Abstract
Tissue Plasminogen Activator (tPA) is a serine protease expressed in different areas of the mammalian brain. It has been used clinically to dissolve clots and shown to have a role in neurodegeneration. Early studies suggested that tPA plays an important role in the processes of learning and memory, demonstrated at the level of behavior and synaptic plasticity. Herein, we extend the behavioral characterization of these mice to the related dimension of exploratory-related behavior using an extensive battery of behavioral tests as well as the neurotransmitter metabolism associated with the behavioral measures. Our results indicate a behavior tendency in these mice consistent with “impulsivity” or reduced exploratory inhibition. These patterns are accompanied by decreased levels of serotonin in several brain regions important in behavioral regulation in the tPA−/− mice compared to control animals. Systemic administration of fluoxetine reversed the behavioral disinhibition of tPA−/− mice, further supporting an important alteration in behavior regulation mediated by serotonin systems as underappreciated but important element of the behavioral phenotype of these animals.
Introduction
tPA converts inactive plasminogen to plasmin, which in turn degrades fibrin and other extracellular matrix (ECM) components (Zhang et al., 2005), and as such is used clinically to treat myocardial infarctions (Vassali et al., 1991). tPA is synthesized in the mammalian brain (Tsirka et al., 1997) and stored in vesicles (Parmer et al., 1997) in areas such as the prefrontal cortex, striatum, hippocampus, amygdala, and the cerebellar Purkinje cells (Rickles and Strickland, 1988; Sappino et al., 1993). The tPA/plasmin system can also promote neuronal degeneration following excitotoxin injury (Tsirka et al., 1995; 1997).
After initial reports that neuronal activity in rat and mouse hippocampi and cerebella induces tPA mRNA expression (Qian et al., 1993; Caroll et al., 1994; Seeds et al., 1995), several studies investigated the role of tPA in learning and memory. While not entirely uniform, the results of these studies are generally consistent with the fact that tPA−/− performance is inferior to that of WT control animals across learning and memory tasks (Huang et al., 1996; Calabresi et al., 2000) and that tPA overexpression is at times associated with better performance than WT controls (Madani et al., 1999).
However, the examination of this literature also suggested that another important phenotypic characteristic is suggested by the results of several of these studies. For example, in a step-down inhibitory avoidance task, tPA−/− mice performed worse than WT controls as early as 90 min after training and up to 7 days later (Pawlak et al., 2002) and tPA−/− mice were found to exhibit impaired inhibitory behavior in operant conditioning tasks (Ripley et al., 2001; Horwood et al., 2004). This appears not to be the result of general hyperactivity, because homecage locomotor activity was not different for tPA−/− mice, as they were observed in their cages for 12-hours (Pawlak et al., 2002), in a novel plexiglass cage for 10min (Calabresi et al., 2000), and a circular runway for 30min (Ripley et al., 1999). Furthermore, when the elevated plus maze was used to determine anxiety-like behaviors for the tPA−/− mice, no differences were observed compared to control mice (Pawlak et al., 2003).
Taken together, these studies suggest that a trait that might be described as “impulsivity” may also be a significant component of the behavioral phenotype of these animals. This term, however, is not a unitary construct and has at least two sub-components when applied in various definitions to humans and rodents: (1) action in response to a proximal stimulus or patterns of action to molar reinforcement contingencies that reflect a lack of sensitivity to the negative consequences of that particular action or pattern of actions or (2) deficits in the ability to inhibit an ongoing or already initiated response (see reviews by Beauchaine & Neuhaus, 2008; Dalley et al, 2008; Sagvolden et al., 2005). Under definition (1), this can be reflected in relatively discrete actions resulting in potential aversive outcomes (e.g. danger) or patterns of action that produce lower long-term reinforcement rates (e.g. consistently choosing a smaller, immediate reinforcer over a larger, delayed reinforcer). It is this aspect that we have focused on presently.
Accordingly, we sought to examine this consequence-insensitivity aspect of the behavioral phenotype of the tPA−/− mice line by testing them in several settings usually used to assess aspects of anxiety, hyperactivity and spatial learning in rodents. However, we supplement standard measures with several non-standard measures in these tests, and together they are suggestive of impulsivity generally conforming to the characteristics described under the first definition. Because of the theoretical context that suggests opposing dopaminergic approach systems and serotonergic inhibition systems, a relevant to trait impulsivity (e.g. Soubrie, 1986; Gainetdinov et al., 1999; Daw et al., 2002), we profiled monoaminergic neurotransmitters levels in major brain regions of interest of the tPA−/− mice, and a pharmacological reversal manipulation was undertaken. Though exploratory, this study represents to our knowledge the first targeted investigation of a potentially substantial phenotypic characteristic of this broadly applied mouse line.
