Abstract
Understanding the molecular basis and target of traditional medicine is critical for drug development. Celastrol, derived from Trypterygium wilfordii Hook F. (“Thunder of God Vine”), a traditional Chinese medicine plant, has been assigned anticancer activities but its mechanism is not well understood. Here, we investigated whether Celestrol could inhibit angiogenesis-mediated tumor growth and if so through what mechanism. When administered subcutaneously to mice bearing human prostate cancer (PC-3 cell) xenografts, Celastrol (2 mg/kg/d) significantly reduced the volume and the weight of solid tumors and decreased tumor angiogenesis. We found that this agent inhibited vascular endothelial growth factor (VEGF)-induced proliferation, migration, invasion, and capillary-like structure formation by primary cultured human umbilical endothelial cells (HUVECs) in a dose-dependent manner. Further, Celastrol abrogated VEGF-induced sprouting of the vessels from aortic rings and inhibited vascular formation in the Matrigel plug assay in vivo. To understand the molecular mechanism of these activities, we next examined the signaling pathways in treated HUVECs and PC-3 tumor cells. Celastrol suppressed the VEGF-induced activation of AKT, mammalian target of rapamycin (mTOR), and ribosomal protein S6 kinase (P70S6K). Additionally, we found that Celastrol inhibited the proliferation of prostate cancer cells and induced apoptosis, and these effects correlated with the extent of inhibition of AKT/mTOR/P70S6 kinase signaling. Taken together, our results suggest that Celastrol targets the AKT/mTOR/P70S6K pathway, which leads to suppression of tumor growth and angiogenesis.
Keywords: Celastrol, tumor angiogenesis, mTOR, Akt, p70S6 kinase, prostate tumor
Introduction
Traditional medicines provide highly fertile ground for modern drug development, but first they must proceed along a pathway of discovery, isolation, and mechanistic studies before eventual deployment in the clinic (1). Celastrol, known as a tripterine, is a functional ingredient that was originally identified and extracted from traditional Chinese medicine named Trypterygium wilfordii Hook F. (“Thunder of God Vine”) and shows potential in treatment of chronic inflammatory disorders, such as arthritis (2, 3), lupus erythematosus (4), lateral sclerosis (5) and Alzheimer’s disease (6). It also potentiates apoptosis in numerous tumor cells through inhibition of IκBα kinase (7, 8), proteasome (9), topoisomerase (10), heat shock protein (11-13), and VEGF receptor expression (14). Although Celastrol has already shown promising in tumor prevention, direct targets remain elusive. Whether Celastrol could modulate tumor angiogenesis has not been validated yet.
It is estimated more than 90% cancer deaths that occur are due to angiogenesis, invasion, and metastasis of cancer to vital organs. Angiogenesis is one of the key processes that mediate metastasis, in part through the interaction of human vascular endothelial cells (HVEC) with vascular endothelial cell growth factor (VEGF). Although antibodies against VEGF (called avastin) have already been approved for human use by the Food and Drug Adminsitration (FDA), safer and more efficient inhibitors are still needed. VEGF mediates its signals through the interaction with three distinct receptors, VEGFR1, VEGFR2 and VEGFR3. These receptors mediate their signals through activation of various cell-signaling intermediates, including the mammalian target of rapamycin (mTOR). The latter has become an extremely important drug target in cancer therapy recently (15). The mTOR, a serine/threonine kinase, stands in a central position on the crossroad of various signal pathways (Ras, PI3K/AKT, HIF, NF-κB) towards mRNA, ribosome, protein synthesis and translation of significant molecules (16). Through activation of its downstream ribosomal p70S6 kinase (S6K1) and hyperphosphorylation of the 4E binding protein (4E-BP1), mTOR is critical for cellular proliferation and growth in endothelial cells and in various tumor cells (17, 18). Over-activation in mTOR signaling is frequently associated with tumorigenesis, angiogenesis, tumor growth, and metastasis (15, 19, 20). In recent years, drugs known as mTOR inhibitors, rapamycin, and the newly developed rapamycin analogs, have shown promise in ongoing and recently completed cancer trials of treatments for hematological malignancy and transplant rejection (21, 22). And temsirolimus (Torisel), an mTOR inhibitor, is currently approved by FDA as clinical anti-cancer therapy. Discovering additional safe and affordable inhibitors of the mTOR pathway is of great attraction. Here, we report that Celastrol effectively inhibited tumor angiogenesis and tumor growth by targeting the AKT/mTOR/P70S6K signaling pathway.
