Abstract
Both underloading and overloading of joints can lead to articular cartilage degradation, a process mediated in part by matrix metalloproteinases (MMPs). Here we examine the effects of reduced loading of rat hindlimbs on articular cartilage expression of MMP-3, which not only digests matrix components but also activates other proteolytic enzymes. We show that hindlimb immobilization resulted in elevated MMP-3 mRNA expression at 6 hours that was sustained throughout the 21 day immobilization period. MMP-3 upregulation was higher in the medial condyle than the lateral, and was greatest in the superficial cartilage zone, followed by middle and deep zones. These areas also showed decreases in safranin O staining, consistent with reduced cartilage proteoglycan content, as early as 7 days after immobilization. One hour of daily moderate mechanical loading, applied as passive joint motion, reduced the MMP-3 and ADAMTS-5 increases that resulted from immobilization, and also prevented changes in safranin O staining. Intra-articular injections of an MMP-3 inhibitor, N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid (NNGH), dampened the catabolic effects of a 7 day immobilization period, indicating a likely requirement for MMP-3 in the regulation of proteoglycan levels through ADAMTS-5. These results suggest that biomechanical forces have the potential to combat cartilage destruction and can be critical in developing effective therapeutic strategies.
Keywords: MMP-3, Immobilization, Passive motion, Cartilage
1. Introduction
Joints have evolved to adequately handle a range of mechanical loading consistent with their physiologic function; however, both reduced loading and overloading of joints may have deleterious effects, particularly on their cartilaginous components (Saxon, et al. 1999, Vanwanseele, et al. 2002). Both acute and chronic high-intensity loads cause cartilage degeneration, which may eventually lead to osteoarthritis (Buckwalter and Martin 2006, Saxon, et al. 1999). Likewise, reduced loading, occurring commonly as a result of joint immobilization following spinal cord injuries or secondary to treatments for acute musculoskeletal injury (Jones and Amendola 2007, McCarthy and Oakley 2002), or as a result of joint diseases such as arthritis (Fontaine, et al. 2004), also creates catabolic responses within articular cartilage. Previous studies have demonstrated that in vivo immobilization causes cartilage thinning (Haapala, et al. 1999, Jurvelin, et al. 1986), tissue softening (Haapala, et al. 2000, Jurvelin, et al. 1986), and reduced proteoglycan content (Haapala, et al. 1999, Haapala, et al. 1996), leading to cartilage matrix fibrillation, ulceration, and erosion (Evans, et al. 1960, Hagiwara, et al. 2009). In patients with spinal cord injury, articular cartilage atrophies at a rate greater than that reported in age-associated osteoarthritis (Vanwanseele, et al. 2003). Despite numerous studies on articular cartilage changes following immobilization, the early stages of the degradative process in particular remain poorly understood. Elucidating these early events therefore remains an unfulfilled goal essential for the development of treatment strategies to prevent or limit cartilage degradation in joint diseases.
The articular cartilage consists of chondrocytes embedded in an abundant extracellular matrix (ECM) composed primarily of type II collagen and the proteoglycan aggrecan. Classic features of cartilage destruction include the degradation of both collagen fibrils and proteoglycans (Aigner and McKenna 2002). Catabolism of the ECM involves a variety of degradative enzymes, many of which are members of the matrix metalloproteinase family (MMP) (Cawston and Wilson 2006). MMPs vary in substrate specificity, primary structure and cellular localization, but many are first synthesized as proenzymes, that themselves require proteolytic cleavage for activation (Chakraborti, et al. 2003). Certain MMPs have been shown to activate others, and so may play key initiating roles in both normal ECM remodeling and pathological degradation.