Methods
Animals
Wild type (tPA+/+ or WT, control) C57Bl/6 and homozygous tPA−/− (tPA−/−) male mice, back-crossed to C57Bl/6 for twelve generations, were used for these experiments. In the plus maze task, both male and female mice were used for both groups. The animals were between three and eight months old. The mice remained in their vivarium for at least 2 weeks maintained on a 12-h light/dark cycle (7 a.m. lights on), in individual, standard laboratory cages, in temperature and humidity- controlled conditions (22 ± 1 °C, 70% relative humidity), and with access to food and water ad libitum (except when a water restriction schedule was applied, as indicated in the specific experiments). All behavioral experiments were conducted in the same room adjacent to the room that housed the animals, during the light cycle between the times 10 a.m. – 6 p.m. The animals have derived from at least 6 different litters of each genotype.
Open field activity (large arena) and novel object exploration
Mice (N = 7 tPA−/− and N = 8 WT) were tested in this apparatus, which was square and made of wood covered with vinyl (60 × 60 cm) and the walls surrounding it were 25 cm high. The arena was divided in 36 squares (10 × 10 cm) and the external 20 squares were considered the periphery of the arena while the internal 16 squares were the central area. Mice were given one daily 10 min trial for three consecutive days. The trials were videotaped. During the first two days the mice were left to explore the open arena. The time spent and entries in the central area were measured, as well as rearing events and number of squares visited. On the third day a small cup taped in the central area of the arena was introduced. A circle was drawn 2.5 cm around the cup and the number of approaches and the time for the first approach to the cup were measured. An approach was defined when the mouse was entering within the circle around the cup.
Plus maze task
Mice (N =8 male and 4 female tPA−/−, and N = 5 male and 9 female WT) were tested on a variation of the elevated plus maze for two days. Initial comparisons of males and females revealed no significant differences in the performance in these experiments, and mean performances of the groups were comparable. This maze was designed to study the exploratory behavior of the mice. The maze was not as anxiogenic as the typical elevated maze, because all four arms had walls that prevented the animals from getting exposed to heights. However, two of the four arms had wooden covers that made the arms completely enclosed and dark and allow them to be compared to the open-top arms. Mice were left at the central area of the cross-shaped maze and were allowed to explore the maze for 10 min. All trials were videotaped. During analysis we determined that there were no differences between the groups in the number of entries to open arms from the closed arms (p = 0.172) and from open to open arms (p = 0.676), and entries to the open arms were combined. Eventually, we measured the time spent in the open arms and the number of entries in the open arms.
Elevated “zero” maze
Mice (N = 8 tPA−/− and N = 8 WT) were tested in this task, which was a variation of the elevated “plus” maze and was shown to be sensitive to anxiety-like behaviors of the animal (Shepherd et al., 1994). It consisted of a circular wooden ring (inner diameter 45 cm, outer diameter 50 cm) divided in four quadrants. The ring sat on top of four wooden legs, which were 62 cm high. Plexiglas walls (24 cm high) protected two opposite quadrants on both the inner and outer sides of the ring. The other two quadrants were open with no protection. This type of maze, compared to the elevated “plus” maze, offered the advantage that it eliminated the hard-to-interpret central area of the maze, such that the animal was forced to spend its time either in the closed (protected) or the open (unprotected) quadrants. The mouse was put at the edge of the closed quadrant and was left in the maze for 7.5 min for one daily trial on two consecutive days. The mice were videotaped during their trials and time spent in the open area, number of entries in the open area, and number of unprotected head dips were measured. The latter is a trait that has been associated with lack of anxiety-like behavior; it was defined as a dip of the mouse’s head below the level of the maze while the whole body remained in the unprotected areas of it.
Radial arm maze
Mice (N = 8 tPA−/− and N = 9 WT) were tested in this maze made of polyurethane-sealed wood. The maze consisted of eight arms radiating out of an octagonal center, which was 22 cm in diameter. The arms were 35 cm long, 10 cm wide, and 25 cm high. Wooden blocks could block the arm entrances. Sweetened milk (2 parts) diluted in water (8 parts) was placed in wells at the end of the arms. The mice were trained to collect all eight rewards. A day of habituation allowed the mice to explore the baited RAM for 10 min. Following the habituation day the mice (which were age- and weight-matched) were placed on a water restriction schedule (for 22 hours) to ensure enough motivation to visit all arms. No differences were obvious in the behavior of the two genotypes of mice regarding their motivation to drink water following the restriction. After the end of each trial mice were allowed to drink water ad libitum for one hour, at which point access to water was again restricted till the trial of the following day. During the training period the animals were left in the maze for 10 min or until all rewards were consumed, whichever came first. An error was counted when the mouse entered an arm from which it had already retrieved the reward. Entry into an arm was counted when all four paws entered into the arm. Training lasted for 10 days. The maze environment did not provide to the animals with explicit additional spatial cues. However major landmarks were present, i.e. the room door, a table, and the experimenter.
Microdissection of brain structures and sample preparation for HPLC
Brains were removed (N = 5/group, 3–5 months old male WT and tPA−/−), flash frozen in isopentane mixed with dry ice (−40°C) and then stored in -80°C. One brain hemisphere was used for the HPLC experiment. To analyze the specific regions the brains were allowed to gradually thaw for 4 min before they were sectioned on a cold stage with a cold apparatus in slices of 0.7 mm thickness. Microdissection of eight regions of interest was performed under a microscope in a cold room using cold micropunches. The eight structures dissected were: olfactory bulb, cingulate, frontal cortex, caudate-putamen, thalamus, hypothalamus, hippocampus and cerebellum (Ase et al., 2000).
