Abstract
Tissue macrophage inflammatory pathways contribute to obesity-associated insulin resistance. Here, we have examined the efficacy and mechanisms of action of a novel anti-inflammatory compound (HE3286) in vitro and in vivo. In primary murine macrophages, HE3286 attenuates LPS- and TNFα-stimulated inflammation. In Zucker diabetic fatty rats, inflammatory cytokine/chemokine expression was downregulated in liver and adipose tissue by HE3286 treatment, as was macrophage infiltration into adipose tissue. In line with reduced inflammation, HE3286 treatment normalized fasting and fed glucose levels, improved glucose tolerance, and enhanced skeletal muscle and liver insulin sensitivity, as assessed by hyperinsulinemic euglycemic clamp studies. In phase 2 clinical trials, HE3286 treatment led to an enhancement in insulin sensitivity in humans. Gluconeogenic capacity was also reduced by HE3286 treatment, as evidenced by a reduced glycemic response during pyruvate tolerance tests and decreased basal hepatic glucose production (HGP) rates. Since serum levels of gluconeogenic substrates were decreased by HE3286, it indicates that the reduction of both intrinsic gluconeogenic capacity and substrate availability contributes to the decrease in HGP. Lipidomic analysis revealed that HE3286 treatment reduced liver cholesterol and triglyceride content, leading to a feedback elevation of LDL receptor and HMG-CoA reductase expression. Accordingly, HE3286 treatment markedly decreased total serum cholesterol. In conclusion, HE3286 is a novel anti-inflammatory compound, which displays both glucose-lowering and cholesterol-lowering effects.
Keywords: HE3286
obesity and insulin resistance are dominant features in most patients with type 2 diabetes mellitus, and recent data have established the concept that chronic, low-grade tissue inflammation is an important etiological cause of decreased insulin sensitivity (34, 44). Recent studies have shown that rodent and human adipose tissues are infiltrated with macrophages and that this phenomenon is exacerbated in obesity (57, 58). Thus, the number of proinflammatory M1-like macrophages increases by severalfold in obesity, and compelling evidence indicates that these M1-like adipose tissue macrophages (ATMs) are an important component of the inflammation/insulin resistance in obesity (26).
Strong genetic evidence supporting these concepts exists. When major proinflammatory pathways within macrophages, the chemotactic ability of macrophages, or secretion or action of TNFα and monocyte chemotactic protein-1 (MCP-1) are blocked, high-fat-diet/obesity induced insulin resistance is largely prevented (2, 8, 16, 46, 52). These studies show that inhibition of macrophage-mediated inflammatory responses results in beneficial effects on glucose metabolism and insulin resistance and suggests that anti-inflammatory therapy might be useful for the treatment of insulin resistance and diabetes.
Dehydroepiandrosterone (DHEA) is a natural steroid that serves as a precursor of male and female sex hormones (21). Produced from the adrenal gland, DHEA, together with its sulfated form (DHEA-S), is the most abundant steroid hormone in humans (21). DHEA has been postulated to have a variety of biological actions, including anti-inflammatory and antidiabetic effects (36, 49). Despite these well-documented activities in animal models, the value of DHEA replacement in humans is controversial. Most human studies failed to show improved glucose tolerance or insulin action (5, 17, 31, 53). Moreover, the potential therapeutic use of DHEA is limited by its side effects due to its conversion to sex hormones (3).
In the current study, we have examined the efficacy and mechanism of HE3286 (17α-ethynyl-5-androstene-3β, 7β, 17β-triol), an analog of a human DHEA metabolite, in cultured cell systems and in insulin resistant Zucker diabetic fatty (ZDF) rats. HE3286 does not bind to or transactivate sex hormone or PPAR receptors and exhibits anti-inflammatory activities (4, 35). Here, we show that HE3286 inhibits macrophage inflammatory pathways and decreases macrophage chemotaxis in vitro and in vivo. HE3286 effectively suppresses systemic inflammation and normalizes hyperglycemia by lowering hepatic glucose production and increasing whole body insulin sensitivity. In addition, HE3286 modulates lipid metabolism in the liver to lower circulating cholesterol levels. Preliminary clinical studies suggested that HE3286 treatment improved insulin sensitivity in humans. In summary, we conclude that HE3286 may serve as a drug candidate for treatment of obesity-related metabolic disorders.
MATERIALS AND METHODS
Materials and cell culture.
LPS and recombinant TNFα were obtained from Sigma. Anti-sterol regulatory element-binding protein-2 (SREBP-2) and anti-Mac2 were purchased from Abcam (Cambridge, MA). All other primary antibodies were purchased from Cell Signaling Technology (Danvers, MA). HE3286 and rosiglitazone were obtained from Hollis Eden Pharmaceuticals (San Diego, CA).
Murine primary macrophages were elicited by intraperitoneal injection of thioglycollate (3 ml/mouse) in C57BL/6J mice. Macrophages were obtained from intraperitoneal lavage and washed twice. Cells were cultured in RPMI supplemented with 10% fetal bovine serum (FBS) for 3 days and then starved in RPMI supplemented with 0.5% FBS for overnight before the treatment.
RAW 264.7 cells and 3T3-L1 cells were cultured as described previously (33, 60).
Animals.
The study was staggered into six cohorts conducted on different days. Forty-two male ZDF rats and six Zucker fatty rats at 7 wk of age were received from Charles River laboratories (Wilmington, MA). The rats were housed individually on a 12:12-h light-dark cycle with the lights on at 0600 and were fed ad libitum except during the experiments. After 1 wk of acclimation, the ZDF rats began daily oral treatment for 32–35 days with vehicle (n = 18), 100 mg·kg−1·day−1 HE3286 (n = 15), or 10 mg·kg−1·day−1 rosiglitazone (n = 9).
Rat procedures conformed to the Guide for Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and were approved by the Animal Subjects Committee of the University of California, San Diego, California.
Metabolic studies.
Glucose, insulin, and pyruvate tolerance tests were performed on 6-h-fasted rats. For glucose tolerance test (GTT), animals were orally gavaged with glucose (1 g/kg), whereas for insulin tolerance test (ITT), 0.35 U/kg insulin (Novolin R; Novo-Nordisk) was injected intraperitoneally.
Rat hyperinsulinemic euglycemic clamp studies were performed as described previously (43) with modifications. Briefly, dual jugular venous cannulae and one carotid arterial cannula were implanted in rats. After 4–5 days of recovery, the hyperinsulinemic euglycemic clamp experiments were begun with a priming injection (7.5 μCi/0.2 ml) and constant infusion (0.25 μCi/min) of d-[3-3H]glucose (Du Pont-NEN, Boston, MA). After 60 min of tracer equilibration and basal sampling at t = −10 and 0 min, glucose (50% dextrose, variable infusion; Abbott) and tracer (0.25 μCi/min) plus insulin (20 mU·kg−1·min−1) were infused into the jugular vein. The achievement of steady-state conditions (100 mg/dl ± 5 mg/dl) was confirmed at the end of the clamp by measuring blood glucose every 10 min and ensuring that steady state for glucose infusion and plasma glucose levels was maintained for a minimum of 30 min. Blood samples were taken at t = −60 (start of experiment), −10, 0 (basal), 110, and 120 min (end of experiment) to determine glucose-specific activity and insulin and free fatty acid (FFA) levels. All blood samples were immediately centrifuged, and plasma was stored at −80°C for subsequent analysis.
