Abstract
Dentin sialophosphoprotein (DSPP) is critical for proper mineralization of tooth dentin, and understanding its structure and function should yield important insights into how dentin biomineralization is controlled. During the recent six years, I have focused on characterizing DSPP-derived proteins isolated from developing porcine teeth. Porcine DSPP is expressed and secreted by odontoblasts and is processed by BMP-1, MMP-20 and MMP-2 into three main parts: dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP). We have learned that DSP is a proteoglycan that forms covalent dimers, DGP is a phosphorylated glycoprotein, and DPP is a highly phosphorylated intrinsically disordered protein that shows extensive length polymorphisms due to the genetic heterogeneity of its coding region.
Keywords: dentin dysplasia, dentinogenesis imperfecta, dentin sialoprotein, dentin glycoprotein, dentin phosphoprotein
Introduction
Dentin is the mineralized tissue comprising the body of a tooth. It protects the pulp within, and supports the overlying enamel and cementum. On a weight basis, dentin is about 70% mineral, 20% organic matrix, and 10% water. Type I collagen and dentin sialophosphoprotein (DSPP)-derived proteins are the most abundant proteins in dentin. Collagen constitutes about 90% of the dentin organic matrix1). The non-collagenous proteins in dentin are dominated by DSPP-derived proteins, which are generated by proteolysis of dentin sialophosphoprotein (DSPP)2), a multidomain protein with hundreds of posttranslational modifications3). Porcine DSPP is processed by proteases into three protein products: dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP)4–7). Expression of a DSP transgene in the DSPP null background indicated distinct roles of DSP and DPP in dentin mineralization, with DSP regulating initiation of dentin mineralization, and DPP being involved in the maturation of mineralized dentin8). Genetic studies have shown that type I collagen and DSPP are critical for proper human dentin formation. Inherited dentin defects have been historically classified as either dentin dysplasia (DD) types I and II, or dentinogenesis imperfecta (DGI) types I, II, or III9). Twenty one different DSPP mutations have been reported in kindreds with inherited dentin defects10–17). Mutations in the DSPP gene have been shown to cause DD type II, DGI type II and DGI type III. Although there are several other potential candidate genes for these disorders, only DSPP mutations have been found in DD or DGI kindreds. Tooth defects in the Dspp null mouse resemble human DGI-III, which is a rarest and most severe phenotype among the three forms (DD-II, DGI-II and DGI-III) of inherited dentin defects caused by DSPP mutations18). The significance of research that advances our understanding of the structure and function DSPP-derived proteins and their interactions with collagen is apparent.
1) Dentin Sialoprotein (DSP)
When we initiated our investigation, no genomic or cDNA sequences for porcine DSPP were available in the databases. This information is important for protein studies because it provides the deduced amino acid sequence. To obtain the porcine DSPP cDNA sequence, we constructed and screened a unidirectional cDNA library derived from the pulp organ of developing porcine teeth and isolated cDNA clones encoding DSP-only, as well as two DSPP clones that were probably affected by artifactual deletions involving part of the coding sequence for the highly redundant region of DPP in exon 519). The DSP-only transcript was completely unexpected, but is important because it helps us define the C-terminus of DSP. The DSP-only transcript was biologically generated through the use of a polyadenylation signal in intron 4, thereby terminating transcription and truncating the transcript upstream of exon 5, which encodes DGP and DPP. An in-frame translation termination signal (TGA) appears just four codons after the end of exon 4, so only three amino acids are added to the C-terminus of the DSP-only protein relative to the DSP domain included in the DSPP chimera. The DSP-only open reading frame has 386 codons, with the first 15 encoding the signal peptide. (In this report we will number the amino acids in DSPP starting with Met1, so the numbers are inclusive of the signal peptide.) We expressed recombinant porcine DSP in bacteria as a GST fusion product and raised polyclonal antibodies that allowed us to isolate DSP from developing porcine teeth20). Using this antibody we are able to track DSP and DSP-derived cleavage products throughout our purification procedures20–22).
