Abstract
Bacground & Aims
Hepatitis C virus (HCV) infection is associated with an increased prevalence of diabetes and insulin resistance (IR); whether this is a causal relationship has not been established.
Methods
We performed a longitudinal study within the lead-in phase of the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) Trial to evaluate whether suppression of hepatitis C is associated with improvement in IR. Participants had advanced hepatic fibrosis and carried non-3 HCV genotypes (n=96). Patients underwent 24 weeks of pegylated interferon (PEG IFN) and ribavirin therapy and were categorized into HCV clearance groups at week 20 based on HCV RNA levels: null responders (NR) had < 1 log10 decline (n=38); partial responders (PR) had ≥1 log10 decline (n=37), but detectable HCV RNA, and complete responders (CR) had no detectable HCV RNA (n=21). The primary outcomewas change (week 20 minus week 0) in IR using the homeostasis model assessment (HOMA2-IR).
Results
Adjusting only for baseline HOMA2-IR, mean HOMA2-IR differences were −2.23 (CR), −0.90 (PR), and +0.18 (NR) (p =0.036). The observed differences in mean HOMA2-IR scores were ordered in a linear fashion across response groups (p=0.01). The association between HCV clearance and improvement in HOMA2-IR could not be accounted for by adiponectin or tumor necrosis alfa, and was independent of potential confounders including age, gender, ethnicity, BMI, duration of infection, medications used, and fibrosis.
Conclusion
HCV suppression correlates with improvement in insulin resistance. These data provide further support for a role of HCV in the development of insulin resistance.
Keywords: HALT-C, Cirrhosis, HOMA, Interferon, Adiponectin
Introduction
A number of studies have demonstrated a strong association between hepatitis C virus (HCV) infection and insulin resistance 1–3, providing a possible link between this infection and diabetes mellitus. Indeed, findings of a correlation between increasing HCV RNA levels and greater insulin resistance have suggested that HCV may directly or indirectly induce insulin resistance 4–7.
Despite the evidence for relatively higher than expected levels of insulin resistance in HCV infection, causality remains to be established. The cross-sectional nature of most of the relevant literature and the presence of multiple confounders have precluded accurate interpretations regarding cause and effect. Using a mouse model, Shintani et al. demonstrated a contribution of HCV to the development of insulin resistance 7. However, the question of whether HCV directly contributes to insulin resistance in a human cohort remains unanswered.
The study of insulin resistance in the setting of de novo HCV infection may be most informative. However, given the limited number of acute infections, a much more practical means of addressing causality in a clinical study is to determine whether insulin resistance improves with clearance of HCV infection. If HCV is a causal factor, then clearance of viremia should be associated with reduced insulin resistance.
Thus, the question of whether insulin resistance improves with clearance of HCV infection merits further investigation. In order to address this question, we performed a nested study within the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) Trial comparing complete responders with partial responders and null responders to antiviral therapy during the lead-in phase of treatment. Furthermore, the possible role of adipocytokines as mediators in the association between HCV and insulin resistance was explored.
Patients and Methods
Study Subjects
Patients who participated in the lead-in phase of the HALT-C Trial were considered for inclusion. HALT-C participants were non-responders to previous round(s) of interferon treatment (with or without ribavirin) for HCV infection with advanced hepatic fibrosis or cirrhosis. The lead-in-phase was a 24-week period of treatment with PEG IFN-alfa 2a (180 μg/week) and ribavirin (1000–1200 mg/day depending on body weight) and has been described in previous HALT-C publications 8. Only HALT-C participants who were documented to be fasting at the time of blood sample collections and who were not taking glucose lowering medications (or who did not have a history of diabetes mellitus) (n=108) were included. Subjects with HCV genotype 3 and those with undetectable insulin levels at any timepoint were excluded (n=12). A total of 96 subjects from the two centers with documented fasting status were included (Saint Louis University School of Medicine (SLU), n=55, and Massachusetts General Hospital (MGH), n=41). All patients were followed longitudinally from week 0 until week 20 of the lead-in-phase.
Subject Characteristics
Baseline demographic characteristics included age, gender, race and history of diabetes mellitus. Waist circumference, weight, height, systolic and diastolic blood pressure at week 0 and at week 20 of the study were reviewed. Liver biopsies were evaluated by a team of 12 pathologists. Hepatic steatosis was graded on a scale of 0 – 4 9 and fibrosis was staged based on the scale described by Ishak et al. 10. Patients with a fibrosis stage 5 or 6 were considered as having cirrhosis.