Following a 3-day recovery after clamp, rats were fasted for 6 h and euthanized. Tissues were harvested at basal state or acute insulin-stimulated state (5 U/kg).
Glucose, insulin, and FFA levels were measured as described previously (37). Glycerol levels were measured using an assay kit from Sigma. Lactate and pyruvate assay kits were obtained from Biovision. TNFα and IL-1β were measured by ELISA assays (Biosource).
Quantitative lipidomics.
Quantitative lipidomic studies were performed by Lipomics Technologies, as described previously (6). Briefly, lipids from serum and tissues were extracted in the presence of internal standards with chloroform-methanol (2:1 vol/vol) (13). Individual lipid classes were separated by high-performance liquid chromatography. Each lipid class fraction was transesterified in 1% sulfuric acid in methanol in a sealed vial under nitrogen at 100°C for 45 min. The fatty acid methyl esters were extracted from the mixture with hexane containing 0.05% butylated hydroxytoluene and prepared for gas chromatography under nitrogen. Fatty acid methyl esters were then separated and quantified by capillary gas chromatography equipped with a 30-m DB-88MS capillary column and a flame ionization detector.
Immunohistochemistry.
Immunohistochemistry studies were conducted by the University of California San Diego histology core at Moores Cancer Center, as described previously (37). Paraffin-embedded epididymal adipose tissue sections were incubated with Mac-2 antibody with a 1:100 dilution overnight at 4°C.
Gene expression analyses.
Total RNA was extracted from tissue using the Purelink total RNA purification system (Invitrogen, Calsbad, CA). PCR was carried out on an MJ Research Chromo4 real-time PCR system (Bio-Rad Laboratories, Hercules, CA). Primer sequences are available upon request. The mRNA expression of all genes reported was normalized to multiple housekeeping genes (34B4, RNA polymerase II, and cyclophilin A), and comparable results were observed.
For quantitative nuclease protection assay, cells were lysed in lysis buffer after various treatments. The quantitative nuclease protection assay ArrayPlate assays were performed by High Throughput Genomics.
Macrophage chemotaxis assay.
Differentiated 3T3-L1 adipocytes (day 11 postdifferentiation) were incubated for 24 h with compounds (10 ng/ml TNFα with or without 100 nM HE3286) or vehicle in DMEM with 0.2% FFA- and endotoxin-free BSA. Chemotaxis assay was performed as described previously (38).
Western blotting.
Cells were serum starved and pretreated with DMSO or HE3286 (100 nM) overnight before LPS (100 ng/ml) or TNFα (10 ng/ml) stimulation for the indicated times. Cells were lysed, and total cell lysates were subjected to Western blotting, as described previously (25).
Human studies.
The study was performed in accordance with the guidelines of the International Conference on Harmonization and the Declaration of Helsinki and approved by both the RCRC Institutional Review Board (Austin, TX) and the Pennington Biomedical Research Center Institutional Review Board (Baton Rouge, LA).
Statistical analysis.
Unless otherwise noted, data were analyzed by ANOVA followed by Tukey post hoc tests. Individual pairwise comparisons were performed using Student's t-test. Analysis was performed using Excel (Microsoft, Redmond, WA) or Prism (GraphPad Software, San Diego, CA).
RESULTS
HE3286 inhibits LPS effects.
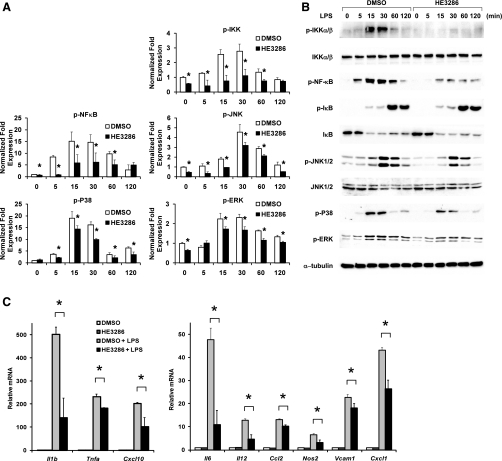
Recent findings indicate an important contribution of macrophage inflammatory pathways in causing the obesity-related decrease in insulin sensitivity (44). HE3286 has potential anti-inflammatory actions, and therefore, we tested its effects in primary murine intraperitoneal macrophages. Treatment with the Toll-like receptor 4 ligand LPS broadly activates proinflammatory signaling cascades, including phosphorylation of IKK and MAPKs such as JNK, p38, and extracellular signal-regulated kinases (ERK) (Fig. 1A). As a result, various inflammatory genes were upregulated (Fig. 1B), as determined by a quantitative nuclease protection assay array. Pretreatment with HE3286 partially, but significantly, blocked the activation of IKK, JNK, p38, and ERK (Fig. 1A). Although IκB phosphorylation and degradation were not influenced by HE3286 treatment, NF-κB phosphorylation was attenuated. Consistent with this, we performed chromatin immunoprecipitation studies and found that drug treatment led to decreased NF-κB occupancy of the κB site in the IL-6 promoter (Supplemental Fig. S1; Supplemental Material for this article can be found at the AJP-Endocrinology and Metabolism web site). Concordantly, LPS-induced transcription of Il1b, Il6, Il12, Tnfa, Nos2, Cxcl10, and Cxcl1 was significantly reduced (Fig. 1B). These results suggest that HE3286 can ameliorate intracellular inflammatory responses elicited by Toll-like receptor 4 signaling. When macrophages were treated with HE3286 alone, no activation of phospho-JNK was detected (Supplemental Fig. S2), demonstrating that the inhibition of LPS-induced inflammation by HE3286 is unlikely due to macrophage tolerance caused by preconditioning.
Fig. 1.
HE3286 attenuates LPS-induced inflammation in murine macrophages. A: murine intraperitoneal macrophages (IP Mac) pretreated with HE3286 (100 nM) or vehicle (DMSO) were treated with 100 ng/ml LPS for the times indicated. Relative phosphoproteins levels normalized to respective kinase or β-tubulin are shown as means ± SD. B: phosphoproteins and total proteins were detected by immunoblotting. C: IP Mac pretreated with HE3286 (100 nM) or DMSO for overnight were stimulated with 50 ng/ml LPS for 6 h. Total RNA extracted from cells was subjected to quantitative nuclease protection assay (qNPA) analysis. Data shown are the fold induction of gene expression normalized with housekeeping genes (means ± SD) from 3 experiments. *Statistical significance (P < 0.05) between conditions connected by bars.