In cartilage, MMP-3 not only digests many cartilage ECM components, but can also activate the pro-forms of several MMPs and also contribute to the activation of aggrecanase II (ADAMTS-5) (Cawston and Wilson 2006, Echtermeyer, et al. 2009). MMP-3 appears to be one of the few genes upregulated very early in the degeneration process (Aigner, et al. 2001), and multiple studies have shown that MMP-3 levels are elevated in various joint pathologies including osteoarthritis and acute injury (Lohmander, et al. 1993, Lohmander, et al. 1993, Walakovits, et al. 1992). Furthermore, aged MMP3-/- mice show a 67% reduction in cartilage damage occurring through spontaneous osteoarthritis (Blom, et al. 2007), suggesting an important role for MMP-3 in pathological cartilage matrix degradation. Therefore, reducing pathologic MMP-3 activity could translate into substantial clinical benefit, but there is still no effective MMP inhibitor for treating arthritis.
Mechanical stimulation of human articular chondrocytes in vitro was shown to decrease MMP-3 mRNA expression (Millward-Sadler, et al. 2000); however, the effects of mechanical loading on MMP-3 expression in joint articular cartilage have not been explicitly investigated in vivo. In this study, we tested the hypothesis that joint immobilization in rats would lead to an upregulation of MMP-3, and that experimental joint mobilization would mitigate this response, indicating a chondroprotective effect.
2. Results
2.1. Immobilization induces an early and sustained increase in expression and activity of MMP-3
To determine the effect of reduced loading on MMP-3 expression in vivo, we immobilized the right hind limb of rats, which fixed the knee in full flexion, and found a significant increase of MMP-3 mRNA expression 6 hours after immobilization. Elevated levels of MMP-3 were sustained until at least 21 days after immobilization, with a 10-fold increase over unimmobilized controls (Fig. 1A). As gene expression does not always correlate with protein expression, MMP activity was also measured. Similar expression patterns were observed in enzyme activity, as activity increased approximately 4.7 times the control level at 21 days (Fig. 1B).
Figure 1.

MMP-3 mRNA and activity changes in articular cartilage during immobilization. Levels of MMP-3 mRNA by RT-PCR (A) and total MMP-3 activity (B) expressed relative to levels in unimmobilized controls. Whole condyles, rather than tissue sections, were used as tissue sources for MMP-3 activity assays. Data show mean ± SEM (n=5). * = P < 0.05 versus unimmobilized control.
2.2. Spatial and temporal changes in MMP-3 expression and safranin-O staining in articular cartilage following immobilization
Morphological changes due to immobilization have previously been reported to affect the lateral and medial compartments differently (Vanwanseele, et al. 2002). Therefore, we analyzed MMP-3 expression from cartilage extracts dissected from the lateral and medial condyles. In control joints, no differences in MMP-3 gene expression or enzyme activity were found between the lateral and medial femoral condyles (Figs. 2B and 2C). Following immobilization, MMP-3 mRNA and activity increased significantly above controls in both condyles; however, levels in the medial condyle were approximately 60% higher than in the lateral condyle after 21 days (Fig. 2B). Similar differences between the two condyles were also seen in MMP-3 activity; levels were 62% higher in medial than lateral condyle (Fig. 2C).
Figure 2.
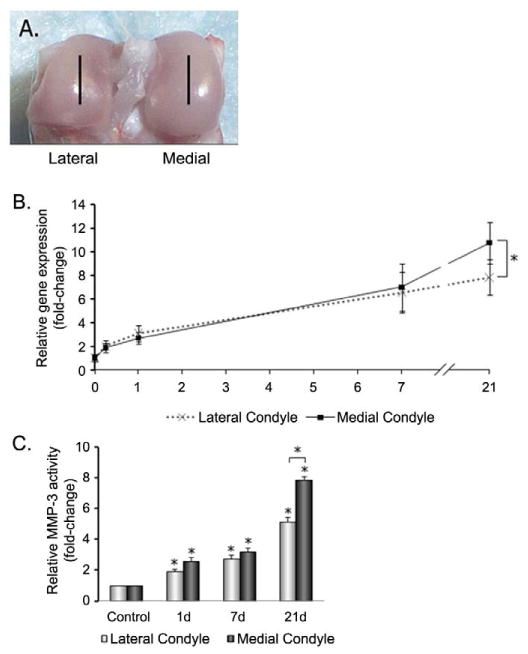
MMP-3 expression and activity in medial and lateral condyles. (A) Sites of tissue analysis by laser capture microdissection. Sagittal sections were taken for LCM from the mid-regions of each condyle as indicated by lines. (B) MMP-3 mRNA levels expressed relative to levels in the lateral condyle of non-immobilized controls. (C) MMP-3 total activity expressed relative to activity in lateral condyles of non-immobilized controls. Data in show mean ± SEM (n = 5). * = P < 0.05 versus unimmobilized control or indicated comparison.
