Abstract
Objectives
To determine whether DNA mismatch repair (MMR) modifies the response to chemotherapy or radiotherapy in patients with endometrial cancer.
Methods
Immunohistochemistry (IHC) for the DNA MMR proteins MLH1, MSH2, MSH6, and PMS2 was performed on a tissue microarray of specimens of primary endometrial cancer. MMR deficiency was defined as lack of expression of one or more proteins. Expression of all proteins classified a tumor as having an intact MMR system. Recurrence rates were calculated for women treated with platinum-based chemotherapy or pelvic external beam radiation. Comparisons were made using the log-rank test. Multiple comparisons were controlled for by utilizing the Bonferroni correction method.
Results
Four hundred seventy-seven cases of endometrial cancer were evaluated on a tissue microarray (TMA). One hundred fifty-eight patients (41%) received chemotherapy. Sixty-six patients (17%) received pelvic teletherapy. Overall and progression-free survival were not different between patients whose tumors had intact MMR and those with defective MMR when stratified by adjuvant treatment with radiation or chemotherapy. Subgroup analyses stratified by histology (non-endometrioid versus endometrioid) and stage did show significant survival differences. There was a significant increase in overall (p=0.003) and progression-free (p=0.004) survival in those with MMR-deficient, non-endometrioid tumors treated with teletherapy compared to those with an intact MMR system. Improved progression-free survival was noted in patients with intact MMR with stage III/IV disease treated with adjuvant chemotherapy (p=0.031).
Conclusions
Subgroups of patients with non-endometrioid endometrial cancer and defective MMR may have improved survival after adjuvant radiotherapy. Patients with advanced stage endometrial cancer and defects in mismatch repair may receive less benefit from adjuvant chemotherapy.
Keywords: Endometrial cancer, Lynch syndrome, Mismatch repair
Introduction
Endometrial cancer is the most common malignancy of the female genital tract in the United States with an estimated 42,160 cases expected to be diagnosed in 2009 [1]. The most frequently identified molecular change in endometrial cancer is a defect in DNA mismatch repair (MMR). Although approximately 30% of endometrial cancers demonstrate-deficient DNA MMR, only rarely is this related to a mutation in one of the genes encoding the MMR proteins, MLH1, MSH2, MSH6, and PMS2, the cause of Lynch syndrome. The majority of cases of deficient MMR are related to the epigenetic methylation of the MLH1 promoter.
A number of studies have evaluated the prognostic implications of DNA MMR in endometrial cancer. Although there is no consensus as to the influence of MMR on survival, our group has recently reported worse survival in patients with endometrial cancers with deficient DNA MMR system [2].
Colorectal cancer is perhaps the best-studied model for mismatch repair. Approximately 15% of these cancers are characterized by defects in mismatch repair. In contrast to endometrial cancer, studies with colon cancer have shown an improved cancer outcome associated with tumors with deficient MMR [3]. Furthermore, colorectal cancers with deficient DNA MMR have been demonstrated to be more responsive to treatment with 5-fluorouracil based chemotherapy regimens compared with tumors with an intact MMR system [3,4].
To date, there is no clear consensus on how MMR status may modulate the response to ionizing radiation and chemotherapy in endometrial cancer. While there is an abundance of in vitro data regarding these questions, our objective was to examine the relationship between mismatch repair status, adjuvant therapy, and clinical outcome in a large cohort of surgically staged endometrial cancer patients.
Methods
Patient selection
Following approval from the institutional review board of the Ohio State University College of Medicine, all patients with primary uterine cancer surgically managed by the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology at The Ohio State University Medical Center from January 1997 to July 2003 were identified from a clinical database. Approximately 90% of endometrial cancer patients at The Ohio State University Medical Center are surgically staged. Generally, these patients undergo extrafascial or radical hysterectomy, bilateral salpingo-oophorectomy, and bilateral pelvic and para-aortic lymphadenectomy. The extent of surgical staging was left to the discretion of the surgeon with full staging omitted in those with either gross extra-uterine disease or confined disease in those patients with a high operative risk. Patients who underwent their primary surgery at an outside institution were excluded from the study. Tumors were both endometrioid (EC) and non-endometrioid histology (NEC). NEC tumors were comprised of serous, clear cell, mixed-epithelial, and uterine carcinosarcomas. Data regarding patient demographics, operative findings, tumor pathology, and survival were recorded from the source data. Patients typically undergo surveillance with vaginal cytology and pelvic examination every 3 months for 2 years and then every 6 months for 3 years.
