Abstract
No studies have yet examined whether there are prognostic factors for survival for cats undergoing splenectomies. The medical records of 19 cats that had complete splenectomy were reviewed for information on preoperative, intraoperative, and postoperative factors. The most common presenting signs were a palpable abdominal mass in 58% and anorexia in 47% of the cats. Mast cell tumors were the most common reason for splenectomy and were found in 10/19 cats (53%); followed by hemangiosarcoma in 4/19 (21%); and lymphoma in 2/19 (11%). The Kaplan–Meier median survival time (MST) was 197 days, with a range from 2 days to 1959 days. Three cats were noted to have preoperative weight loss, and this was the only factor that had prognostic significance for survival following surgery. For cats with weight loss the MST was 3 days, for cats with no weight loss noted the MST was 293 days (P=0.008).
Complete splenectomy is indicated to treat suspected malignant neoplasia, ischemic obstruction, or generalized splenic enlargement secondary to infiltrative diseases of the spleen. It may also be used as part of the treatment for some immune-mediated diseases. 1 The types, treatments, and prognoses of splenic diseases in cats are not well described. The most common splenic diseases in cats include mast cell tumors (MCTs), lymphoma, and myeloproliferative disease. 2,3
Several papers have examined prognostic indicators for survival time in dogs following splenectomy, and have found that dogs with non-neoplastic and benign splenic diseases survive for a longer period after a splenectomy than dogs with neoplastic splenic disease. 4–6 To date, no studies have looked at survival of cats after a splenectomy with delineation of predictors of survival. The purpose of this study was to identify common reasons and clinical predictors of survival for cats undergoing splenectomy.
Materials and methods
The medical records of 73 cats undergoing splenectomies between July 1999 and 2005 were reviewed. Fifty-four cases were excluded based on missing or incomplete medical records or if partial splenectomy was performed. Nineteen cats that had complete splenectomy between July 1999 and July 2005 met the inclusion criteria. Data were collected through record review, veterinarian contact, or owner contact. Data were grouped into three categories: preoperative, intraoperative, and postoperative factors.
Preoperative evaluation
Preoperative factors included age, breed, gender, presenting clinical signs (collapse, lethargy, anorexia, vomiting, or weight loss), presenting physical examination findings (palpable abdominal mass, or hemoabdomen), and hematological factors (packed cell volume (PCV), reticulocyte percentage and count, and platelet count). Weight loss was documented through serial weights or owner report. Presence of a hemoabdomen was either found preoperatively through abdominocentesis or intraoperatively. A red blood cell count of less than 32% was considered anemic (reference range 32–45%). Thrombocytopenia was diagnosed if the platelets were less than 150,000/μl (reference range 150,000–700,000/μl) and platelet clumping was not detected.
Intraoperative evaluation
Intraoperative factors included peri-operative antibiotic administration, evidence of abdominal metastases at the time of surgery, additional surgical procedures performed, whether a transfusion was given and total operative time (min). Evidence of metastasis was confirmed by histological examination of other organs (ie, liver, lymph nodes), which were biopsied during the splenectomy procedure.
Postoperative evaluation
Postoperative factors included histopathological findings and survival time. Survival time was defined as the time from surgery to euthanasia or death. When the information was available, the cause of death was described as related to the splenic disease or not. Some of the cats were euthanased at another veterinary facility and no further information as to the cause of death was available. The histopathological sections of the spleen from all 19 cases were reviewed retrospectively by one pathologist (SL) who was blind to the original diagnosis for confirmation of lesion categorization.
Median survival time (MST) in days postoperatively was calculated via Kaplan–Meier life table analysis. Associations of recorded variables with survival were performed by log-rank analysis for categorical variables and by Cox proportional hazards analysis for numerical and categorical variables. If univariate prognostic factors were found (any variable on univariate with a P-value≤0.2), step-wise multivariate analysis by Cox proportional hazards was completed. The different variables were examined for significant relationships using Fisher's exact test and the Mann–Whitney U test. Analyses were performed with standard software (Statview, version 5.0.1, SAS Institute, Cary, NC). For survival analysis, cats were censored if they were still alive at the end of the study or died as a result of another disease. P<0.05 was considered significant for all tests.