Laboratory Determinations
All sample collections were taken following at least an 8 hour fast at week 0 and week 20. Routine chemistries including lipids, glucose, liver enzymes, bilirubin levels, and prothrombin time were obtained in local laboratories within the participating clinical centers by standard assays. HCV RNA testing was performed at the HALT-C central virology laboratory (University of Washington, Seattle, WA) using commercial assays (quantitative and qualitative COBAS Amplicor Monitor HCV assay version 2.0). The Virology lab subsequently tested all week 20 samples with the VERSANT HCV RNA Qualitative Assay (Bayer Diagnostics, Berkeley, CA), based on transcription-mediated amplification (TMA) technology (lower limit of HCV RNA detection of 10 IU/mL),11. Fasting insulin levels were tested at the University of Michigan, Ann Arbor, MI, by a radioimmunoassay 12. Frozen samples were shipped to Massachusetts General Hospital, in Boston, MA, for measurement of adipocytokines. Adiponectin levels were quantified using ELISA (Linco Research, St. Charles, MO) while tumor necrosis factor alpha (TNF-α) levels were measured using a highly sensitive ELISA assay (R & D Systems, Minneapolis, MN). Manufacturers’ instructions were followed for both adipocytokine measures, and all adipocytokine samples were measured in duplicate.
Definitions and calculations
Final adiponectin and TNF-α levels represented an average of the duplicate values obtained per sample. Body mass index (BMI) was calculated as weight (Kg) divided by height squared (m2). Insulin resistance (IR) was calculated using the homeostasis model assessment 2 (HOMA2) from the publicly available software provided by its original authors (http://www.dtu.ox.ac.uk/homa) 13. We did not exclude or truncate any insulin or glucose values in this IR cohort 9. Eradication of viremia was represented as a categorical variable in which subjects were classified as either “complete responders” (no detectable HCV RNA at week 20), “partial responders” (drop in HCV RNA of >=1 log10 from week 0) or “null responders” (decline in HCV RNA < 1 log10 ). Complete responders were further evaluated to confirm their status using the more sensitive transcription-mediated amplification (TMA) assay 14. Among complete responders, only one was positive by TMA assay. This subject was then recategorized as a partial responder.
Statistical Analysis
In order to evaluate the association between response to HCV therapy and change in insulin resistance over time, a multiple linear regression model was constructed in which change in HOMA2-IR (week 20 minus week 0) was the dependent and response to therapy categories the independent effect. In order to avoid confounding from baseline insulin resistance, ln HOMA2-IR at week 0 (because of lack of normality) was included in the initial model. In the second part of the analysis, the potential role of AN and TNF-α in the association between HCV and insulin resistance was explored in univariate and multivariate models. Correlations between each of these adipokines and HCV infection were evaluated. Baseline AN and TNF-α entered into the regression model. Then, a more comprehensive model was built for identification of potential confounders by analysis of clinically relevant variables. Variables associated with the outcome at a significance level (p value) of <=0.1 were selected for inclusion in the more parsimonious final model. Finally, center and a center by HCV treatment response interaction were evaluated in the final model to exclude the possibility of confounding based on participating institution. JMP Statistical Software version 7 (SAS Institute, Cary, NC) was used for all statistical analyses. All analyses were performed at the University of Miami using a dataset provided by the HALT-C data coordinating center (New England Research Institutes).
Results
Baseline characteristics
As mentioned previously, our cohort represents a sub-group within the HALT-C Trial. Descriptive statistics for the cohort are presented in Table 1. The median age was 49 years and the majority of participants were male Caucasians. The median duration of infection for our group was 29 years. Mean body mass index (BMI) was 30.4 Kg/m2. The majority of subjects had advanced fibrosis or cirrhosis while 34% percent had moderate or advanced steatosis. Mean baseline glucose was 107.3 mg/dL and mean HOMA 2-IR at week 0 was 4.8. Potential differences between the two centers were evaluated. No statistically significant differences between the centers were noted in any of the variables except for triglyceride levels at week 0 (means: 147 (SLU) vs. 100 (MGH) mg/dL, p=0.0025).
TABLE 1.