In osteoarthritis, MMP-3 expression has been shown to be depth-dependent (Tetlow, et al. 2001), but whether a similar pattern is exhibited in reduced loading conditions is unknown. Further analysis of MMP-3 mRNA levels in chondrocytes isolated by LCM from different zones within each condyle revealed depth-dependent changes in gene expression 21 days after immobilization. The highest level of expression was detected in the superficial zones, followed by the middle and deep zones of the medial condyle (Fig. 3B). In the lateral condyles, no significant zonal differences were observed. Depth-dependent expression pattern of MMP-3 protein was also observed with time after immobilization, as assessed by immunohistochemical staining (Fig. 4A).
Figure 3.

Zonal analysis of MMP-3 mRNA expression following 21 days of immobilization. (A) Safranin O stained section of cartilage indicating zones selected for analysis by Laser Capture Microdissection. (B) MMP-3 mRNA levels (expressed as copy number/ng RNA). Data show mean and SEM (n = 5). * = P < 0.05.
Figure 4.

MMP-3 protein expression in medial and lateral condyles following immobilization. (A). Immunohistochemistry of MMP-3 and (B) Safranin O-fast green staining of medial and lateral condyles. Bar = 100μm.
The spatial pattern of changes in MMP-3 expression and activity in articular cartilage corresponded with changes in safranin O staining. Cartilage from immobilized limbs showed irregular patches of reduced staining, compared to stronger, more uniform staining in naïve controls (Fig. 4B). Moreover, the reduction in staining was more evident in the medial than lateral condyles. The earliest differences in safranin O staining appeared after 7 days of immobilization, and were clearly evident by 21 days (Fig. 4B). As safranin O staining in cartilage reflects tissue proteoglycan content (Rosenberg 1971), this pattern is consistent with early stages of matrix degeneration.
2.3. Passive motion loading suppresses immobilization-induced MMP-3 upregulation and histological changes in articular cartilage
Accumulating studies suggest mechanical loading at physiological levels is beneficial and might limit cartilage degradation in arthritic conditions (Ferretti, et al. 2005, Salter 2004). Therefore, we tested whether intervals of passive joint motion during hind limb immobilization would reduce cartilage degradation and inhibit MMP-3 upregulation. In this experiment, immobilized rat hind limbs were subjected to 1 hour of passive motion in the middle of 6 or 24 hour immobilization periods, or to daily 1 hour periods of passive motion during 7 days of immobilization. MMP-3 mRNA levels in the immobilized cartilage were upregulated; however, these increases were prevented in limbs subjected to passive motion (Fig. 5A). Passive motion loading also completely blocked the increase in total articular cartilage MMP-3 activity (Fig. 5B). Histological evaluation of cartilage using safranin O-fast green staining revealed that the reduced staining intensity resulting from immobilization, particularly in the superficial and middle zones, was markedly diminished in animals that had undergone passive motion loading (Fig. 5C). Since ADAMTS-5 contributes to the depletion of proteoglycans and is activated by MMP-3 (Echtermeyer, et al. 2009), we investigated the effect of loading on ADAMTS-5 in immobilized cartilage. ADAMTS-5 was increased due to 7 days of immobilization and its expression was dampened upon passive motion loading (Fig. 5C).
Figure 5.