HE3286 inhibits TNFα signaling.
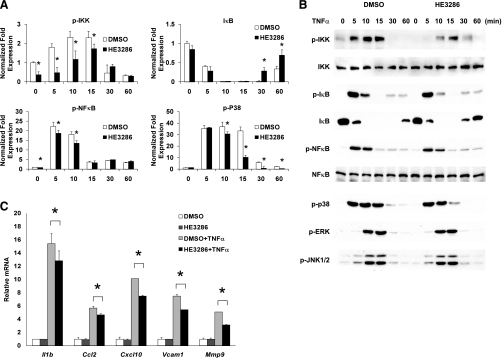
TNFα stimuates inflammation and activates NF-κB, Akt, and MAPKs by binding to TNF receptor 1 and 2. Of note, LPS-activated macrophages secrete TNFα, which then stimulates macrophages in an autocrine or paracrine fashion (24, 42). When TNFα was immunodepleted in the culture media, the effect of LPS to stimulate inflammatory signaling was reduced significantly (Supplemental Fig. S3). At the same time, when TNFα was neutralized, no additional inhibition of phospho-JNK and phospho-NF-κB by HE3286 treatment was observed. Since LPS-induced TNFα secretion was not altered by HE3286 (Supplemental Fig. S4), we speculated that the action of TNFα might be inhibited by HE3286. To test this, murine macrophages were pretreated with DMSO or HE3286, followed by TNFα stimulation. We found that HE3286 significantly decreased phosphorylation of IKK, NF-κB, and p38 but not JNK (Fig. 2A). Although changes in phosphorylation or degradation of IκB with HE3286 treatment were not observed, the recovery of IκB appeared to be enhanced (compare DMSO and HE3286-treated cells at 30 and 60 min; Fig. 2A). HE3286 attenuated TNFα-stimulated expression of Il1b, Cxcl10, Vcam1, Mmp9, and Ccl2 (Fig. 2B). Therefore, it appears that HE3286 causes inhibition of the macrophage inflammatory program primarily by inhibiting TNFα action.
Fig. 2.
HE3286 ameliorate TNFα-induced inflammation in murine macrophages A: murine IP Mac pretreated with HE3286 (100 nM) or vehicle (DMSO) were treated with 100 ng/ml LPS for the times indicated. Relative phosphoprotein levels normalized to respective kinase or β-tubulin are shown as means ± SD. B: phosphoproteins and total proteins were detected by immunoblotting. C: IP Mac pretreated with HE3286 (100 nM) or DMSO were stimulated with 10 ng/ml TNFα for 3 h. Total RNA extracted from cells was subjected to qNPA analysis. Data shown are the fold induction of gene expression normalized with housekeeping genes (means ± SD) from 3 experiments. *Statistical significance (P < 0.05) between conditions connected by bars.
Effects on macrophage chemotaxis.
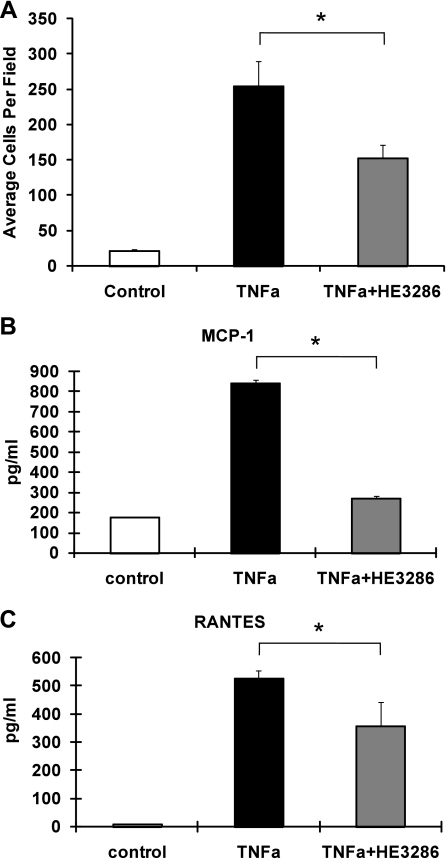
Increased macrophage infiltration and accumulation in adipose tissue occurs in obesity (57, 58), and therefore, we determined whether HE3286 could regulate macrophage chemotaxis. We used conditioned media (CM) from 3T3-L1 adipocytes to induce chemotaxis of RAW 264.7 monocyte/macrophages. Figure 3A shows that CM from TNFα-treated adipocytes markedly stimulated macrophage migration, which was reduced by ∼30% (P < 0.05) when adipocytes were pretreated with HE3286. In addition, adipocyte secretion of inflammatory cytokines, such as MCP-1/CCL2 and chemokine (C-C motif) ligand 5 (CCL5/regulated upon activation, normal T cell expressed and secreted), was augmented in TNFα-treated adipocyte CM and was significantly impaired by HE3286-pretreatment of the adipocytes (Fig. 3, B and C).
Fig. 3.
HE3286 impairs adipocyte conditioned media-induced macrophage chemotaxis. A: 3T3-L1 adipocytes were treated with vehicle and TNFα (10 ng/ml) in the absence or presence of HE3286 (100 nM). Conditioned media were then collected to induce chemotaxis of suspended RAW 264.7 macrophages. Migrated RAW 264.7 cells were quantified, and data are shown as means ± SE. B and C: 3T3-L1 adipocytes were treated with vehicle and TNFα (10 ng/ml) in the absence or presence of HE3286 (100 nM). Conditioned media were collected to induce cytokine release from RAW 264.7 macrophages. Concentrations of monocyte chemoattractant protein-1 (MCP-1; B) and regulated upon activation, normal T cell expressed and secreted (RANTES; C) in adipocyte-conditioned media were measured by ELISA. Data are shown as means ± SD. *Statistical significance (P < 0.05) between conditions connected by bars.
In vivo studies in ZDF rats.
