sagittal incision was made in the skin, and burr-holes were made in the skull by a drill, 0.5 mm anterior to the bregma and 3 mm to the right and left of the midline. All infusions were performed by CED (Hadaczek et al., 2006). To minimize trauma and reflux, silica cannulae (O.D. 235 μm; I.D. 100 μm) were used for all infusions (Polymicro Technologies, Phoenix, AZ). The cannulae were attached directly to Nanofill-100 syringes placed in the pumps controlled by a Micro4™ MicroSyringe Pump Controller (World Precision Instruments Inc., Sarasota, FL).
Recombinant human GDNF and NTN proteins were purchased from R&D Systems, Minneapolis, MN. GDNF was resuspended in PBS (pH 7.4) at a concentration of 1.5 mg/ml. Due to poor solubility of NTN, it was resuspended in PBS equilibrated with HCl to pH 5.25. The final concentration of NTN in solution was 1.0 mg/ml. Both growth factors were infused into the striatum (AP: +0.5mm; ML: ± 3 mm relative to bregma; DV: −5 mm, at rate 0.5 μl/min; 10 μl total volume). The coordinates were taken from the rat brain atlas (Paxinos, 2005). The infusion cannulae were lowered manually.
Enzyme-linked immunosorbent assay (ELISA)
Tissue concentrations of GDNF were determined with a commercially available kit: DuoSet® Human GDNF (R&D Systems, Minneapolis, MN). After dissection, whole striata were stored at −80°C. We routinely take whole striata for such analyses. This averages the final reading of the assay, but we avoid accidental differences from individual punch collections. We define such results as “gross striatal values”. Samples were later homogenized with a model 100 Fisher Science Dismembrator® in 250 μl of ice-cold buffer containing 100 mM potassium phosphate (pH 7.8), 0.2% Triton X-100 and protease inhibitors (Complete Mini®; Roche, Palo Alto, CA), and homogenates were centrifuged at 13,000 × g for 15 min. The supernatant solutions were collected and GDNF concentrations measured by ELISA. Chemiluminescence was detected on a Flx800 microplate reader (Biotek, Winooski, VT) and data expressed in relative light units. The final data were expressed as ng of GDNF per mg of total tissue protein (DC Protein Assay kit from Bio-Rad, Hercules, CA).
Analyses of DA and metabolites by high-performance liquid chromatography (HPLC)
After euthanasia, rat brains were removed, and whole striata isolated and stored at −80°C. These samples were later placed in 250 μl 0.4 N perchloric acid, sonicated, and centrifuged for 15 min at 13,000 × g at 4°C. The supernatant solution (30 μl) was injected into a high-performance liquid chromatography system (Coularray; ESA, Chelmsford, MA) for the detection of dopamine (DA) and its metabolites, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). The Coularray software calculated the amount of analyte in each sample compared to external standards: DA, DOPAC, and HVA – 100 ng/ml (using integration). We ran a standard every six injections of samples to control for slight changes in retention time. Protein content was determined in pellet fractions as described (Lowry et al., 1951). Data are expressed as dopamine turnover (DOPAC/DA and HVA/DA ratios).
Immunohistochemistry
After euthanasia, rats were transcardially perfused with cold PBS and then 4% paraformaldehyde (PA). The brains were removed and post-fixed in PA overnight. Brains were washed briefly in PBS, transferred to a 30% w/w sucrose solution in 0.01 M PBS for cryopreservation and then cut into 40-μm serial coronal sections on a cryostat. Frozen sections were collected in a series in antifreeze solution and stored at −80°C. Every fifth section was immunostained for GDNF and NTN. After 3 washes in PBS, the sections were blocked in 1% v/v H2O2 for 20 min. After another extensive wash in PBS, sections were incubated in blocking solution (2% v/v normal horse serum and 0.01% v/v Tween-20 in PBS) for 30 min, followed by incubation in primary antibody solution (a goat polyclonal anti-GDNF or anti-NTN, R&D Systems; 1:300 and 1:100 respectively in blocking solution) overnight at room temperature. After three washes in PBS with 0.01% Tween-20, sections were allowed to react for 1 h with biotinylated horse anti-goat IgG (concentration 1:300; Vector Laboratories). A 1-h incubation in Streptavidin-HRP (1:300; Vector Laboratories) followed another brief washing step in PBS. Immunoreactivity was visualized by DAB/H2O2 reaction. Sections were mounted onto slides, dried and covered with glass coverslips.