Having obtained the porcine DSPP cDNA and deduced amino acid sequence, and having raised antibodies specific for DSP, we advanced to characterizing DSPP-derived proteins in porcine dentin. In vivo, the first proteolytic cleavage releases DPP from the C-terminus of DSPP. Subsequent cleavages release DGP or extended DGP from the C-terminus of DSP-DGP. The C-terminus of porcine DSP varies depending upon the cleavage site used. The main cleavage site is on the C-terminal side of Arg391, which was confirmed by demonstrating that Ser392 is at the N-terminus of DGP20,21). The genetic and proteolytic cleavage data are consistent, and show that DSP is the N-terminal domain of DSPP and that DSP is encoded by exons 2 through 4 of the DSPP gene19). Based upon the characterization of DSPP cleavage products isolated from developing porcine teeth, DSP from DSPP has 376 amino acids, extending from Ile16 to Arg391. Despite this clear definition of the boundaries of DSP, our research shows that the proteolytic processing of porcine DSPP does not simply generate three cleavage products (DSP, DGP, and DPP) that accumulate to serve separate functions. Instead these domains are only somewhat stable intermediates in what appears to be the extracellular degradation of DSPP.
Computer analyses of the porcine DSP deduced amino acid sequence identified potential post-translational modification sites, which are a major and important feature of DSP. Porcine DSP contains 8 putative N-glycosylation sites19). There are several predicted O-glycosylation sites as well as four SerGly (SG) pairs that could potentially be targeted by xylosyltransferase to introduce glycan attachments23). We devised efficient extraction and purification methods to isolate DSPP-derived proteins in quantity from developing porcine teeth. Unerupted molars at the late crown formation stage are extracted from 5-month-old pigs as they are slaughtered at the Michigan State Meat Laboratory. The soft tissues and enamel are removed, and the dentin is pulverized using a jaw crusher. Dentin powder (40 g) is sequentially extracted with guanidine (G), acetic acid (A) and acetic acid/NaCl (AN). DGP and low molecular weight (LMW) DSP cleavage products are in the A extract, while DSP, DSP-DGP, and DPP are in the AN extract (Fig 1). These extracts are each fractionated by size exclusion chromatography and reversed phase HPLC.
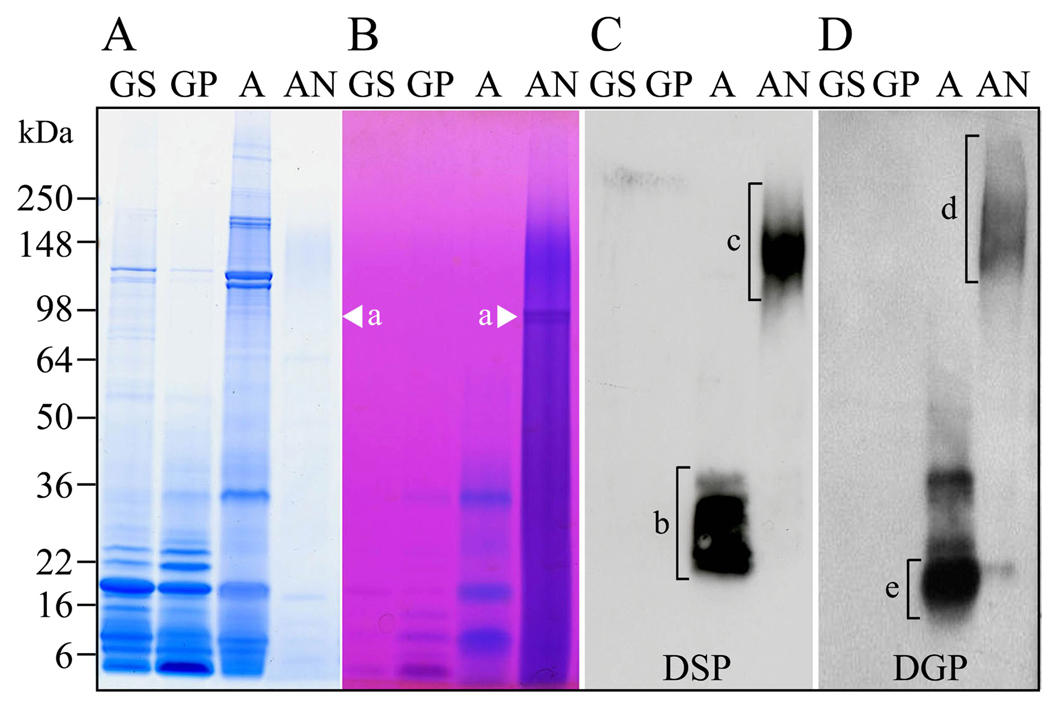
Figure 1. DSPP derived proteins in porcine dentin extracts.
