Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is an integral membrane glycoprotein which functions as an anion channel and influences diverse cellular processes. We studied its role in the development of epithelial tightness by expressing wild-type (WT-CFTR) or mutant (ΔF508-CFTR) CFTR in human airway epithelial cell monolayers cultured at the air–liquid interface. Green fluorescent protein (GFP)-tagged WT or ΔF508 constructs were expressed in the CF bronchial cell line CFBE41o− using adenoviruses, and the results were compared with those obtained using CFBE41o− lines stably complemented with wild-type or mutant CFTR. As predicted, GFP-WT-CFTR reached the apical membrane whereas GFP-ΔF508-CFTR was only detected intracellularly. Although CFTR expression would be expected to reduce transepithelial resistance (TER), expressing GFP-CFTR significantly increased the TER of CFBE41o− monolayers whilst GFP-ΔF508-CFTR had no effect. Similar results were obtained with cell lines stably overexpressing ΔF508-CFTR or WT-CFTR. Preincubating ΔF508-CFTR monolayers at 29°C reduced mannitol permeability and restored TER, and the effect on TER was reversible during temperature oscillations. Expression of GFP-ΔF508-CFTR or GFP-WT-CFTR in a cell line already containing endogenous WT-CFTR (Calu-3) did not alter TER. The CFTR- and temperature-dependence of TER were not affected by the CFTR inhibitor CFTRinh172 or low-chloride medium; therefore the effect of CFTR on barrier function was unrelated to its ion channel activity. Modulation of TER was blunted but not eliminated by genistein, implying the involvement of tyrosine phosphorylation and other mechanisms. Modulation of CFTR trafficking was correlated with an increase in tight junction depth. The results suggest that CFTR trafficking is required for the normal organisation and function of tight junctions. A reduction in barrier function caused by endoplasmic reticulum retention of ΔF508-CFTR may contribute to fluid hyperabsorption in CF airways.
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) is an anion channel and member of the ATP-binding cassette (ABC) transporter superfamily. It is expressed in a wide range of tissues including brain (Hincke et al. 1995), heart (Nagel et al. 1992) and smooth muscle (Robert et al. 2004); however, its best known role is to mediate anion movements across epithelia (Riordan, 2008). The loss of CFTR function leads to dramatically impaired ion and fluid movement, which produces the respiratory and digestive tract abnormalities that characterize cystic fibrosis (CF). The most common CFTR mutation is the deletion of phenylalanine at position 508 (ΔF508). This mutant is partially misfolded, accumulates in the endoplasmic reticulum (Cheng et al. 1990; Gilbert et al. 1998), and is targeted for degradation rather than being trafficked to the apical membrane (Puchelle et al. 1992). As CFTR is an anion channel, it has been suggested that the respiratory symptoms observed in CF are caused by the loss of apical CFTR chloride (Cl−) conductance and dysregulation of ion and fluid transport across the epithelium, leading to the accumulation of dehydrated mucus and bacteria (Boucher, 2007). CF epithelia have impaired regeneration and differentiation (Dupuit et al. 1995; Hajj et al. 2007), therefore ΔF508-CFTR may also cause pathogenesis by disrupting epithelial development and repair, although these mechanisms remain to be identified.
Barrier function plays a crucial role in airway host defences. Epithelial cells prevent the airborne contaminants that are carried into the lungs from entering the submucosa. Airway epithelia also have specialised defence mechanisms, such as the secretion of antimicrobial peptides, and mucociliary clearance which transports harmful substances out of the airways. Both these host defence mechanisms require epithelial differentiation and barrier function to prevent the entry of toxins and pathogens into the submucosa (Humlicek et al. 2007), and to minimize alterations in airway surface liquid volume or composition that might compromise mucociliary clearance (Morimoto et al. 1998).
Tight junctions (TJs) restrict paracellular diffusion of small solutes and fluid across the epithelium (Cereijido et al. 1989) and are tightly coupled to the sub-apical actin network. They delimit the apical from basolateral membrane and determine the tightness of the epithelium and therefore strongly influence the transepithelial resistance (TER). Normal polarisation and differentiation of airway epithelial cells is dependent on the integrity of intercellular junctions (Farquhar & Palade, 1963; Wang & Margolis, 2007), and a link between CFTR and epithelial cell polarisation has been reported, although the relationship is not well understood (Hollande et al. 1998).
Here we study the role of CFTR in barrier function under well-defined conditions using air–liquid interface cultures of human bronchial epithelial cell lines as models for CF and non-CF absorptive airway surface epithelium.
Methods
Culture media
Cell lines were maintained in Eagle's minimum essential medium (EMEM, Wisent, St-Bruno, QC, Canada) plus 10% fetal bovine serum (FBS). For low chloride experiments, medium containing 1.3 mm Cl− was prepared based on EMEM: 1.36 mm calcium gluconate, 5.37 mm potassium gluconate, 811.5 μm magnesium sulfate, 116.4 mm sodium gluconate, 1.01 mm sodium dihydrogen phosphate monohydrate, 20.76 mm sodium bicarbonate, 5.55 mm d-glucose and 28.2 μm phenol red, supplemented with MEM non-essential amino acids without glutamine (Wisent), pH 7.2. CFTRinh172 (3-[ (3-trifluoromethyl)phenyl ]-5- [ (4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone) (Ma et al. 2002) was used for pharmacological inhibition of CFTR. Genistein was used at 10 μm, which inhibits tyrosine kinases (IC50= 2.6 μm) but does not significantly activate ΔF508-CFTR current in CFBE cells (Bebok et al. 2005) or alter its processing and trafficking in BHK cells (Schmidt et al. 2008). Live cell imaging was performed in EMEM without phenol red (Wisent). Forskolin (10 μm) was used to elevate intracellular cAMP.
Cell culture
The CFBE41o− and Calu-3 cell lines were cultured initially under liquid–liquid conditions, then allowed to polarise at the air–liquid interface. CFBE41o− stable cell variants expressing wild-type CFTR (CFBE-WT) or the mutant ΔF508 CFTR (CFBE-ΔF) kindly provided by J. P. Clancy (University of Alabama, Birmingham, USA; Bebok et al. 2005) were also used in some experiments. For TER measurements, 300,000 cells cm−2 were seeded on cell culture inserts (Transwell-clear, diameter 12 mm, 0.4 μm pores; Corning, Acton, MA, USA) in multi-well plates and kept submerged (i.e. under liquid–liquid conditions) until they were confluent, after which the apical medium was removed from the upper compartment to allow polarisation at the air–liquid interface (ALI). Cells were used for adenoviral infections, TER measurements, backflux studies and immunohistological analyses after 1 week at the ALI.
CFBE and Calu-3 cells were cultured at the air–liquid interface under conditions that have been shown previously to produce well-developed tight junctions, as indicated by zona occludens 1 (ZO-1) immunostaining, and polarisation, as shown by apical immunostaining of CFTR (Bebok et al. 2005). In pilot experiments in Calu-3 and CFTR-transfected CFBE cells, we found a large response to forskolin (10 μm) after permeabilizing the basolateral membrane with nystatin (200 μg ml−1) and imposing a transepithelial chloride gradient (11.6 to 119.8 mm), whereas this forskolin response was lost when the apical membrane was permeabilized under the same conditions. Further evidence for apical CFTR expression was obtained using the antagonist CFTRinh172 (10 μm), which inhibited the forskolin-stimulated current when added apically but not basolaterally. Thus, ΔF508-CFTR was functionally polarized in CFBE monolayers after low temperature (29°C) rescue. In other experiments, ouabain (1 mm) inhibited the forskolin-stimulated short-circuit current when added to the basolateral side but had no effect when added apically, consistent with polarization of the CFBE cell monolayers with respect to Na+/K+ ATPase. Finally, using Alcian blue colourimetric assays and MUC5AC Western blots we detected the release of high molecular weight glycoconjugates including the major airway mucin MUC5AC from CFBE and Calu-3 monolayers exclusively on the apical side, consistent with polarized mucus secretion.