Results
Pharmacokinetics of GDNF protein in the rat brain
To determine the tissue clearance of GDNF from the rat brain, we infused two doses of GDNF protein into the striatum and measured GDNF content at various times after injection. We observed a dose-dependent correlation between the striatal concentration of GDNF and the amount infused (Fig. 2). Striatal infusion of 15 μg GDNF protein resulted in detection of approximately 60 ng/mg tissue protein on day 3. Infusion of a five-fold lower dose (3 μg) resulted in a tissue GDNF concentration of 13 ng/mg protein, effectively maintaining proportionality between the two infused doses. For both doses, there was a rapid decrease in GDNF tissue level observed between day 3 and 7 post-infusion. The high-dose group (15 μg) showed elevated levels of GDNF even after one month (compared to the baseline level), whereas the low-dose group (3 μg) reached a baseline concentration one week earlier (21 days after infusion). The endogenous level of GDNF in normal rat striatum detected by our ELISA kit was 0.04 – 0.05 ng/mg protein. GDNF immunohistochemistry (IHC) showed the same trend – i.e. GDNF signal in the low-dose group was weaker and disappeared sooner than seen in the high-dose group (Fig. 3). Immunohistochemistry is a less sensitive technique than ELISA and there was, therefore, little detectable signal beyond day 14 after infusion. Interestingly, aside from the targeted striatum, IHC also revealed the presence of GDNF in the septum and the base of the brain (including suprachiasmatic nucleus, retrochiasmatic area, arcuate nucleus dorsal/lateral part as well as median eminence). A strong GDNF signal was also detectable in dorsal and ventral endopiriform nucleus up to at least 7 days post-infusion. This signal was always found in the vicinity of a blood vessel (Fig. 4) suggesting active perivascular transport of GDNF (Hadaczek et al., 2006).
Fig. 2.
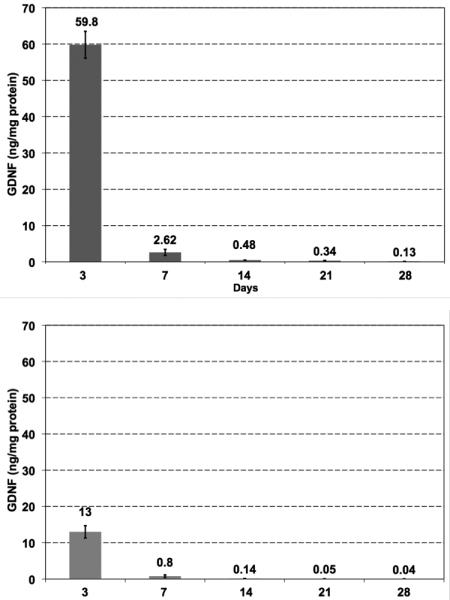
The tissue clearance of GDNF protein from the rat brain after a single GDNF infusion. The upper panel with darker columns represents the high dose group (rats were infused with 15 μg GDNF). The lower panel with lighter columns shows the results from the low dose group (rats were infused with 3 μg GDNF).
Fig. 3.