Each sample was rapidly homogenized by sonication (Fisher Scientific, Sonic Dismembrator 550) in 0.1 M of perchloric acid and centrifuged at 15,000 rpm for 25 min at 4°C. The pellets were resuspended in 1 M NaOH overnight to be used for the protein assay (DC Protein Assay, BIORAD and Spectrophotometer Molecular Devices, Spectra Max 340), and the supernatants were stored at −80 °C until the HPLC analysis was carried out.
The HPLC system carried a normbore column (300×5 mm C18 column; BAS, model MF 8954), and a BAS LC-4C electrochemical transducer with a dual glassy carbon electrode set at 650 mV. The column was kept at 35.4 °C. The mobile phase (flow rate 1.0 ml/min) consisted of 25g Na2HPO4, 50 mg EDTA, 3 g sodium octyl sulfate, 200 ml MeOH, pH to 3.4 and QS to 4L.
The heights of analyte peaks were calculated and compared them to the standards peaks (Chromgraph 2.34). Standards of norepinephrine (NE), dopamine (DA), serotonin (5-HT) were analyzed prior to and following the experimental samples to correct for drift. Values were normalized for the amount of protein in each sample and reported in ng/mg of protein.
Fluoxetine administration
Mice (N = 11 for tPA−/− and WT injected with fluoxetine; N = 10 for tPA−/− and N = 9 for WT injected with saline) were injected with fluoxetine (20 mg/kg, i.p.) or saline and were left in their cages for 30 min before being tested on the open field.
Statistical analyses
All statistical analyses were performed using the SPSS program (version 13.0 for Windows). The α level for all statistical analyses was 0.05. Mixed ANOVA was used for the comparisons in the behavioral tests using days as the within subjects factor and group (WT and tPA−/−) as the between subjects factor. Independent t-tests were used for the HPLC data.
Results
tPA−/− mice show altered exploratory behaviors and reactivity to novel objects
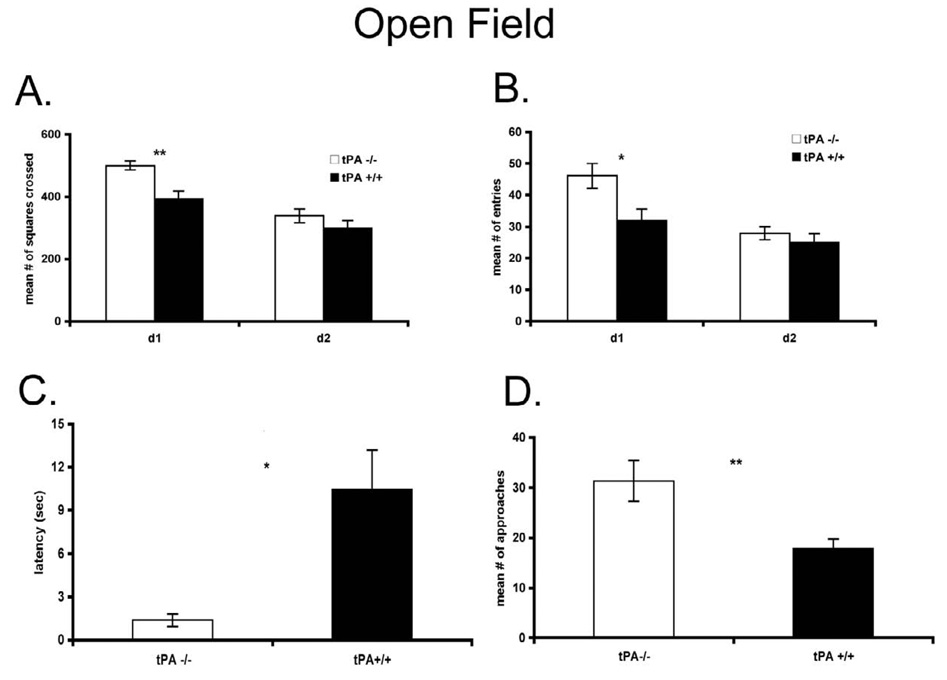
The tPA−/− mice demonstrated an enhanced tendency to actively explore and engage in behaviors involving more exposure in the open field, O-maze and elevated plus maze across a variety of measures. When a large open field arena was used, tPA−/− mice showed significantly higher locomotor rates (P < 0.005, Fig. 1A) and entered the central, presumably anxiogenic, area more often on the first day of testing (P < 0.02, Fig. 1B). Neither measure differed on the second day of testing, as both groups were habituated to the basic arena environment. The most striking finding is latency to approach the novel object (P < 0.015, Fig. 1C) and the number of approaches (P < 0.007, Fig. 1D) on the third day of testing.
Figure 1.