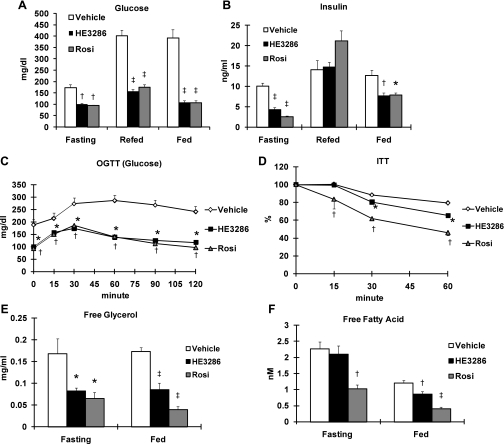
Given the anti-inflammatory effects of HE3286 and the known role of systemic inflammation in insulin resistance, we assessed whether HE3286 could improve glucose metabolism in vivo. We treated ZDF rats with placebo vehicle, HE3286 (100 mg·kg−1·day−1), or rosiglitazone (10 mg·kg−1·day−1) for 4 wk. The ZDF rat, a model of obesity, insulin resistance, and diabetes, is known to develop hyperinsulinemia at 8–9 wk of age and hyperglycemia after 9–10 wk of age (11, 47). The treatment was initiated at 8 wk of age so that the progression of diabetes in the animals could be evaluated. We found that HE3286 completely normalized fasting and fed glucose levels throughout the study. Thus, 1 wk of treatment with HE3286 was sufficient to normalize fasting and fed glucose levels as well as plasma insulin levels (Fig. 4, A and B). Rosiglitazone, a known insulin sensitizer, was used as a positive control and had the expected effects to ameliorate hyperglycemia and hyperinsulinemia (Fig. 4, A and B). However, rosiglitazone treatment also caused increased weight gain, whereas body weight of the vehicle- and HE3286-treated rats was comparable (Supplemental Table S1).
Fig. 4.
HE3286 improves glucose tolerance in Zucker diabetic fatty (ZDF) rats. Eight-week-old male ZDF rats were administered orally with vehicle (n = 12), HE3286 (100 mg·kg−1·day−1; n = 11), or rosiglitazone (Rosi; 10 mg·kg−1·day−1; n = 8) for 30 days. On day 7, glucose (A) and insulin levels (B) were measured at fed, 6-h fasting, and refed state (6-h refeeding after overnight fasting). C: on day 14, rats were subjected to oral glucose tolerance tests (OGTT). D: on day 21, rats were subjected to insulin tolerance tests (ITT). Data shown in D are the percentages compared with glucose levels at 0 min. E and F: on day 14, plasma glycerol (E) and free fatty acid (F) levels were measured by colorimetric assay kits. For A, B, E, and F: *statistical significance vs. vehicle-treated rats (P < 0.05); †P < 0.01; ‡P < 0.001. For C and D: *statistical significance (P < 0.05) between vehicle-treated and HE3286-treated rats; †statistical significance (P < 0.05) between vehicle-treated and Rosi-treated rats. All data are shown as means ± SE.
Oral GTTs and ITTs.
To further investigate the effect of HE3286 on insulin secretion and glucose utilization, oral GTTs (OGTT) were performed. At 10 wk of age (day 14 during treatment), vehicle-treated ZDF rats began to exhibit fasting hyperglycemia, whereas HE3286-treated animals had reduced glycemia both in the fasting state and after glucose administration (Fig. 4C). Glucose-induced insulin levels were also reduced (Supplemental Fig. S5). In these studies, the effects of HE3286 were comparable with those of rosiglitazone administration (Fig. 4, A and C, and Supplemental Fig. S5).
To determine whether HE3286 affects insulin-induced glucose clearance, ITTs were performed on a subset of the treated rats on day 21. Upon insulin injection, both HE3286- and rosiglitazone-treated rats showed enhanced glucose clearance (Fig. 4D), consistent with improved insulin sensitivity.
Studies of gluconeogenesis.
Since it has been suggested that fasting glucose level and the area under the curve for the first 30 min during a GTT largely represent insulin effects on the liver (1), we speculated that HE3286 could regulate hepatic glucose production (HGP). We reasoned that HE3286 treatment reduces gluconeogenic substrate levels. In ZDF rats, glycerol is a key gluconeogenic substrate for increased HGP, whereas other substrates such as lactate and pyruvate also contribute (50). To address this issue, plasma levels of glycerol, lactate, and pyruvate were measured on day 14. In agreement with our hypothesis, both fasting and fed glycerol levels were markedly reduced by HE3286 treatment (Fig. 4E). Likewise, lactate and pyruvate levels were also decreased (Supplemental Figs. S6 and S7). As published previously, rosiglitazone also effectively reduced the level of gluconeogenic substrates (Fig. 4E and Supplemental Figs. S5 and S6). In addition, we measured FFA levels since FFAs can contribute indirectly to gluconeogenesis by interfering with insulin suppression on HGP and by providing an energy source for ATP generation (18). As seen in Fig. 4F, fed FFA levels were decreased in the HE3286-treated group.
Glucose clamp studies.
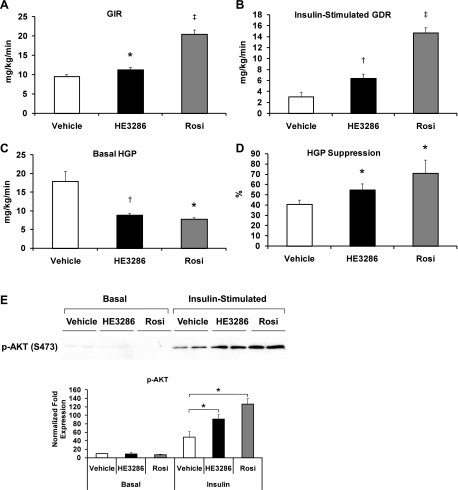
Euglycemic hyperinsulinemic clamp studies provide a quantitative measurement of in vivo insulin sensitivity. As summarized in Fig. 5, after 4 wk of treatment, both HE3286 and rosiglitazone treatment led to an increase in the glucose infusion rate and insulin-stimulated glucose disposal rate (IS-GDR), with the effects of rosiglitazone being more robust (Fig. 5, A and B). Since 70–80% of IS-GDR is attributable to skeletal muscle, this implies that HE3286 treatment improves skeletal muscle insulin action. In support of this, Akt phosphorylation was enhanced in insulin-stimulated skeletal muscle and liver (Fig. 5E and Supplemental Fig. S9). Importantly, basal rates of HGP were markedly and equally reduced by HE3286 and rosiglitazone treatment (Fig. 5C), and since basal HGP is the major contributor to basal hyperglycemia, these results are fully consistent with the marked reduction in basal glucose levels observed in these animals. In addition, we also found that the ability of insulin to suppress HGP was enhanced in the treated rats (Fig. 5D and Supplemental Fig. S8). Taken together, these in vivo results support the conclusion that HE3286 treatment leads to robust effects on hepatic glucose metabolism, which result in a marked reduction in gluconeogenic flux, and near normalization of hyperglycemia and enhanced hepatic insulin sensitivity.
Fig. 5.
HE3286 enhances insulin sensitivity in vivo. A–D: ZDF rats treated with vehicle (n = 14), HE3286 (n = 11), or Rosi (n = 8) for 4 wk were subjected to euglycemic hyperinsulinemic clamps. Glucose infusion rate (GIR; A), insulin-stimulated glucose disposal rate (GDR; B), basal hepatic glucose production (HGP; C), and HGP suppression by insulin (D) are shown as means ± SE. Statistical significance vs. vehicle-treated rats is indicated by *P < 0.05, †P < 0.01, or ‡P < 0.001. E: protein extracted from the skeletal muscle of treated rats was subjected to SDS-PAGE. Phospho-Akt (Ser473) was detected by immunoblotting. Relative protein levels normalized to respective kinase are shown as means ± SE. *Statistical significance (P < 0.05) between conditions connected by bars.