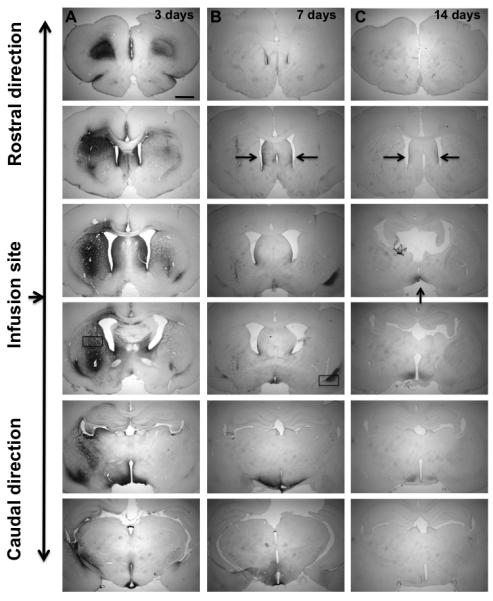
Immunostaining for GDNF infused into the rat brain – time course. Columns A, B, and C show the time points after striatal infusions – 3, 7, and 14 days, respectively. GDNF was infused bilaterally – 15 μg into the left and 3 μg into the right hemisphere. No signal was detected 21 and 28 days after infusion. The arrows in column C show residual GDNF staining (septum and the base of the brain including retrochiasmatic area). The size bar = 1.5 mm.
Fig. 4.
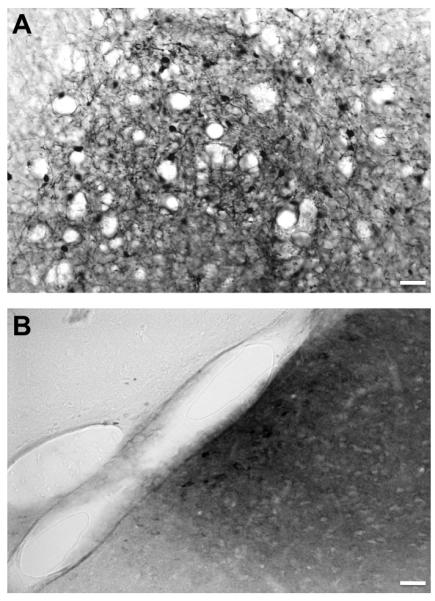
Immunostaining for GDNF infused into the rat brain. (A) Higher magnification from the frame in column A – striatal neurons positive for GDNF; size bar = 100 μm. (B) Higher magnification from the frame in column B – GDNF staining in the vicinity of a blood vessel. After striatal infusion, GDNF was transported to a different brain region (in this case - dorsal endopiriform nucleus) via perivascular transport, phenomenon we often observe during cerebral infusion of vectors and other molecules; size bar = 100 μm.
Turnover of dopamine after GDNF infusion
Striatal infusion of GDNF protein in rats induces robust increases in dopamine turnover indicated by increases in DOPAC and HVA with little change in DA (Hebert et al., 1996). To determine the effects of GDNF on dopamine turnover, we measured the ratio of DOPAC and HVA to DA after 3, 7, 14, 21, and 28 days after a single striatal infusion of GDNF (same schedule of sample collection as for the tissue clearance of GDNF). Also, the same doses were used (15 μg and 3 μg per hemisphere). The metabolic turnover of dopamine was significantly increased for more than a week after GDNF infusion; this effect was dose-dependent and reversible. DOPAC/DA and HVA/DA ratios returned to baseline 2 weeks after GDNF infusion (Fig. 5). As shown by Hebert and colleagues, DA levels were not changed in the rats infused with GDNF or NTN. Thus, we expressed the results in dopamine turnover (DOPAC/DA and HVA/DA ratios) as a readout of the response of the dopaminergic system to the infused growth factors.
Fig. 5.