Results
Preoperative evaluation
Nineteen cats were included in this study (Table 1). The mean age at the time of splenectomy was 12.2 years (median, 12.0 years; range, 3.0–18.0 years). There were 11 neutered males (58%) and eight spayed females (42%). Sixteen of the cats were domestic shorthair, two were domestic longhair, and one was a Russian Blue. Presenting signs and findings included a palpable abdominal mass in 11/19 (58%); anorexia in 9/19 (47%); lethargy in 6/19 (32%); vomiting in 6/19 (32%); hemoabdomen in 4/19 (21%); weight loss in 3/19 (16%); and collapse in 3/19 (16%). Sixteen of the cats evaluated had multiple presenting signs. There was no significant relationship between signalment, presenting complaint or physical examination findings and postoperative survival except for the presence of weight loss. On multivariate analysis, presence of preoperative weight loss (either documented through serial weights or owner report) was significantly associated with survival time. For cats with weight loss the MST was 3 days, while for cats with no weight loss noted the MST was 293 days (P=0.008) (Fig. 1). Cats with weight loss prior to surgery were nearly six times more likely to die, compared with cats that did not lose weight (odds ratio=5.95, 95% confidence interval (CI): 1.30–27.0, P=0.02). When the data was analyzed using Cox proportional hazards step-wise multivariate, weight loss was the only significant variable, with cats that had evidence of preoperative weight loss having a 10.75 times greater chance of dying (95% CI=1.38–83.3, P=0.02).
Table 1.
Preoperative, intraoperative, and postoperative data on 19 cats that had complete splenectomies performed. Reference ranges are provided in paranthesis were appropriate.
| Age at surgery (yr) | Sex* | Breed† | Diagnosis‡ | Surgical time (min) | Abdominal metastasis | Transfusions§ | Hemoabdomen | Palpable abd mass | Vomiting | Anorexia | Weight loss | Lethargy | Collapse | Thrombocyte count (/μl) (300–700 thousand/μl) | Reticulocyte count (/μl) (0–500,000/mm3) | Preoperative PCV or Hct\ (32–45%) | Outcome | Survival time (days) | Chemotherapy∥ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | SF | Russian Blue | MCT | 44 | Y | WB, FFP, Ox | Y | N | N | N | N | N | Y | 87,000 | 93,240 | 21 | Alive | 1789 | No |
| 8 | SF | DSH | MCT | 112 | Y | Ox | N | N | Y | Y | N | Y | N | 32 | Dead | 3 | No | ||
| 12 | CM | DLH | MCT | 90 | Y | None | N | Y | N | Y | N | N | N | 19 | Alive | 854 | No | ||
| 12 | CM | DSH | MCT | 75 | Y | None | N | Y | N | N | N | N | N | 114,000 | 22,050 | 23 | Alive | 1959 | No |
| 12 | SF | DSH | MCT | 100 | Y | WB | N | Y | Y | N | N | N | N | 225,000 | 36 | Dead | 2 | No | |
| 14 | CM | DSH | MCT | 89 | Y | WB | N | Y | Y | N | N | N | N | 188,000 | 83,980 | 28 | Dead | 132 | CCNU, vinblastine |
| 16 | CM | DSH | MCT | 93 | Y | WB | N | N | N | Y | N | N | N | Dead | 4 | No | |||
| 16 | CM | DSH | MCT | 55 | Y | Ox | N | N | N | Y | Y | N | N | 20 | Dead | 2 | No | ||
| 16 | SF | DSH | MCT | 85 | Y | PRBCs, WB | N | Y | Y | N | N | N | N | 51,000 | 137,700 | 18 | Alive | 343 | No |
| 18 | SF | DSH | MCT | 185 | Y | WB | N | Y | Y | Y | N | N | N | 44,200 | 12 | Dead | 322 | Vinblastine | |
| 3 | CM | DSH | HAS | 55 | WB | Y | Y | N | N | N | Y | N | 72,000 | 132,480 | 18 | Dead | 197 | Adriamycin | |