Baseline characteristics of cohort
| Variable | Frequency n (%), Median (range), or Mean (SD) n= 96 |
|---|---|
| Age (years) | 49 (33 – 73) |
| Male gender | 73 (76%) |
| Race | |
| Caucasian | 87 (91%) |
| African American | 9 (9%) |
| Estimated duration of infection (years) | 29 (7 – 49) |
| Mean systolic/Mean diastolic blood pressure (mm Hg) | 133 (16.7) / 81 (9.0) |
| Waist circumference (cm) | 99 (13.4) |
| Body Mass Index (Kg/m2) | 30.4 (5.3) |
| Log HCV RNA | 6.5 (0.4) |
| Fibrosis (Ishak score) | |
| 2–4 | 52 (54%) |
| 5–6 | 44 (46%) |
| Steatosis (Brunt scale) | |
| 0–1 | 63 (66%) |
| 2–3 | 33 (34%) |
| Glucose (mg/dL) | 107.3 (32.1) |
| Insulin (μIU/mL) | 42.5 (41.5) |
| HOMA2-IR | 4.8 (3.2) |
| Adiponectin (ng/mL) | 42.5 (15.6) |
| Tumor necrosis factor (pg/mL) | 3.0 (2.1) |
Clearance of HCV correlates with improvement of insulin resistance
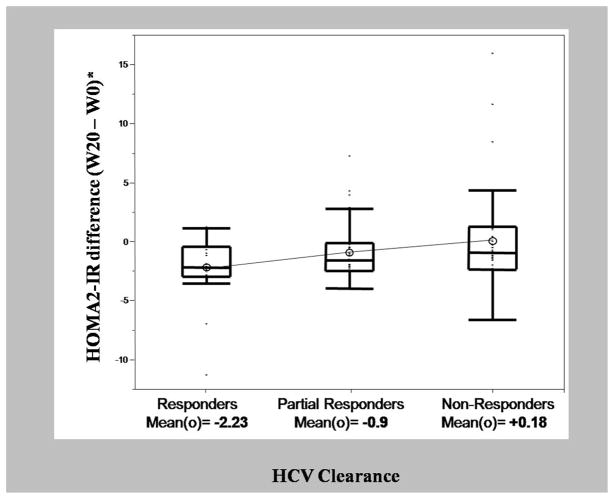
Clearance of HCV was established based on week 20 HCV response categories. In our cohort, there were 21 complete responders, 37 partial responders and 38 non-responders to HCV therapy. Our initial model evaluated the correlation between eradication of the virus (represented by HCV clearance categories) and change in HOMA2-IR (week 20-week 0) while controlling for baseline HOMA2-IR. A ten percent increase in baseline HOMA2 was associated with a reduction in HOMA2 by 0.4 units (p=<0.01). The adjusted mean HOMA2-IR differences (week 20 – week 0) across the three virological clearance categories were −2.23 for complete responders, −0.90 for partial responders, and 0.18 for non-responders (p=0.036). The observed mean HOMA2-IR difference scores were ordered in a linear fashion across response groups and this linear trend was statistically significant (F(1,92)=6.8, p=0.01) (Figure 1). The linear trend accounted for virtually all of the variation observed in the HOMA2-IR difference scores.
Figure 1.
Side-by-side quartile box and whisker plots of difference in HOMA2-IR by HCV clearance categories. Box plots represent median (center line in boxes), means (open circles), 25th to 75th percentiles (box ends) and 5th to 95th percentiles (whisker ends) of HOMA2-IR difference scores, n=96. Mean HOMA2-IR differences are −2.23 (CR), −0.90 (PR), and +0.18 (NR), (p =0.036), adjusted for natural log of baseline HOMA2-IR. HOMA2-IR difference scores were ordered in a linear fashion across response groups and this linear trend was statistically significant (F(1,92)=6.8, p=0.01).