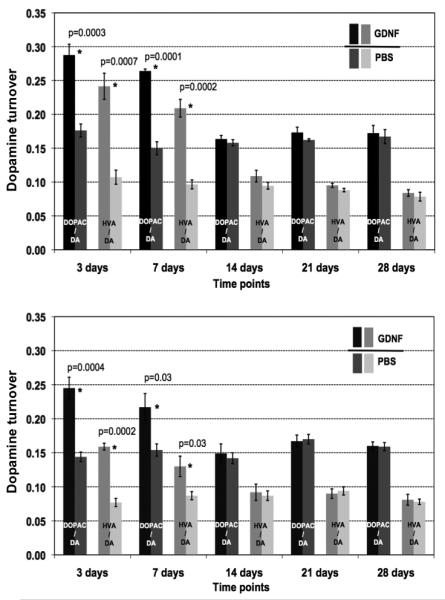
Turnover of dopamine (DA) after a single infusion of GDNF protein into the rat striatum. The upper panel shows the results for the high dose group (15 μg GDNF into the left hemisphere), the lower panel represents the low dose group (3 μg GDNF into the left hemisphere). The turnover of dopamine is expressed as the ratio of dopamine metabolites, (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) to DA itself. Darker columns represent results from the GDNF-infused hemispheres, lighter – from the control, right, hemispheres infused with PBS. Statistically significant differences are marked as * and p values are given.
Pharmacokinetics of infused NTN in rat brain
In contrast to the broad distribution of GDNF in striatum, NTN infusion resulted in an intense, relatively focal distribution pattern (Fig. 6). In other experiments (data not shown), we found that bacterially synthesized NTN precipitates at neutral pH. Therefore, it seems reasonable to assume that infusion of acidified, soluble NTN results in significant precipitation at the site of infusion as it equilibrates to neutral pH in the parenchyma. This focal distribution of NTN was confined to the striatum and, in contrast to GDNF, did not appear in other structures like septum or the base of the brain consistent with lack of transport of precipitated NTN. The kinetics of elimination of NTN from the striatum was otherwise similar to that of GDNF. Thus, NTN was detected until day 21 post-infusion (high-dose group), at least one week longer than for GDNF (Fig. 6), and the intensity of NTN signal was proportional to the dose infused.
Fig. 6.
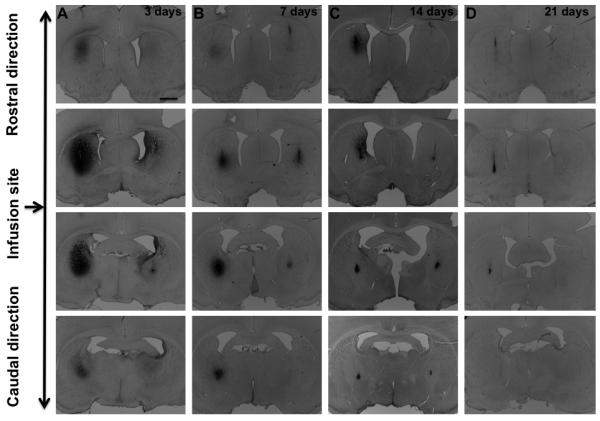
Immunostaining for NTN infused into the rat brain – time course. Columns A, B, C, and D show the time points after striatal infusions – 3, 7, 14, and 21 days respectively. NTN was infused bilaterally – 10 μg into the left and 2 μg into the right hemisphere. No signal was detected 28 days after infusion. The left hemisphere was infused with a higher dose (10 μg) and the right one with a lower dose (2 μg).
Turnover of dopamine after NTN infusion
A single infusion of NTN into the striatum increased the metabolic turnover of DA for at least 21 days post-infusion in the high-dose group, 2 weeks longer than for GDNF (Fig. 7); however, the peak response was lower. This correlated well with the duration of NTN staining. The low-dose group showed a significant increase in DA turnover only during the first week after infusion.
Fig. 7.