Changes in cartilage mRNA, enzyme activity and histology due to immobilization and motion loading. (A) Relative MMP-3 mRNA gene expression after 6 and 24 hours, and 7 days after immobilization, with and without motion loading. (B) MMP-3 activity after 7 days immobilization with and without motion loading. (C) Immunohistochemistry of MMP-3, ADAMTS-5 and Safranin-O staining of control, 7day immobilized, immobilized + passive motion loaded cartilage, and NNGH treated cartilage from medial condyles. Data show mean + SEM (n = 5). * = P < 0.05 versus unimmobilized control; + = P < 0.05 versus immobilization. Bar = 100μm.
To determine the relationship between MMP-3, ADAMTS-5, and proteoglycan content, we injected an MMP-3 inhibitor, N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid (NNGH), in 50% PBS/50% ethanol into the joint of immobilized hind limbs to suppress MMP-3. Inhibiting MMP-3 prevented increases in ADAMTS-5 as well as proteoglycan depletion, (Fig. 5C), while injections of PBS/ethanol alone did not (data not shown). This demonstrates that the downregulation of MMP-3 is required for the load-induced attenuation of ADAMTS-5 and the prevention of cartilage degradation.
3. Discussion
The concepts that physiologic loading is necessary for proper joint maintenance, that joint immobilization leads to cartilage degradation, and that joint motion can protect cartilage from degradation due to immobilization or other causes, are generally accepted (Ferretti, et al. 2005, Hagiwara, et al. 2009, Salter 1989). The results of this study are consistent with those concepts, and further support a role for MMP-3 as a mediator of cartilage breakdown resulting from immobilization. MMP-3 gene expression and enzyme activity in articular cartilage were upregulated within 6 hour following immobilization, remained elevated during a 21 day immobilization period, and exhibited a spatial distribution that was mirrored by increases in ADAMTS-5 and histological changes in cartilage suggestive of proteoglycan loss. Inhibition of MMP-3 by NNGH treatment prevented these immobilization-induced catabolic changes, demonstrating the requirement of MMP-3 in regulating proteoglycan homeostasis through ADAMTS-5.
Notably, MMP-3 mRNA levels increase within 6 hours following the onset of immobilization, suggesting that immobilization may trigger a catabolic gene expression response at an early phase under reduced loading conditions. Since MMP-3 regulates proteoglycan homeostasis, the fact that increased MMP-3 expression both precedes and spatially corresponds to the apparent proteoglycan loss in cartilage further supports that MMP-3 contributes to the initial molecular events underlying pathologic cartilage degradation. Previously, the earliest changes reported after immobilization were an increase of MMP-1 within 24 hours in antigen-induced-arthritis rabbits (Ferretti, et al. 2005), and a loss of luster on the cartilage surface after 7 days of immobilization in rats (Hagiwara, et al. 2009).
Spatial analysis of changes in MMP-3 gene and protein levels during immobilization revealed that upregulation varied not only among zones within a region of articular cartilage, but also differed between condyles. Using laser capture microdissection to precisely isolate target cells, and immunolocalization, our findings that MMP-3 is elevated mainly in the superficial zone after 21 days of immobilization, reveal a pattern that has been reported in OA cartilage, suggesting that reduced and overloading may share a common progression of cartilage degradation (Okada, et al. 1992, Tetlow, et al. 2001, Tetlow and Woolley 2001). This finding also agrees with the concept that the initial stages of OA are characterized by disorganization and loosening of collagen fibers from the superficial and upper middle zones (Arokoski, et al. 2000). The pattern of increased MMP-3 mRNA expression and activity in the medial compared to the lateral condyle expectedly corresponded to regions of reduced safranin O staining. Interestingly, morphological changes due to immobilization have previously been reported to affect the lateral and medial compartments differently. The mean articular cartilage thickness in patients who experienced complete, traumatic spinal cord injury, decreased significantly in the medial but not lateral tibia 6 months after injury (Vanwanseele, et al. 2002). In animal models of immobilization, the cartilage thickness of the medial femoral condyle in dogs was more substantially affected compared to the lateral compartment (Haapala, et al. 2000, Haapala, et al. 1999). Moreover, in OA, which is believed to be initiated by abnormal loading (Roos 2005), there are also reports that cartilage degeneration occurs more commonly in the medial femoral condyle than the lateral condyle (Ledingham, et al. 1993). The reason why the medial compartment may be more mechanically sensitive to reduced loading is unknown, but differences in contact forces or pressures (Andriacchi, et al. 2004, Bullough 2004), and differential contact with the menisci between the lateral and medial femoral condyles (Freeman and Pinskerova 2005) may be a major causal factor for condyle-dependent susceptibility to cartilage degradation. Since the load distribution in the joint is primarily transmitted through the medial compartment during weight-bearing activities (Thambyah 2007), in reduced loading conditions, this compartment may see a greater percent decrease in stress, resulting in the greater rate of degradation that we observed.