Association between HCV clearance and improvement in insulin resistance appears to be independent of serum adiponectin and/or TNF-α
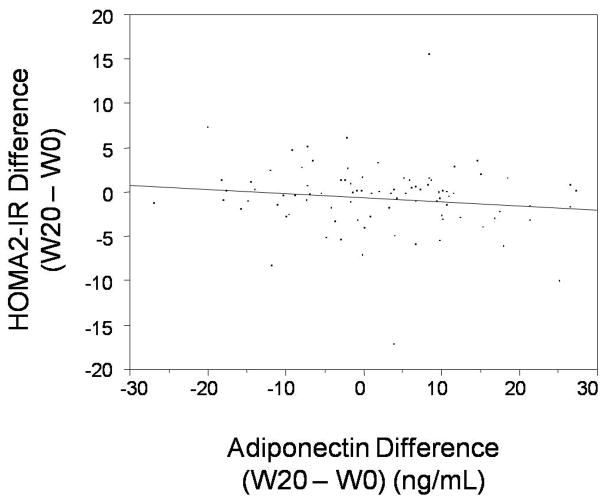
Analyses were conducted to evaluate whether the association between HCV and IR could be explained, at least in part, by respective changes in AN and/or TNF-α. We found no significant association in our cohort between HCV clearance or change in HCV RNA levels (week 20 – week 0) and change in AN. A trend towards a negative correlation between change in AN and change in HOMA2-IR (week 20 – week 0) was noted, but this did not achieve statistical significance (r= −0.14, p=0.19) (Figure 2). Baseline AN and change in AN were entered into the regression model described above and we found that a one unit increase in baseline AN correlated with a reduction in insulin resistance of 0.05 HOMA2-IR units (p=0.02), while a one unit change in AN (week 20 – week 0) did not have a statistically significant correlation with change in insulin resistance (r= −0.05, p=0.07). We found that males (n=71) had statistically lower mean baseline AN levels (40.1 ng/mL) than females (n=21, 50.4 ng/mL), but entering gender in muiltivariate models did not change our findings. No significant correlations were noted between TNF-α and HCV clearance or change in HOMA2-IR in our cohort. Evaluating TNF-α in the regression model did not change the previous results as only HCV clearance, baseline HOMA2-IR, and baseline AN were significantly associated with improvement in HOMA2-IR. Thus, the association between HCV clearance and improvement in HOMA2-IR was independent of AN and TNF-α in our cohort.
Figure 2.
Plot of change in Adiponectin (X-axis) versus change in HOMA2-IR (Y-axis) (week 20 – week 0). A trend towards decreasing HOMA2-IR with increasing adiponectin level is noted (R2= − 0.14) but this did not achieve statistical significance (p= 0.19).
Correlation between HCV clearance and reduction in insulin resistance is not explained by confounders
A number of additional variables were then thoroughly evaluated in an effort to address potential confounding. Initially, univariate associations between HCV clearance and change (week 20 – week 0) in BMI, chemistries, AN, and TNF-α were analyzed and none achieved statistical significance in our cohort (data not shown). This suggested that these factors were not likely to account for the association between HCV clearance and HOMA2-IR. As expected from our study design using relatively uniform histology, the correlation between degree of fibrosis and HCV clearance was not significant since most had advanced fibrosis. Similarly, the correlation between steatosis at baseline and HCV clearance was not significant. Thus, changes in HOMA2-IR were not attributable to differences in baseline histology. To further reduce the likelihood of confounding, a multivariate model was constructed. Based on well established clinical associations, a model was built in which change in HOMA2-IR (week 20 minus week 0) was predicted from HCV clearance categories while controlling for age, gender, baseline BMI, BMI difference (week 20 – week 0), duration of infection, steatosis, fibrosis, as well as AN and baseline HOMA2-IR. From this model, the variables selected (p<=0.1) were HCV clearance, baseline HOMA2-IR, baseline AN, and baseline BMI. A final model with only the selected variables showed that, once again, HCV clearance, baseline HOMA2-IR, and baseline AN correlated statistically with change in insulin resistance while BMI did not achieve statistical significance in our model. Once the final model was selected, center as well as center by HCV clearance interaction were entered into the model but the results remained unchanged as these last two variables did not achieve statistical significance. Results from the final multivariate model are summarized in Table 2.
Table 2.
Multivariate regression model
| Variable | Effect on change in HOMA2-IR (W20-W0) | P- value |
|---|---|---|
| HCV clearance | Complete responders=− 2.42 | 0.02 |
| Partial responders=− 0.88 | ||
| Non-responders=+ 0.03 | ||
| Baseline HOMA2-IR (10% increment) | − 0.49 | <0.01 |
| Baseline AN | − 0.05 | 0.05 |
| Baseline BMI | 0.11 | 0.14 |
Discussion
Most clinical studies published on the association between HCV and insulin resistance have been cross-sectional in nature and confounded by a number of factors. Potential confounders have not been evaluated thoroughly in the literature, and multifactorial models simultaneously analyzing their independent effects have been lacking.
