Abstract
Context: Sclerostin is a negative regulator of bone formation.
Objective: The aim of the study was to compare serum sclerostin levels in premenopausal and postmenopausal women and evaluate its relationship to estrogen, TH, bone turnover, and bone mass.
Design, Setting, and Participants: We conducted a cross-sectional observational study of healthy community-dwelling pre- and postmenopausal women.
Intervention(s): There were no interventions.
Main Outcome Measure(s): We compared serum sclerostin levels in pre- and postmenopausal women and correlated sclerostin levels with female sex hormones, calciotropic hormones, bone turnover markers, and bone mineral density.
Results: Premenopausal women were 26.8 yr old, and postmenopausal women were 56.8 yr old. Postmenopausal women had lower values for estradiol (30 ± 23 vs. 10 ± 4 pg/ml; P < 0.001), estrone (61 ± 24 vs. 29 ± 10 pg/ml; P <0.001), and free estrogen index (FEI) (6 ± 4 vs. 3 ± 2 pmol/nmol; P = 0.008) and significantly lower bone mineral density at all sites compared to premenopausal women, with no significant differences in levels of PTH, 25-hydroxy or 1,25-dihydroxy vitamin D levels. Postmenopausal women had significantly higher serum sclerostin levels (1.16 ± 0.38 ng/ml vs. 0.48 ± 0.15 ng/ml; P < 0.001). Because most of the premenopausal women were on oral contraceptives, subsequent analyses were limited to postmenopausal women. There were significant negative correlations between sclerostin and FEI and sclerostin and PTH in this group. Using multiple regression analysis, both FEI (β = −0.629; P = 0.002) and PTH (β = −0.554; P = 0.004) were found to be independent predictors of sclerostin levels in postmenopausal women.
Conclusions: Our findings suggest that serum sclerostin levels are regulated by both estrogens and PTH in postmenopausal women. These findings need to be explored further in larger prospective studies.
Serum sclerostin level is regulated by estrogen and parathyroid hormone in postmenopausal women.
Osteoporosis is a major cause of morbidity and mortality, particularly in postmenopausal women and older men (1). Although the pathogenesis of bone loss and skeletal fragility in this disease is not well understood, estrogen deficiency clearly plays a role in its development in both sexes (2). In addition, in many older patients, secondary hyperparathyroidism, associated with calcium or vitamin D deficiency, may accelerate bone loss and increase the risk of developing osteoporosis (2). Both estrogen deficiency and secondary hyperparathyroidism are associated with a primary increase in bone resorption and an impaired bone formation response (2). This imbalance between bone formation and resorption produces bone loss and a deterioration of the skeletal microarchitecture, which in turn leads to height loss, skeletal deformities, and fracture (1).
Recently, the Wnt/β-catenin signaling pathway and its inhibitor sclerostin were found to be involved in the control of bone mass in a number of genetic studies of experimental animals and humans (3,4,5). Activation of the pathway results in increased proliferation and differentiation of osteoblast precursor cells as well as reduced apoptosis of mature osteoblasts. These changes favor the deposition of new bone and an increase in bone density (6,7,8). In addition, Wnt signaling may inhibit osteoclastogenesis (9). Activation of the Wnt/β-catenin canonical signaling pathway in osteoblasts occurs upon binding of any of multiple Wnt ligands to a seven-transmembrane domain—spanning frizzled receptor and either of two coreceptors, low-density lipoprotein receptor-related proteins 5 or 6 (LRP5/6) (10).