The delivery of adjuvant chemotherapy and radiation therapy was at the discretion of the treating physician. In general, patients with uterine-confined disease were observed following comprehensive staging. Patients with metastatic disease were treated with adjuvant external beam radiation or chemotherapy. Prior to 2003, chemotherapy treatment was generally with cisplatin and adriamycin. Following 2003, chemotherapy treatment was generally platinum and a taxane. There was a portion of patients who received both chemotherapy and radiation. Due to the small number of events taking place, these patients were unable to be statistically analyzed as a separate group and were analyzed as part of both the chemotherapy and radiation cohorts.
Tissue microarray
Tissue cores from formalin-fixed paraffin blocks from 477 patients with primary endometrial cancer were arrayed into a 35×20-mm recipient paraffin block with a custom-built precision instrument (Beecher Instruments, Silver Springs, MD). Four cores from each block were used to create a tissue microarray of 0.6-mm cores, including both tumor specimens and controls including normal endometrium, brain, and pancreas tissue. Tumor grading was performed according to current International Federation of Gynecology and Obstetrics (FIGO) standards. All cases were reviewed by one of the authors (C.D. M). The presence of tumor tissue on the microarray was verified with hematoxylin and eosin staining.
Immunohistochemistry
Immunoperoxidase staining was performed on formalin-fixed, paraffin-embedded tissue cuts at four microns and placed on positively charged slides. Slides were then placed in a 60 °C oven for 1 h, cooled, then deparaffinized and rehydrated through xylenes and graded ethanol solutions to water. All slides were quenched for 5 min in a 3% hydrogen peroxide solution in methanol to block for endogenous peroxidase. Antigen retrieval was performed by a steamer method in which the specimens were placed in a citric acid solution (Target Retrieval Solution, pH 6.1, Dako Cytomation, Carpinteria, CA) for 30 min at 94 °C using a vegetable steamer. Slides were then placed on a Dako autostainer for use with immnohisto-chemistry and stained with MLH1 (clone G168-15, 1:40, BD Pharmingen, San Diego, CA), MSH2 (clone FE11, 1:200, Oncogene Research Products, Cambridge, MA), MSH6 (clone 44, 1:200, Transduction Laboratories, Lexington , KY), and PMS2 (clone SC-618, 1:200, Santa Cruz Biotechnology, Incorporated, Santa Cruz, CA) antibodies. The detection system used was labeled streptavidin– biotin complex. This method is based on the consecutive application of (1) a primary antibody against the antigen to be localized, (2) biotinylated linking antibody, (3) enzyme conjugated streptavidin, and (4) substrate chromogen. Tissues were avidin and biotin blocked prior to the application of the biotinylated secondary reagent. Slides were then counterstained in hematoxylin, dehydrated through graded ethanol solutions. For the above antibodies, staining was considered negative only if all 4 cores on the TMA were without immunoreactivity. A single pathologist (W.L.F.) and an author (K.E.R.) reviewed each TMA.
Definition of mismatch repair (MMR)
Tumors that lacked staining of any one of the four MMR proteins were classified as having deficient MMR. Tumors with positive staining for all four proteins were classified as having an intact MMR system.