A, The locomotor rates of the tPA−/− (n = 7) and control wt (n = 8) mice were measured and B, the entry to the central (more anxiogenic) area of a large open field arena were also compared. C, Latency and the numbers of approaches D to a novel object in the open field on the third day were recorded for tPA−/− mice and control wt mice, For all figures, * p < 0.05 versus control mice, ** p < 0.01 versus control mice, and mean +/− s.e.m. shown.
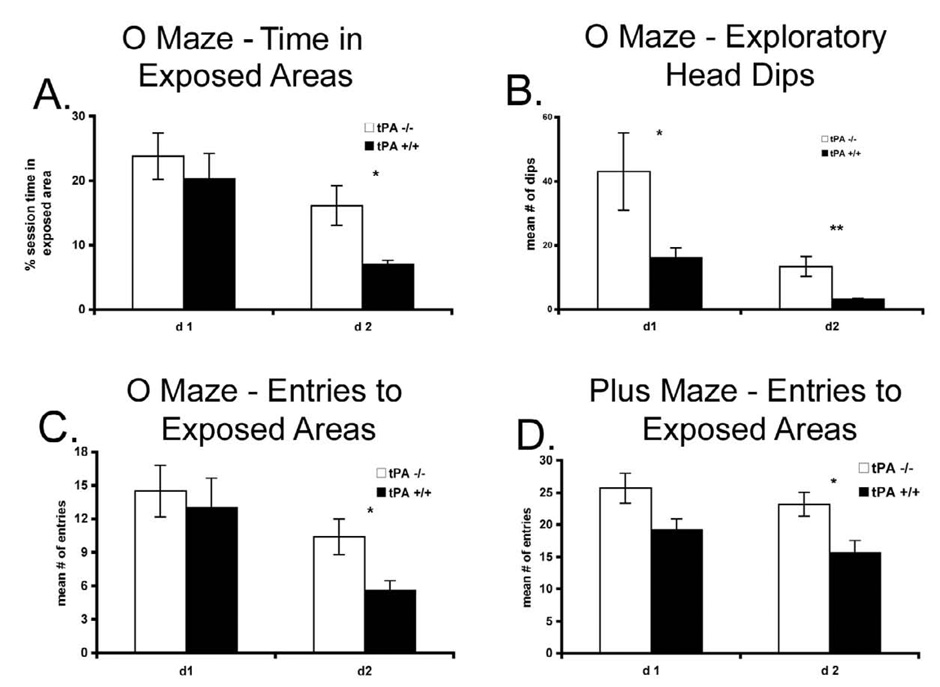
In the elevated “zero” maze task, they entered the open areas more often and spent more time in them. These differences became significant on the second day of testing, as the animals were consistently exploring the open areas (P < 0.02, Fig. 2A and P < 0.01, Fig. 2B for percentage of total session time and the number of entrances in the open area, respectively). However, what was most revealing was the number of head dips below the maze level while in the open areas which was significantly higher for the tPA−/−mice on both days of testing (P < 0.048 for day 1 and P < 0.006 for day 2, Fig. 2C). In the plus maze, tPA−/− mice entered the open arms more often on the second day (P < 0.02, Fig. 2D), similar to the open field.
Figure 2.
A, the latency of the first entries in the open areas of an elevated “zero” maze were measured for the tPA−/− (n = 7) and wt (n = 8) mice, B, the number of entries to the exposed areas during the second day of testing was also quantified, and C, the number of head dips below the maze level on both days of testing. D, In the plus maze, the entry of tPA−/− mice (n = 12) into the open arms on the second day of testing were measured and compared to control mice (n = 14).