The four primary dentin extracts are GS, GP, A, and AN extract. A and B, porcine dentin extracts analyzed by SDS-PADE stained with CBB and Stains-all. DPP is the CBB-negative, Stains-all-positive band migrating at 98 kDa in the AN extract (a, arrowheads). C and D, Western blots of SDS-PAGE using the DSP, and DGP antibodies. Lower molecular weight (LMW) DSP cleavage products from 22 to 38 kDa are observed in the A extract (b, bracket). Higher molecular weight (HMW) DSP-positive (c, bracket) and DGP-positive (d, bracket) proteins are observed in the AN extract. Most of lower molecular weight DGP-positive bands in the A extract are also DSP-positive except for the only DGP-positive proteins from 16 to 21 kDa (e, bracket).
The complexity of DSP glycosylation is evident on SDS-PAGE. DSP extracted from porcine teeth migrates as a smear extending from 100 to 280-kDa on Western blots20). In contrast, the apparent molecular mass of recombinant DSP expressed in bacteria is 65-kDa and the predicted molecular mass of unmodified DSP is only 39-kDa. To gain information about carbohydrate attachments on the purified porcine DSP glycoprotein, we conducted a series of deglycosylation digestions. First, N-linked oligosaccharide chains were removed by glycopeptidase A digestion; second, O-linked oligosaccharide chains were removed by O-glycosidase digestion; third, glycan attachments were removed by protease-free chondroitinase ABC digestion. Carbohydrates were released from DSP in each step, so DSP has N- and O-linked glycosylations as well as glycan attachments20). We also determined that the glycan attachments are comprised exclusively of chondroitin 6-sulfate20). This was accomplished by HPLC analysis and by digesting N- and O-deglycosylated DSP with four glycosaminoglycanases (protease-free chondroitinase ABC, heparitinase I, keratanase, and keratanase II) and then assaying the products on Western blots using a panel of monoclonal antibodies that recognize specific glycans. The results indicated that porcine DSP is a proteoglycan with a large and variable amount of chondroitin 6-sulfate.
The more we worked with DSP the more familiar it became. On SDS-PAGE and Westerns, DSP always appeared as two smear bands extending from 280-kDa to 100-kDa, so we decided to compare its migration on SDS-PAGE under oxidizing and reducing conditions to check for disulfide bridging. Under reducing conditions (with β-mercaptoethanol) the higher molecular weight smear disappears, as it is comprised of DSP dimers covalently linked by a disulfide bridge. Porcine DSP has only one cysteine residue per molecule, so the disulfide connection must join two DSP proteins at Cys205.
Our next objective was to determine the positions of the glycosylations on DSP. We used several strategies based upon the fact that amino acids carrying a post-translational modification show a “blank cycle” when sequenced by Edman degradation, that is, no chromatographic peak is observed at any of the expected RP-HPLC retention times for normal amino acids in the cycle that releases a modified amino acid. We digested DSP with lysylendopeptidase or pronase and also isolated smaller DSP-derived cleavage products from dentin extracts. The DSP-derived peptides were assayed for the presence of glycosylations using the phenol sulfuric acid method, and characterized by N-terminal sequencing. We also exploited the slight change in mass (1 Da) caused by the deamidation of Asn at an N-glycan attachment site to Asp following digestion by glycopeptidase A. We digested LMW DSP cleavage products with V8 endoproteinase and glycopeptidase A, and characterized N-glycan-released peptides by LC/MS/MS analysis, which is a technique that combines the physical separation capabilities of HPLC (LC) with the mass analysis capabilities of mass spectrometry (MS) to determine the amino acid sequence of a short peptide. The results show that DSP has six N-glycan, three O-glycan, and two chondroitin 6-sulfate attachment sites (Fig. 2A).