For live cell imaging experiments, cells were cultured in a modified (upside-down) ALI system by seeding them at the same density on the bottom side of Transwell inserts. Cells were allowed to adhere overnight, then the culture medium was replaced and the inverted cells were cultured to confluence under liquid–liquid conditions. The medium was then removed from the bottom compartment of the well (i.e. apical side) and the inverted cells were allowed to polarise at the ALI for 1 week before observation.
Transepithelial resistance (TER) measurements
TER was measured using chop stick electrodes and an ohmmeter (EVOM, World Precision Instruments, Sarasota, FL, USA) after adding 500 μl of medium to the apical surface. The medium was removed immediately after measuring TER.
Western blotting
Polarised CFBE41o− monolayers were scraped into radioimmunoprecipitation assay (RIPA) buffer and lysates were loaded on a 10% polyacrylamide gel (10 μg of total protein per lane). After migration at 100 V for 1 h, proteins were transferred to a nitrocellulose membrane (BioRad Laboratories, Hercules, CA, USA) and occludin was detected using polyclonal rabbit antiserum (71-1500, Invitrogen) at 1/1000 dilution.
Immunostaining
Cell monolayers were fixed in paraformaldehyde (4% in phosphate buffered saline: PBS) for 1 hour, then rinsed with PBS. Cultures were cut from the inserts and embedded in paraffin. Sections 5 μm thick were laid on microscope slides and rehydrated by successive immersion in xylene (3 baths), ethanol (2 baths), 70% ethanol, 50% ethanol and water. Non-specific binding was inhibited by 1 h incubation in PBS containing 3% bovine serum albumin (BSA). The primary antibodies (anti-CFTR C-terminal, clone 24-1, R&D Systems, Minneapolis, MN, USA or anti-ZO-1, clone A12, Invitrogen, Carlsbad, CA, USA), were diluted 1/50 or 1/200, respectively, in PBS–1% BSA and incubated overnight at 4°C. Sections were rinsed three times in PBS and incubated with secondary goat anti-mouse Cy3-conjugated antibody (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h. After rinsing twice with PBS, cells were counterstained with Mayer's haematoxylin (Sigma-Aldrich, St Louis, MO, USA), rinsed with water and then again with BSA solution, and analysed by confocal microscopy (Carl Zeiss, Confocor LSM 510 META, ×63, NA 1.4, oil immersion).
Adenovirus infection and membrane co-localisation
Monolayers were infected with adenoviruses directing the expression of GFP-CFTR or GFP-ΔF508-CFTR (Vais et al. 2004). Infections were performed by incubating overnight in EMEM at a multiplicity of infection of 100 virus particles per cell. The cultures were rinsed with PBS and allowed to express the construct for 5 days prior to imaging. After measuring TER, cells were loaded for 15 min with the membrane dye FM4-64 (Molecular Probes, Invitrogen) and placed in a glass-bottom dish for analysis by confocal microscopy. All procedures were approved by the Environmental Safety Office of McGill University.
Mannitol backflux
To measure mannitol backflux, 14C-mannitol (6.55 μm, 14.8 kBq) was added to the basal compartment and 100 μl of medium were added to the apical chamber of ALI cultures that had been kept at 37°C or 29°C for 24 h. After 1 h exposure to tracer at 37°C or 29°C, 20 μl were collected from the basal (i.e. input) and apical (output) compartments and measured using a scintillation counter. Background counts from cultures without 14C-mannitol were subtracted, the unidirectional mannitol flux was determined as nmol cm−2 h−1, and the permeability coefficient *P was calculated as *P=Jb→a/[mannitol] where Jb→a is the flux of mannitol from the basolateral to apical compartment. The effect of cAMP stimulation on mannitol permeability was studied by adding forskolin to the culture medium during the entire experiment (i.e. during the 24 h preincubation period + 1 h exposure to radiolabelled mannitol during flux measurements).
Image analysis and statistics
Confocal images were analysed using ImageJ (http://rsb.info.nih.gov/ij/) and membrane colocalisation was determined by analysing the whole acquired images using the bidimensional correlation function from the SIVP toolbox (http://sivp.sourceforge.net/) for SciLab (http://www.scilab.org/). Briefly, grey-level images of the membrane and CFTR signals were imported as bidimentional matrixes and the coefficient of cross-correlation was determined as
 |
where R(xi, yj) and G(xi, yj) are the matrices obtained from the images acquired in the red and green channels, respectively, and  and
and  are the corresponding means. At least nine image stacks spanning the whole thickness of the monolayers were used for each condition. Results were subjected to the Mann–Whitney non-parametric test, with an error probability lower than 5% (P < 0.05) or 0.5% (P < 0.005) considered as significant or highly significant, respectively. The data are presented as non-parametric variables, i.e. as the medians [first quartile; third quartile].
are the corresponding means. At least nine image stacks spanning the whole thickness of the monolayers were used for each condition. Results were subjected to the Mann–Whitney non-parametric test, with an error probability lower than 5% (P < 0.05) or 0.5% (P < 0.005) considered as significant or highly significant, respectively. The data are presented as non-parametric variables, i.e. as the medians [first quartile; third quartile].
Results
CFTR localization and transepithelial resistance (TER)
To study the influence of CFTR and its trafficking on barrier function we transiently expressed WT-CFTR or ΔF508-CFTR as GFP-tagged fusion proteins in polarised monolayers of CFBE41o− cells, which already express low levels of endogenous ΔF508-CFTR, and Calu-3 cells, which express high levels of endogenous WT-CFTR. The amphiphilic styryl dye FM4-64 was used to visualize the plasma membrane when localizing the GFP-tagged constructs by confocal microscopy.
In all monolayers examined, the dye FM4-64 produced a fine outline of cell contours with little background intracellular staining (Fig. 1A). A significant fraction of the GFP signal was co-localized with the FM4-64 dye in polarised CFBE41o− cells, suggesting localization in the apical membrane or a vesicle compartment very close to the membrane although some GFP-CFTR signal was also observed intracellularly (Fig. 1A). By contrast, GFP-ΔF508-CFTR was situated deep within the cells and there was little co-localization with the membrane dye (Fig. 1B). Similar results were obtained when GFP fusion proteins were expressed in Calu-3 monolayers; GFP-CFTR was partially co-localized with FM4-64 (Fig. 1C) whereas GFP-ΔF508-CFTR was only detected intracellularly (Fig. 1D).
Figure 1. Localisation of adenovirally expressed GFP-CFTR and GFP-ΔF508-CFTR in polarised epithelial cell lines.