Statistics
All statistical tests were performed with SAS (v.9.1) (SAS Institute Inc., Cary, North Carolina). The rates of recurrence between deficient and intact MMR were compared using the chi-square test. Data were collected from the date of surgery to last known follow-up. Those that died from other causes or were lost to follow-up were censored at the either death or at the last known follow-up date. Kaplan–Meier estimates of overall survival and progression-free survival were calculated, when possible, and time to progression and death was compared using the log-rank test. The assumption of proportional hazards was verified for each comparison. We also stratified the analysis by histology (endometrioid and non-endometrioid) and stage (stages I/II and III/IV). A Bonferroni-corrected alpha level of 0.025 was used for the multiple comparisons of histology and stage strata.
Results
There were 477 patients evaluated on the TMA. The characteristics of the patients are summarized in Tables 1 and 2. MMR-deficient tumors are summarized by staining profile in Table 3.
Table 1.
Surgical pathologic factors in the patients represented on the tissue microarray.
| Variable | Number (%) |
|---|---|
| Median age (range), years | 62 (17–89) |
| Race | |
| White | 414 (87) |
| African American | 38 (8) |
| Other or unknown | 25 (5) |
| Median BMI (range), kg | 33.5 (16–77) |
| Histologic type | |
| Endometrioid | 364 (76) |
| Non-endometrioid (Mixed epithelial, carcinosarcoma serous, clear cell) | 113 (23) |
| Tumor grade | |
| 1 | 262 (55) |
| 2 | 95 (19) |
| 3 | 119 (25) |
| Stage | |
| I | 292 (61) |
| II | 41 (8.5) |
| III | 106 (22) |
| IV | 35 (7.3) |
Table 2.
Clinical and pathologic characteristics based on mismatch repair (MMR) status.
| Variable | MMR+ (n = 322) | MMR– (n = 155) |
|---|---|---|
| Median age (years) | 63 | 68 |
| BMI | 33.8 kg/m2 | 30.5 kg/m2 |
| Grade | ||
| 1 | 178 (55%) | 47 (24%) |
| 2 | 112 (34%) | 48 (31%) |
| 3 | 32 (9%) | 60 (39%) |
| Stage | ||
| Ia | 78 (24%) | 22 (4%) |
| Ib | 90 (27%) | 41 (26%) |
| Ic | 44 (13%) | 21 (13%) |
| IIa | 12 (3%) | 5 (3%) |
| Iib | 9 (2%) | 11 (7%) |
| IIIa | 23 (7%) | 13 (8%) |
| IIIb | 8 (2%) | 2 (1%) |
| IIIc | 35 (10%) | 33 (21%) |
| Iva | 4 (1%) | 0 (0%) |
| Ivb | 19 (5%) | 7 (4%) |
| Histology | ||
| Endometrioid | 189 (58%) | 124 (80%) |
| Non-endometrioid | 133 (42%) | 31 (20%) |
| Adjuvant therapy | ||
| Chemotherapy | 53 (33%) | 105 (67%) |
| Radiation | 47 (71%) | 19 (29%) |
Table 3.
Breakdown of MMR-deflcient tumors by negative staining.a
| MLH1/MSH2/PMS2/MSH6 | 12 (8.0%) |
| MLH1/PMS2/MSH6 | 1 (.7%) |
| MLH1/MSH2/PMS6 | 1 (.7%) |
| MSH2/PMS2/MSH6 | 3 (2.2%) |
| MLH1/MSH2 | 3 (2.2%) |
| MLH1/PMS2 | 89 (63.1%) |
| MSH2/MSH6 | 3 (2.2%) |
| MSH2/PMS2 | 1 (.7%) |
| MLH1/MSH6 | 1 (.7%) |
| MLH1 | 8 (5.9%) |
| PMS2 | 12 (8.8%) |
| MSH2 | 1 (.7%) |
Six patients were lacking at least one MMR gene but had indeterminate staining for the remaining 3 MMR genes.