Sclerostin is a secreted Wnt antagonist, which appears to regulate bone mass by binding to LRP5 and LRP6 and inhibiting canonical Wnt/β-catenin signaling (11,12,13). In vitro studies have shown that sclerostin inhibits osteoblast proliferation, promotes osteoblast apoptosis, and suppresses mineralization of osteoblastic cells (14). Sclerostin-deficient mice have increased bone mass and bone strength (15), whereas overexpression of normal human SOST alleles (the gene for sclerostin) in mice causes osteopenia (16). Similarly, other conditions associated with defective sclerostin production such as sclerosteosis and Van Buchem’s disease are also associated with high bone mass (17,18). Sclerosteosis is caused by a mutation in the SOST gene, resulting in an improperly spliced SOST mRNA (17), whereas Van Buchem’s disease is caused by a deletion of an enhancer element that is normally downstream of the SOST gene (18). Bone biopsy specimens from these patients show more activated osteoblasts compared with normal controls (19,20). The findings from these genetic conditions associated with sclerostin deficiency suggest that inhibition of sclerostin may have therapeutic potential for the treatment of low bone mass disorders. In confirmation of this hypothesis, studies using antisclerostin neutralizing antibodies in rats (21), and primates (22) have demonstrated increased bone density and bone strength. More recently, it was shown that treatment of postmenopausal women with an antisclerostin antibody resulted in an increase in markers of bone formation (23).
The bone loss in women after menopause has been attributed to estrogen deficiency (2). The purpose of our study was to determine whether sclerostin circulates in the serum and to examine the relationship between serum sclerostin levels and levels of circulating female sex steroid hormones, PTH, and other factors in premenopausal and postmenopausal women.
Subjects and Methods
Twenty premenopausal women and 20 postmenopausal women were evaluated in a cross-sectional observational study. The groups were evaluated for serum levels of sclerostin, sex hormones, calciotropic hormones, bone density, and bone turnover markers. All research was conducted at the University of Connecticut Health Center’s (UCHC) General Clinical Research Center (GCRC) in Farmington, Connecticut. The Institutional Review Board at UCHC and the Scientific Advisory Committee of the GCRC approved all aspects of this study. Subject recruitment for the study was done by open advertisement in the institution’s broadcast e-mail system. Subjects had an initial phone screening and, if eligible, were scheduled for a study visit. Screening and study labs were all drawn at the same visit. Bone density measurement was scheduled for a later date, and the full panel of study labs was evaluated only if subjects met the laboratory screening criteria.
For inclusion in the study, premenopausal women had to be 20–30 yr of age with a body mass index (BMI) of 28 kg/m2 or less and regular menstrual cycles. Sixteen of the 20 premenopausal women who were enrolled in the study were on oral contraceptives. Study labs were drawn in the initial 5–10 d of the menstrual cycle to minimize variability in bone markers that might be due to changes in sex hormone levels during the menstrual cycle. The postmenopausal women were 55 yr of age or older with a BMI of 30 kg/m2 or less and no history of prior treatment for osteoporosis.
All subjects had a brief baseline history obtained at the time of initial phone screening. Once deemed eligible by phone screening, subjects came in for full screening and to have study labs drawn. Subjects were excluded if they had a history of an eating disorder; Paget’s disease of bone; primary hyperparathyroidism; osteomalacia; untreated hyperthyroidism; multiple myeloma; chronic kidney, gastrointestinal, or liver diseases; prior cancer diagnosis (other than skin cancer); recent (within 1 yr) unstable angina; congestive heart failure; or recent myocardial infarction. They were also excluded if they received treatment with medications that interfere with bone metabolism within the last 6 months, such as chronic corticosteroids (>3 months duration), seizure medications (phenytoin, phenobarbital), selective estrogen receptor modulators (raloxifene, tamoxifen), calcitonin, calcitriol, estrogen (hormone replacement therapy), aromatase inhibitors (letrozole, anastrozole, or exemestane), or chronic heparin therapy. Women were also excluded if they had any prior treatment with bisphosphonates (alendronate, risedronate, ibandronate, zoledronic acid). Smokers were excluded from participation in the study. Premenopausal women were also excluded if they had been pregnant in the last 6 months or were breastfeeding. Serum ionized calcium, creatinine, phosphorous, and TSH, and urinary calcium, creatinine, and phosphorous were obtained for screening. Baseline FSH levels, obtained in postmenopausal women only, had to be above 30 IU/liter. Further exclusions were made if any screening labs were outside the normal range or urine calcium/creatinine ratio was above 300 mg calcium/g creatinine.