In this study we found that insulin resistance improves with clearance of hepatitis C viremia. Overall, non-responders exhibited negligible change in HOMA2-IR during the course of HCV therapy, while partial and complete responders experienced a reduction in HOMA2-IR levels. An important result of our study was the linear trend noted, whereby HOMA2-IR decreased gradually across the three HCV clearance categories. Detailed multivariate analyses did not reveal confounders in the association between HCV and IR. Our findings provide support for a causal role of HCV in the induction of insulin resistance. Although this evidence is indirect, it represents the most direct human data to date to address the relationship between HCV and insulin resistance.
In 2005, Romero-Gomez et al. found that among 50 patients, HOMA IR decreased during the first 6 months of therapy in those who achieved HCV RNA clearance at 6 months, but remained unchanged in patients that did not eradicate viremia 16. This study included subjects with heterogeneous liver histologies and more than one HCV genotype. Kawaguchi et al. 17 found that HCV clearance improved insulin resistance among 89 subjects. Although this was an interesting study, there were a number of limitations. Subjects had mild to moderate fibrosis and responders had statistically lower levels of fibrosis than relapsers or nonpresponders. In addition, baseline insulin resistance was not considered in the analysis, subjects underwent different treatment modalities, and change in IR was not evaluated in a single statistical model. Nevertheless, our results are consistent with those of Kawaguchi et al. 17.
In our cohort we did not find evidence to support a role for adiponectin or TNF-α as possible mediators in the effect of HCV on insulin resistance. However, the significant fibrosis of our cohort may have impaired hepatic clearance of adipokines 19, 20, which limits possible interpretability. In addition, the lower adiponectin levels observed among males who represented the majority of our subjects may also limit our findings. Our results suggest that higher baseline AN correlates with improvement in insulin resistance independently of HCV clearance. A trend towards an inverse correlation between change in adiponectin and change in IR raises the possibility of a lack of power for this particular analysis. We believe the role of adipokines in mediating the effect of HCV on insulin resistance merits additional study.
One potential limitation of our findings is that the effects noted on insulin resistance across the different response categories could be interpreted as secondary to varying levels of response to the medications themselves. However, it is unlikely that the improvement in insulin resistance noted with higher degrees of response to therapy could be secondary to the use of interferon, which would be expected to cause the opposite effect 21, 22.
The concept that HCV may induce insulin resistance in vivo has important clinical consequences. Insulin resistance and diabetes mellitus lead to a number of serious systemic complications and adversely impact the natural history of HCV and its response to antiviral therapy. Based on our findings, improvement of insulin resistance might be an added benefit of treatment of HCV infection. In addition, early identification and management of insulin resistance should have beneficial health effects overall, and would be expected to have a positive impact on HCV liver disease, although further prospective studies will be required to support this premise.
In summary, our results demonstrate a correlation between clearance of HCV RNA levels and improvement of insulin resistance. These findings provide additional in vivo support for a causal role of HCV in the development of insulin resistance. Further study will be necessary to clarify the mechanisms of HCV-associated insulin resistance and to evaluate the effects of early identification and management of insulin resistance in HCV infection.
Acknowledgments
This work was supported by: NIH K08 DK070022 (ADB), NIH DK078772 (RTC), the Robert Wood Johnson Foundation (ADB), NIH P60 DK020572 (Michigan Diabetes Research and Training Center), NIH Contract N01-DK-9-2323 (ASL).