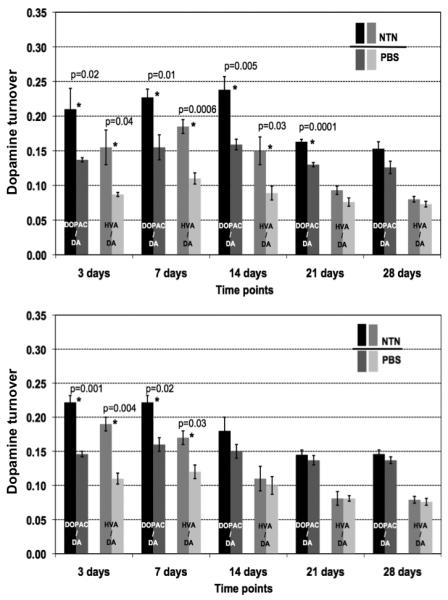
Turnover of dopamine (DA) after a single infusion of NTN protein into the rat striatum. The upper panel shows the results for the high dose group (10 μg NTN into the left hemisphere), the lower panel represents the low dose group (2 μg NTN into the left hemisphere). The turnover of dopamine is expressed as the ratio of dopamine metabolites, (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) to DA itself. Darker columns represent results from the NTN-infused hemispheres, lighter – from the control, right, hemispheres infused with PBS. Statistically significant differences are marked as * and p values are given.
Discussion
GDNF and NTN are potent neurotrophic factors that protect catecholaminergic neurons from degeneration (Airaksinen and Saarma, 2002; Airaksinen et al., 1999; Horger et al., 1998; Lin et al., 1993; Oiwa et al., 2002; Pascual et al., 2008; Rosenblad et al., 1999). More recently, we have shown that GDNF can also induce significant up-regulation of dopaminergic function in non-human primates many months after MPTP treatment, suggesting that GDNF (and probably NTN) have the capacity not only to protect these vulnerable neurons from insult but also to restore at least some lost innervation (Su et al., 2009). Demonstration of neuroprotection in animal models of PD served as a foundation for GDNF-based therapies (Akerud et al., 2001; Choi-Lundberg et al., 1997; Gash et al., 1996; Kirik et al., 2004; Rosenblad et al., 1998; Tomac et al., 1995). Two early open-label clinical trials were conducted with very promising clinical results (Gill et al., 2003; Slevin et al., 2005), but a double-blind Phase 2 study showed little clinical benefit to PD patients (Lang et al., 2006). These somewhat contradictory data suggest a need to critically re-assess all the important factors contributing to the successful design of clinical trials. Designing new treatments for the CNS involving direct cerebral infusions of proteins requires a very precise understanding of pharmacokinetics of the therapeutic to be used – its optimal dose, distribution, clearance, and effects on the targeted biochemical pathway. Most studies dating from the early-to-mid 1990's describe bioavailability of GDNF when infused ICV or into substantia nigra (Lapchak et al., 1997) but not into parenchyma even though the phase 2 study of GDNF infusion for PD was intra-putaminal (Lang et al., 2006). Therefore, we undertook this study to elucidate more definitively the pharmacokinetic properties of GDNF and NTN infused into the striatum by CED that affords effective distribution into large volumes of parenchyma.