| 8 | CM | DSH | HAS | 90 | N | None | N | N | N | N | N | N | N | 40.9 | Dead | 25 | Mitoxantrone intracavitary | ||
| 11 | SF | DSH | HAS | 100 | N | Ox | Y | N | N | N | N | N | Y | 85,000 | 97,920 | 12 | Died | 259 | No |
| 11 | CM | DLH | HAS | 70 | N | P, WB | Y | N | N | Y | N | Y | Y | 85,000 | 115,200 | 12 | Alive | 1270 | No |
| 16 | CM | DSH | LSA | 96 | Y | None | N | Y | N | N | N | Y | N | 131,000 | 28 | Dead | 415 | Madison–Wisconsin | |
| 17 | CM | DSH | LSA | 81 | Y | PRBCs | N | Y | N | N | Y | Y | N | 37,600 | 17 | Dead | 3 | No | |
| 9 | SF | DSH | EMH | 44 | WB | N | Y | N | Y | Y | Y | N | 36 | 401,850 | 12 | Dead | 53 | No | |
| 13 | CM | DSH | Myelolipoma and lymphoid hyperplasia | 140 | None | N | N | Y | Y | N | N | N | 4440 | 41 | Alive | 339 | No | ||
| 14 | SF | DSH | Perisplenic mass – sarcoma | 85 | WB | N | Y | N | Y | N | N | N | 36.2 | Dead | 293 | No |
CM=castrated male, SF=spayed female.
DSH=domestic shorthair, DLH=domestic longhair.
LSA=lymphoma, HAS=hemangiosarcoma, EMH=extramedullary hematopoeisis.
Ox=oxytocin; WB=whole blood; P=plasma; PRBCs=packed red blood cells; FFP=fresh frozen plasma; PCV = Packed Cell Volume; Hct=hematocrit.
CCNU = N-(2-chloroethyl)-N’-cyclohexyl-N-nitrosourea (Lomustine).
Fig 1.

Kaplan–Meier survival curve comparing cats with reported weight loss (red line) to cats without reported weight loss (blue line).
Eighteen cases had a preoperative PCV or hematocrit reported. The average PCV was 23.7 (median, 20.5; range, 12.0–41.0). Thirteen of the 18 (72%) had anemia. A platelet count was available for 16 of the cases. The average platelet count was 107,400/μl (median, 86,000/μl; range, 36,000–225,000/μl). Eight of the 16 (50%) of the cases had thrombocytopenia. Mann–Whitney analysis showed a significant relationship between increased reticulocyte number and thrombocytopenia (P=0.04); and preoperative anemia with increased surgical time (P=0.05). A significant relationship was demonstrated between hemoabdomen and collapse (P<0.01); between hemoabdomen and regenerative anemia (P=0.02); and between age at surgery and hemoabdomen at the time of surgery (P=0.02, increased age with no hemoabdomen). No significant relationship was found between any preoperative clinicopathological factors with survival following splenectomy.
Intraoperative evaluation
Sixteen patients received peri-operative antibiotics. Twelve patients (63%) had visible abdominal metastasis at the time of surgery. Seventeen patients (89%) underwent additional surgical procedures at the time of splenectomy. Frequently these additional surgical procedures consisted of biopsies of suspect metastatic lesions. Fourteen patients (73%) required a transfusion of a blood product. The average surgical time was 89 min (median, 89 min; range, 44–185 min). Analysis of relevant data revealed a significant relationship between the presence of abdominal metastases and hemoabdomen (P=0.02). For cats receiving a blood transfusion during hospitalization the Kaplan–Meier MST was 132 days versus 1959 days (median not reached) for those that did not receive a transfusion during hospitalization, however, this difference was not statistically significant (P=0.07). There was no significant relationship between any intraoperative variables assessed and survival time following surgery.