Glucose clamp studies in humans.
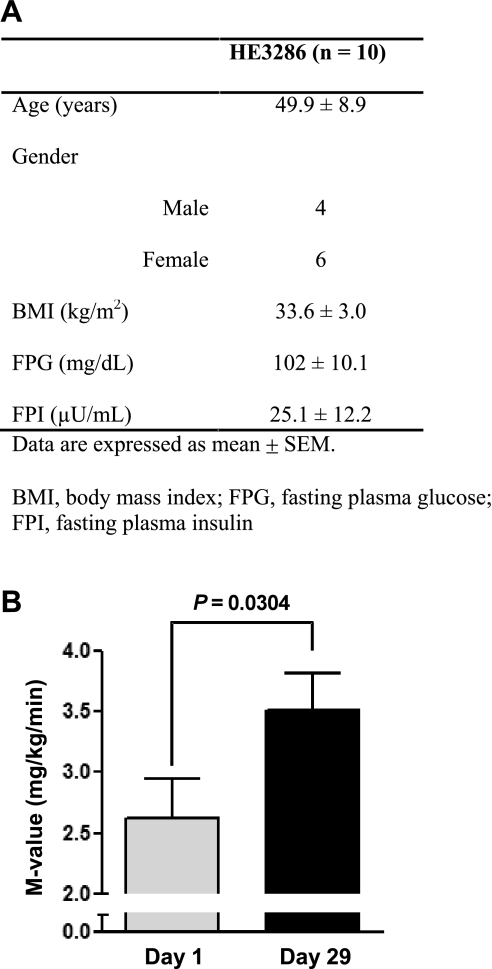
In preliminary clinical studies, HE3286 is effective at improving insulin sensitivity in insulin-resistant, obese patients. In these studies, 10 obese, insulin-resistant subjects with impaired glucose tolerance (according to standard American Diabetes Association OGTT criteria) were treated with HE3286 (5 mg BID, n = 5, or 10 mg BID, n = 5; total, n = 10) for 4 wk (Fig. 6A). Hyperinsulinemic euglycemic glucose clamp studies were performed on the day prior to treatment and on day 28 of treatment. The results are seen in Fig. 6B, which showed a significant 34% increase (P = 0.0304) in the glucose infusion rate (M value) in these patients. Thus, these results in insulin-resistant subjects are fully consistent with the in vitro results and the data in ZDF rats.
Fig. 6.
HE3286 enhances insulin sensitivity in humans. A: clinical characteristics of obese, insulin-resistant subjects treated with HE3286 (5 mg BID, n = 5, or 10 mg BID, n = 5; total, n = 10) for 4 wk. B: whole body insulin sensitivity was determined by hyperinsulinemic euglycemic clamps, and the results are expressed in terms of M values. Statistical significance was calculated via a paired (2-tailed) t-test.
Inflammation and macrophages in vivo.
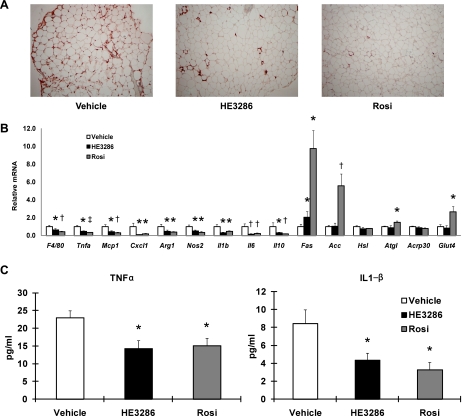
Increased ATM content is the central component of the chronic tissue inflammatory state in obesity (34, 44). To determine whether HE3286 modifies macrophage-mediated inflammation in vivo, we performed histological analysis of adipose tissue sections from control and treated rats. As seen in Fig. 7A, there was a marked reduction in ATM content, as measured by staining for the macrophage-specific marker Mac-2 in the treated rats. This directly demonstrates decreased ATM accumulation, and, consistent with this, we also found a decrease in F4/80 mRNA expression in both groups of treated animals (Fig. 7B). Moreover, we observed a striking decrease in a variety of inflammatory markers, including TNFα and MCP-1, in adipose tissue from the HE3286- and rosiglitazone-treated rats. Both M1 macrophage markers (NOS2, CXCL1, and IL-1) and M2 markers (arginase-1 and IL-10) were comparably reduced by HE3286 treatment (Fig. 7B), and the ratio of M1/M2 markers remained the same (Supplemental Fig. S10). In contrast to these broad changes in adipose tissue inflammatory markers, expression of genes such as glucose transporter 4, adiponectin, adipose triglyceride lipase, and hormone-sensitive lipase was not altered by HE3286 treatment (Fig. 7B). In contrast, rosiglitazone treatment led to an increase in glucose transporter 4 and adipose triglyceride lipase expression as well as very large increases in the lipogenic program, as measured by fatty acid synthase and acetyl-CoA carboxylase expression. Consistent with the decrease in inflammatory gene expression profile in the adipose tissue, circulating TNFα and IL-1β levels were also markedly reduced in the treated rats (Fig. 7C). We measured adipocyte size and found no significant difference after HE3286 treatment, in contrast to the effects of rosiglitazone, which decreased adipocyte size (Supplemental Fig. S11). On the basis of these findings, it seems likely that HE3286 exerts potent anti-inflammatory effects in vivo.
Fig. 7.
HE3286 regulates gene expression involved in inflammation and metabolism. A: tissue sections of epididymal white adipose tissue were subjected to Mac-2 staining (brown). B: total RNA extracted from epididymal white adipose tissue of treated ZDF rats was subjected to quantitative PCR analysis. Data shown are the fold induction of gene expression normalized with housekeeping genes and expressed as means ± SE. C: serum levels of TNFα and IL-1 were measured by ELISA. Data are shown as means ± SE. Statistical significance vs. vehicle-treated rats is indicated by *P < 0.05, †P < 0.01, or ‡P < 0.001.
To determine whether HE3286 treatment also produces anti-inflammatory effects in the liver, we measured the hepatic expression of inflammatory cytokines such as TNFα, IL-1β, IL-6, CXCL1, and MCP-1. As in adipose tissue, HE3286 led to suppression of inflammatory programs (Fig. 8D). Rosiglitazone treatment led to qualitatively similar, but quantitatively less marked, effects (Fig. 8D).