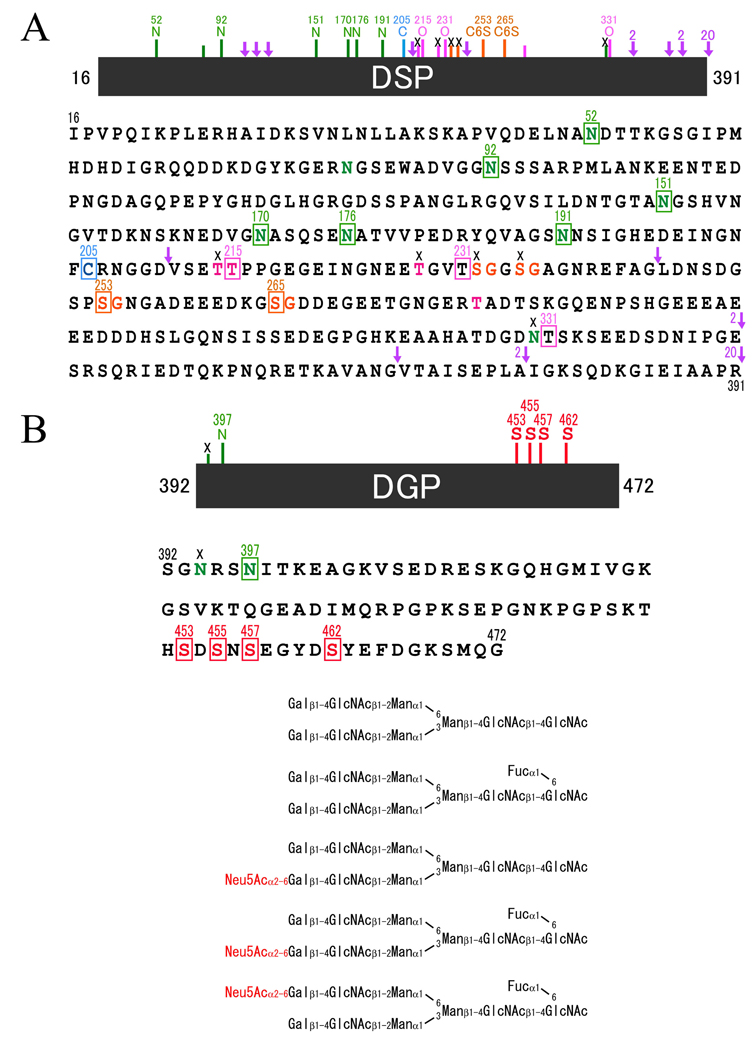
Figure 2. Amino acid sequence and posttranslational modification sites in porcine DSP and DGP.
A, potential and known carbohydrate attachment and cleavage sites by MMP-2 and MMP-20 in porcine DSP. In the primary amino acid sequence of porcine DSP there are eight predicted N-linked glycosylation (N at positions 52, 82, 92, 150, 170, 176, 191 and 330) (green), four potential O-linked glycosylations (T at positions 214, 215, 228, and 279) (pink), and four potential glycan attachment sites (S at positions 232, 235, 253, and 265) (orange). We have now demonstrated at the protein level that six of the potential N-linked sites (N52, N92, N151, N170, N176 and N191) (green squares) are glycosylated, but one (N330) is not (black cross). Two of the four predicted O-linked glycosylation sites (T214 and T228) are not glycosylated (black crosses), but one predicted site (T215) and two unpredicted sites (T231 and T331) are O-glycosylated (pink squares) ; and two of the four potential glycan attachment sites (S253 and S265) (orange squares) are used, but other two (S232 and S235) are not. The arrows indicate the locations of cleavage sites by proteinases. We have demonstrated that three sites are cleaved by MMP-2 (2) and MMP-20 (20). B, amino acid sequence and structures of oligosaccharides of DGP. DGP is consisted of 81 amino acids. We have demonstrated that one potential N-linked oligosaccaride binding site (N397) is glycosylated (green square) and four serines (S453, S455, S457 and S462) are phosphorylated (red squares). We also have identified biantennary glycan structures by analyses of oligosaccharides released from DGP and fluorescent labeled.
2) Dentin Glycoprotein (DGP)
We discovered the middle portion of DSPP as a prominent stains-all positive protein in porcine dentin extracts, and designated it dentin glycoprotein, or DGP21). DGP is a glycoprotein that migrates at 19-kDa on SDS-PAGE, and is reduced to 16-kDa following deglycosylation with glycopeptidase A. DGP is the 81-amino acid segment of DSPP (Ser392 to Gly472) between the DSP and DPP domains. DGP is easy to isolate from porcine dentin, allowing us to characterize its entire amino acid sequence by protein methods. DGP has four phosphorylated serine residues (Ser453, Ser455, Ser457, and Ser462) and one glycosylated asparagine (Asn397). Any of five different complex biantenniary structures, both sialidated and unsialidated can be attached to Asn397 (Fig. 2B). DGP has a novel amino acid sequence and only shares significant amino acid identity with homologous regions of other DSPP sequences. Porcine DGP shares 81% amino acid identity with human DGP, and all of the five modified amino acids are conserved. Although the main DGP protein has 81 amino acids, we also identified several larger versions of the protein (“extended DGPs”) that are generated by cleavage of DSP-DGP at alternative sites. A 22-kDa band, for instance has 105 amino acids (Val368-Gly472) and is 24 amino acids longer than DGP22).