Postoperative evaluation
Metastasis was confirmed on histopathology in 12/19 (63%) of the cases. Histopathological examination of the submitted spleens revealed 10 cases with MCT (53%), four with splenic hemangiosarcoma (21%), two with lymphoma (11%), and one each (5%) of myelolipoma with lymphoid hyperplasia, extramedullary hematopoeisis, and perisplenic sarcoma. The median survival of cats with neoplastic disease was 197 days and with non-neoplastic disease it was 339 days (P=0.60). MST for cats with mast cell disease was 132 days and hemangiosarcoma was 197 days. The overall Kaplan–Meier MST was 197 days, with 25.3% of the cats still alive at 1 year. None of the cats alive at 1 year postoperatively died in the following 4 years (Fig. 2).
Fig 2.
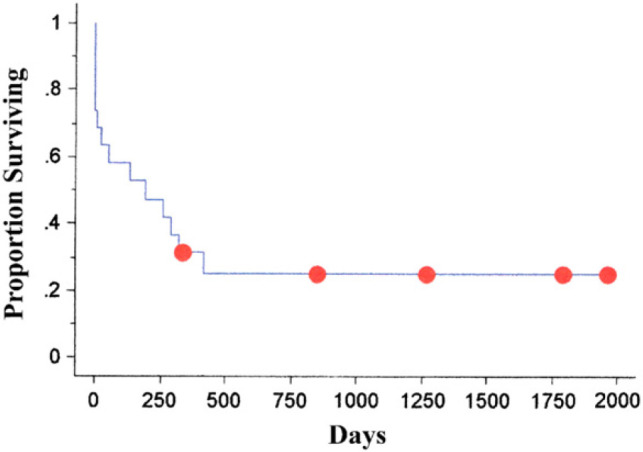
Overall Kaplan–Meier survival time for all cats undergoing splenctomies.
Discussion
This study included 19 cats that had undergone splenectomies for a variety of diseases. The variety of splenic diseases in cats found in this study corresponds to the splenic diseases found in cats in other studies. 2,3 In the current study, the three most common diseases resulting in splenectomy were MCT (53%), hemangiosarcoma (21%), and lymphoma (11%). In one previous study, lymphoma was the most common (30%, 30/101) followed by MCT and extramedullary hematopoeisis and/or lymphoid hyperplasia (both 27%, 27/101). Only 2% (2/101) of the cases had hemangiosarcoma. 3 In another study, MCT (15%, 66/455), lymphoma (9%, 40/455), myeloproliferative disease (6%, 28/455) and hemangiosarcoma (3%, 13/455) comprised 33% of the total splenic disease from a population of cats that had surgical specimens submitted for pathologic evaluation. 2 In that study, the pathology records were searched for diagnoses that included ‘spleen’ or ‘splenic’. This might have included animals that were euthanased for other reasons but had clinically silent splenic lesions found at necropsy, and thus many of the splenic lesions found could have been incidental findings during necropsies. This increased number of incidental splenic lesions would then decrease the prevalence of surgically relevant splenic lesions. It is difficult to make accurate comparisons between the current study and past studies as the past studies have included greater case numbers than the present study. In addition, not all the cats in the previous studies had undergone splenectomies as some of the splenic diseases would have been managed medically. However, according to the previous studies and the current study, systemic mastocytosis with splenic involvement appears to be the most common cause of splenomegaly and splenic disease in domestic cats. 7–10
Previously reported MSTs of cats that have had splenectomies for MCT is 360–570 days, with a range of 60–1140 days. Death is usually due to euthanasia after return of clinical signs. 7,8,10–12 The current study found the MST in cats with mast cell disease to be 132 days. Splenectomy is the recommended treatment even though metastasis is usually present at the time of diagnosis. Anorexia, significant weight loss and male gender have been shown to be negative prognostic indicators for cats with splenic MCT. 13
Splenectomy is also the primary method of treatment of splenic hemangiosarcoma in cats. 14 A study of the prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone found no prognostic factors for survival following splenectomy. In a recent study of hemangiosarcoma in cats, it was shown that cats with visceral hemangiosarcoma had a significantly shorter MST when compared to cats with cutaneous or subcutaneous hemangiosarcoma. 15 For cats with visceral hemangiosarcoma, the MST was 77 days (range, 23–296 days). 16 The MST of cats undergoing splenectomy for hemangiosarcoma has been reported to be 140 days, ranging from 42 to 245 days and in the current study it was 197 days. 17
There are no previous reports of splenectomies performed in cats with splenic lymphoma. Splenectomy has been reported as a treatment for splenomegaly in various types of lymphoma in humans, dogs, and ferrets. 18–21 Splenectomy has been shown to be of benefit when treating splenic lymphomas in humans. 18,22 In a study of 16 dogs with lymphoma that were treated with splenectomy, five died within 42 days due to disseminated intravascular coagulation and sepsis while the remaining 11 dogs underwent chemotherapy. In the seven dogs that were evaluated until death the mean survival time was 402 days. 20 In another study of dogs receiving splenectomies due to splenic lymphoma, 75% (15/20) dogs were dead at 1 year with a mean survival of 84 days with 90% mortality 6 months postoperatively for dogs with a diffusely enlarged spleen. Dogs with nodular splenic lymphoma survived a mean of 252 days with 28.6% mortality in 6 months. 5 For the two cats in the present study, one had a 3 days survival time and the other had a 415 days survival time postoperatively. Further research needs to be undertaken to investigate whether performing a splenectomy is beneficial in treating cats with splenic lymphoma.