Finally, our study indicated that a brief (1 hour) period of joint mobilization per day was able to suppress the upregulation of MMP-3 and ADAMTS-5 as well as histological changes suggesting cartilage breakdown. Mechanical stimulation of chondrocytes in vitro has been demonstrated to decrease MMP-3 mRNA levels within one hour of loading, a pathway involving integrins, stretch-activated channels, and IL-4 (Millward-Sadler, et al. 2000). Similarly, mechanically responsive genes such as CITED2 were shown to downregulate MMP-1 and MMP-13 expression in response to moderate fluid shear (Yokota, et al. 2003), identifying a possible intracellular signaling pathway for the chondroprotective effects of joint loading. In vivo, the anti-catabolic effects of physiologic loading, in the form of passive motion and moderate mechanical loading on articular cartilage have been clearly demonstrated (Salter 1989, Torzilli, et al. 2009). However, the molecular mechanisms through which this mechanical loading modality exerts chondroprotective effects are unknown. The best pathophysiologic estimates suggest that passive motion can inhibit the action and/or release of pro-inflammatory cytokines, such as IL-1β and TNF-α amongst others, in the joint space (Ferretti, et al. 2006, Ferretti, et al. 2005). In this respect, it is particularly interesting that we observed the duration of loading required to suppress the catabolic effects of immobilization in cartilage to be relatively short. Clinically, passive motion therapies have been reported to have low compliance because they nominally involve 4+ hours of treatment, while the mean duration of therapy patients endure was 126 minutes (Kasten, et al. 2007). Our study suggests that shorter periods of loading might be sufficient for chondroprotective effects, and thus improve compliance.
In summary, we demonstrated that MMP-3 expression in rat articular cartilage undergoes a rapid and sustained upregulation during limb immobilization. The pattern of MMP-3 upregulation is non-uniform, being concentrated in the medial condyle in the superficial zone of the articular cartilage. Increased MMP-3 levels consequently contributed to increases in ADAMTS-5 expression and reduced safranin O staining. Finally, a short duration of joint loading suppressed MMP-3 and ADAMTS-5 upregulation and decreased safranin O staining in response to immobilization. Taken together, our findings provide evidence that a lack of biomechanical signals may be critical in the induction of MMP-3-mediated cartilage destruction, whereas physiologic joint loading could be used for developing effective therapeutic interventions to halt cartilage degradation in arthritic diseases. Furthermore, the application of laser capture microdissection and gene expression analysis to the model of immobilization and passive motion loading in rats may be particularly useful to reveal molecular pathways and mechanisms underlying cartilage degeneration.
4. Experimental Procedures
4.1. Animals
Male Sprague-Dawley rats (5-6 months old, weighing 580 ± 35g) were used in this study. Rats were housed in a 12 hour light/dark environment and were given free access to food and water. Following experiments, all animals were euthanized with CO2. All procedures were approved by the IACUC of Mount Sinai School of Medicine.