We were surprised to find that in spite of a rapid initial clearance, GDNF remained within the brain for an extended period. In our experiments, we observed a very robust GDNF signal within the striatum. However, we also detected signal in the septum and the base of the brain. Most likely this was caused by the leakage of GDNF into the ventricle and, via CSF flow, was transported to the neighboring (septum) or more distant (base of the brain) structures. It is likely that, in addition to GDNF leakage into the CSF, perivascular trafficking of GDNF was also involved in dissemination of GDNF beyond the striatum. We have previously described the phenomenon of the perivascular pump powered by cardiac contractions that generate arterial pulsation (Hadaczek et al., 2006). This contributes to the distribution and transport of parenchymally infused molecules within perivascular spaces around the brain blood vessels, indicated by detection of infusate in the vicinity of arteries and arterioles. Indeed, we also observed this phenomenon in our rats with GDNF signal found in the dorsal endopiriform nucleus around one of the blood vessels permeating this region. Thus, an awareness of the exact organization of brain vasculature and its connections within different brain regions is important in being able to predict the spread of therapeutic agents (such as AAV2-GDNF or GDNF protein) beyond the infused structure. Such spillovers to distant brain areas must be also taken into consideration for possible adverse effects caused by GDNF affecting the structures outside the motor pathways. GDNF-based treatments can cause weight loss (Johnston et al., 2009; Manfredsson et al., 2009; Su et al., 2009) and/or degeneration of Purkinje cells within the cerebellum (Hovland et al., 2007). Therefore, it is of utmost importance to determine optimal, safe doses of the infused growth factors. In our studies, we did not observe even a transient weight loss in the rats infused with both high and low doses of GDNF or NTN (data not shown). We believe that targeting striatum (as opposed to substantia nigra or intraventricular infusions) is probably the safest approach. Striatal delivery resulted in quite localized distribution of GDNF and NTN. We did see slight leakage of GDNF into the ventricles and perivascular transport to other brain structures; however, it did not affect regions like the nucleus accumbens or SN believed to trigger weight loss. More detailed studies addressing safety issues would be required to avoid such complications.
Direct infusion of GDNF into the brain caused expected DA changes in the dopaminergic system in line with previous findings (Gill et al., 2003; Miyoshi et al., 1997). The metabolic turnover of DA was significantly increased (p≤ 0.03) for more than 7 days after infusion regardless of the infused dose. When a larger dose of GDNF was infused (15 μg), the magnitude of effect but not duration was more pronounced (p≤ 0.0002).
Discovered in 1996 (Kotzbauer et al., 1996), NTN is expressed and developmentally regulated in the nigrostriatal system. Like GDNF, it exhibits potent actions on the survival and function of midbrain DA neurons (Horger et al., 1998) (Kordower et al., 2006). Horger's studies indicated that midbrain DA neurons are as responsive to NTN as to GDNF, and it is therefore possible that these homologous factors may act together to regulate the development and/or function of midbrain DA neurons. Rosenblad et al. showed that striatal infusion of NTN provided partially effective (72%) protection for the 6-OHDA-lesioned nigral DA neurons, lower than the almost complete protection (90-92%) when GDNF was used (Rosenblad et al., 1999). However, in contrast to recombinant GDNF, Oiwa et al. reported that NTN in a rat model of PD showed neuroprotective and regenerative effects (Oiwa et al., 2002), a finding supported in MPTP-lesioned monkeys where putaminal infusion of exogenous NTN protein restored motor and dopaminergic function in the globus pallidus (Grondin et al., 2008). However, the degree of benefit of NTN varied among different studies. Different doses, delivery conditions, and treatment regimens have made it difficult to draw broad conclusions.
In our study, we observed a significant difference between the distribution of NTN and GDNF within the rat brain. The infusion of NTN resulted in a more confined spread as opposed to a diffuse distribution of GDNF protein. NTN was restricted to the striatum and did not appear in distal brain structures. NTN is known to distribute less efficiently than GDNF (Gash et al., 2005; Grondin et al., 2008), and is perhaps explained by poor solubility of NTN at neutral pH. We used PBS at pH 5.25 for our NTN infusions. Higher pH values (pH > 5.5) resulted in precipitation of NTN (data not shown). It is very likely that, after cerebral infusion, NTN was gradually deposited in a solid form when in contact with the physiologically neutral extracellular brain matrix and CSF. When Rosenblad et al. directly compared GDNF and NTN infused into the rat lateral ventricle, neuronal protection was observed for GDNF but not NTN (Rosenblad et al., 1999). They attributed this to the poor solubility of NTN in neutral pH solutions, just as we found. Our data confirmed poorer overall striatal distribution for NTN, although focal immunohistochemical signal could be still detected even 21 days after a single high dose (10 μg) infusion. It is also important to mention that glycosylated forms of NTN produced in eukaryotic systems (our system was prokaryotic, hence NTN was non-glycosylated) might have different properties and solubility.