Chemotherapy patients
We were able to obtain data for all variables of interest for 385 (80.7%) of the 477 eligible patients for the chemotherapy subgroup. A total of 158/385 (34%) patients received adjuvant chemotherapy. Fifty-six tumors of those treated with chemotherapy (21.8%) were non-endometrioid (NEC). Intact MMR was seen in 53/158 (33.5%) tumors from patients treated with chemotherapy while 105 (66.5%) were MMR deficient. Twelve recurrences (22.6%) were noted among these 53 patients. Among the 105 patients with tumors that were MMR deficient, 28 (26.7%) recurrences were noted.
Median overall survival for those patients receiving chemotherapy and with intact MMR was 13 years. Median overall survival for those with defects in the MMR system could not be estimated due to small number of events. However, no difference in overall or progression-free survival between these groups was seen (data not shown).
Radiation
We were able to obtain data for all variables of interest for 383 (80.3%) of the 477 eligible patients when looking at the radiation therapy subgroup. A total of 66/383 (17.2%) patients received radiation therapy. Forty-seven (71.2%) of the 66 tumors were MMR deficient. Eight recurrences (17.0%) were noted in patients with deficient MMR treated with teletherapy. Of the 19 (28.8%) with intact MMR, 5 (26.3%) had a recurrence.
Median overall survival for those patients receiving radiation therapy and with an intact MMR was 13 years. Median overall survival for those with defective MMR could not be estimated due to small number of events. However, no difference in overall or progression-free survival between these groups was seen (data not shown).
Combined adjuvant therapy
Of the 66 patients who received radiation therapy, 30 patients (53%) received both chemotherapy and radiation. Of the group that received both, endometrioid histology comprised the largest percentage of histologic subtype (70%).
Histology and stage
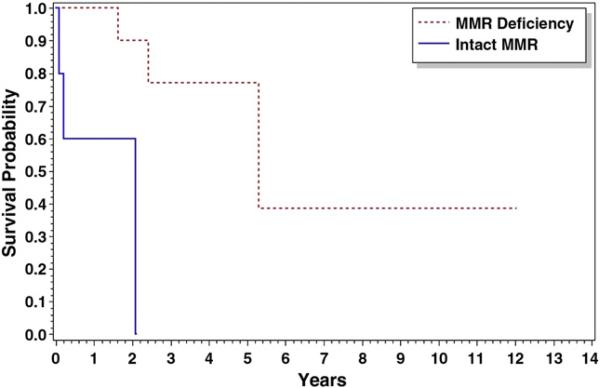
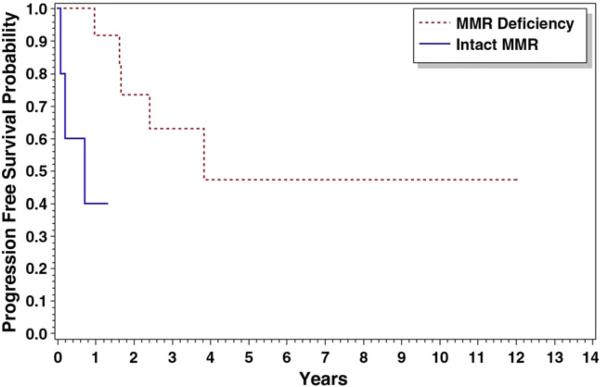
When stratified by histology (NEC versus EC), survival differences between MMR-deficient and MMR-intact patients were seen. Patients with NEC tumors with defective MMR (n=14) who received radiation therapy demonstrated improved overall survival (p=0.003) and progression-free survival (p=0.004) compared with those with an intact MMR system (n=5). The median time to death (5 years) and progression (5 years) was higher in the MMR-deficient group of tumors than that in patients with an intact MMR system (2 and 2 years, respectively) (Figs. 1 and 2).
Fig. 1.
Overall survival for non-endometrioid patients receiving radiation therapy: Median overall survival was 5.30 vs. 2.09 years for MMR(−) and MMR(+), respectively, p=0.003.
Fig. 2.
Progression-free survival for non-endometrioid patients receiving radiation therapy: Median overall survival was 5.30 vs. 2.09 years for MMR(−) and MMR(+), respectively, p=0.004.