Previous studies of prognostic factors for survival time in dogs undergoing splenectomy have shown that the only prognostic indicator was whether the splenic lesion was neoplastic or not. In one study, the 2-month postoperative survival of dogs with splenic hematomas associated with non-neoplastic conditions was 83% whereas it was only 31% for dogs with hemangiosarcoma, with or without associated hematomas. Twelve month survival times were 64% and 7%, respectively. 5 Another study found that the mean survival time for patients with neoplastic splenic disease was 94 days and for non-neoplastic splenic disease it was 212 days. 23
Dogs with anemia, increased nucleated red blood cells in circulation, abnormal red blood cell morphology, or splenic rupture have been found to have a significantly greater chance of having splenic neoplasia. 4 However, that particular study did not identify whether these clinical findings had any prognostic significance. Median survival after splenectomy for all stages of splenic hemangiosarcoma was 56 days and for all other splenic malignancies was 91 days. In 24 dogs with non-neoplastic diseases the MST was greater than 252 days with 12 dogs still alive at the time of data collection. 4
The only factor identified as a prognostic indicator in the current study was weight loss. Weight loss was only noted in three cases in this study; this weight loss was sometimes only reported by the referring veterinarian. In addition, two of the cats with weight loss were geriatric so there is a possibility that there were concurrent, undiagnosed diseases causing the weight loss. However, numerous studies in human medicine have shown that weight loss and a poor body condition score can negatively affect the outcome of various types of diseases including chronic heart failure, 24,25 chronic obstructive pulmonary disease, 26 chronic kidney disease, 27 and numerous cancers. 28 A recent veterinary study demonstrated that feline cancer patients with a body condition score of <5 on a scale of nine had a MST of 3.3 months in contrast to cats that had a body condition score of ≥5 and a MST of 16.7 months. 29 No veterinary studies could be found that examined the relationship between weight loss and prognosis in other disease processes, although nutritional status of patients is recognized as an important prognostic factor. 30
Weight loss has been shown to be a prognostic indicator in human cancer patients. 31–34 There are numerous factors that contribute to weight loss in cancer patients including decreased energy intake, increased energy expenditure, and changes in nutrient metabolism. Much of the weight loss appears to be due to mediators produced by the tumor or by the body in response to the tumor, but local obstruction and maldigestion and malabsorbtion due to obstruction of biliary and pancreatic ducts and their secretions also play a role. 35,36
This study has a number of limitations. One limitation is its retrospective nature and lack of control over the data that had been recorded. Another major limitation was that there were only 19 cases included in this study. The relationship between hemabdomen and metastases and hemoabdomen and age could be type I errors due to the low case numbers. Due to the limited number of cases in this study and the resulting low power this should be viewed as a factor warranting further investigation before changes in clinical decision making can be made.
Conclusion
While feline splenic disease is much rarer than its canine counterpart, it is still an important entity in veterinary medicine. Little is known about the various types, treatments, and prognoses of feline splenic diseases. The most common type of feline splenic disease resulting in splenectomy is MCT. The only prognostic factor for survival time following a splenectomy in cats that was identified in this study was presence of preoperative weight loss.