Subjects had fasting serum and urine collected for screening labs, bone turnover markers, sex hormones, calciotropic hormones, and sclerostin level. Serum and urine samples were collected between 0700 and 0930 h after a 10- to 12-h fast. Freshly separated serum was then divided into 0.5-ml aliquots and stored at −70 C. Bone marker assays were run in duplicate after one thaw, and all samples were assayed using the same kit. All bone marker assays were performed in the core laboratory of the GCRC at the UCHC, unless otherwise specified. Serum and urine creatinine and phosphorous levels were measured by calorimetric assay. Urine calcium was measured using an indirect ion-specific electrode and calcium ionophore membrane. TSH was measured using a one-step sandwich immunoassay in the clinical lab. Ionized calcium was measured with an ion selective electrode with pH correction.
Bone turnover markers
Bone formation markers included bone-specific alkaline phosphatase (BSAP) (ELISA; Quidel Inc., Mountain View, CA), and N-terminal propeptide of type 1 collagen (P1NP) (RIA; Orion Diagnostica, Espoo, Finland). The within-run and between-run intraassay coefficient of variability (CV) was below 6% for BSAP and below 9% for P1NP. Bone resorption markers included urinary N-telopeptide collagen crosslinks (NTX; Zeus Scientific, Princeton, NJ), serum C-telopeptide of collagen cross-links (CTX; Nordic Bioscience Diagnostics, Herlev, Denmark), and serum osteocalcin. Interassay CV was below 4% for NTX and below 7% for CTX. Osteocalcin was measured by enzyme immunoassay (Diagnostic Products Corporation, Los Angeles, CA) with a within-run intraassay CV of 2.5%.
Hormone measurements
Sex hormone measurements included estradiol (E2), estrone (E1), and SHBG. E2 and E1 were measured by RIA (Diagnostic Systems Laboratories, Inc., Webster, TX), with a within-run intraassay CV of 7.5% for E2 and 6.5% for E1. The between-run interassay CV was 9.4% for E2 and 9.1% for E1. The sensitivity of the assay was 2.2 pg/ml for E2, and 1.2 pg/ml for E1. SHBG was measured using an enzyme immunoassay (Diagnostic Products Corporation, Los Angeles, CA) with a within-run intraassay CV of 2.9%. The free estrogen index (FEI) was calculated as follows: E2 + E1 (in picomoles)/SHBG. FSH was measured in postmenopausal women by a two-step sandwich immunoassay.
Calciotropic hormones included intact PTH, 25-hydroxyvitamin D [25(OH) vit D], and 1,25-dihydroxyvitamin D [1,25(OH)2 vit D]. PTH and 25(OH) vit D were measured in the GCRC core laboratory at UCHC by enzyme immunoassay (Diagnostic Products Corporation, with a within-run intraassay CV of 3.4%; and Immunodiagnostic Systems Limited, Fountain Hills, AZ, with a within-run and between-run intraassay CV of <7%, respectively). The 1,25(OH)2 vit D was measured by RIA (Associated Regional and University Pathologists’ laboratory, Salt Lake City, UT). Serum sclerostin levels were measured on coded specimen by Amgen (Thousand Oaks, CA) using a chemiluminescence sandwich assay, which employed separate capture and detection antibodies. The assay had a range of 0.1–3.2 ng/ml. None of the measured values of sclerostin in our subjects were below the limits of detection for this assay. In validation studies for the assay, serum samples with endogenous high sclerostin were used for quality control; the interassay CV was set at below 25%, and the total error was set at below 30%. However, for this particular study, the assay performed much better with a CV of less than 15% and a total error of less than 20%. Serum samples with high sclerostin were used as quality control to confirm the specificity for the endogenous molecule, and these endogenous controls were quantified reproducibly over time as a measure of the assay reproducibility.
Bone density measurements
Bone mineral density (BMD) was measured by dual-energy x-ray absorptiometry (Lunar Prodigy, Madison, WI). The CV of BMD measurement, based on reproducibility scans, were 1.5% for femoral neck, 1% for total hip, 2% for L1–L2 spine, and 4.4% for L2–L4 spine.
Statistical analysis
Student’s t test was used to compare the groups for differences in baseline characteristics, calciotropic hormones, bone turnover hormones, and BMD. Pearson product moment correlation coefficients were calculated between the sclerostin level and E2, E1, and FEI, as well as between sclerostin level and BMD and bone turnover markers. The relationship between sclerostin, estrogen, and PTH was evaluated further using multivariate analysis.