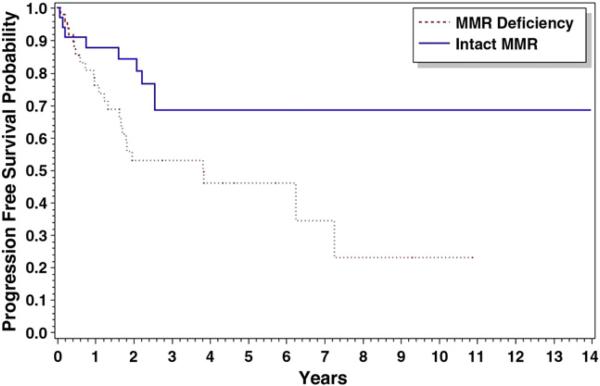
Additionally, there was improvement in progression-free survival in those MMR-intact patients with stage III/IV disease receiving chemo-therapy. Median progression-free survival was 3.81 years for MMR-deficient advanced stage cancers treated with chemotherapy (n=50). The median progression-free survival was unable to be calculated for similarly treated cancers with intact MMR secondary to the low number of events (Fig. 3). There was overall improvement (p=0.031) in progression-free survival in advanced stage cancers with an intact MMR system treated with chemotherapy (n=33). Overall survival was unable to be calculated secondary to the low number of events.
Fig. 3.
Progression-free survival for stage III/IV patients receiving chemotherapy: Median overall survival was 3.81 years for MMR(−) but was not calculable for MMR (+), p=0.031.
Discussion
In women with endometrial cancer, DNA mismatch repair appears to modulate response to both chemotherapy and radiation in certain subsets of patients. In endometrial cancer, Cohn et al. [2] reported on the prognostic significance of MMR status in a large group of comprehensively staged patients. In this previous study, endometrial tumors with deficient DNA MMR were associated with poor prognostic factors including lympho-vascular space involvement and cervical involvement. Tumors with deficient DNA MMR were associated with a worse survival when compared with tumors with an intact MMR system. This study, however, did not examine the role of adjuvant therapy in outcome or specifically how MMR status influences the response to adjuvant treatment. The patient population described here includes the 336 patients described previously by Cohn et al. [2]
The results of our current study force us to consider the role adjuvant chemotherapy in those patients with defective mismatch repair. In our patients, adjuvant chemotherapy was almost universally platinum based. The mechanism of action of both cisplatin and carboplatin includes the formation of inter- and intra-strand DNA crosslinks as well as adduct formation [4]. While bulky DNA adducts are likely repaired by the nucleotide excision repair pathway, MMR deficiency is now being seen as a potential explanation for platinum resistance. Previously, platinum resistance in MMR-deficient cell lines was explained using the “futile cycling” model by which platinum-induced DNA damage in MMR-deficient cells was unable to be repaired; thus, mutated cells persisted in the cell cycle. Currently, it is believed that an intact MMR system may directly signal the cellular apoptotic machinery [6]. With defects in the MMR system the damaged cell will lack this signal and apoptosis will not occur [7]. Additionally, cisplatin sensitivity has been amplified by restoring MMR in deficient cell lines [8,9].
The relationship between MMR status and response to platinum may also be related to the status of MLH1 promoter methylation. When A2780, an ovarian cancer cell line with methylation of MLH1 promoter, was treated with 5-AZA, an inhibitor of methylation, cells showed increased sensitivity to all concentrations of cisplatin [10]. Given that the majority of the deficient MMR seen in endometrial cancer is due to methylation of the MLH1 promoter, it stands to reason that worse survival may be expected in MMR-deficient tumors treated with cisplatin as was seen in our study.