References
- 1.Tillson D.M. Spleen. Slatter D.H. Textbook of Small Animal Surgery, 3rd edn, 2003, Saunders: Philadelphia, PA, 1046–1062. [Google Scholar]
- 2.Spangler W.L., Culbertson M.R. Prevalence and type of splenic diseases in cats: 455 cases (1985–1991), J Am Vet Med Assoc 201, 1992, 773–776. [PubMed] [Google Scholar]
- 3.Hanson J.A., Papageorges M., Girard E., Menard M., Hebert P. Ultrasonographic appearance of splenic disease in 101 cats, Vet Radiol Ultrasound 42, 2001, 441–445. [DOI] [PubMed] [Google Scholar]
- 4.Johnson K.A., Powers B.E., Withrow S.J., Sheetz M.J., Curtis C.R., Wrigley R.H. Splenomegaly in dogs. Predictors of neoplasia and survival after splenectomy, J Vet Intern Med 3, 1989, 160–166. [DOI] [PubMed] [Google Scholar]
- 5.Spangler W.L., Kass P.H. Pathologic factors affecting postsplenectomy survival in dogs, J Vet Intern Med 11, 1997, 166–171. [DOI] [PubMed] [Google Scholar]
- 6.Hosgood G. Splenectomy in the dog: a retrospective study of 31 cases, J Am Anim Hosp Assoc 23, 1987, 275–283. [Google Scholar]
- 7.Liska W.D., MacEwen E.G., Zaki F.A., Garvey M. Feline systemic mastocytosis: a review and results of splenectomy in seven cases, J Am Anim Hosp Assoc 15, 1979, 589–597. [Google Scholar]
- 8.Confer A.W., Langloss J.M., Cashell I.G. Long-term survival of two cats with mastocytosis, J Am Vet Med Assoc 172, 1978, 160–161. [PubMed] [Google Scholar]
- 9.Garner F.M., Lingeman C.H. Mast-cell neoplasms of the domestic cat, Pathol Vet 7, 1970, 517–530. [DOI] [PubMed] [Google Scholar]
- 10.Guerre R., Millet P., Groulade P. Systemic mastocytosis in a cat: remission after splenectomy, J Small Anim Pract 20, 1979, 769–772. [DOI] [PubMed] [Google Scholar]
- 11.Madewell B.R., Gunn C., Gribble D.H. Mast cell phagocytosis of red blood cells in a cat, Vet Pathol 20, 1983, 638–640. [DOI] [PubMed] [Google Scholar]
- 12.Feinmehl R., Matus R., Mauldin G.N. Splenic mast cell tumors in 43 cats (1975–1992), Annu Vet Conf of Vet Cancer Soc, 1992, 50.
- 13.Thamm D.H., Vail D.M. Chapter 19. Mast cell tumors. Withrow S.J., Vail D.M. Small Animal Clinical Oncology, 3rd edn, 2007, W B Saunders: Philadelphia, 402–424. [Google Scholar]
- 14.Thamm D.H. Chapter 32. Miscellaneous tumors. Withrow S.J., Vail D.M. Small Animal Clinical Oncology, 4th edn, 2007, W B Saunders: Philadelphia, 785–823. [Google Scholar]
- 15.Johannes C.M., Henry C.J., Turnquist S.E., et al. Hemangiosarcoma in cats: 53 cases (1992–2002), J Am Vet Med Assoc 231, 2007, 1851–1856. [DOI] [PubMed] [Google Scholar]
- 16.Culp W.T., Drobatz K.J., Glassman M.M., Baez J.L., Aronson L.R. Feline visceral hemangiosarcoma, J Vet Intern Med 22, 2008, 148–152. [DOI] [PubMed] [Google Scholar]
- 17.Scavelli T.D., Patnaik A.K., Mehlhaff C.J., Hayes A.A. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases, J Am Vet Med Assoc 187, 1985, 817–819. [PubMed] [Google Scholar]
- 18.Thieblemont C., Felman P., Berger F., et al. Treatment of splenic marginal zone B-cell lymphoma: an analysis of 81 patients, Clin Lymphoma 3, 2002, 41–47. [DOI] [PubMed] [Google Scholar]
- 19.Adler S., Stutzman L., Sokal J., Mittelman A. Splenectomy for hematologic depression in lymphocytic lymphoma and leukemia, Cancer 35, 1975, 521–528. [DOI] [PubMed] [Google Scholar]