Learning and memory of a maze task
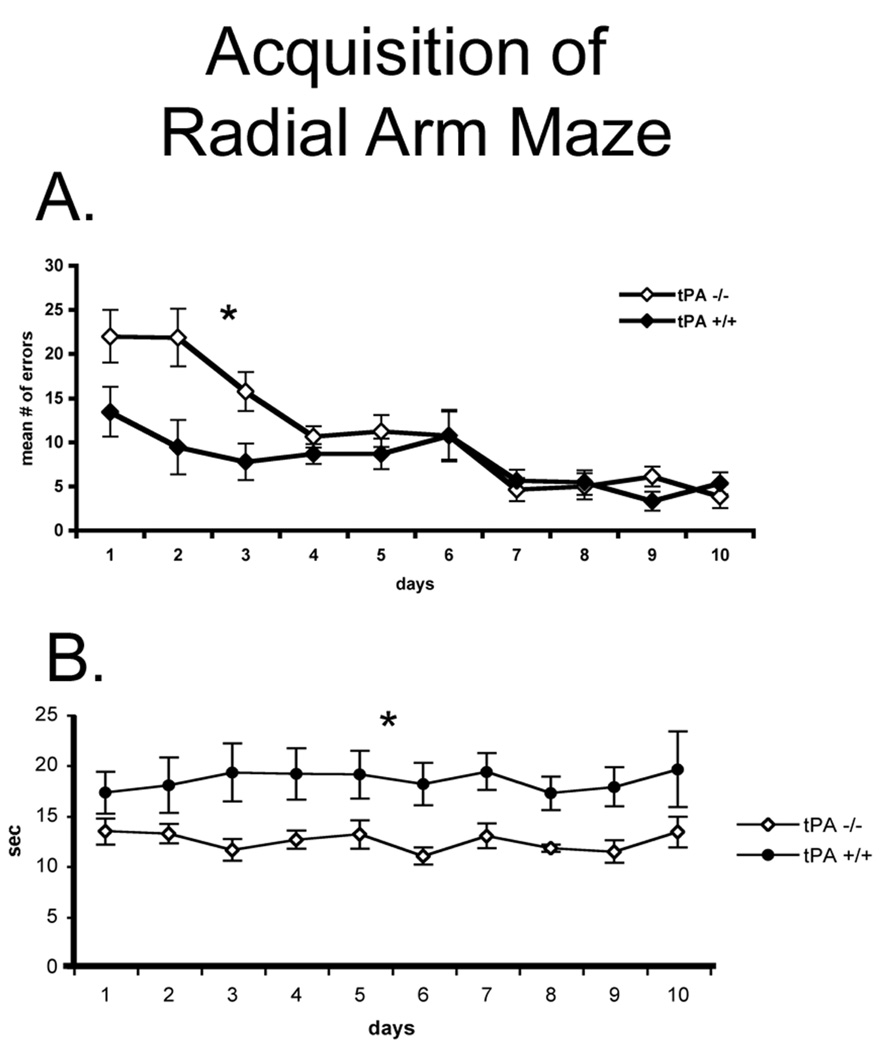
We used an eight-arm radial maze (RAM) with no additional explicit visual or spatial cues, as described in Methods, and no internal maze cues. During the first three days of task acquisition the tPA−/− mice committed more errors compared with the control animals (P < 0.012, Fig. 3A). However, tPA-deficient mice also spent significantly less time in each arm visit for the entire period of acquisition (P < 0.05, Fig. 3B), again supporting the observations in the other tasks of increase impulsivity-like behavior. It should be noted that the initial starting difference between the two groups may not reflect a difference in learning ability as much as another initial difference in exploration between the tPA-deficient and controls.
Figure 3.
A, the number of errors during the acquisition phase of the task was compared between the tPA−/− mice (n = 8) and the wt control mice (n = 9). B, Moreover, the time spent in each arm visit while learning the radial arm maze was measured.
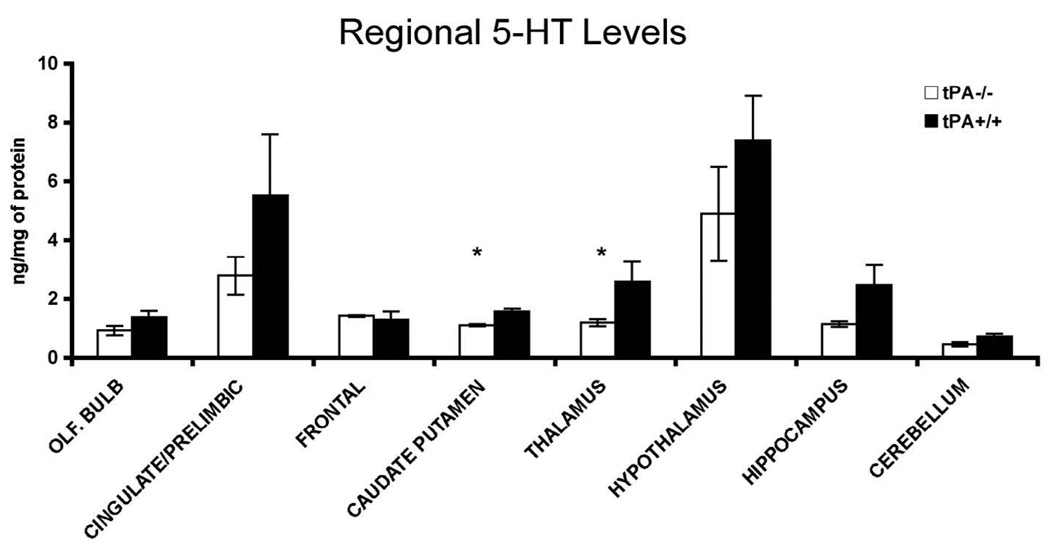
Lower serotonin levels in the thalamus and the caudate putamen
The behavioral phenotype of the tPA−/− mice bears similarities with that of mice, which have a deficiency in the dopamine transporter and the 5-HT1b receptor genes (Dulawa et al., 1997). Specifically, 5-HT1b receptor-knock out mice exhibited higher locomotor rates, were exploring more readily novel stimuli introduced in a familiar environment and were completing the tasks faster than the control mice. These observations led us to investigate potential parallels between the two phenotypes by microdissecting brain regions from frozen brains of animals that had not undergone any behavioral testing, and using high performance liquid chromatography (HPLC) with electrochemical detection to measure monoamine levels. Significantly lower serotonin levels were detected in the thalamus (P < 0.033) and the caudate putamen regions (P < 0.045; Fig. 4). No significant differences were evident in other regions, even though there was a trend for lower serotonin levels in the hippocampus and the cingulate/prelimbic cortices. The DA levels were also quantified in the caudate putamen [control animals 10.75 ng/mg protein (± 2.73 sem) and tPA−/− mice 11.9 ng/mg protein (± 1.49 sem)], and the thalamus [control animals 0.86 ng/mg protein (± 0.13 sem) and tPA−/− mice 0.59 ng/mg protein (± 0.13 sem)], thus indicating slightly higher levels of DA in the caudate of tPA−/− mice. Norepinephrine levels did not differ by region between the tPA deficient mice and wild type controls.