There is a lack of consensus regarding the response of MMR-deficient cells to ionizing radiation. Ionizing radiation creates a variety of lesions within DNA. While base pair mismatch does occur, the majority of DNA damage is caused by single- and double-stranded breaks in the DNA [5]. Until now, the majority of studies examining the response to ionizing radiation have involved cell lines derived from explants of knockout mice or cell lines known to be deficient in one of the MMR genes. Fritzell et al. [11] demonstrated that cell lines derived from PMS2, MLH1, and MSH2 nullizygous mice all demonstrated higher levels of survival following radiation doses up to 1200 cGy. In contrast, Franchitto et al. [12] demonstrated that MSH2-deficient cells are slightly more sensitive to ionizing radiation and that MSH2 may modulate genes involved in the double-stranded repair process. Additionally, it has also been reported that Myc down-regulation induces down-regulation of MHL1 and MSH2 in melanoma cells, in the process increasing cell sensitivity to radiation [13].
One explanation to our finding of increased sensitivity to radiation in MMR-deficient tumors may be additional DNA repair modulation by the tumor suppressor gene p53. p53 has been implicated in the cellular response to radiation by activating cell cycle checkpoints and apoptosis [14]. Double knockout cells deficient in both Pms2 and p53 demonstrated increased sensitivity to radiation when compared to either Pms2-null or p53-null cells, indicating mutually exclusive roles for the MMR system and p53 [15]. Previously, our group has shown that in NEC, MMR-intact tumors are more likely to demonstrate strong over-expression of p53 than MMR-deficient tumors (47% vs. 17%, p=0.025) [16]. Perhaps the improved survival seen in the NEC, MMR-deficient patients treated with radiation is secondary to “rescue” by a functional p53 allowing apoptosis to occur. Follow-up, multi-institutional studies examining clinical outcomes associated with MMR status in conjunction with p53 status will be needed in order to determine if our findings are reproducible.
Our current study suffers from the common weaknesses of an observational study: adjuvant therapy was prescribed at the discretion of the treating physician, non-standardized radiation fields and chemo-therapeutic agents were administered, and patient follow-up was not always complete. However, there are a number of strengths of this study. We report on a very large series of consistently treated patients, with a high proportion of patients undergoing surgical staging. In addition, our patient population was not selected for family history; thus, our results should not be enriched for patients with Lynch syndrome. However, the patients reported in the current study were a population intentionally enriched for non-endometrioid histology so as to maximize the number of patients receiving adjuvant therapy. A potential criticism of our study is that we have chosen to screen our patients for defects in MMR with IHC rather than MSI testing. Defective MMR is typically defined as lack of staining of any single MMR protein. Hampel et al. [17] recently published the results of prospective Lynch screening in 500 newly diagnosed colon cancer patients. The sensitivity of IHC in this population was 94%. The positive predictive value of an abnormal IHC for detecting Lynch syndrome was 23.89%; significantly higher than both age b50 (10.3%) and first degree relative with colon cancer or EMCA (8.8%). Vasen et al. [18] described a 93% concordance between IHC and MSI testing in colon tumors when all 4 MMR proteins were examined. We have previously demonstrated that positive staining for MLH1 or MSH2 predicted an intact mismatch repair system in 95% of cases [2].
As well, there was a subset of patients who did receive both chemotherapy and radiation. Secondary to the small number of events occurring in this population of endometrial cancer patients, this population was unable to be analyzed separately. As well, multivariate analysis was unable to be performed. The small samples sizes would have likely led to unstable estimates. While this will likely bias the results, given that there was no statistical significance with chemo-therapy treatment when stratified by histology, we do not feel this greatly affects the results in the NEC radiation cohort. Interestingly, in the original report by Cohn et al. [2], a higher proportion of grade 2/3 tumors was seen in the MMR-deficient tumors although this trend did not reach statistical significance. In our current report, we do note a significantly higher proportion of grade 2/3 tumors among the MMR-deficient tumors. While this certainly may indicate an inherent difference in the tumor biology and aggressiveness of MMR-deficient tumors, our study is not powered to detect such a difference. We feel strongly that practice patterns should not be based on the results of a subgroup analysis and prospective studies with standard treatment protocols would be needed in order to avoid the bias inherent in retrospective studies.