Side view (X–Z) of a z-stack of confocal images. Red: FM4-64. Green: GFP signal. A, GFP-CFTR and FM4-64 signals are co-localised apically in CFBE41o− monolayers, indicating membrane trafficking of GFP-CFTR (arrowheads). B, most GFP-ΔF-CFTR is localised intracellularly. Similar results were obtained using Calu-3 cells. C, the GFP-CFTR signal detected at the apical membrane of Calu-3 cells. D, most GFP-ΔF-CFTR is localised intracellularly. Representative of 9 experiments.
These results were quantified by determining the bi-dimensional correlation between the FM4-64 and GFP signals (Fig. 2A). According to this analysis the fraction of GFP-CFTR co-localized with FM-64 was significantly higher compared to GFP-ΔF508-CFTR (correlation: 0.57 [0.47; 0.69]vs. 0.11 [0.03; 0.26], P < 0.005, no units). Data are expressed as median [first quartile; third quartile]. 2-D correlation confirmed that the GFP-CFTR signal was also significantly more co-localized with the membrane dye compared to GFP-ΔF508-CFTR in Calu-3 monolayers (0.53 [0.49; 0.69]vs. 0.35 [0.34; 0.43], P < 0.005) (Fig. 2B). These results suggest that heterologously expressed wild-type GFP-CFTR is trafficked to the plasma membrane as expected, and its steady-state expression at the apical membrane is higher than that of GFP-ΔF508-CFTR, consistent with the cellular phenotype in cystic fibrosis.
Figure 2. Membrane localisation of adenovirally expressed CFTR and the effect on trans-epithelial resistance.
A and B, two dimensional correlation of the membrane dye FM4-64 and the GFP signal in adenovirus-infected monolayers. C and D, transepithelial resistance (TER, Ω cm2) of adenovirus-infected CFBE41o− monolayers. A and C, CFBE41o− monolayers expressing GFP-ΔF508CFTR or GFP-CFTR. C, control monolayers were infected using an empty adenovirus. B and D, CALU-3 monolayers expressing GFP-ΔF508CFTR or GFP-CFTR. D, control: empty adenovirus. A, in CFBE41o− cell monolayers 2-D correlation of the green and red signals is significantly higher for GFP-CFTR than for GFP-ΔF508CFTR, in agreement with the increased TER shown in C. In CALU-3 cells, 2-D correlation of the green and red signals is significantly higher for GFP-CFTR than for GFP-ΔF508CFTR (B); however, the increase in TER is not significant. Results are presented as medians ± quartiles (n≥ 9, *P < 0.005).
Airway epithelia are classified as moderately leaky because most transepithelial diffusion of ions occurs via the paracellular pathway (70–82% in nasal epithelial primary cultures and 80% in Calu-3 monolayers according to Willumsen & Boucher, 1989, and Tamada et al. 2001, respectively). A large increase in transepithelial resistance (TER) is therefore likely to reflect a decrease in the shunt conductance and tight junction permeability. Surprisingly, the TER of CFBE41o− monolayers expressing GFP-CFTR was significantly higher than non-infected control monolayers (638 [474; 807]vs. 380 [350; 386]Ω cm2, P < 0.005). This was not an artifact of the virus because infection with empty adenovirus or a virus directing the expression of GFP-ΔF508-CFTR had no effect on electrical resistance (348 [297; 418]Ω cm2, P= 0.7) (Fig. 2C). When GFP-CFTR was expressed in cells that already contained endogenous CFTR (Calu-3) there was a trend towards a higher TER, but in contrast to CFBE41o− it was highly variable and did not reach statistical significance. Expression of GFP-ΔF508-CFTR had no effect on the TER of Calu-3 monolayers (Fig. 2D).
These results indicate that GFP-CFTR is correctly trafficked to the apical membrane of polarised CF cells, and that improved trafficking is correlated with an increase in transepithelial resistance. Since transepithelial resistance is mainly determined by the shunt, this suggests that CFTR modulates paracellular permeability, presumably at the level of the tight junctions as they form the rate-limiting barrier. Since ΔF508-CFTR did not affect transepithelial resistance, we next examined whether the increase in transepithelial resistance induced by wild-type CFTR depends on its trafficking to the plasma membrane.
Low temperature rescue of ΔF508-CFTR trafficking restores high TER
To determine if low transepithelial resistance is due to defective trafficking of ΔF508-CFTR and to further exclude artifacts that might be due to the use of an adenoviral vector, we studied the effect of rescuing stably expressed ΔF508-CFTR by exposure to low temperature. Parental CFBE41o− cells and cells stably transduced with ΔF508-CFTR (CFBE-ΔF) or wild-type CFTR (CFBE-WT) were incubated at 29°C for 24 h to rescue trafficking to the apical membrane (Denning et al. 1992).
First we localized ΔF508-CFTR by immunostaining CFBE41o− monolayers that had been cultured at 37°C (Fig. 3A and C) or 29°C (Fig. 3B and D) for 24 h. At 37°C, ΔF508-CFTR was detected throughout the cell and as perinuclear staining (Fig. 3C). After 24 h at 29°C the perinuclear staining was no longer seen and ΔF508-CFTR could be detected at the apical pole of the cells (arrowheads; Fig. 3D), confirming that low temperature rescues ΔF508-CFTR trafficking to the membrane in this model cell line (Denning et al. 1992; Zeitlin et al. 2002; Dudez et al. 2008; Luo et al. 2009).
Figure 3. Low temperature rescue of ΔF508-CFTR trafficking to the apical pole in CFBE41o− monolayers.
CFBE41o− monolayers were incubated at 37°C or 29°C for 24 h, then embedded in paraffin. Sections were immunostained for CFTR and observed by confocal microscopy. ΔF508-CFTR is mainly detected within the cells at 37°C, often in perinuclear bodies (A and C, empty arrowheads). Some intracellular staining remains in cells incubated at 29°C for 24 h; however, ΔF508-CFTR is also detected at the apical surface (B and D, filled arrowheads). Bas, basal compartment; Api, apical compartment. Representative of 6 experiments.
We then compared the resistance of monolayers that had been incubated at 29°C or 37°C for 24 h prior to electrical measurements. The TER of CFBE-ΔF monolayers was significantly higher after cells were exposed to 29°C than at 37°C (489 [378; 613]vs. 320 [263; 374]Ω cm2; Fig. 4). Low temperature caused a more pronounced increase in apical expression in parental CFBE41o− cells, which express ΔF508-CFTR at low (endogenous) levels; TER more than doubled after exposure to 29°C (1005 [863; 1101]Ω cm2, P < 0.005) compared to 37°C (494 [401; 584]Ω cm2). By contrast, lowering temperature had no effect on the resistance of monolayers expressing wild-type CFTR, which had a TER of 868 [771; 1022]Ω cm2 at 37°C and 825 [711; 967]Ω cm2 after 24 h at 29°C.
Figure 4.
Effect of preincubation temperature on transepithelial resistance (TER). TER was monitored in parental CFBE41o− cells (centre bars) and cell variants stably expressing ΔF508-CFTR (CFBE-ΔF) or wild-type CFTR (CFBE-WT) after they had been cultured at 37°C or 29°C for 24 h. Low temperature preincubation increased the trans-epithelial resistance in CFBE-ΔF and completely rescued the TER of CFBE41o− monolayers. TER was not altered by low temperature in monolayers that had been complemented with wild-type CFTR. Results are presented as medians ± quartiles (n= 8, *P < 0.005).