Results
Premenopausal women were 26.8 ± 2.6 yr old, and postmenopausal women were 58.6 ± 3.4 yr old. BMI was 23.5 ± 2.5 kg/m2 for premenopausal and 25.4 ± 3.2 kg/m2 for postmenopausal women (P = 0.04). There were no significant differences between the two groups in their screening labs (Table 1), except for urinary calcium excretion per gram of creatinine on the morning fasting specimen, which was about 2-fold higher in the postmenopausal women (P < 0.001). As expected, E2, E1, and FEI were significantly lower in postmenopausal women. There were no significant differences in serum SHBG, PTH, 25(OH) vit D, or 1,25(OH)2 vit D levels between the two groups (Table 1). The bone formation marker serum BSAP and the bone resorption markers urine NTX and serum CTX were significantly higher (25 to 40%) in postmenopausal women compared with premenopausal women (Table 2). BMD was also significantly lower at the lumbar spine, femoral neck, total hip, and greater trochanter in postmenopausal women (Table 2).
Table 1.
Baseline chemistries, female sex hormones, and calciotropic hormones
| Premenopausal women | Postmenopausal women | P value | |
|---|---|---|---|
| TSH (μU/ml) | 2 ± 1 | 1.9 ± 0.68 | 0.603 |
| FSH (IU/liter) | Not done | 84 ± 32 | |
| lonized Ca (mmol/liter) | 1.25 ± 0.02 | 1.26 ± 0.03 | 0.159 |
| Cr (mg/dl) | 0.8 ± 0.15 | 0.8 ± 0.1 | 0.632 |
| Phosphorous (mg/dl) | 3.8 ± 0.5 | 4.1 ± 0.5 | 0.125 |
| Urine Ca/Cr (mg/g) | 68 ± 35 | 128 ± 57 | 0.001 |
| E2 (pg/ml) | 30 ± 23 | 10 ± 4 | <0.001 |
| E1 (pg/ml) | 61 ± 24 | 29 ± 10 | <0.001 |
| FEI (pmol/nmol) | 6 ± 4 | 3 ± 2 | 0.008 |
| SHBG (nmol/liter) | 78 ± 38 | 61 ± 23 | 0.091 |
| Intact PTH (pg/ml) | 51 ± 19 | 54 ± 21 | 0.647 |
| 25(OH) vit D (ng/ml) | 30 ± 14 | 34 ± 9 | 0.387 |
| 1,25(OH)2 vit D (pg/ml) | 65 ± 25 | 62 ± 24 | 0.772 |
| Sclerostin (ng/ml) | 0.48 ± 0.15 | 1.16 ± 0.38 | <0.001 |
Data are expressed as mean ± sd. Cr, Creatinine.
Table 2.
Serum and urine bone turnover markers and BMD
| Premenopausal women | Postmenopausal women | P value | |
|---|---|---|---|
| BSAP (μg/liter) | 21 ± 6 | 29 ± 9 | 0.001 |
| P1NP (μg/liter) | 52 ± 25 | 65 ± 28 | 0.121 |
| Osteocalcin (ng/ml) | 7 ± 5 | 8 ± 5 | 0.660 |
| CTX (ng/ml) | 0.59 ± 0.26 | 0.80 ± 0.27 | 0.018 |
| NTX (nmol/mmol Cr) | 43 ± 17 | 57 ± 19 | 0.021 |
| L1–L4 BMD (g/cm2) | 1.182 ± 0.093 | 1.086 ± 0.143 | 0.017 |
| Mean femoral neck BMD (g/cm2) | 1.030 ± 0.118 | 0.884 ± 0.074 | <0.001 |
| Mean trochanter BMD (g/cm2) | 0.805 ± 0.109 | 0.736 ± 0.073 | 0.027 |
| Mean total hip BMD (g/cm2) | 1.035 ± 0.116 | 0.924 ± 0.084 | 0.002 |
Data are expressed as mean ± sd.