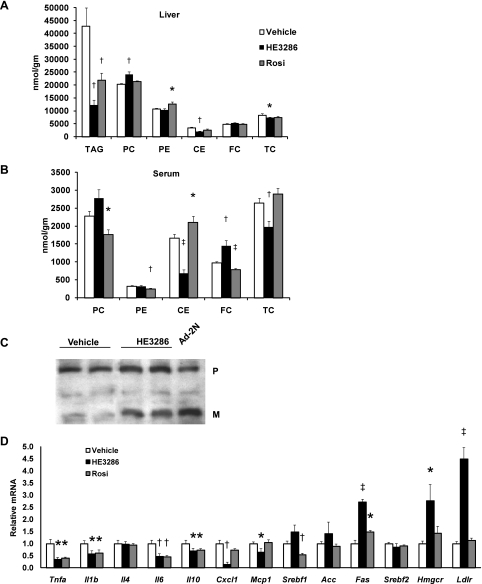
Fig. 8.
HE3286 regulates lipid metabolism in vivo. A and B: lipid classes in the liver (A) and serum (B) were quantified by lipidomic analysis. Data are expressed as means ± SE. C: protein extracted from the livers of treated rats was subjected to SDS-PAGE. Sterol regulatory element-binding protein-2 (SREBP-2) precursor (P) and mature form (M) were detected by immunoblotting. Culture cells infected with adenovirus expressing SREBP-2 NH2 terminus (Ad-2N) were used as a positive control for blotting. D: total RNA extracted from the liver of treated ZDF rats was subjected to quantitative PCR analysis. Data shown are the fold induction of gene expression normalized with housekeeping genes and expressed as means ± SE. Statistical significance vs. vehicle-treated rats is indicated by *P < 0.05, †P < 0.01, or ‡P < 0.001. TAG, triacylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; CE, cholesteryl ester; FC, free chholesterol; TC, total cholesterol.
In vivo lipidomic studies.
Dyslipidemia usually coexists with insulin resistance/diabetes, and increased tissue lipid accumulation has been demonstrated in several animal models of obesity and diabetes, including ZDF rats (7). Therefore, we, assessed lipid profiles in three key insulin-responsive tissues: liver, fat, and skeletal muscle. Figure 8A shows that HE3286 treatment led to a marked decrease in intracellular triacylglycerol content in livers, as did rosiglitazone. HE3286 significantly reduced hepatic cholesteryl ester (CE) levels by 73%, and as a result, total cholesterol content in the liver was reduced by 15%. Interestingly, HE3286 treatment led to a marked reduction in serum CEs and total cholesterol levels (by 59 and 25%, respectively) despite an increase in serum free cholesterol levels (Fig. 8B). The opposite is true for rosiglitazone treatment (Fig. 8B). Gene expression measurements demonstrated enhanced low-density lipoprotein receptor (LDLR) and HMG-CoA reductase expression with HE3286 treatment (Fig. 8D). On the basis of this, we questioned whether hepatic LDLR and HMG-CoA reductase expression was increased by activation of SREBP-2 secondary to the reduced hepatic cholesterol content. Since the mRNA level of SREBP-2, a key transcription factor regulating both HMG-CoA reductase and LDLR, remained unchanged (Fig. 8D), we speculated that HE3286 treatment leads to inhibition of cholesterol synthesis with proteolytic activation of SREBP-2 as a feedback response to low intrahepatic cholesterol. To assess this idea, we measured protein levels of SREBP-2 in treated rats. Figure 8C shows that HE3286 treatment enhanced the level of nuclear SREBP-2 (nuclear form) in the liver, indicating an increase in SREBP-2 protein cleavage and activation.
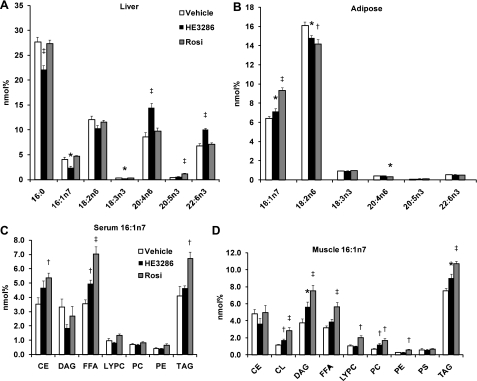
To further understand the effect of HE3286 and rosiglitazone on lipid metabolism, we examined tissue fatty acid composition. Figure 9A demonstrates that HE3286 decreased the levels of palmitate (16:0), palmitoleate (16:1n7), and linolenate (18:3n3) in the liver, whereas arachidonic acid (20:4n6) and docosahexaenoic acid (22:6n3) were increased. Interestingly, treatment with rosiglitazone increased only the level of eicosapentaenoic acid (20:5n3) but not docosahexaenoic acid (Fig. 9A).
Fig. 9.
HE3286 modulates fatty acid composition in vivo. A and B: mole percentages (nmol%) of palmitate (16:0), palmitoleate (16:1n7), linoleic acid (18:2n6), α-linolenic acid (18:3n3), arachidonic acid (20:4n6), eicosapentaenoic acid (20:5n3), and docosahexaenoic acid (22:6n3) in the liver (A) and white adipose tissue (B) were measured. C and D: nmol% of palmitoleate in lipid classes (16:1n7 compared with total fatty acids in indicated lipid classes) were calculated in serum (C) and muscle (D). Data are expressed as means ± SE. Statistical significance vs. vehicle-treated rats is indicated by *P < 0.05, †P < 0.01, or ‡P < 0.001. CL, cardiolipin; DAG, diacylglycerol; FFA, free fatty acid; LYPC, lysophosphatidylcholine; PS, phosphatidylserine.
Figure 9B summarizes the fatty acid levels in epididymal white adipose tissue. It has been suggested that palmitoleate (16:1n7) is a lipokine that can promote insulin sensitivity (6), and we found an elevation of palmitoleate levels in both HE3286- and rosiglitazone-treated animals (Fig. 9B). Since 16:1n7 is an indicator of de novo lipogenesis (6, 56), these results imply enhanced adipocyte lipogenesis in the treated rats.
We also measured the effects of HE3286 and rosiglitazone treatment on serum fatty acid profiles, as shown in Fig. 9C. Since fatty acids released by adipose tissue are the dominant source of circulating fatty acids, one would expect serum FFA patterns to largely mirror adipose tissue. Thus, we observed an increase in palmitoleate levels in FFAs in HE3286- and rosiglitazone-treated rats (Fig. 9C), reflecting the corresponding changes in adipose tissue. De novo fatty acid synthesis in muscle is negligible, and therefore, the fatty composition of muscle is determined largely by circulating FFAs and VLDL (6). Indeed, we found an increase in palmitoleate levels in several lipid classes in skeletal muscle (Fig. 9D).