Figure 4.
The levels of 5-HT were measured in different regions of the tPA−/− and control mice.
Fluoxetine treatment reverses the exploratory behaviors and reactivity to novel objects in tPA−/− mice
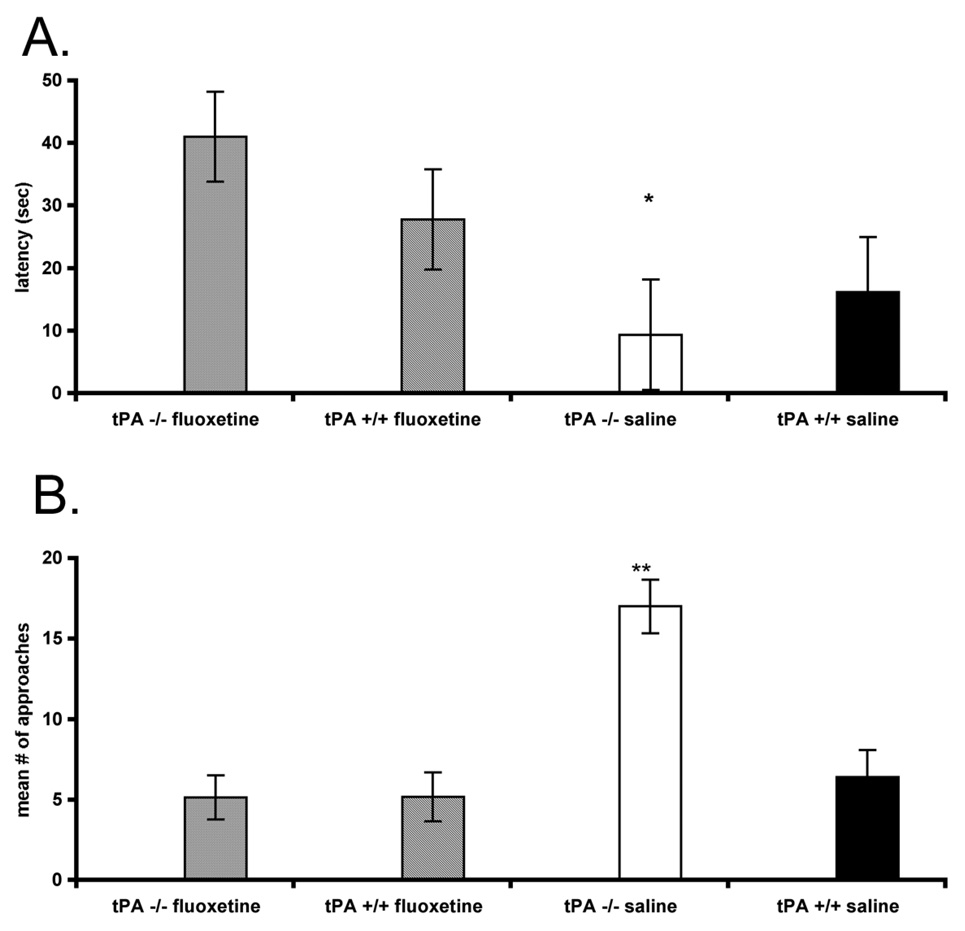
We hypothesized that the lower serotonin levels of the tPA−/− mice could be associated with the behavioral characteristics we had detected. To pharmacologically interfere with the potentially critical lower levels of serotonin, we administered systemically fluoxetine, and then used the large open field and novel object tests as before. The fluoxetine-treated tPA−/− mice exhibited significantly slower latencies to approach the novel object compared to the saline-treated tPA−/− mice (P < 0.038), and these latencies were similar to those of the controls (Fig. 5A). Further they had significantly fewer approaches towards the novel object compared to the saline-treated tPA−/− mice (P < 0.001), and were comparable to the controls (Fig. 5B).
Figure 5.
Effects of fluoxetine on exploratory behaviors and reactivity to novel objects. A, Fluoxetine was delivered systemically in tPA−/− mice and the latencies to approach a novel object in a large open field arena were measured and compared to tPA−/− mice treated with saline and control mice (n = 9–11 per group). B, The number of approaches towards the novel object were also quantified.