4.2. Immobilization
The right limbs of rats were immobilized as previously described (Coutinho, et al. 2002). Briefly, the rats were anesthetized with isoflurane and were fitted with a cast made of steel mesh and cotton materials which fixed the knee in full flexion. The rats were immobilized for 6 and 24 hours, and 7 and 21 days. A separate group of rats was used as naïve controls. For rats treated with NNGH during immobilization, intra-articular injections of 50μL of 5mM NNGH (Enzo Life Sciences International, Plymouth Meeting, PA) in 50% PBS/50% ethanol were given in the immobilized hind limb with a 28½ gauge needle via the patella tendon on days 1, 3, and 5 of the experiment under anesthesia.
4.3. Passive motion loading
Three groups of rats were subjected to immobilization as described above for 6 or 24 hours, or 7 days. During the middle of each immobilization period (for 6 and 24 hour groups), and daily, 12 hours after the initial application of the casts (for 7 day group), rats were anesthetized, the casts were removed, and the rats were placed on a joint loading system (Gu, et al. 2009) for 1 hour. During that period, these rats were subjected to motion loading at a frequency of two cycles per minute, with a range of motion between 65° and 115°, while a separate group of rats were maintained in the device without motion, at 115° of knee flexion (sham group). After the experimental period, all rats were either sacrificed or re-cast until the next motion loading session.
4.4. Laser capture microdissection
Distal femora were dissected, decalcified with Morse's solution (20% formic acid, 10% sodium citrate) for 2 days at 4°C, fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid) for 1 hour at 4°C (Shao, et al. 2006), embedded in paraffin, cut into 5-7μm thick sections and mounted on Superfrost/Plus slides. After deparaffinization, slides were air-dried and laser capture microdissection (LCM) was performed with the Arcturus Pixcell IIe (Mountain View, CA). Approximately 500 chondrocytes were microdissected from each of the superficial, middle, and deep zones of the lateral and medial condyles using the following instrument settings: Power – 85mW, Spot size – 7.5μm, Duration – 600μs-2.5ms. Captured cells were collected in microtubes containing lysis buffer (Qiagen, Valencia, CA).
4.5. RNA isolation, cDNA synthesis, real-time PCR
Total RNA was extracted using the RNeasy mini kit (Qiagen) with DNase treatment. RNA was quantitated with a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE), then reverse transcribed (RT) using oligo(dT) primers. Two nanograms of total RNA were analyzed by real-time PCR with SYBR Green to assess MMP expression, as well as GAPDH and β-actin as housekeepers. PCR primers pairs used were: MMP-3, forward 5′-TCAGCGGATCTTCACAGTTG-3′, reverse 5′-ACTTCAGTGCGCCAAGTTTC-3′; GAPDH, forward 5′-GAGGACCAGGTTGTCTCCTG-3′, reverse 5′-ATGTAGGCCATGAGGTCCAC-3′; β-actin, forward 5′-TTGCTGACAGGATGCAGAAG-3′, reverse 5′-ACATCTGCTGGAAGGTGGAC-3′. Expression values of GAPDH and ß-actin for each treatment condition were averaged and used as a denominator to determine the relative expression level of MMP-3 (Yokota, et al. 2003).
4.6. MMP activity assay
Tissues were dissected, flash-frozen in liquid nitrogen and stored at -80°C. Frozen tissue samples were pulverized (Dismembrator, B Braun Biotech, Germany) and enzyme activity was quantitated in tissue extracts using fluorogenic substrates specific for individual MMP's as described previously (Lee, et al. 2009). To measure total MMP levels in the tissue, assays were carried out in the presence of the activator 4-aminophenylmercuric acetate (APMA); endogenous active MMP levels were determined in assays carried out in the absence of APMA.
4.7. Immunohistochemistry and Safranin O staining
Immunostaining of histological sections prepared as described above was performed using monoclonal antibodies against MMP-3 and ADAMTS-5. Endogenous peroxidase activity was blocked with 3% (vol/vol) H2O2 for 5 minutes. Tissues were incubated with primary antibody overnight at 4°C with anti-MMP-3 (1:100 dilution, Abcam, Cambridge, MA) and anti-ADAMTS-5 (1:100 dilution, Abcam) followed by a 30 minute incubation with anti-rabbit secondary antibody (Dako, Carpinteria, CA) and visualization with TrueBlue (KPL, Gaithersburg, MD) for 10 minutes or DAB chromagen for 3 minutes. Negative control sections were prepared using irrelevant isotype-matched antibodies (Dako) in place of the primary antibody. Safranin O-fast green staining was carried out to demonstrate glycosaminoglycans in the articular cartilage.