3) Dentin phosphoprotein (DPP)
DPP is the most acidic protein ever discovered, having an isoelectric point near 1.1, partly because it is highly phosphorylated (porcine DPP averages 155 phosphates per molecule). Due to such extensive phosphorylation, DPP stains very strongly with stains-all24), but does not stain with Coomassie Brilliant Blue (CBB). Porcine DPP isolated from our large-scale dentin extractions is glycosylated at N- and C-terminal domains (bracketing the acidic repeat region) and shows up as multiple (usually 4 bands) bands migrating between 96-kDa and 100-kDa on SDS-PAGE25). We determined that each of these bands has the same N-terminus (AspAspProAsn; DDPN). Dephosphorylation reduces the apparent size of the DPP bands on SDS-PAGE, but does not alter the pattern of multiple bands. Deglycosylation does not noticeably affect the mobility or pattern of the multiple bands. To elucidate the cause of its size heterogeneity, we isolated DPP separately from each of 22 pigs and observed DPP size variations on SDS-PAGE25). We amplified the DPP coding region (exon 5 of DSPP) by polymerase chain reaction (PCR) and cloned and characterized the DPP coding region from selected pigs. We discovered that the length variations in the DPP code closely correlated with the length variations observed at the protein level (Fig. 3). Characterization of the DPP coding region identified 4 allelic variants. Among the 4 alleles, 27 sequence variations were identified, including 16 length polymorphisms ranging from 3 to 63 nucleotides25). None of the length variations shifted the reading frame, and all localized to the highly redundant region of the DPP code. The four alleles encode DPP domains having 551, 575, 589 or 594 amino acids and completely explain the DPP size variations (Fig. 3). We also observed that porcine DPP is unstable at low pH and high temperatures and found that complexing DPP with collagen improves its stability25).
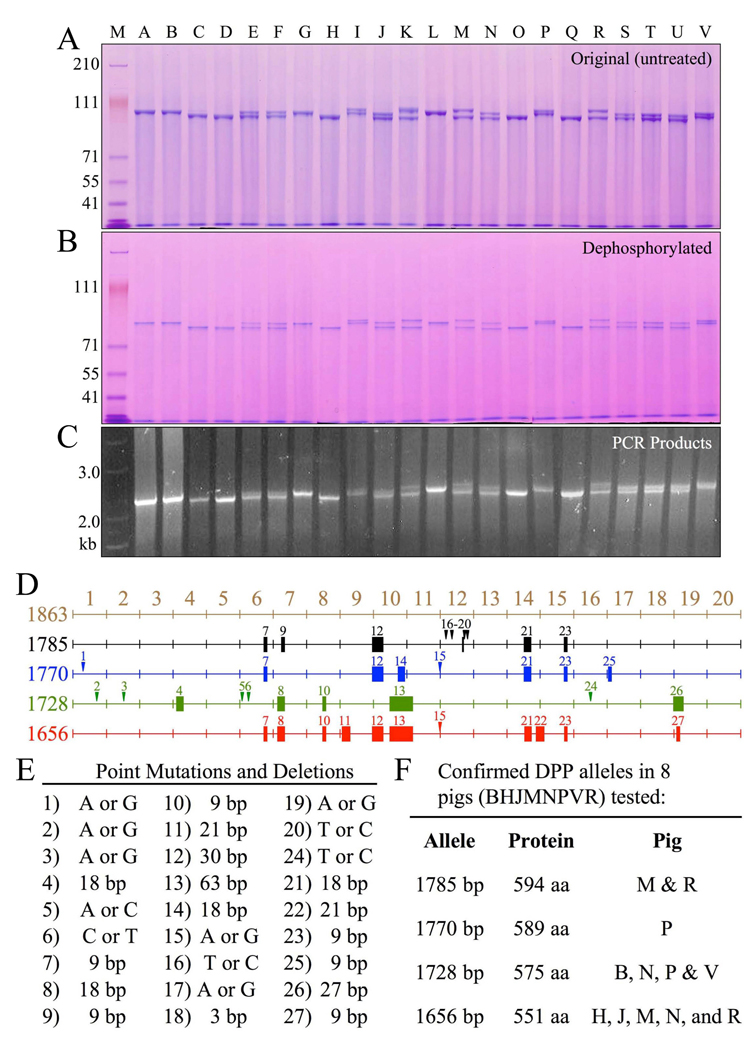
Figure 3. Length polymorphisms of DPP protein and coding region, and DPP allelic variations.