Discussion
Tissue plasminogen activator (tPA), expressed in the mouse brain by neurons and microglia, has been associated with events that are activity dependent (Melchor and Strickland, 2005; Samson and Medcalf, 2006). tPA is normally stored in vesicles and released from dendritic spines upon synaptic activity (Lochner et al., 2006). It acts as a modulator of glutamatergic transmission, and as such has been shown to potentiate calcium signaling after activation of NMDA receptors (Nicole et al., 2001; Sampson et al., 2008). Moreover, it contributes to the activation of BDNF (Pang et al., 2004). It also affects dopaminergic transmission, as striatal spiny neurons from tPA−/− mice show wild-type physiological and pharmacological EPSP properties but display a severe impairment in corticostriatal LTP (Centonse et al., 2002).
We report here two new significant insights into the behavior of the tPA−/− mice. First, these animals showed a pattern of behavioral disinhibition/impulsivity in several tasks. Second, decreased serotonin levels were detected in thalamus and caudate putamen of these animals. We found that tPA−/− mice were exploring at higher rates a new environment, or a stimulus introduced in a familiar environment, had attenuated anxiety-like behaviors. Interestingly, administration of fluoxetine resulted in elimination of the differences between tPA−/− and control mice in an open field task with the subsequent introduction of a novel object.
The most salient characteristic of tPA−/− mice was their tendency to move faster in the maze or the arena in which they were placed combined with a willingness to explore, take apparent risks and eventually react more readily to novel stimuli when they were habituated to the environment. It has to be noted that this augmented, stereotypic locomotion was apparent in tasks that involved mazes that were lending themselves for exploration (such as the radial maze with 8 arms and the elevated “zero” maze, where the tPA−/− mice spent less time in each arm visit while learning the radial arm maze) and involved a significantly large area (such as the large open field with darkly delineated squares contrasting on a white background). Our observations of exploratory differences in the open field arena differ on the surface with those of one previous report who did not observe any differences between the two groups in activity in a novel Plexiglas cage (Huang et al. 1996). However, this discrepancy perhaps highlights an important characteristic of the environment: The cage activity environment is physically constraining and offers less anxiety-provoking stimulation, thus either inhibiting the exploration by controls less, or arousing the tPA−/− mice more. Indeed, it is also possible that the tPA−/− behavioral differences result from “self-stimulation” provided by fast, and “risky” movements to compensate for less arousal experienced in novel environments. In this interpretation then, rapid approaches to the open field novel object, head-dips from the O-maze, and less visiting time in the radial arm maze may all serve to provide arousal rather than be in response to it. While our data support a momentary stimulus-setting driven “risk-taking” in the animals, additional evaluation of molar consequence-insensitivity aspect of this apparent impulsivity trait should employ classic operant paradigms such as differential reinforcement of low response rate (DRL) and fixed-consecutive number (FCN) schedules, delayed reinforcement “self-control” paradigms, or the five-choice serial reaction time test would be a compelling next step (Dalley et al., 2008).
Our results show that the tPA−/− mice also have decreased 5-HT levels in certain brain regions. While this evidence does not offer in any way a complete mediation mechanism for the observed behavioral differences, this finding is the first report of this difference and represents an additional relevant neurochemical phenotypic characteristic of these mice. However, it is possible that the lower serotonin level contributes in part to the behavioral phenotypic differences. Serotonin is known to have a modulatory role in the brain (Jacobs and Azmitia, 1992), and in particular serotonergic input from the dorsal raphe nuclei towards the striatal neurons promotes excitation (Vandermaelen et al., 1979; Park et al., 1982). The output from the neostriatum (to other basal ganglia structures initially and then indirectly to the thalamus and cortex) is inhibitory, as most of the principal neurons and interneurons are GABAergic in nature (Wilson, 2004). Therefore the decreased 5-HT levels in the caudate putamen of tPA−/− mice could be leading to attenuation of inhibitory actions in the striatum. Our data resemble those of a study where the 5-HT levels were depleted centrally in rats; the depletion resulted in disinhibited behaviors in certain simple tasks, but did not affect the more cognitively taxing decision-making required for a delay-discounting task (Winstanley et al., 2004). Both studies underline the significant effect that decreased 5-HT levels can have on the speed of execution of certain simple actions.
The data from the systemic administration of fluoxetine also supports this notion, that serotonin potentially plays a role for the behavioral phenotype of tPA−/− mice. Although we cannot unequivocally determine that fluoxetine affected the brain areas that showed decreased 5-HT levels in the tPA-deficient animals, the fact that their behavior became similar to that of control animals supports this speculation. A possibility that has been proposed over the years is that 5-HT and DA oppose each other in the brain: 5-HT offers a tonic inhibitory effect, and DA provides the phasic excitatory element (e.g. Soubrie, 1986; Gainetdinov et al., 1999; Daw et al., 2002). If that is the case, it is possible that the attenuated 5-HT levels in brain regions of the tPA−/− mice result in an imbalance such that the DA effects are amplified. For example, it has been reported that tPA−/− mice exhibit increased sensitivity to cocaine that was reflected in their locomotor rates, and over-responded while performing an operant task coupled to the self-administration of the drug (Ripley et al., 1999). Cocaine can bind at the serotonin and dopamine transporters, thereby blocking reuptake and increasing the extracellular levels of the neurotransmitters to produce its effects on behavior (e.g. Ritz et al., 1990).