For GDNF, at an even a higher dose (15 μg), the signal from immunostaining disappeared from the striatum almost completely at day 7 with slight residual signal in the base of the brain and septum persisting 14 days after infusion. It is possible that NTN persisted in the striatum in a solid form after injection and precipitation at neutral pH, thereby retarding NTN clearance from the parenchyma. Concomitantly, our results from the analysis of DA turnover after NTN infusion showed that a single dose of 10 μg NTN caused long lasting effects (>21 days) on DA metabolism, closely correlating with the immunohistochemical staining. Due to its poor solubility, we infused maximum possible dose of 10 μg of NTN (for the NTN high dose group) vs. 15 μg of GDNF (for the GDNF high dose group) and, despite that, observed longer lasting effects on the dopamine turnover after NTN infusion.
Based on the long tissue clearance of GDNF and NTN protein, it is likely that continuous infusion of these growth factor may not be desirable since unacceptable accumulation in the striatum may take place. A separate issue that should be considered is the method of striatal administration required for efficient dispersion of the solution containing growth factor within the striatum. CED is based on infusion under pressure, which drives efficient spread of fluid within the target region (Bobo et al. 1994). However, once the fluid front reaches the outer boundaries of the striatum, infusion should be terminated to prevent unwanted leakage of the solution outside of the striatum. For these reasons, we believe that, in contrast to a previous GDNF clinical trial design, continuous infusions may be neither necessary nor desirable (Gill et al., 2003). A dosing regimen, based on an understanding of pharmacokinetics and clearance of GDNF and infusion parameters, should be undertaken. It is also imperative to deliver GDNF in a targeted manner. Any spillover beyond the targeted brain structure could lead to potentially dangerous adverse effects (Manfredsson et al., 2009; Su et al., 2009). Other published data have identified two safety concerns with respect to recombinant GDNF infusion: 1) development of neutralizing (anti-GDNF) antibodies (Lang et al., 2006; Slevin et al., 2005; Tatarewicz et al., 2007) and 2) cerebellar toxicity (Hovland et al., 2007). Our delivery method (CED) has proven efficacious in delivering GDNF and NTN into the rat striatum. Although clearly important, the physical aspects of the delivery protocol are quite often ignored. The failure of the phase 2 GDNF multicenter clinical trial (putaminal infusion) was very likely caused by insufficient distribution of GDNF within the putamen (Lang et al., 2006). The infusion rate in that study was only 6.25 μl/h. This is a very slow rate (good for diffusion only) and the large tip diameter of the catheter did not generate enough pressure to displace the extracellular fluid in the parenchyma sufficient to engage the convective mechanism. These two factors are crucial for achieving effective distribution (Krauze et al., 2005).
CED has been proven to be a safe delivery method (Johnston et al., 2009; Krauze et al., 2005; Su et al., 2009). The slow infusion rate (not exceeding 0.5 μl/min) and a very thin silica cannula (inner and outer diameters of 100 μm and 235 μm, respectively) allowed us to distribute both growth factors without noticeable reflux and a major tissue damage caused by a high build-up of pressure often seen with other non-CED infusions (Hamilton syringe). Such injuries can indeed occasionally accompany striatal infusions. And although we saw some damage at the site of infusion with GDNF, we did not consider it as a major injury. For larger animals and humans, our group developed a new, reflux-free, stepped-design cannula that permits CED with a higher flow rate (up to 5 μl/min) with minimal, if any, tissues damage (Krauze et al., 2005). Concerns might be raised against repeated infusions. In clinical settings though, we would consider a cannula that is permanently placed within the striatum (as in the clinical trials) to avoid injuries caused by multiple insertions/removals of such cannulae.