DISCUSSION
Numerous studies have shown that chronic, low-grade tissue inflammation is a key component of obesity-induced insulin resistance (2, 44, 57, 58). Several reports have found that anti-inflammatory salicylate treatment improves insulin sensitivity (19), and the discovery that proinflammatory macrophages accumulate in adipose tissue has further indicated that anti-inflammatory therapies can have beneficial effects in insulin resistance/diabetes (57, 58). Here, we show that a novel anti-inflammatory compound, HE3286, can inhibit IKKβ/NF-κB and JNK signaling, reduce cytokine release, and decrease ATM infiltration. As a result, glucose tolerance and insulin sensitivity are substantially improved in ZDF rats treated with HE3286. These findings support the concept that inflammation is an important cause of insulin resistance and that anti-inflammatory therapy can be an effective measure to treat this class of metabolic disorders.
Despite positive findings in rodent models, indicating that DHEA treatment improves glucose tolerance and insulin sensitivity, DHEA supplementation in humans has yielded inconclusive results (5, 10, 17, 53). One possibility to explain these discrepancies is that a metabolite(s) of DHEA, rather than DHEA itself, may be necessary for its full action in human physiology. DHEA undergoes extensive conversion and derivatization to multiple products by phase 1 reactions involving the cytochrome P450 system (22, 28, 29), and studies have shown that these phase 1 products can be more potent than parental DHEA (22, 23, 27–29). Phase 1 reactions frequently decline in elderly subjects (45), and since such subjects have been the major participants in human DHEA treatment studies, it is possible that biologically active metabolites of DHEA were not produced in adequate amounts in previous human studies. It is also possible that qualitative changes in DHEA metabolism between rodents and humans may account for these differences (12). HE3286 is a synthetic analog of a DHEA metabolite, it is chemically designed to resist microsomal oxidation, and it is metabolically stable (35). HE3286 is readily bioavailable and does not bind to or activate sex hormone receptors or PPAR family receptors (54). The hydroxyl group at the carbon 7 position of HE3286 prevents its conversion to testosterone, preventing generation of unwanted metabolic byproducts of DHEA. Consistent with our findings in ZDF rats, preliminary results from ongoing clinical trials in type 2 diabetic subjects have shown that HE3286 exerts beneficial effects on glucose metabolism and improves insulin sensitivity in humans.
Our in vivo studies showed that basal hyperglycemia was largely normalized by HE3286 treatment and that this was due to correction of the basal rate of HGP. Since the elevated HGP in ZDF rats is due largely to increased gluconeogenesis (51), these results indicate that treatment with this agent markedly inhibits hepatic gluconeogenesis, leading to the reduction in HGP and normalization of basal glycemia. HE3286 treatment significantly decreased plasma levels of a wide range of gluconeogenic substrates, including glycerol, pyruvate, and lactate. Compared with FFAs, glycerol released by adipose tissue is considered a more accurate reflection of lipolysis since FFA levels can be influenced by both the lipolytic and FFA clearance pathways. Given that plasma glycerol has been shown to be a key driving force for HGP in ZDF rats (50), the marked reduction in adipose tissue lipolysis with a decrease in circulating glycerol levels is likely to be an important factor in reducing HGP.
Hyperinsulinemic euglycemic clamp studies demonstrated further that HE3286 treatment leads to systemic insulin sensitivity with improved insulin action in muscle and liver (Figs. 4 and 5). Thus, drug treatment caused an increase in the insulin-stimulated GDR and an improved ability of insulin to suppress HGP and to lower FFA levels, indicating a broad systemic increase in insulin sensitivity. These results are qualitatively comparable with the effects of rosiglitazone treatment. However, quantitative differences were apparent in that the effects of rosiglitazone on muscle insulin sensitivity (IS-GDR) were greater, whereas the hepatic effects were comparable between the two drugs (Fig. 5).
The mechanisms by which HE3286 improves systemic insulin sensitivity are of high interest and are likely multifaceted. For example, HE3286 treatment attenuates JNK/AP1 and IKK/NF-κB signaling in macrophages, and it has been documented that genetic deletion of these pathways in myeloid cells is sufficient to alleviate systemic insulin resistance (2, 46). Inflammatory cytokines such as TNFα, IL-6, and IL-1β may be key mediators linking local tissue inflammation and increased ATM content to systemic insulin resistance (34, 44). HE3286 treatment inhibited macrophage TNFα mRNA expression and also led to a decrease in circulating TNFα and IL-1 levels in treated rats. Clearly, the latter could be largely a result of the reduced number of ATMs. These findings support the conclusion that an important aspect of HE3286-induced increased insulin sensitivity is related to its anti-inflammatory effects. Another component of HE3286 in vivo action relates to inhibition of hepatic lipogenesis. Although it is still unclear whether hepatic lipogenesis and steatosis can directly cause insulin resistance (30, 40, 59), ample evidence exists showing that amelioration of hepatic lipid accumulation is associated with an improvement in insulin sensitivity (9, 32). Therefore, the drug-induced reduction of hepatic triglyceride and cholesterol content may participate in the effects on hepatic insulin sensitivity. Lastly, palmitoleate, a product of de novo lipogenesis, has recently been identified as a lipokine secreted by adipose tissue, which can lead to peripheral insulin sensitization (6). We observed an increase in palmitoleate levels in the serum FFA fraction and in adipose tissue after HE3286 treatment. Close inspection shows that 16:1n7 does not increase in all serum lipid classes, and the changes in fatty acid composition in the specific lipid classes have potential physiological implications. Serum and muscle lipid profiles largely reflect events occurring in the liver and adipose tissue. For example, fasting serum phosphatidylcholine (PC), trigylceride (TG), and CE arise mainly from the liver, whereas serum FFAs come from adipose tissue (15, 20, 48). Therefore, 16:1n7 in serum PC, TG, or CE reflects enhanced hepatic lipogenesis, whereas increased 16:1n7 in FFA reflects adipose tissue lipogenesis (6, 20, 39, 55). Drug treatment caused a significant increase in 16:1n7 FFA but not in the other serum lipid classes, suggesting a specific increase in de novo lipogenesis in fat. The reduction in hepatic TG and 16:1n7 levels by HE3286 treatment suggests decreased hepatic lipogenesis. The changes in 16:1n7 in blood are consistent with the findings in liver and adipose tissue, and together these results suggest that HE3286 treatment led to decreased lipogenesis in the liver and increased lipogenesis in the fat.
Cardiovascular disease is a major complication of type 2 diabetes and is closely associated with dyslipidemia and inflammation (41). In the current study, we observed novel effects of HE3286 on intracellular lipid metabolism. Thus, drug treatment led to a decrease in intracellular cholesterol concentration that could account for the feedback elevation of hepatic LDLR and HMG-CoA reductase expression. Elevation of LDLR-mediated clearance would in turn lead to the reduction in circulating cholesterol levels in the treated rats. Many previous reports have confirmed that reduced intrahepatic cholesterol content will activate the key cholesterogenic transcription factor SREBP-2 via proteolytic cleavage and nuclear translocation (14). Subsequently, key enzymes involved in cholesterol biosynthesis (HMG-CoA reductase) and cholesterol uptake (LDLR) are upregulated. Our findings show that this sequence of events is triggered by HE3286 treatment, and this is a potentially novel mechanism that may have additional cardiovascular benefits.