In conclusion, we report here novel behavioral and neurochemical characteristics of the tPA−/− mouse, in particular that they exhibit a pattern of behavioral disinhibition and attenuated 5-HT levels. These results expand the perspective from which to develop new questions regarding the tPA in relation to neurochemistry to illuminate the multifaceted role of this protein in the brain.
Acknowledgments
This work was supported by National Institutes of Health RO1NS42168 (to S.E.T.), and R01DA15041 and the Department of Energy (to S.L.D). The authors would like to thank members of the Tsirka lab for suggestions during the course of the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E. Impulsivity and vulnerability to psychopathology. In: Beauchaine TP, Hinshaw S, editors. Child psychopathology. Hoboken, NJ: Wiley; 2008. pp. 129–156. [Google Scholar]
- Calabresi P, Napolitano M, Centonze D, Marfia GA, Gubellini P, Teule MA, Berretta N, Bernardi G, Frati L, Tolu M, Gulino A. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- Carroll PM, Tsirka SE, Richards WG, Frohman MA, Strickland S. The mouse tissue plasminogen activator gene 5' flanking region directs appropriate expression in development and a seizure-enhanced response in the CNS. Development. 1994;120:3173–3183. doi: 10.1242/dev.120.11.3173. [DOI] [PubMed] [Google Scholar]
- Centonze D, Napolitano M, Saulle E, Gubellini P, Picconi B, Martorana A, Pisani A, Gulino A, Bernardi G, Calabresi P. Tissue plasminogen activator is required for corticostriatal long-term potentiation. Eur. J. Neurosci. 2002;16:713–721. doi: 10.1046/j.1460-9568.2002.02106.x. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology (Berl) 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Ripley TL, Stephens DN. Evidence for disrupted NMDA receptor function in tissue plasminogen activator knockout mice. Behav Brain Res. 2004;150:127–138. doi: 10.1016/S0166-4328(03)00248-1. [DOI] [PubMed] [Google Scholar]
- Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, Schoonjans L, Kandel ER, Godfraind JM, Mulligan R, Collen D, Carmeliet P. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci U S A. 1996;P93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, Vassalli JD. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor JP, Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb Haemost. 2005;93:655–660. doi: 10.1160/TH04-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nature Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Park MR, Gonzales-Vegas JA, Kitai ST. Serotonergic excitation from dorsal raphe stimulation recorded intracellularly from rat caudate-putamen. Brain Res. 1982;243:49–58. doi: 10.1016/0006-8993(82)91119-2. [DOI] [PubMed] [Google Scholar]
- Parmer RJ, Mahata M, Mahata S, Sebald MT, O'Connor DT, Miles LA. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J Biol Chem. 1997;272:1976–1982. doi: 10.1074/jbc.272.3.1976. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Nagai N, Urano T, Napiorkowska-Pawlak D, Ihara H, Takada Y, Collen D, Takada A. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113:995–1001. doi: 10.1016/s0306-4522(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Rickles RJ, Strickland S. Tissue plasminogen activator mRNA in murine tissues. FEBS Lett. 1988;229:100–106. doi: 10.1016/0014-5793(88)80806-8. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Rocha BA, Oglesby MW, Stephens DN. Increased sensitivity to cocaine, and over-responding during cocaine self-administration in tPA knockout mice. Brain Res. 1999;826:117–127. doi: 10.1016/s0006-8993(99)01280-9. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Horwood JM, Stephens DN. Evidence for impairment of behavioural inhibition in performance of operant tasks in tPA−/− mice. Behav. Brain Res. 2001;125:215–227. doi: 10.1016/s0166-4328(01)00303-5. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Samson AL, Nevin ST, Croucher D, Niego B, Daniel PB, Weiss TW, Moreno E, Monard D, Lawrence DA, Medcalf RL. Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J Neurochem. 2008;107:1091–1101. doi: 10.1111/j.1471-4159.2008.05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli JD. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;192:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds NW, Williams BL, Bickford PC. Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. doi: 10.1126/science.270.5244.1992. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology (Berl) 1994;16:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Soubrie P. Serotonergic neurons and behavior. J Pharmacol. 1986;17:107–112. [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermaelen CP, Bonduki AC, Kitai ST. Excitation of caudate-putamen neurons following stimulation of the dorsal raphe nucleus in the rat. Brain Res. 1979;175:356–361. doi: 10.1016/0006-8993(79)91016-3. [DOI] [PubMed] [Google Scholar]
- Vassali JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Basal Ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. 5th edition. New York: Oxford UP; 2004. pp. 361–413. [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pothakos K, Tsirka SA. Extracellular proteases: biological and behavioral roles in the mammalian central nervous system. Curr Top Dev Biol. 2005;66:161–188. doi: 10.1016/s0070-2153(05)66005-x. [DOI] [PubMed] [Google Scholar]