A, SDS-PAGE showing variations in the mobility of DPP protein isolated from 22 individual pigs. Nine of the 22 pigs showed only a single band of DPP (A, B, C, D, G, H, L, O, Q). Thirteen pigs showed two DPP bands (E, F, I, J, K, M, N, P, R, S, T, U, V). Although no individual pig showed more than 2 DPP bands, six bands of non-identical size could be discerned among the 22 pigs. B, Dephosphorylation of DPP from the 22 pigs shifted the bands lower on the gel but did not alter the pattern of bands. C, DPP PCR products using genomic DNA as template generated an identical pattern of 1 or 2 bands. D, Mapping of sequence variations in the porcine DPP coding region. Point mutations are indicated by numbered arrowheads; deletions are indicated by solid rectangles. On the left is the number of basepairs in each DPP allele. The first map (1863 bp, brown) is not an allele, but a merged reference sequence that contains all of the DPP code found on the four DPP alleles characterized. The four DPP alleles had 1785 (black), 1770 (blue), 1728 (green) and 1656 (red) basepairs. E, Key to the 27 sequence variations, the number of coding basepairs (bp) in each allele, the number of amino acids (aa) in the DPP protein, and the pigs shown to host each allele. The predominant alleles in the 22 pigs were 1656 and 1728 bp. Based upon the mobility of the proteins (A), and the cloning results, these alleles were likely present in 15 or 14 of the 22 pigs, respectively (1656: C2, D2, E, F, H2, J, K, M, N, O2, Q2, R, S, T, U; 1728: A2, B2, E, F, G2, I, J, L2, N, P, S, T, U, V). Only 6 of the 22 pigs hosted other alleles (I, K, M, P, R, V). The 1770 allele in pig P also appeared to be present in pigs I and V. The 1785 allele in pigs M and R also appeared to be in pig K. The frequency of the four characterized alleles in the 22 pigs (44 alleles) were 1656: 20 alleles; 1728: 18 alleles; 1770: 3 alleles; 1785: 3 alleles.
4) Proteolysis of DSPP
Intact DSPP has never been isolated (or even detected with certainty) from dentin extracts. Very rapidly following its synthesis, DSPP is cleaved by an unidentified protease after Gly472, into DSP-DGP (Ile16 to Gly472) and DPP (Asp473 to C-terminus). We also have successfully isolated these two cleavage products (DSP-DGP and DPP) from porcine dentin22). The amino acid context surrounding the initial cleavage site that releases DPP from the DSPP chimera (between Gly472 and Asp473) matches the target site consensus sequence for bone morphogenetic protein 1 or BMP-126). We designed fluorescence resonance energy transfer (FRET)-DSPP peptide containing Gly472-Asp473 and found that the recombinant human BMP-1 catalyzed cleavage of the FRET-DSPP peptide exactly at that DPP cleavage site.
Even in molars at an early stage of development (before the onset of root formation), a large number of other DSPP cleavage products are observed in the extracts. There is intact DSP (Ile16 to Arg391), various N-terminal pieces of DSP, a “DSP proteoglycan core” comprised of short DSP peptides starting after the disulfide bridge at Cys205 that contains the two glycan attachments on Ser253 and Ser265, extended DGPs (S345-G472 and I377-G472) and DGP (S392-G472). The DPP domain appears to be degraded almost at random, leaving a homogeneous smear of degradation products containing no discernible bands on SDS-PAGE.
Zymogram analyses of porcine dentin extracts identified three major proteases in dentin: gelatinase A (MMP-2), gelatinase B (MMP-9), and enamelysin (MMP-20). We isolated MMP-2 and MMP-20 from dentin and incubated them with DSP-DGP or DPP22). These enzymes showed no activity against DPP. MMP-20 cleaved DSP-DGP to generate DSP and DGP (S392-G472), and also cleaved DSP at multiple sites to generate the DSP proteoglycan core. MMP-2 generated both extended DGP forms. Clearly these two proteases play an important part of the process/degradation of DSPP (Fig. 4).