In conclusion, we find that in this large series of patients with surgically staged endometrial cancer, deficient DNA mismatch repair does appear to modify the response to adjuvant chemotherapy and radiation. Our findings, which have biologic relevance based on the in vitro data, need further examination in a large, multi-institutional study. The clinical utility of our findings is apparent; a significant portion of endometrial cancer patients will require adjuvant radiation therapy. Information such as this may be useful in the assignment of these treatments in order to maximize clinical outcomes.
Footnotes
This manuscript was presented in abstract form at the 37th Annual Meeting of the Society of Gynecologic Oncology in Palm Springs California, March 22–26, 2006.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics. A Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cohn DE, Frankel WL, Resnick KE, et al. Improved survival with an intact DNA mismatch repair system in endometrial cancer. Obstet Gynecol. 2006;108:1208–15. doi: 10.1097/01.AOG.0000239097.42987.0c. [DOI] [PubMed] [Google Scholar]
- 3.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumor site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–50. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 4.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsattelite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Eng J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers M, Hwang A, Wagner MW, Boothman DA. Special section: impact of the environment on colon cancer (II), Role of DNA mismatch repair in apoptotic responses to therapeutic agents. Environ Mol Mutagen. 2004;44:249–64. doi: 10.1002/em.20056. [DOI] [PubMed] [Google Scholar]
- 6.Brabec V, Kasparkova J. Molecular aspects of resistance to antitumor platinum drugs. Drug Resist Updat. 2002:147–61. doi: 10.1016/s1368-7646(02)00047-x. [DOI] [PubMed] [Google Scholar]
- 7.Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-abl regulates p73 in apoptotic response to cisplatin induced DNA damage (see comments). Nature. 1999;399(6738):806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 8.Aebi S, Kurdi-Haidar B, Gordon R, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res. 1996;56:3087–90. [PubMed] [Google Scholar]
- 9.Fink D, Nebel S, Aebi S, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- 10.Strathdee G, MacKean MJ, Illand M, Brown R. A role for methylation of the hMLH1 promoter in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene. 1999;18:2335–41. doi: 10.1038/sj.onc.1202540. [DOI] [PubMed] [Google Scholar]
- 11.Fritzell JA, Narayanan L, Baker SM, et al. Role of DNA mismatch repair in the cytotoxicity of ionizing radiation. Cancer Res. 1997;57:5143–7. [PubMed] [Google Scholar]
- 12.Franchitto A, Pichierri P, Piergentili R, Crescenzi M, Bignami M, Palitti F. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene. 2003;57:5143–7. doi: 10.1038/sj.onc.1206254. [DOI] [PubMed] [Google Scholar]
- 13.Bucci B, D'Agnano, Amendola D, et al. Myc down-regulation sensitizes melanoma cells to radiotherapy by inhibition of MLH1 and MSH2 mismatch repair proteins. Clin Cancer Res. 2005;7:2756–67. doi: 10.1158/1078-0432.CCR-04-1582. [DOI] [PubMed] [Google Scholar]
- 14.Giaccia A, Kastan M. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–83. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 15.Zeng M, Narayanan L, Xu XS, Prolla TA, Liskay RM, Glazer PM. Ionizing radiation-induced apoptosis via separate Pms2- and p53-dependent pathways. Cancer Res. 2000;60:4889–93. [PubMed] [Google Scholar]
- 16.Cohn DE, Resnick KE, Morrison CD, et al. DNA mismatch repair and molecular alterations in non-endometrioid endometrial cancers. Abstract Gynecol Oncol. 2006;101:S2–S177. [Google Scholar]
- 17.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for lynch syndrome among patients with colon cancer. J Clin Oncol. 2008;26:5783–6. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasen HFA, Hendriks Y, de Jong AE, et al. Identification of HNPCC by molecular analysis of colorectal and endometrial tumors. Dis Markers. 2004;20:207–13. doi: 10.1155/2004/391039. [DOI] [PMC free article] [PubMed] [Google Scholar]