List of Abbreviations
- AN
adiponectin
- HALT C
Hepatitis C Antiviral Long-Term Treatment against Cirrhosis
- HCV
hepatitis C virus
- HOMA2-IR
homeostasis model assessment
- IFN
interferon
- IR
insulin resistance
- IRS-1
insulin resistance substrate 1
- IU
International Units
- NHANES
National Health and Nutrition Examination Survey
- NR
null responders
- PCR
polymerase chain reaction
- PEG IFN
pegylated interferon
- PR
partial responders
- CR
complete responders
- RNA
ribonucleic acid
- TMA
transcription-mediated amplification
- TNF-α
tumor necrosis factor-α
Footnotes
Disclosures: No conflicts of interest exist
Authors’ involvement in manuscript: ADB (concept, design, acquisition of data, analysis, interpretation of data, drafting of manuscript, funding, study supervision); SHJ (study concept, acquisition of data, technical support, analysis and interpretation of data, critical revision of manuscript); BN (analysis and interpretation of data, revision of manuscript); DH (acquisition of data, technical support); WL (study concept, technical support); YK (study concept and design, acquisition of data, technical support); MC (technical support); DAL (statistical support, critical revision of manuscript); ASFL (critical revision of manuscript, funding); RTC (study concept, design, analysis and interpretation of data, critical revision of the manuscript, funding, study supervision).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Narita R, Abe S, Kihara Y, Akiyama T, Tabaru A, Otsuki M. Insulin resistance and insulin secretion in chronic hepatitis C virus infection. J Hepatol. 2004;41:132–8. doi: 10.1016/j.jhep.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, Bhan A, Baid S, Cosimi AB, Pascual M, Chung RT. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77:703–10. doi: 10.1097/01.tp.0000114283.04840.3a. [DOI] [PubMed] [Google Scholar]
- 3.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–44. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. Gastroenterology. 2003;125:1695–704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Borrego A, Liu YS, Jordan SH, Agrawal S, Zhang H, Christofi M, Casson D, Cosimi AB, Chung RT. Prospective study of liver transplant recipients with HCV infection: Evidence for a causal relationship between HCV and insulin resistance. Liver Transpl. 2008;14:193–201. doi: 10.1002/lt.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, Sata M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–8. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 8.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG, Morishima C, Wright EC, Everhart JE. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–92. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Lok AS, Everhart JE, Chung RT, Padmanabhan L, Greenson JK, Shiffman ML, Everson GT, Lindsay KL, Bonkovsky HL, Di Bisceglie AM, Lee WM, Morgan TR, Ghany MG, Morishima C. Hepatic steatosis in hepatitis C: comparison of diabetic and nondiabetic patients in the hepatitis C antiviral long-term treatment against cirrhosis trial. Clin Gastroenterol Hepatol. 2007;5:245–54. doi: 10.1016/j.cgh.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Ishak KG. Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol. 2000;113:40–55. doi: 10.1309/42D6-W7PL-FX0A-LBXF. [DOI] [PubMed] [Google Scholar]
- 11.Hendricks DA, Friesenhahn M, Tanimoto L, Goergen B, Dodge D, Comanor L. Multicenter evaluation of the VERSANT HCV RNA qualitative assay for detection of hepatitis C virus RNA. J Clin Microbiol. 2003;41:651–6. doi: 10.1128/JCM.41.2.651-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi M, Floyd JC, Jr, Pek S, Fajans SS. Insulin, proinsulin, glucagon and gastrin in pancreatic tumors and in plasma of patients with organic hyperinsulinism. J Clin Endocrinol Metab. 1977;44:681–94. doi: 10.1210/jcem-44-4-681. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.Morishima C, Morgan TR, Everhart JE, Wright EC, Shiffman ML, Everson GT, Lindsay KL, Lok AS, Bonkovsky HL, Di Bisceglie AM, Lee WM, Dienstag JL, Ghany MG, Gretch DR. HCV RNA detection by TMA during the hepatitis C antiviral long-term treatment against cirrhosis (Halt-C) trial. Hepatology. 2006;44:360–7. doi: 10.1002/hep.21265. [DOI] [PubMed] [Google Scholar]
- 15.Yoon EJ, Hu KQ. Hepatitis C virus (HCV) infection and hepatic steatosis. Int J Med Sci. 2006;3:53–6. doi: 10.7150/ijms.3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, Corpas R, Cruz M, Grande L, Vazquez L, Munoz-De-Rueda P, Lopez-Serrano P, Gila A, Gutierrez ML, Perez C, Ruiz-Extremera A, Suarez E, Castillo J. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–41. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, Sata M. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–6. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 18.Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, George J. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66–73. doi: 10.1002/hep.21703. [DOI] [PubMed] [Google Scholar]
- 19.Tietge UJ, Boker KH, Manns MP, Bahr MJ. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287:E82–9. doi: 10.1152/ajpendo.00494.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kaser S, Moschen A, Kaser A, Ludwiczek O, Ebenbichler CF, Vogel W, Jaschke W, Patsch JR, Tilg H. Circulating adiponectin reflects severity of liver disease but not insulin sensitivity in liver cirrhosis. J Intern Med. 2005;258:274–80. doi: 10.1111/j.1365-2796.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- 21.Kanai F, Omata M. Glucose intolerance caused by interferon therapy. Nippon Rinsho. 2005;63 (Suppl 2):315–9. [PubMed] [Google Scholar]
- 22.Sasaoka T. Glucose intolerance induced by interferon therapy. Nippon Rinsho. 2002;60 (Suppl 7):760–5. [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]