These results indicate that low temperature rescue of ΔF508-CFTR trafficking increases the resistance of CF (i.e. CFBE41o−) cell monolayers, and this is only detectable when the temperature-sensitive folding mutant ΔF508-CFTR is expressed.
Mannitol backflux is reduced after rescue of ΔF508-CFTR trafficking
To confirm that CFTR-induced changes in transepithelial resistance were mediated by the paracellular pathway, we measured the permeability of monolayers to 14C-mannitol. There are no known transporters for mannitol, a small (∼4 Å hydrodynamic radius) and relatively inert molecule that is thought to diffuse across epithelia through the paracellular pathway and is often used as a marker of paracellular permeability (Madara, 1998). The permeability of parental CFBE41o− monolayers to radiolabelled mannitol was 35.7 × 10−7[28.4 × 10−7; 41.6 × 10−7] cm−2 h−1 when cells were kept at 37°C but this decreased 6-fold to 4.8 × 10−7[3.42 × 10−7; 7.14 × 10−7] cm−2 h−1 after preincubation at 29°C (Fig. 5A). An even larger decrease was observed in CFBE-ΔF monolayers (19.2 × 10−7[3.76 × 10−7; 30.5 × 10−7] cm−2 h−1vs. 1 × 10−7[0.8 × 10−7; 3.0 × 10−7] cm−2 h−1, P < 0.005). The permeability measured after low temperature rescue was similar to that of CFBE-WT monolayers (4 × 10−7[0.2 × 10−7; 1.9 × 10−7] cm−2 h−1), which was not altered significantly by low temperature (1.7 × 10−7[0.8 × 10−7; 4.6 × 10−7] cm−2 h−1, P= 0.46).
Figure 5. Reduced paracellular permeability to mannitol after low temperature rescue of ΔF508-CFTR in CFBE41o− monolayers.
A, unidirectional flux of 14C-mannitol was used to monitor the paracellular permeability of CFBE41o− monolayers cultured at 37°C or 29°C for 24 h then exposed to 14C-mannitol on the basolateral side for 1 h. Note that preincubation at low temperature significantly reduced paracellular mannitol leakage. B, to monitor the effects of cAMP on paracellular permeability, forskolin (10 μm) or the vehicle alone (control) were added to CFBE41o− monolayers cultured as in A. Preincubating monolayers at 29°C caused a dramatic decrease in paracellular permeability. Forskolin produced small reductions in mannitol permeability at both temperatures that were not statistically significant. Results are presented as medians ± quartiles (n= 3–12, *P < 0.005).
To study the effect of cAMP on paracellular permeability, we added forskolin (10 μm) or vehicle (DMSO, Control) to the basolateral compartment of CFBE41o− monolayers and incubated them at 29°C or 37°C for 24 h (Fig. 5B). Mannitol permeability was 21.0 × 10−7[15.9 × 10−7; 22.2 × 10−7] cm−2 h−1 at 37°C, and was dramatically reduced after 24 h at 29°C (0.14 × 10−7[0.07 × 10−7; 0.52 × 10−7] cm−2 h−1, P < 0.05). There was a trend at both temperatures towards lower mannitol permeability after forskolin exposure; however, this did not reach statistical significance (13.4 × 10−7[11.1 × 10−7; 18.5 × 10−7] cm−2 h−1, P= 0.15, and 0.08 × 10−7[0.07 × 10−7; 0.19 × 10−7] cm−2 h−1, P= 0.49 at 37°C and 29°C, respectively).
The higher mannitol backflux of CFBE-ΔF monolayers compared to CFBE-WT monolayers provides independent evidence that the barrier function of CF monolayers depends on CFTR trafficking to the cell surface.
Reversible changes in TER during temperature oscillations
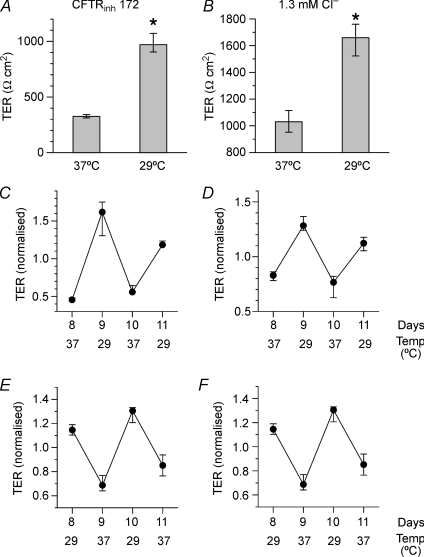
To further establish CFTR trafficking as a determinant of barrier function and determine if its effects are reversible, we subjected CFBE41o−, CFBE-ΔF and CFBE-WT monolayers to temperature oscillations between 37°C and 29°C at 24 h intervals. To eliminate bias, the monolayers were divided into two groups and subjected to opposite protocols. Thus, after culture at 37°C, one group was kept at 37°C, switched to 29°C, to 37°C again and finally back to 29°C (i.e. 37 → 29 → 37 → 29) whereas the opposite sequence was used for the other group (i.e. 29 → 37 → 29 → 37). The short-term effects of temperature were superimposed on a slowly changing baseline; therefore each value was normalized to the mean of all measurements made at the same time point (Fig. 6).
Figure 6. Reversible changes in transepithelial resistance (TER) during temperature oscillations.
Polarised monolayers were subjected to successive 24 h incubation periods that alternated between 37°C and 29°C. TER was measured at the end of each period and normalized to the resulting mean to compensate for long-term changes in resistance. TER is reversibly increased after incubation at 29°C and decreased by culture at 37°C in CFBE-ΔF cells expressing both endogenous and heterologous ΔF508-CFTR (A), and in parental CFBE41o− monolayers (B) that express only endogenous ΔF508-CFTR. Similar responses were obtained whether the starting temperature was 37°C (left panels) or 29°C (right panels). C, changes in TER are not statistically significant in CFBE-WT monolayers and trends are in the opposite direction. Results are presented as medians ± quartiles. n= 4 per point. P < 0.05 between temperatures, except for CFBE-WT.
TER increased when CFBE-ΔF monolayers were shifted from 37°C to 29°C for 24 h, then decreased again after 24 h at 37°C and increased after a second 24 h exposure to 29°C (Fig. 6A, left panel). Equivalent temperature-dependent changes were observed with the opposite sequence of temperatures (Fig. 6A, right panel). Interestingly, CFBE41o− cells expressing low levels of endogenous ΔF508-CFTR exhibited a qualitatively similar dependence of TER on temperature (Fig. 6B) whereas the TER of CFBE-WT monolayers was not altered significantly and showed a trend in the opposite direction; i.e. decreasing TER after exposure to low temperature (Fig. 6C). The latter observation suggests that low temperature may have non-specific actions (e.g. on metabolism) that partially counteract the effect of rescuing ΔF508-CFTR; therefore it is possible that the effects of temperature on TER were underestimated. Regardless, the results indicate that barrier function depends on temperature only if the cells exclusively express ΔF508-CFTR.