In summary, we have identified HE3286 as a novel compound that exerts potent and broad anti-inflammatory effects in macrophages both in vitro and in vivo. This compound subsequently leads to improved glucose tolerance and systemic insulin sensitivity. Coupled with its effects to lower serum cholesterol levels in rats, HE3286 could ultimately prove to be a useful therapeutic in the treatment of metabolic diseases.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-033651 (J. M. Olefsky) and DK-063491 (J. M. Olefsky) and a grant from Hollis Eden Pharmaceuticals (J. M. Olefsky). This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through Cooperative Agreement U54-HD-012303-25 as part of the specialized Cooperative Centers Program in Reproduction and Infertility Research.
DISCLOSURES
The authors have declared that no conflict of interest exists.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Dorothy Sears, J. Bruce German, and Maziyar Saberi for helpful discussion. We thank Jachelle O. Pimentel, Arezou Amidi, and Sarah Nalbandian for technical support.
REFERENCES
- 1.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30: 89–94, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Arnold JT. DHEA metabolism in prostate: for better or worse? Mol Cell Endocrinol 301: 83–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auci D, Kaler L, Subramanian S, Huang Y, Frincke J, Reading C, Offner H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: androstene hormones as regulators of regulatory T cells. Ann NY Acad Sci 1110: 630–640, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes 56: 753–766, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134: 933–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirieac DV, Collins HL, Cianci J, Sparks JD, Sparks CE. Altered triglyceride-rich lipoprotein production in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 287: E42–E49, 2004 [DOI] [PubMed] [Google Scholar]
- 8.De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-α contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab 293: E713–E725, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 55: 2159–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dhatariya K, Bigelow ML, Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes 54: 765–769, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism 49: 684–688, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick JL, Ripp SL, Smith NB, Pierce WM, Jr, Prough RA. Metabolism of DHEA by cytochromes P450 in rat and human liver microsomal fractions. Arch Biochem Biophys 389: 278–287, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 14.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 29: 431–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47: 348–380, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, Miyamoto S, Nakano M, Ogawa H. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab 88: 3190–3195, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kehlenbrink S, Tonelli J, Koppaka S, Chandramouli V, Hawkins M, Kishore P. Inhibiting gluconeogenesis prevents fatty acid-induced increases in endogenous glucose production. Am J Physiol Endocrinol Metab 297: E165–E173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunesova M, Hainer V, Tvrzicka E, Phinney SD, Stich V, Parizkova J, Zak A, Stunkard AJ. Assessment of dietary and genetic factors influencing serum and adipose fatty acid composition in obese female identical twins. Lipids 37: 27–32, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol 187: 169–196, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lardy H, Marwah A, Marwah P. C(19)-5-ene steroids in nature. Vitam Horm 71: 263–299, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lardy H, Partridge B, Kneer N, Wei Y. Ergosteroids: induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone. Proc Natl Acad Sci USA 92: 6617–6619, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J Immunol 178: 3731–3739, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol 760–771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56: 16–23, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Marwah A, Gomez FE, Marwah P, Ntambi JM, Fox BG, Lardy H. Redox reactions of dehydroepiandrosterone and its metabolites in differentiating 3T3-L1 adipocytes: A liquid chromatographic-mass spectrometric study. Arch Biochem Biophys 456: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Matsuzaki Y, Honda A. Dehydroepiandrosterone and its derivatives: potentially novel anti-proliferative and chemopreventive agents. Curr Pharm Des 12: 3411–3421, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Miller KK, Cai J, Ripp SL, Pierce WM, Jr, Rushmore TH, Prough RA. Stereo- and regioselectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab Dispos 32: 305–313, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Hevener AL, Farese RV., Jr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355: 1647–1659, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2: 55–65, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract 4: 619–626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offner H, Firestein GS, Boyle DL, Pieters R, Frincke JM, Garsd A, White SK, Reading CL, Auci DL. An orally bioavailable synthetic analog of an active dehydroepiandrosterone metabolite reduces established disease in rodent models of rheumatoid arthritis. J Pharmacol Exp Ther 329: 1100–1109, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Padgett DA, Loria RM. Endocrine regulation of murine macrophage function: effects of dehydroepiandrosterone, androstenediol, and androstenetriol. J Neuroimmunol 84: 61–68, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8: 301–309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patsouris D, Neels JG, Fan W, Li PP, Nguyen MT, Olefsky JM. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J Biol Chem 284: 31223–31235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Königsrainer A, Königsrainer I, Häring HU, Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem 55: 2113–2120, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118: 829–838, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am 37: 603–621, viii, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruan H, Miles PD, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 51: 3176–3188, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes 54: 1304–1313, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118: 2992–3002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmucker DL. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging 18: 837–851, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6: 386–397, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Sparks JD, Phung TL, Bolognino M, Cianci J, Khurana R, Peterson RG, Sowden MP, Corsetti JP, Sparks CE. Lipoprotein alterations in 10- and 20-week-old Zucker diabetic fatty rats: hyperinsulinemic versus insulinopenic hyperglycemia. Metabolism 47: 1315–1324, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest 115: 1139–1142, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol 151: 1–14, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Terrettaz J, Jeanrenaud B. Contribution of glycerol and alanine to basal hepatic glucose production in the genetically obese (fa/fa) rat. Biochem J 270: 803–807, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Poelje PD, Potter SC, Chandramouli VC, Landau BR, Dang Q, Erion MD. Inhibition of fructose 1,6-bisphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes 55: 1747–1754, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Verschuren L, Kooistra T, Bernhagen J, Voshol PJ, Ouwens DM, van Erk M, de Vries-van der Weij J, Leng L, van Bockel JH, van Dijk KW, Fingerle-Rowson G, Bucala R, Kleemann R. MIF deficiency reduces chronic inflammation in white adipose tissue and impairs the development of insulin resistance, glucose intolerance, and associated atherosclerotic disease. Circ Res 105: 99–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 292: 2243–2248, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Wang T, Villegas S, Huang Y, White SK, Ahlem C, Lu M, Olefsky JM, Reading C, Frincke JM, Alleva D, Flores-Riveros J. Amelioration of Glucose Intolerance by the Synthetic Androstene HE3286: Link to Inflammatory Pathways. J Pharmacol Exp Ther In press [DOI] [PubMed] [Google Scholar]
- 55.Warensjö E, Rosell M, Hellenius ML, Vessby B, De Faire U, Risérus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis 8: 37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res 43: 1809–1817, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yahagi N, Shimano H, Hasty AH, Matsuzaka T, Ide T, Yoshikawa T, Amemiya-Kudo M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga J, Harada K, Gotoda T, Nagai R, Ishibashi S, Yamada N. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem 277: 19353–19357, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29: 1363–1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.