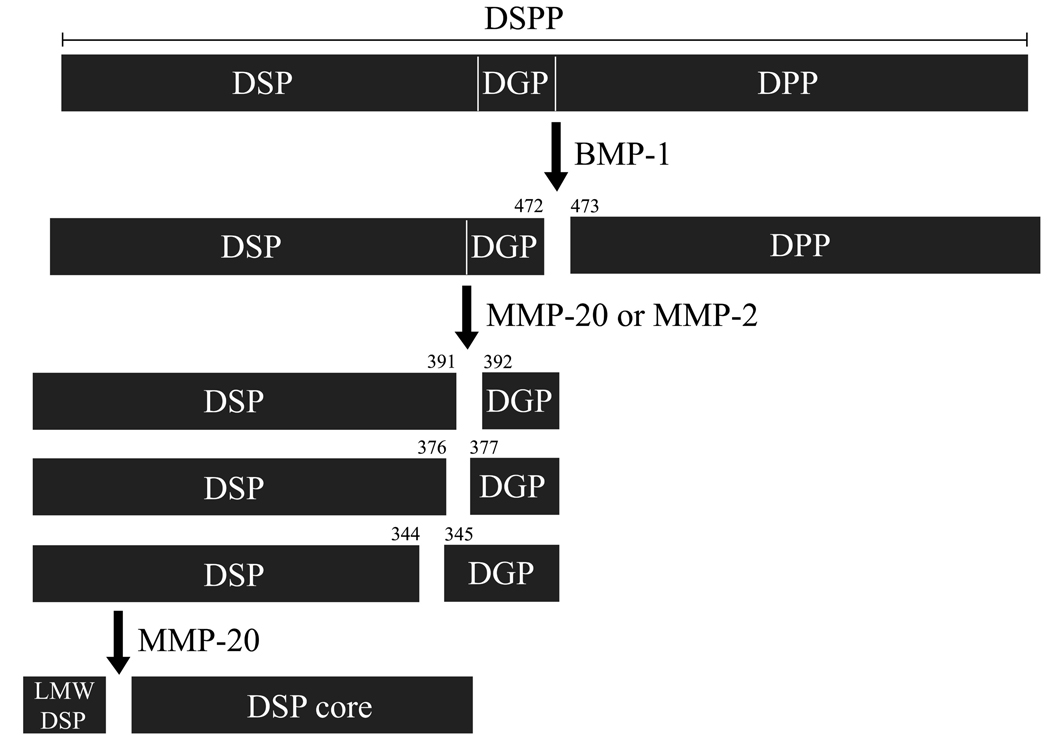
Figure 4. Proteolysis of porcine DSPP.
Numebers indicate cleavage sites discovered by characterizing DSPP-derived cleavage products from developing porcine molars or by analyzing FRET-DSPP peptide. DSPP is first processed by bone mophogenetic protein-1 (BMP-1) to generate DSP-DGP complex and DPP. Then, DSP-DGP is further cleaved in the dentin extracellular matrix by MMP-2 and MMP-20, generating DSPs and DGPs. MMP-20 performs the cleavage that separates DSP and DGP (at Ser392) and generates N-terminal DSP cleavage products. On the other hand, MMP-2 cleaves DSP-DGP primarily within the C-terminal region of the DSP (at Ser345 and Ile377), releasing DGP-containing cleavage products. The mechanism of DPP degradation in vivo is unknown.
Concluding Remarks
The DSPP gene has been implicated in the etiologies of Dentin Dysplasia type II and dentinogenesis imperfecta (types II and III). These autosomal dominant disorders affect about 1 in every 6000 to 8000 individuals. There is much interest in what DSPP-derived proteins do and how they function. DSPP represents 90% of the noncollagenous proteins in dentin. We discovered that DSP is a proteoglycan that forms covalent dimers, which significantly alters our concepts of its likely role in dentin formation. We pioneered use of the porcine animal model, allowing us to isolate hundreds of milligrams of specific proteins like DSP or DPP, which will facilitate subsequent studies. Our future work involves continuing the structural characterization of dentin matrix proteins, but more challenging, investigating the functions of DSP as a proteoglycan and DPP as an intrinsically disordered protein, and explaining the functional importance of the processing of DSPP by proteinases.
Acknowledgements
I thank the National Institute of Craniofacial and Dental Research of the National Institutes of Health (NIDCR/NIH) for supported my research (Yamakoshi, DE018020), as well as the research of my collaborators Drs. Bartlett (DE016276), Hu (DE011301), Margolis (DE016376), and Simmer (DE015846).