CFTR channel activity is not required for modulation of the paracellular pathway
It has been shown that rescued ΔF508-CFTR retains significant channel function in vitro (Denning et al. 1992) and in vivo (Zeitlin et al. 2002). We therefore investigated whether the increase in TER after ΔF508-CFTR rescue was dependent on restoration of CFTR channel function by subjecting CFBE41o− monolayers to temperature oscillations in the presence of a specific CFTR inhibitor (CFTRinh172; 40 μm; Ma et al. 2002), or in culture medium containing negligible (1.3 mm) chloride. When monolayers were cultured in the presence of CFTRinh172 at 37°C, TER was 314 [305; 332]Ω cm2 and this increased to 918 [859; 1000]Ω cm2 (n= 7, P < 0.005) when the temperature was reduced to 29°C (Fig. 7A). Similar results were obtained when cells were kept in low chloride solution (Fig. 7B). Imposing temperature oscillations revealed that TER changes were reversible and reproducible for four test temperatures regardless of the sequence, and this was true for both CFTRinh172 (Fig. 7C and E) and low chloride (1.3 mm Cl−) conditions (1031 [953; 1115]vs. 1659 [1523; 1761]Ω cm2, n= 12, P < 0.005; Fig. 7D and F).
Figure 7. Mechanisms of temperature-induced changes in TER.
A–F, polarised CFBE41o− monolayers incubated with (A, C, E) CFTRinh-172 (40 μm), a CFTR inhibitor (Ma et al. 2002) or (B, D, F) low-chloride medium ([Cl−]= 1.3 mm) for the entire duration of the experiment (i.e. 24 h), both manoeuvers that should strongly inhibit CFTR-mediated chloride conductance. Correction of ΔF508-CFTR trafficking by low temperature increases the TER of CFBE41o− monolayers significantly despite the presence of inhibitor (A) or the near-absence of Cl− (B). TER was measured during oscillations in temperature and plotted as in Fig. 6. Note that TER increases reversibly after exposure to 29°C for 24 h despite the presence of CFTRinh-172 (C and E), and in low-Cl− medium (D and F). Results are presented as medians ± quartiles (n= 7–12, *P < 0.005).
We conclude that the increase in TER following rescue of ΔF508-CFTR is independent of its chloride channel activity and that trafficking of the ΔF508-CFTR protein to the membrane is sufficient for epithelial barrier function.
CFTR modulation of the transepithelial resistance is diminished by a tyrosine kinase inhibitor
It has been shown previously that CFTR is required for the normal regulation of gap junction permeability by tumour necrosis factor-α (TNF-α), and this effect is mediated by a Src family tyrosine kinase (SFK) and recruitment of CFTR into detergent-resistant membrane (DRM) domains (Chanson et al. 1999; Dudez et al. 2008). Moreover, inhibiting the SFK c-Yes can impair TJ formation by preventing its association with occludin (Chen et al. 2002), and an association of the CFTR binding partner EBP50 with c-Yes has also been demonstrated (Mohler et al. 1999). We therefore examined the role of tyrosine kinases in CFTR-dependent modulation of epithelial tightness using the broad-spectrum tyrosine kinase (TK) inhibitor genistein (Akiyama et al. 1987).
Control CFBE41o− monolayers had a TER of 668 [642; 770]Ω cm2 when cultured at 37°C, and this increased to 1163 [1093; 1268]Ω cm2 (P < 0.05) after 5 h at 29°C in the absence of genistein but was only 793 [626; 867]Ω cm2 (n= 4, P < 0.05 relative to control 29°C cultures) in the presence of 10 μm genistein (Fig. 8). Interestingly, genistein treatment further decreased the TER of monolayers cultured at 37°C (470 [463; 479]Ω cm2).
Figure 8. Effect of the tyrosine kinase (TK) inhibitor genistein (10 μm) on the increase in TER induced by low temperature.
Both basal TER and its elevation by low temperature were inhibited when polarised CFBE41o− monolayers were incubated with the TK inhibitor genistein (gen, 10 μm) for the entire duration of the experiment (i.e. 5 h). Low temperature (29°C) increased the TER and genistein inhibited this effect (29°C + gen). Medians ± quartiles. n= 4, *P < 0.05.
These results provide evidence that the CFTR dependence of barrier function is reduced though not eliminated by tyrosine kinase inhibition, implicating TK activity in the coupling between ΔF508-CFTR trafficking and junctional tightness. The ability of TK inhibition to lower the transepithelial resistance of monolayers maintained at 37°C suggests that a small amount of ΔF508-CFTR may still reach the apical membrane and modulate tight junction permeability under these conditions.
CFTR trafficking modulates tight junction morphology
Paracellular permeability is mainly regulated by the tight junctions (TJs). We therefore examined the expression and localisation of TJ components in this epithelial cell model. Occludin mRNA transcript levels were decreased in CFBE41o− monolayers cultured at 29°C for 24 h compared to those kept at 37°C (F. Ciciriello and D. Y. Thomas, personal communication), and this was the only temperature-induced change in mRNA level amongst TJ proteins. However, occludin protein expression level was not modified in CFBE41o− monolayers according to Western blot analysis (Fig. 9A). Tight junctions are dynamic structures and are regulated by the energy-dependent exchange of ZO-1 protein between cytoplasmic and membrane pools (Shen et al. 2008). We therefore examined ZO-1 localization at the TJs of CFBE41o− monolayers by immunostaining (Fig. 9B and C). In monolayers kept at 37°C, ZO-1 staining was detectable as small dots at the apical pole near cell–cell junctions (Fig. 9B, arrowheads). When the cells were cultured for 24 h at 29°C, ZO-1 staining was stronger and extended towards the basolateral side of the epithelium, suggesting a wider zonula occludens (Fig. 9C).
Figure 9. Effect of CFTR trafficking on the localization of tight junction (TJ) proteins.
A, occludin protein expression was not altered noticeably despite a 2-fold decrease in the mRNA level when CFBE monolayers were cultured for 24 h at 29°C vs. 37°C (F. Ciciriello and D. Y. Thomas, personal communication). ZO-1 (green) had a broader distribution at the apical junctions (arrowheads) when monolayers were cultured at 29°C (C) rather than at 37°C (B). Nuclei are shown in blue. Each panel shows a monolayer that is representative of 3 independent experiments.
Discussion
In this study we report that CFTR modulates paracellular permeability in airway epithelial cell (CFBE) monolayers. Thus, in addition to its well established role as a plasma membrane channel mediating cAMP-stimulated anion secretion, the presence of CFTR at the membrane seems to be required for normal epithelial barrier function. Mislocalization of ΔF508-CFTR in human airway surface epithelium may increase fluid absorption through its effect on the paracellular shunt. Since hyperabsorption of airway surface liquid is thought to contribute to defective mucociliary clearance in CF, alterations in paracellular permeability may have important implications for the pathogenesis of cystic fibrosis.
CFTR increases transepithelial resistance and reduces mannitol permeability
When wild-type GFP-CFTR was transiently expressed in parental CFBE cells, a fraction was trafficked to the apical membrane and this was correlated with an increase in transepithelial resistance. This increase in TER was unexpected, since CFTR is a channel and any increase in membrane conductance should lower transepithelial resistance. We did not routinely expose cells to forskolin + genistein, therefore the currents carried by CFTR and rescued ΔF508-CFTR were probably small. Nevertheless, if there had been any change in TER due to CFTR, we would have expected it qualitatively to be a decrease rather than an increase. The electrophysiological effects of GFP-CFTR were not due to adenoviral infection because there was no resistance change if cells were infected with an empty adenovirus or adenovirus containing GFP-tagged ΔF508-CFTR. Moreover similar effects on resistance were observed when adenoviruses were not used; i.e. a CFBE cell line stably expressing wild-type CFTR had significantly higher resistance compared to both the parental cell line and one stably expressing heterolgous ΔF508-CFTR. The surface expression of wild-type CFTR and mislocalisation of the mutant are consistent with other cell types, and agree with studies of CFBE cells that involve surface biotinylation and streptavidin pull-down after labelling oligosaccharides on CFTR (Vais et al. 2004) or the polypeptide (Luo et al. 2009).