4.8. Statistical Analysis
Results are expressed as the mean ± SEM. Statistical analysis was carried out using a one-way ANOVA and Tukey's test for post hoc analysis with significance set at P < 0.05.
Acknowledgments
This study was supported by grants from the National Institutes of Health to H.S. (AR47628 and AR52743) and L.C. (AG34198 and HL101157) and from the National Science Foundation to L.C. (0723027).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci. 2002;59:5–18. doi: 10.1007/s00018-002-8400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–2789. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- Blom AB, van Lent PL, Libregts S, Holthuysen AE, van der Kraan PM, van Rooijen N, van den Berg WB. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56:147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58:150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Bullough PG. The role of joint architecture in the etiology of arthritis. Osteoarthritis Cartilage. 2004;12 A:S2–9. doi: 10.1016/j.joca.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Cawston TE, Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol. 2006;20:983–1002. doi: 10.1016/j.berh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Coutinho EL, Gomes AR, Franca CN, Salvini TF. A new model for the immobilization of the rat hind limb. Braz J Med Biol Res. 2002;35:1329–1332. doi: 10.1590/s0100-879x2002001100010. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- Evans EB, Eggers GWN, Butler JK, Blumel J. Experimental Immobilization and Remobilization of Rat Knee Joints. J Bone Joint Surg Am. 1960;42:737–758. [Google Scholar]
- Ferretti M, Gassner R, Wang Z, Perera P, Deschner J, Sowa G, Salter RB, Agarwal S. Biomechanical signals suppress proinflammatory responses in cartilage: early events in experimental antigen-induced arthritis. J Immunol. 2006;177:8757–8766. doi: 10.4049/jimmunol.177.12.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti M, Srinivasan A, Deschner J, Gassner R, Baliko F, Piesco N, Salter R, Agarwal S. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J Orthop Res. 2005;23:1165–1171. doi: 10.1016/j.orthres.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine KR, Heo M, Bathon J. Are US adults with arthritis meeting public health recommendations for physical activity? Arthritis Rheum. 2004;50:624–628. doi: 10.1002/art.20057. [DOI] [PubMed] [Google Scholar]
- Freeman MA, Pinskerova V. The movement of the normal tibio-femoral joint. J Biomech. 2005;38:197–208. doi: 10.1016/j.jbiomech.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Gu XI, Leong DJ, Guzman F, Mahamud R, Li YH, Majeska RJ, Schaffler MB, Sun HB, Cardoso L. Development and Validation of a Motion and Loading System for a Rat Knee Joint In Vivo. Ann Biomed Eng. 2009 doi: 10.1007/s10439-009-9865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala J, Arokoski J, Pirttimaki J, Lyyra T, Jurvelin J, Tammi M, Helminen HJ, Kiviranta I. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000;21:76–81. doi: 10.1055/s-2000-8860. [DOI] [PubMed] [Google Scholar]
- Haapala J, Arokoski JP, Hyttinen MM, Lammi M, Tammi M, Kovanen V, Helminen HJ, Kiviranta I. Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res. 1999:218–229. [PubMed] [Google Scholar]
- Haapala J, Lammi MJ, Inkinen R, Parkkinen JJ, Agren UM, Arokoski J, Kiviranta I, Helminen HJ, Tammi MI. Coordinated regulation of hyaluronan and aggrecan content in the articular cartilage of immobilized and exercised dogs. J Rheumatol. 1996;23:1586–1593. [PubMed] [Google Scholar]
- Hagiwara Y, Ando A, Chimoto E, Saijo Y, Ohmori-Matsuda K, Itoi E. Changes of articular cartilage after immobilization in a rat knee contracture model. J Orthop Res. 2009;27:236–242. doi: 10.1002/jor.20724. [DOI] [PubMed] [Google Scholar]