- 20.Brooks M.B., Matus R.E., Leifer C.E., Patnaik A.K. Use of splenectomy in the management of lymphoma in dogs: 16 cases (1976–1985), J Am Vet Med Assoc 191, 1987, 1008–1010. [PubMed] [Google Scholar]
- 21.Flock M. Splenic lymphosarcoma in a ferret, Can Vet J 30, 1989, 597. [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagawa M., Suzumiya J., Ohshima K., Kanda M., Tamura K., Kikuchi M. Splenic lymphoproliferative disorders in human T lymphotropic virus type-I endemic area of Japan: clinicopathological, immunohistochemical and genetic analysis of 27 cases, Leuk Lymphoma 41, 2001, 593–605. [DOI] [PubMed] [Google Scholar]
- 23.Spangler W.L., Culbertson M.R. Prevalence, type, and importance of splenic diseases in dogs: 1,480 cases (1985–1989), J Am Vet Med Assoc 200, 1992, 829–834. [PubMed] [Google Scholar]
- 24.Anker S.D., Ponikowski P., Varney S., et al. Wasting as independent risk factor for mortality in chronic heart failure, Lancet 349, 1997, 1050–1053. [DOI] [PubMed] [Google Scholar]
- 25.Anker S.D., Negassa A., Coats A.J. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study, Lancet 361, 2003, 1077–1083. [DOI] [PubMed] [Google Scholar]
- 26.Schols A.M., Broekhuizen R., Weling-Scheepers C.A., Wouters E.F. Body composition and mortality in chronic obstructive pulmonary disease, Am J Clin Nutr 82, 2005, 53–59. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K., Kopple J.D., Humphreys M.H., Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients, Nephrol Dial Transplant 19, 2004, 1507–1519. [DOI] [PubMed] [Google Scholar]
- 28.Fearon K.C., Voss A.C., Hustead D.S. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis, Am J Clin Nutr 83, 2006, 1345–1350. [DOI] [PubMed] [Google Scholar]
- 29.Baez J.L., Michel K.E., Sorenmo K., Shofer F.S. A prospective investigation of the prevalence and prognostic significance of weight loss and changes in body condition in feline cancer patients, J Feline Med Surg 9, 2007, 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauldin G.E., Davidson J.R. Nutritional support of hospitalized cats and dogs. Hosgood G., Slatter D. Textbook of Small Animal Surgery, 3rd edn, 2003, Saunders: Philadelphia, 87–113. [Google Scholar]
- 31.Pater J.L., Loeb M. Nonanatomic prognostic factors in carcinoma of the lung: a multivariate analysis, Cancer 50, 1982, 326–331. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen H., Hansen H.S., Cederqvist C., Lober J. The prognostic significance of weight loss and its integration in stage-grouping of oesophageal cancer, Acta Chir Scand 148, 1982, 363–366. [PubMed] [Google Scholar]
- 33.Dewys W.D., Begg C., Lavin P.T., et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern cooperative oncology group, Am J Med 69, 1980, 491–497. [DOI] [PubMed] [Google Scholar]
- 34.Vigano A., Bruera E., Jhangri G.S., Newman S.C., Fields A.L., Suarez-Almazor M.E. Clinical survival predictors in patients with advanced cancer, Arch Intern Med 160, 2000, 861–868. [DOI] [PubMed] [Google Scholar]
- 35.Barber M.D., Ross J.A., Fearon K.C. Cancer cachexia, Surg Oncol 8, 1999, 133–141. [DOI] [PubMed] [Google Scholar]
- 36.DeVita V.T., Hellman S., Rosenberg S.A. Cancer, Principles and Practice of Oncology, 7th edn, 2005, Lippincott Williams & Wilkins: Philadelphia, PA. [Google Scholar]