Epithelia have cellular and paracellular pathways for transepithelial diffusion and both routes determine transepithelial resistance, although their relative contributions vary in different epithelia. Airway epithelial cell monolayers are moderately leaky, and this is reflected by the intermediate transepithelial resistance of CFBE cells (∼350 Ω cm2). Permeability of the paracellular pathway can also be assessed by measuring transepithelial permeability to 14C-mannitol. CFTR expression increased transepithelial resistance and decreased mannitol flux, both of which are consistent with a reduction in paracellular permeability (Madara, 1998), although we note that electrical resistance and mannitol flux are not always correlated (Hasegawa et al. 1999). Wild-type CFTR probably exerts its effects at the level of the tight junctions, since they limit paracellular permeation in most epithelia and form a signalling complex (Zahraoui et al. 2000) that could potentially be influenced by the presence of CFTR in the plasma membrane.
Amiloride (10 μm) did not affect the short-circuit current when CFBE monolayers were cultured under these conditions (data not shown); therefore, the epithelial sodium channel (ENaC) probably did not influence TER significantly. Moreover, if ENaC had been expressed we would have expected TER to be dominated by the paracellular pathway as in other moderately leaky epithelia, minimizing the impact of ENaC. We cannot formally exclude contributions by other membrane conductances; however, the 14C-mannitol permeability measurements provide strong evidence for our interpretation that the large changes in TER are mostly in the paracellular pathway.
CFTR must traffic to the apical membrane to affect barrier function
The role of trafficking in CFTR modulation of paracellular permeability was shown by monitoring changes in electrical resistance when monolayers that express ΔF508-CFTR were subjected to temperature oscillations. It is well established that the processing of this mutant and its trafficking is partially corrected in most cell types by lowering the temperature from 37°C to 26–29°C (Denning et al. 1992). When monolayers were exposed to 29°C for 24 h (conditions that rescued ΔF508-CFTR trafficking) transepithelial resistance increased and became comparable to that observed for WT-CFTR cells. By contrast, low temperature had the opposite effect on cells expressing WT-CFTR, although the changes were not statistically significant. This indicates that the increase in TER at 29°C specifically requires correction of ΔF508-CFTR trafficking. It has been suggested that accumulation of misfolded ΔF508-CFTR in the endoplasmic reticulum may cause stress by triggering the unfolded protein response (UPR; Rab et al. 2007); however, this is unlikely to be the basis of altered barrier function in the present study since ΔF508-CFTR coexpression did not affect the TER of WT-CFTR monolayers. There was no indication that ΔF508-CFTR alters barrier function, as overexpressing GFP-ΔF508-CFTR did not reduce transepithelial resistance. The increase in resistance after low temperature incubation was less dramatic in cells that stably overexpress ΔF508-CFTR (CFBE-ΔF) compared to those expressing only endogenous ΔF508-CFTR, as would be expected when there is a low but finite leakage of overexpressed protein from the endoplasmic reticulum (Dalemans et al. 1991). A small amount of membrane-localized CFTR (or ΔF508-CFTR) appears sufficient to maintain low paracellular permeability. The effect of ΔF508-CFTR on transepithelial resistance was confirmed and localised to the paracellular pathway by measuring the backflux of radiolabelled mannitol. Taken together, the results indicate that CFTR must traffic to the plasma membrane and that wild-type and rescued mutant CFTR have similar effects on paracellular permeability.
Channel activity is not required for CFTR-dependent barrier function
The effects of CFTR on paracellular permeability and transepithelial resistance were observed without cAMP stimulation. Since the open probability of the CFTR channel pore is near zero under these conditions, this suggests that CFTR-mediated chloride conductance is not required to modulate barrier function. This was confirmed in CFBE41o− monolayers by the minimal effect of forskolin, which strongly activates CFTR, on mannitol permeability (Fig. 5B) and transepithelial resistance (data not shown). Further evidence for this independence comes from pharmacological and ion substitution experiments. The channel inhibitor CFTRinh172 and nominal removal of chloride (final [Cl−]= 1.3 mm) did not affect the CFTR dependence of TER; therefore, modulation of barrier function does not require CFTR channel activity or membrane anion conductance, and most probably results from CFTR interactions with proteins situated in the cell periphery. Low permeability of the paracellular pathway was reversed during 24 h temperature oscillations, thus CFTR is needed at the apical membrane to preserve the tightness of the epithelium.
Evidence for a role of tyrosine phosphorylation in CFTR regulation of paracellular permeability
The binding of PDZ domain proteins immobilizes CFTR and enables it to interact with other components of the apical macromolecular complex. These and other types of interactions may enable CFTR to interact with tight junction-associated proteins such as the PDZ domain protein ZO-1 (Wang et al. 1998) or regulatory proteins that control the assembly of junctional complexes (Zahraoui, 2004). The tyrosine kinase inhibitor genistein reduced CFTR's effect on barrier function consistent with the involvement of tyrosine phosphorylation e.g. by Src family kinases such as c-Yes (Chen et al. 2002) or c-Src (Basuroy et al. 2003), both of which can associate with CFTR through NHERF-1 (Voltz et al. 2001). Mislocalization of ΔF508-CFTR may prevent the assembly of such complexes and the formation of normal tight junctions, either directly through tyrosine phosphorylation of structural elements of the complex such as occludin (Chen et al. 2002) or indirectly through the loss of Par-3 phosphorylation (Wang et al. 2006). Occludin binds the Src family kinase c-Yes and becomes phosphorylated during tight junction assembly, and its dephosphorylation reduces TER and is associated with a loss of barrier function in MDCK cells (Chen et al. 2002). Occludin and ZO-1 are both dephosphorylated by the tyrosine phosphatase DEP-1, and overexpressing DEP-1 decreases TER and increases paracellular permeability as measured by fluorescein flux (Sallee & Burridge, 2009). Finally, tyrosine phosphorylation is increased under several conditions that enhance barrier function such as treatment with epidermal growth factor or protamine sulphate, and ATP replenishment (see González-Mariscal et al. 2008). We examined if genistein would block the increase in TER induced by rescuing of ΔF508CFTR and found a modest decrease. However, tyrosine phosphorylation of tight junction components is also increased under some conditions that are associated with disruption of barrier function, such as oxidative stress, transfection with v-src, and treatment with either hepatocyte growth factor or tyrosine phosphatase inhibitors (González-Mariscal et al. 2008). Thus, while much evidence implicates tyrosine phosphorylation of tight junction proteins in the regulation of barrier function, the precise effect of phosphorylation may depend on the particular sites, and understanding the mechanisms will require identification and mutagenesis of the target molecules, which is beyond the scope of this paper.