Serum sclerostin levels were significantly higher in postmenopausal women (1.16 ± 0.38 ng/ml) compared with premenopausal women (0.48 ± 0.15 ng/ml) (P < 0.001) (Table 1). There was a significant negative correlation between serum sclerostin level and femoral neck BMD (r = −0.486; P = 0.002) with a trend toward a significant negative correlation between serum sclerostin level and the mean total hip BMD (r = −0.305; P = 0.059) in the entire group. However, these correlations did not persist once the bone density was adjusted for age in the entire group or when the groups were separated into premenopausal and postmenopausal subgroups. No significant correlations were seen between serum sclerostin levels and lumbar spine BMD or bone turnover markers either in the entire group or in the subgroups.
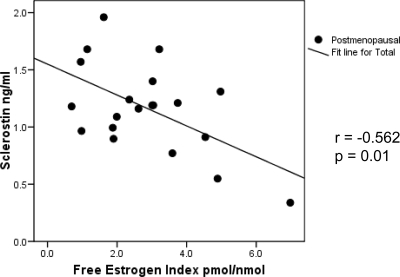
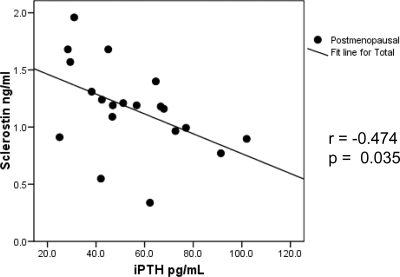
Because the majority of the premenopausal women (16 of 20) were on oral contraceptives containing a variety of sex hormones, further analysis on the relationship between sclerostin and sex hormones was limited to the postmenopausal women only. In the postmenopausal group, there was a significant negative correlation between serum sclerostin level and FEI (r = −0.562; P = 0.01) (Fig. 1). There was also a trend toward a significant negative correlation between serum sclerostin levels and E2 (r = −0.382; P = 0.096) and E1 (r = −0.426; P = 0.06). Serum PTH level also showed a significant negative correlation with serum sclerostin level in postmenopausal women (r = −0.474; P = 0.035) (Fig. 2). In the postmenopausal women, there were no significant correlations between serum sclerostin levels and any of the measured bone turnover markers. Using multivariate analysis, both FEI (β = −0.629; P = 0.002) and PTH (β = −0.554; P = 0.004) were found to be independent predictors of serum sclerostin levels in postmenopausal women, after adjusting for age, BMI, and 25(OH) vit D levels (Table 3).
Figure 1.
Correlation of serum sclerostin levels with FEI in postmenopausal women.
Figure 2.
Correlation of serum sclerostin levels with serum PTH levels in postmenopausal women.
Table 3.
Result for multiple regression in postmenopausal women
| Predictor | B | se | β | t | Sig |
|---|---|---|---|---|---|
| Postmenopausal | |||||
| Age | −0.017 | 0.017 | −0.154 | −1.01 | 0.33 |
| BMI | 0.003 | 0.022 | 0.021 | 0.12 | 0.91 |
| 25(OH) vit D | 0.011 | 0.007 | 0.274 | 1.68 | 0.12 |
| FEI | −0.151 | 0.041 | −0.629 | −3.72 | 0.002 |
| PTH | −0.010 | 0.003 | −0.554 | −3.48 | 0.004 |
Discussion
Our results show that sclerostin circulates in serum and its levels are significantly higher in postmenopausal women compared with premenopausal women (Table 1). We have not found any reports in the literature evaluating differences in serum sclerostin levels between pre- and postmenopausal women. Sclerostin has previously been known to act in a local paracrine fashion in the microenvironment of bone. Our findings demonstrate that sclerostin also enters the circulation and suggest the possibility that, in addition to its local actions, it may regulate bone mass by acting as an endocrine hormone. The higher serum sclerostin levels in postmenopausal women may be a cause or an effect of the increased bone turnover, which occurs in the postmenopausal state.