- Jones MH, Amendola AS. Acute treatment of inversion ankle sprains: immobilization versus functional treatment. Clin Orthop Relat Res. 2007;455:169–172. doi: 10.1097/BLO.0b013e31802f5468. [DOI] [PubMed] [Google Scholar]
- Jurvelin J, Kiviranta I, Tammi M, Helminen JH. Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop Relat Res. 1986:246–252. [PubMed] [Google Scholar]
- Kasten P, Geiger F, Zeifang F, Weiss S, Thomsen M. Compliance with continuous passive movement is low after surgical treatment of idiopathic club foot in infants: a prospective, double-blinded clinical study. J Bone Joint Surg Br. 2007;89:375–377. doi: 10.1302/0301-620X.89B3.18184. [DOI] [PubMed] [Google Scholar]
- Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52:520–526. doi: 10.1136/ard.52.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Taub PJ, Wang L, Clark A, Zhu LL, Maharam ER, Leong DJ, Ramcharan M, Li Z, Liu Z, Ma YZ, Sun L, Zaidi M, Majeska RJ, Sun HB. Identification of CITED2 as a negative regulator of fracture healing. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Hoerrner LA, Dahlberg L, Roos H, Bjornsson S, Lark MW. Stromelysin, tissue inhibitor of metalloproteinases and proteoglycan fragments in human knee joint fluid after injury. J Rheumatol. 1993;20:1362–1368. [PubMed] [Google Scholar]
- Lohmander LS, Hoerrner LA, Lark MW. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993;36:181–189. doi: 10.1002/art.1780360207. [DOI] [PubMed] [Google Scholar]
- McCarthy C, Oakley E. Management of suspected cervical spine injuries--the paediatric perspective. Accid Emerg Nurs. 2002;10:163–169. doi: 10.1054/aaen.2002.0360. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Davies LW, Nuki G, Salter DM. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000;43:2091–2099. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66:680–690. [PubMed] [Google Scholar]
- Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989:12–25. [PubMed] [Google Scholar]
- Salter RB. Continuous passive motion: from origination to research to clinical applications. J Rheumatol. 2004;31:2104–2105. [PubMed] [Google Scholar]
- Saxon L, Finch C, Bass S. Sports participation, sports injuries and osteoarthritis: implications for prevention. Sports Med. 1999;28:123–135. doi: 10.2165/00007256-199928020-00005. [DOI] [PubMed] [Google Scholar]
- Shao YY, Wang L, Hicks DG, Ballock RT. Analysis of gene expression in mineralized skeletal tissues by laser capture microdissection and RT-PCR. Lab Invest. 2006;86:1089–1095. doi: 10.1038/labinvest.3700459. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Woolley DE. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage. 2001;9:423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- Thambyah A. Contact stresses in both compartments of the tibiofemoral joint are similar even when larger forces are applied to the medial compartment. Knee. 2007;14:336–338. doi: 10.1016/j.knee.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Bhargava M, Park S, Chen CT. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwanseele B, Eckstein F, Knecht H, Spaepen A, Stussi E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum. 2003;48:3377–3381. doi: 10.1002/art.11367. [DOI] [PubMed] [Google Scholar]
- Vanwanseele B, Eckstein F, Knecht H, Stussi E, Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46:2073–2078. doi: 10.1002/art.10462. [DOI] [PubMed] [Google Scholar]
- Vanwanseele B, Lucchinetti E, Stussi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage. 2002;10:408–419. doi: 10.1053/joca.2002.0529. [DOI] [PubMed] [Google Scholar]
- Walakovits LA, Moore VL, Bhardwaj N, Gallick GS, Lark MW. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992;35:35–42. doi: 10.1002/art.1780350106. [DOI] [PubMed] [Google Scholar]
- Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–47280. doi: 10.1074/jbc.M304652200. [DOI] [PubMed] [Google Scholar]