It is interesting that CFTR also modulates gap junction communication through a mechanism that involves tyrosine phosphorylation (Huang et al. 2003). In MDCK cells, wild-type CFTR is required for the recruitment of c-Src and tumour necrosis factor alpha (TNF-α) receptors into lipid rafts during TNF-α stimulation, which ultimately leads to tyrosine phosphorylation of connexin-43 and inhibition of cell–cell communication in response to TNF-α (Dudez et al. 2008). An analogous mechanism may mediate the effects of CFTR on tight junctions and paracellular permeability. Alternatively, proinflammatory cytokines have been shown to increase paracellular permeability (Coyne et al. 2002) and there are many reports of elevated cytokine production by CF epithelial cells (Nichols et al. 2008). If endogenous cytokine levels are decreased by partial correction of ΔF508-CFTR trafficking and/or heterologous expression of WT-CFTR this could lead to a reduction in tight junction permeability.
The composition and functional properties of tight junctions vary greatly among cell lines; however, the CFTR dependence of TER and barrier function reported here may not be unique to CFBE. For example, the type I Madin–Darby canine kidney (MDCK) cell line expresses CFTR at much higher levels than the type II MDCK cell line (Mohamed et al. 1997), and has >30-fold higher TER (Stevenson et al. 1988). This difference correlates with the preferential expression of claudin-2 in MDCK-II cells, and expressing claudin-2 heterologously in MDCK-I cells dramatically reduces TER to a value similar to that of MDCK-II (Furuse et al. 2001). Many potential mechanisms and multiple kinases may mediate changes in barrier function. Thus, down-regulation of claudin-2 expression and increased TER by the extracellular signal-regulated kinases 1/2 (ERK1/2) have been reported (Lipschutz et al. 2005), and PKC activation causes a 4-fold increase in barrier function in human nasal epithelial cells (Koizumi et al. 2008). In our cellular model, claudin gene transcription did not change significantly with modulation of CFTR trafficking; however, ZO-1 staining was more extensive when CFTR trafficking was rescued so that it reached toward the basolateral side of the monolayer, indicating a change in TJ morphology. As ZO-1 plays an important role in regulating claudin trafficking to the tight junction (Umeda et al. 2006), further studies of claudin expression, localisation and regulation by kinases may indicate the mechanism of CFTR regulation, and whether the maintenance of barrier function is a general function of CFTR.
Previous studies and implications for pathophysiology
Contradictory results have been reported with respect to CFTR and transepithelial resistance in airway epithelia. A comparison of nasal epithelial cells in primary culture suggested a modest increase in resistance and a reduction in the shunt conductance in CF monolayers compared with wild-type cells (Willumsen & Boucher, 1989). However, shunt conductance was deduced by analysing steady-state electrochemical gradients and currents, and the sensitivity of the method is uncertain since it did not detect any reduction in the apical membrane Cl− conductance of CF cells compared to normal cells, and another study from the same group did not show this increase (Boucher et al. 1988). CFTR-dependent changes in resistance may not have been detected previously due to its variability in primary cell cultures, especially when CF cells originally isolated from infected and inflamed tissues are studied. We used airway epithelial cell lines which, though less differentiated than primary cultures, have been characterized previously and are known to polarise and preserve many features of airway surface epithelium including CFTR channel function (Kunzelmann et al. 1993).
The present results indicate that CFTR trafficking is essential for epithelial tightness, and this effect is independent of its anion channel function. Since permeation through the paracellular pathway accounts for a large fraction of the transepithelial conductance in airway epithelia, this action of CFTR must also be an important determinant of net salt and fluid absorption. Cl− is the main counter-ion during amiloride-sensitive sodium absorption; therefore increased paracellular Cl− conductance could enhance salt and fluid absorption by CF epithelia, thereby contributing to hyperabsorption and defective mucociliary clearance that are hallmarks of cystic fibrosis (Morimoto et al. 1998; Boucher, 2007). Increased paracellular permeability of CF airway epithelia may also allow the entry of bacterial toxins into the submucosa, increasing susceptibility to bacterial infection and exacerbating inflammation (Humlicek et al. 2007). Tight junctions also play an important role during the early stages of epithelial repair and regeneration; therefore the paracellular leakiness described here may help explain the delay and other abnormalities during the repair of CF respiratory epithelium after injury (Cereijido et al. 1989). The present results encourage studies of native epithelia, which may clarify the role of the paracellular pathway in CF pathogenesis.
Acknowledgments
We thank J.P. Clancy (University of Alabama at Birmingham, AL, USA) and J. Wakefield (Tranzyme Inc., Birmingham, AL, USA) for providing the stably transduced cell lines used in this study, and the Penn Vector Core (University of Pennsylvania) for providing adenoviruses that express GFP-CFTR and GFP-ΔF508 CFTR. We thank the staff of the McGill University Life Sciences Complex Imaging Facility and Histology Facility for excellent technical help and discussion. This work was supported by grants from the Canadian CF Foundation (CCFF), the Canadian Institute of Health Research (CIHR), and the CF Foundation Therapeutics Inc. (CFFT) to J.W.H., and from Pennsylvania Cystic Fibrosis, Inc (PACFI) and the Cystic Fibrosis Foundation (CFF) to D.C.G. P.L. is a fellow of the Research Institute of the McGill University Health Centre.
Glossary
Abbreviations
- ALI
air–liquid interface
- CFTR
cystic fibrosis transmembrane conductance regulator
- GFP
green fluorescent protein
- TER
transepithelial resistance
- TJ
tight junction
- WT
wild-type
- ZO-1
zona occludens 1
Author contributions
P.L. and J.W.H. designed the experiments and wrote the manuscript with help from all the co-authors. P.L. developed and performed all the experiments, with the help of J.L. for the cell culture procedures and R.R. for the electrophysiological experiments. D.C.G. developed the CFBE41o− cell line. All authors approved the final version.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, Sorscher EJ, Clancy JP. Failure of cAMP agonists to activate rescued DF508 CFTR in CFBE41o− airway epithelial monolayers. J Physiol. 2005;569:601–615. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Gonzalez-Mariscal L. Development and alteration of polarity. Annu Rev Physiol. 1989;51:785–795. doi: 10.1146/annurev.ph.51.030189.004033. [DOI] [PubMed] [Google Scholar]
- Chanson M, Scerri I, Suter S. Defective regulation of gap junctional coupling in cystic fibrosis pancreatic duct cells. J Clin Invest. 1999;103:1677–1684. doi: 10.1172/JCI5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227–1237. doi: 10.1091/mbc.01-08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, Lazdunski M. Altered chloride ion channel kinetics associated with the DF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Dudez T, Borot F, Huang S, Kwak BR, Bacchetta M, Ollero M, Stanton BA, Chanson M. CFTR in a lipid raft-TNFR1 complex modulates gap junctional intercellular communication and IL-8 secretion. Biochim Biophys Acta. 2008;1783:779–788. doi: 10.1016/j.bbamcr.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuit F, Kälin N, Brézillon S, Hinnrasky J, Tümmler B, Puchelle E. CFTR and differentiation markers expression in non-CF and DF 508 homozygous CF nasal epithelium. J Clin Invest. 1995;96:1601–1611. doi: 10.1172/JCI118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulaie occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A, Jadot M, Leontieva E, Wattiaux-De Coninck S, Wattiaux R. ΔF508 CFTR localizes in the endoplasmic reticulum-Golgi intermediate compartment in cystic fibrosis cells. Exp Cell Res. 1998;242:144–152. doi: 10.1006/excr.1998.4101. [DOI] [PubMed] [Google Scholar]