The novelty of our approach is based on the precise assessment of pharmacokinetics of both growth factors in the brain. Clear understanding of the pharmacokinetics of GDNF and NTN proteins within the brain and infusion parameters may guide adjustment of dose and frequency of administration in order to achieve therapeutic effects. Intermittent delivery by means of CED may not only ensure accurate and efficient spread of GDNF and NTN within the target, but may also provide more uniform tissue levels. These persistent effects of a single dose of GDNF on DA metabolism suggest that intermittent dosing could eliminate the need for a continuously active implantable pump and allow infusions to be conducted at intervals.
Acknowledgements
Funding was provided by NIH U54NS045309 and Kinetics Foundation. The authors of this manuscript have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci. 2001;21:8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann Neurol. 2005;58:224–233. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Ai Y, Ding F, Walton AA, Surgener SP, Gerhardt GA, Gash DM. Intraputamenal infusion of exogenous neurturin protein restores motor and dopaminergic function in the globus pallidus of MPTP-lesioned rhesus monkeys. Cell Transplant. 2008;17:373–381. [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, Park JW, Bankiewicz K. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E, Jr., Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland DN, Jr., Boyd RB, Butt MT, Engelhardt JA, Moxness MS, Ma MH, Emery MG, Ernst NB, Reed RP, Zeller JR, Gash DM, Masterman DM, Potter BM, Cosenza ME, Lightfoot RM. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF in rhesus monkeys. Toxicol Pathol. 2007;35:1013–1029. doi: 10.1177/01926230701481899. [DOI] [PubMed] [Google Scholar]
- Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J, Bankiewicz KS. Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum Gene Ther. 2009;20:497–510. doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr., Milbrandt J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, Berger MS, Bankiewicz K. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Gash DM, Collins F, Hilt D, Miller PJ, Araujo DM. Pharmacological activities of glial cell line-derived neurotrophic factor (GDNF): preclinical development and application to the treatment of Parkinson's disease. Exp Neurol. 1997;145:309–321. doi: 10.1006/exnr.1997.6501. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, Rising AC, Foust KD, Zhang Y, Muzyczka N, Gorbatyuk OS, Scarpace PJ, Mandel RJ. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther. 2009;17:980–991. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Zhang Z, Ovadia A, Lapchak PA, Collins F, Hilt D, Lebel C, Kryscio R, Gash DM. Glial cell line-derived neurotrophic factor-levodopa interactions and reduction of side effects in parkinsonian monkeys. Ann Neurol. 1997;42:208–214. doi: 10.1002/ana.410420212. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, Yoshimura R, Nakai K, Itakura T. Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and histochemical measurements in a model of progressive Parkinson's disease. Brain Res. 2002;947:271–283. doi: 10.1016/s0006-8993(02)02934-7. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Paxinos GW,C. The Rat Brain In Stereotaxic Coordinates. Elsevier Academic Press; 2005. [Google Scholar]
- Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Bjorklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson's disease after administration into the striatum or the lateral ventricle. Eur J Neurosci. 1999;11:1554–1566. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Bjorklund A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson's disease. Neuroscience. 1998;82:129–137. doi: 10.1016/s0306-4522(97)00269-8. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Anderson RB, Kobayashi K, Young HM. Effects of NGF, NT-3 and GDNF family members on neurite outgrowth and migration from pelvic ganglia from embryonic and newborn mice. BMC Dev Biol. 2008;8:73. doi: 10.1186/1471-213X-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, Bringas J, Pivirotto P, Penticuff J, Eberling J, Federoff HJ, Forsayeth J, Bankiewicz KS. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:1627–1640. doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- Tatarewicz SM, Wei X, Gupta S, Masterman D, Swanson SJ, Moxness MS. Development of a maturing T-cell-mediated immune response in patients with idiopathic Parkinson's disease receiving r-metHuGDNF via continuous intraputaminal infusion. J Clin Immunol. 2007;27:620–627. doi: 10.1007/s10875-007-9117-8. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]