Increased bone turnover and the resulting bone loss in women after menopause have been attributed to estrogen deficiency (2). The majority of the premenopausal women that we examined (16 of 20) were on oral contraceptives, which contain a variety of sex hormones. Hence, it was difficult to directly assess the total estrogen status of this group. However, we believe that they were estrogen replete because of their higher measured estrogen levels and significantly lower bone resorption markers, relative to the postmenopausal women. A relationship between sclerostin and estrogen has not previously been demonstrated. In addition, we are unaware of any studies that have examined the effects of estrogens on sclerostin production or release from bone in experimental animal or culture models. In this study, we found a significant negative relationship between serum sclerostin levels and FEI in postmenopausal women (Fig. 1), with a trend toward significant negative relationships between serum sclerostin levels and serum levels of E2 and E1. This negative relationship could be due to an estrogen-mediated inhibition of the production of sclerostin, or its release from the skeleton. It is also possible that the increased bone resorption associated with estrogen deficiency caused a greater release of sclerostin into the circulation. Alternatively, estrogen may regulate the clearance of sclerostin from the circulation.
The role of sclerostin in estrogen deficiency has been implicated in studies where sclerostin antibodies were used to treat bone loss in an aged ovariectomized rat model (21). In this work, ovariectomized 6-month-old female rats were left untreated for 1 yr to produce estrogen deficiency-induced bone loss. The animals were then treated with a neutralizing antisclerostin monoclonal antibody for 5 wk. This intervention caused significant anabolic changes in the bones of the rats with markedly increased bone formation on the trabecular, periosteal, endocortical, and intracortical surfaces, resulting in significantly increased bone mass (21).
Human PTH, given intermittently, has anabolic effects and is currently approved for the treatment of postmenopausal osteoporosis (24). In contrast, continuous exposure to PTH can result in bone loss (25,26). Bellido et al. (27) showed that both chronic and acute injections of PTH in mice decreased SOST expression. They found that continuous infusion of PTH to mice for 4 d decreased SOST mRNA levels in vertebral bone by 80–90%. This was associated with lower levels of sclerostin in these bones as shown by immunocytochemical staining of vertebral bone sections. They also found that acute administration of PTH resulted in a transient (50%) reduction in SOST mRNA at 2 h. Keller et al. (28) have reported similar findings with intermittent PTH injection. They also evaluated SOST mRNA expression in the bones of 11-month-old osteopenic, estrogen-deprived ovariectomized rats after 8 wk of systemic, intermittent administration of 5 μg/kg of PTH (1-34). They found that PTH-treated animals displayed increased BMD as measured by peripheral quantitative computed tomography, whereas SOST mRNA levels were decreased compared with vehicle-treated ovariectomized and sham controls. Our study suggests that the inverse relationship between serum PTH and sclerostin levels, seen in animal studies, also occurs in humans. We found that PTH had a significant negative correlation with serum sclerostin levels that was independent of serum estrogen levels in the multivariate analysis. Curiously, we did not find any correlation between serum sclerostin levels and serum PTH levels in the premenopausal women (data not shown). This suggests that there are complicated relationships between serum sclerostin levels, the estrogen status of women, and serum PTH levels, which prevent serum PTH levels from correlating with serum sclerostin levels in the estrogen-replete women.
Although we found that there was a significant negative correlation between serum sclerostin levels and femoral neck BMD in the combined pre- and postmenopausal groups, this relationship did not persist when the postmenopausal group was analyzed separately. Because BMD reflects the combined effects of peak bone mass and bone loss related to menopause, the absence of a relationship in the postmenopausal group is not surprising. Additionally, these correlations also did not persist when adjustments were made for age in the combined group. We also did not find any significant relationships between serum sclerostin levels and bone turnover markers in any of the groups. To further examine these relationships, there is a need for additional prospective studies with adequate statistical power to compare the rates of bone loss in the postmenopausal population with serum sclerostin, estrogen, and PTH levels.
In conclusion, our study demonstrates that serum sclerostin levels are significantly higher in postmenopausal women compared with premenopausal women. More importantly, we found significant negative correlations between FEI and serum sclerostin levels as well as serum PTH and serum sclerostin levels in postmenopausal women. Both FEI and serum PTH levels appear to independently correlate with serum sclerostin levels in postmenopausal women. Our results imply that sclerostin is an additional serum marker of the effects that estrogen and PTH have on bone mass. Additionally, the relationships between serum sclerostin levels, bone turnover markers and bone density, and the predictive value of serum sclerostin levels in determining postmenopausal bone loss need to be explored further in larger prospective studies. We conclude that measurement of serum sclerostin levels may represent a novel approach to understanding the regulation of bone mass, as well as a potential new marker for assessing skeletal status.