- González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signalling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Hajj R, Lesimple P, Nawrocki-Raby B, Birembaut P, Puchelle E, Coraux C. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol. 2007;211:340–350. doi: 10.1002/path.2118. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Fujita H, Katoh H, Aoki J, Nakamura K, Ichikawa A, Negishi M. Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in Madin-Darby canine kidney cells. J Biol Chem. 1999;274:20982–20988. doi: 10.1074/jbc.274.30.20982. [DOI] [PubMed] [Google Scholar]
- Hincke MT, Nairn AC, Staines WA. Cystic fibrosis transmembrane conductance regulator is found within brain ventricular epithelium and choroid plexus. J Neurochem. 1995;64:1662–1668. doi: 10.1046/j.1471-4159.1995.64041662.x. [DOI] [PubMed] [Google Scholar]
- Hollande E, Fanjul M, Chemin-Thomas C, Devaux C, Demolombe S, Van Rietschoten J, Guy-Crotte O, Figarella C. Targeting of CFTR protein is linked to the polarization of human pancreatic duct cells in culture. Eur J Cell Biol. 1998;76:220–227. doi: 10.1016/S0171-9335(98)80037-X. [DOI] [PubMed] [Google Scholar]
- Huang S, Dudez T, Scerri I, Thomas MA, Giepmans BNG, Suter S, Chanson M. Defective activation of c-Src in cystic fibrosis airway epithelial cells results in loss of tumor necrosis factor-α-induced gap junction regulation. J Biol Chem. 2003;278:8326–8332. doi: 10.1074/jbc.M208264200. [DOI] [PubMed] [Google Scholar]
- Humlicek AL, Manzel LJ, Chin CL, Shi L, Excoffon KJDA, Winter MC, Shasby DM, Look DC. Paracellular permeability restricts airway epithelial responses to selectively allow activation by mediators at the basolateral surface. J Immunol. 2007;178:6395–6403. doi: 10.4049/jimmunol.178.10.6395. [DOI] [PubMed] [Google Scholar]
- Koizumi J-i, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, Harimaya A, Murata M, Osanai M, Chiba H, Himi T, Sawada N. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol. 2008;74:432–442. doi: 10.1124/mol.107.043711. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the DF508 CFTR mutation. Am J Respir Cell Mol Biol. 1993;8:522–529. doi: 10.1165/ajrcmb/8.5.522. [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Li S, Arisco A, Balkovetz DF. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J Biol Chem. 2005;280:3780–3788. doi: 10.1074/jbc.M408122200. [DOI] [PubMed] [Google Scholar]
- Luo Y, McDonald K, Hanrahan JW. Trafficking of immature DF508-CFTR to the plasma membrane and its detection by biotinylation. Biochem J. 2009;419:211–219. doi: 10.1042/BJ20081869. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJV, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Ferguson D, Seibert FS, Cai HM, Kartner N, Grinstein S, Riordan JR, Lukacs GL. Functional expression and apical localization of the cystic fibrosis transmembrane conductance regulator in MDCK I cells. Biochem J. 1997;322:259–265. doi: 10.1042/bj3220259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, Uehara Y, Iwanaga K, Kakemi M, Ohashi Y, Tanaka A, Nakai Y. Influence of absorption enhancers (bile salts) and the preservative (benzalkonium chloride) on mucociliary function and permeation barrier function in rabbit tracheas. Eur J Pharm Sci. 1998;6:225–230. doi: 10.1016/s0928-0987(97)10003-3. [DOI] [PubMed] [Google Scholar]
- Nagel G, Hwang TC, Nastiuk KL, Nairn AC, Gadsby DC. The protein kinase A-regulated cardiac Cl− channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992;360:81–84. doi: 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signalling. Clin Rev Allergy Immunol. 2008;34:146–162. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]
- Puchelle E, Gaillard D, Ploton D, Hinnrasky J, Fuchey C, Boutterin MC, Jacquot J, Dreyer D, Pavirani A, Dalemans W. Differential localization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis airway epithelium. Am J Respir Cell Mol Biol. 1992;7:485–491. doi: 10.1165/ajrcmb/7.5.485. [DOI] [PubMed] [Google Scholar]
- Rab A, Bartoszewski R, Jurkuvenaite A, Wakefield J, Collawn JF, Bebok Z. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol. 2007;292:C756–C766. doi: 10.1152/ajpcell.00391.2006. [DOI] [PubMed] [Google Scholar]
- Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- Robert R, Thoreau V, Norez C, Cantereau A, Kitzis A, Mettey Y, Rogier C, Becq F. Regulation of the cystic fibrosis transmembrane conductance regulator channel by β-adrenergic agonists and vasoactive intestinal peptide in rat smooth muscle cells and its role in vasorelaxation. J Biol Chem. 2004;279:21160–21168. doi: 10.1074/jbc.M312199200. [DOI] [PubMed] [Google Scholar]
- Sallee JL, Burridge K. Density-enhanced phosphatase 1 regulates phosphorylation of tight junction proteins and enhances barrier function of epithelial cells. J Biol Chem. 2009;284:14997–15006. doi: 10.1074/jbc.M901901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hughes LK, Cai Z, Mendes F, Li H, Sheppard DN, Amaral MD. Prolonged treatment of cells with genistein modulates the expression and function of the cystic fibrosis transmembrane conductance regulator. Br J Pharmacol. 2008;153:1311–1323. doi: 10.1038/sj.bjp.0707663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodelling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Anderson JM, Goodenough DA, Mosseker MS. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J Cell Biol. 1988;107:2401–2408. doi: 10.1083/jcb.107.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T, Hug MJ, Frizzell RA, Bridges RJ. Microelectrode and impedance analysis of anion secretion in Calu-3 cells. JOP. 2001;2:219–228. [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Vais H, Gao G, Yang M, Tran P, Louboutin J, Somanathan S, Wilson JM, Reenstra WW. Novel adenoviral vectors coding for GFP-tagged wtCFTR and ΔF508-CFTR: characterization of expression and electrophysiological properties in A549 cells. Pflugers Arch. 2004;449:278–287. doi: 10.1007/s00424-004-1331-0. [DOI] [PubMed] [Google Scholar]
- Voltz JW, Weinman EJ, Shenolikar S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene. 2001;20:6309–6314. doi: 10.1038/sj.onc.1204774. [DOI] [PubMed] [Google Scholar]
- Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72:1448–1458. doi: 10.1038/sj.ki.5002579. [DOI] [PubMed] [Google Scholar]
- Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–108. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, Ullrich A, Lottspeich F, Chen Z. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signalling. EMBO J. 2006;25:5058–5070. doi: 10.1038/sj.emboj.7601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol Cell Physiol. 1989;256:C1054–C1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- Zahraoui A. Tight junctions, a platform regulating cell proliferation and polarity (in French) Med Sci (Paris) 2004;20:580–585. doi: 10.1051/medsci/2004205580. [DOI] [PubMed] [Google Scholar]
- Zahraoui A, Louvard D, Galli T. Tight junction, a platform for trafficking and signalling protein complexes. J Cell Biol. 2000;151:F31–F36. doi: 10.1083/jcb.151.5.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin PL, Diener-West M, Rubenstein RC, Boyle MP, Lee CKK, Brass-Ernst L. Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol Ther. 2002;6:119–126. doi: 10.1006/mthe.2002.0639. [DOI] [PubMed] [Google Scholar]