References
- 1.Linde A, Lussi A, Crenshaw MA. Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int. 1989;44:286–295. doi: 10.1007/BF02553763. [DOI] [PubMed] [Google Scholar]
- 2.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 3.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral Biol. Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 4.Veis A, Perry A. The phosphoprotein of the dentin matrix. Biochemistry. 1967;6:2409–2416. doi: 10.1021/bi00860a017. [DOI] [PubMed] [Google Scholar]
- 5.Leaver AG, Triffitt JT, Holbrook IB. Newer knowledge of non-collagenous protein in dentin and cortical bone matrix. Clin Orthop Relat Res. 1975:269–292. doi: 10.1097/00003086-197507000-00039. [DOI] [PubMed] [Google Scholar]
- 6.Dimuzio MT, Veis A. Phosphophoryns-major noncollagenous proteins of rat incisor dentin. Calcif Tissue Res. 1978;25:169–178. doi: 10.1007/BF02010765. [DOI] [PubMed] [Google Scholar]
- 7.Linde A, Bhown M, Butler WT. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980;255:5931–5942. [PubMed] [Google Scholar]
- 8.Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, Kohler T, Muller R, Goldberg M, Kulkarni AB. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biology. 2009 doi: 10.1016/j.matbio.2009.03.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields ED, Bixler D, el-Kafrawy AM. A proposed classification for heritable human dentine defects with a description of a new entity. Arch. Oral Biol. 1973;18:543–553. doi: 10.1016/0003-9969(73)90075-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, et al. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 11.Xiao S, et al. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 12.Rajpar MH, Koch MJ, Davies RM, Mellody KT, Kielty CM, Dixon MJ. Mutation of the signal peptide region of the bicistronic gene DSPP affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum Mol Genet. 2002;11:2559–2565. doi: 10.1093/hmg/11.21.2559. [DOI] [PubMed] [Google Scholar]
- 13.Malmgren B, Lindskog S, Elgadi A, Norgren S. Clinical, histopathologic, and genetic investigation in two large families with dentinogenesis imperfecta type II. Hum Genet. 2004;114:491–498. doi: 10.1007/s00439-004-1084-z. [DOI] [PubMed] [Google Scholar]
- 14.Kim JW, Nam SH, Jang KT, Lee SH, Kim CC, Hahn SH, Hu JC, Simmer JP. A novel splice acceptor mutation in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet. 2004;115:248–254. doi: 10.1007/s00439-004-1143-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, et al. Mutational hot spot in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet. 2005;116:186–191. doi: 10.1007/s00439-004-1223-6. [DOI] [PubMed] [Google Scholar]
- 16.McKnight DA, Hart PS, Hart TC, Hartsfield JK, Wilson A, Wright JT, Fisher LW. A comprehensive analysis of normal variation and disease-causing mutations in the human DSPP gene. Hum Mutat. 2008;29:1392–1404. doi: 10.1002/humu.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song YL, Wang CN, Fan MW, Su B, Bian Z. Dentin phosphoprotein frameshift mutations in hereditary dentin disorders and their variation patterns in normal human population. J Med Genet. 2008;45:457–464. doi: 10.1136/jmg.2007.056911. [DOI] [PubMed] [Google Scholar]
- 18.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 19.Yamakoshi Y, Hu JC, Liu S, Zhang C, Oida S, Fukae M, Simmer JP. Characterization of porcine dentin sialoprotein (DSP) and dentin sialophosphoprotein (DSPP) cDNA clones. Eur. J. Oral Sci. 2003;111:60–67. doi: 10.1034/j.1600-0722.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J. Biol. Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 21.Yamakoshi Y, Hu JC, Fukae M, Zhang H, Simmer JP. Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J. Biol. Chem. 2005;280:17472–17479. doi: 10.1074/jbc.M413220200. [DOI] [PubMed] [Google Scholar]
- 22.Yamakoshi Y, Hu JC, Iwata T, Kobayashi K, Fukae M, Simmer JP. Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J. Biol. Chem. 2006;281 doi: 10.1074/jbc.M607767200. 38235-28243. [DOI] [PubMed] [Google Scholar]
- 23.Gotting C, Kuhn J, Kleesiek K. Human xylosyltransferases in health and disease. Cell Mol. Life Sci. 2007;64:1498–1517. doi: 10.1007/s00018-007-7069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonsson M, Fredriksson S. Isoelectric focusing of the phosphoprotein of rat-incisor dentin in ampholine and acid pH gradients. Evidence for carrier ampholyte-protein complexes. J Chromatogr. 1978;157:234–242. doi: 10.1016/s0021-9673(00)92338-0. [DOI] [PubMed] [Google Scholar]
- 25.Yamakoshi Y, Lu Y, Hu JC, Kim JW, Iwata T, Kobayashi K, Nagano T, Yamakoshi F, Hu Y, Fukae M, Simmer JP. Porcine dentin sialophosphoprotein; Length polymorphisms, glycosylation, phosphorylation, and stability. J. Biol. Chem. 2008;283:14835–14844. doi: 10.1074/jbc.M800633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1 / Tolloid-like metalloproteinases. Matrix Biology. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]