Footnotes
This study received funding from Amgen.
Disclosure Summary: F.S.M. and J.A.L. owned stock in Amgen within the past 2 yr but do not hold it at present. I.D.P. is a full-time employee of Amgen and owns stock in Amgen as well. L.G.R. has nothing to declare.
First Published Online February 15, 2010
Abbreviations: BMD, Bone mineral density; BMI, body mass index; BSAP, bone-specific alkaline phosphatase; CTX, C-telopeptide of collagen cross-links; CV, coefficient of variability; FEI, free estrogen index; NTX, N-telopeptide collagen crosslinks; 1,25(OH)2 vit D, 1,25-dihydroxyvitamin D; 25(OH) vit D, 25-hydroxyvitamin D; P1NP, N-terminal propeptide of type 1 collagen.
References
- Stepnick LS 2004 The frequency of bone disease. In: MacGowan JALGR, Noonan AS, Elderkin AL, eds. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 68–87 [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 1998 A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13:763–773 [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y 2005 Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750 [DOI] [PubMed] [Google Scholar]
- Rawadi G, Vayssière B, Dunn F, Baron R, Roman-Roman S 2003 BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 18:1842–1853 [DOI] [PubMed] [Google Scholar]
- Baron R, Rawadi G 2007 Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep 5:73–80 [DOI] [PubMed] [Google Scholar]
- Yavropoulou MP, Yovos JG 2007 The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens) 6:279–294 [DOI] [PubMed] [Google Scholar]
- Clevers H 2006 Wnt/β-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- Johnson ML, Kamel MA 2007 The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol 19:376–382 [DOI] [PubMed] [Google Scholar]
- Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG 2006 Wnt signalling in osteoblasts regulates expression of the receptor activator of NFκB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 119:1283–1296 [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X 2004 LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131:1663–1677 [DOI] [PubMed] [Google Scholar]
- Semënov M, Tamai K, He X 2005 SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280:26770–26775 [DOI] [PubMed] [Google Scholar]
- Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R 2006 Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21:1738–1749 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D 2005 Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887 [DOI] [PubMed] [Google Scholar]
- Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, Latham JA 2004 Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 35:828–835 [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C 2008 Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869 [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA 2003 Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W 2001 Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543 [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W 2002 Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW 2004 Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SA, Witkop C, Hill S, Fallon MD, Viernstein L, Gucer G, McKeever P, Long D, Altman J, Miller NR, Teitelbaum SL, Schlesinger S 1983 Sclerosteosis: neurogenetic and pathophysiologic analysis of an American kinship. Neurology 33:267–277 [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C 2009 Sclerostin antibody treatment increases bone formation, bone mass and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588 [DOI] [PubMed] [Google Scholar]
- Ominsky M, Stouch B, Doellgast G, Gong J, Cao J, Gao Y, Tipton B, Haldankar R, Winters A, Chen Q, Graham K, Zhou L, Hale M, Henry A, Lightwood D, Moore A, Popplewell A, Robinson M, Vlasseros F, Jolette J, Smith SY, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C 2006 Administration of sclerostin monoclonal antibodies to female cynomolgus monkeys results in increased bone formation, bone mineral density and bone strength. J Bone Miner Res 21 (Suppl 1):1162 [Google Scholar]
- Padhi D, Stouch B, Jang G, Fang L, Darling M, Glise H, Robinson M, Harris S, Posvar E 2007 Anti-sclerostin antibody increases markers of bone formation in healthy postmenopausal women. J Bone Miner Res 22 (Suppl 37) (Abstract) [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 1976 The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part III of IV parts; PTH and osteoblasts, the relationship between bone turnover and bone loss, and the state of the bones in primary hyperparathyroidism. Metabolism 25:1033–1069 [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Mosekilde L, Melsen F 1986 Trabecular bone remodeling and balance in primary hyperparathyroidism. Bone 7:213–221 [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL 2005 Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- Keller H, Kneissel M 2005 SOST is a target gene for PTH in bone. Bone 37:148–158 [DOI] [PubMed] [Google Scholar]