Materials and Methods
Reagents and antibodies
A 50 mmol/L stock solution of Celastrol (obtained from Cayman Chemicals, Ann Arbor, MI) was prepared and frozen at −20°C in small aliquots until needed. Bacteria-derived recombinant human VEGF (VEGF165) was a gift from the Experimental Branch of the National Institutes of Health (NIH; Bethesda, MD). Growth factor-reduced Matrigel was purchased from BD Biosciences (San Diego, CA). The antibodies against β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies anti-AKT, anti-mTOR, anti-p70S6K1, anti-poly (ADP-ribose) polymerase (PARP), phospho-specific anti-AKT (Ser473), anti-mTOR (Ser2448), anti-p70S6K1 (Thr421/Ser424), anti-p70S6K1 (Thr389) and anti-VEGFR2 (Tyr1175) were purchased from Cell Signaling Technology (Beverly, MA).
Cell lines and cell culture
Primary human umbilical vascular endothelial cells (HUVECs) were kindly provided by Dr. Xinli Wang (Cardiothoracic Surgery Division of Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX). HUVECs were cultured in endothelial cell growth medium (ECGM) as described previously (23). Human prostate cancer (PC-3) cells were purchased from American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). HUVECs and PC-3 cells were cultured at 37°C under a humidified 95%: 5% (v/v) mixture of air and CO2.
Human prostate tumor xenograft mouse model
All the mice used in this study were maintained in a laminar airflow cabinet under specific pathogen-free condition in a 12–h light-dark cycle. Experiments and managements were followed in accordance with Texas A&M University Institutional Animal Care and Use Committee guidelines at M. D. Anderson, ACUC typically approves animal protocols. Xenograft human prostate mouse model was constructed as previously described (24). Six-week-old male BALB/cA nude mice (National Rodent Laboratory Animal Resources, Shanghai Branch, China) weighing about 28 g were randomly divided into 2 groups of 5 each. PC-3 prostate cancer cells were subcutaneously injected into the mice (5×106 cells/mouse). After tumors grew to about 130 mm3, the mice were then subcutaneously treated with or without Celastrol (2 mg/kg/d). The body weight of each mouse was recorded and tumor volume was determined by Vernier caliper every day, following the formula of A×B2×0.52, where A is the longest diameter of tumor and B is the shortest diameter. After 16 d, the mice were killed and solid tumors were removed.
Histology and immunohistochemistry
Solid tumors were fixed with 10% formaldehyde and embedded in paraffin. To identify infiltrating blood vessels, immunohistochemistry was carried out on 5-μm deparaffinized sections using a specific blood vessel staining kit (von Willebrand Factor; Millipore, Billerica, MA). Images of the stained blood vessels were taken using a Leica DM 4000B photo microscope (Magnification, ×200; Solms, Germany). The Image-Pro plus 6.0 software package (Media Cybernetics Inc., Bethesda, MD) was used to determine the mean optical density (MOD) of blood vessel staining.
Wound-healing migration assay
Wound-healing migration assay was performed as described previously (25). Briefly, HUVECs were starved to inactivate cell proliferation and then wounded by pipette tips. ECGM containing 0.5% FBS was added with or without 10 ng/mL VEGF and different dilutions of Celastrol. Images of the cells were taken after about 8–10 h of incubation. Migrated cells were quantified manually, and the percentage inhibition was expressed using untreated wells at 100%. Three independent experiments were performed.
Transwell migration assay
Transwell migration assay was performed as described previously (25). Briefly, HUVECs (2×104 cells/Transwell) along with the indicated concentrations of Celastrol were seeded into the upper chambers. The bottom chambers were filled with 500 μL ECGM supplemented with 10 ng/mL VEGF. After 8-10 h incubation, migrated cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet. Images were taken using an OLYPUS inverted microscope (Magnification, ×100). The percentage inhibition of migrated cells in treated groups was recorded.
Capillary-like tube formation assay
Tube formation was assessed as described previously (25). Briefly, HUVECs were pretreated with various dilutions of Celastrol for 30 min and then seeded onto the Matrigel layer in 24–well plates at a density of 4–7×104 cells. ECGM with or without 10 ng/mL VEGF. After 6 h, tubular structure of endothelial cells was photographed using an inverted microscope (Olympus; magnification, ×100). Three independent experiments were performed.
Cell viability assay
HUVECs or PC-3 cell (5–7×103 cells/well) were treated with or without VEGF (10 ng/mL) and Celastrol for 24 h. Cell viability was determined by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] method, as described previously (24, 25).
Rat aortic ring assay
Rat aortic ring assay was performed as described previously (26). In brief, aortas isolated from Sprague–Dawley rats (NIH) were cleaned of periadventitial fat, and cut into rings 1–1.5 mm in circumference. The aortic rings were randomized into Matrigel-coated wells and sealed with a 100-μl overlay of Matrigel. VEGF, with or without different dilutions of Celastrol, was added to the wells. As a control, medium alone was assayed. After 6 d, we fixed the microvessel sprouting and photographed it using an OLYMPUS inverted microscope (Olympus, Center Valley, PA; magnification, ×100). The assay was scored from 0 (least positive) to 5 (most positive) in a double-blinded manner. Two independent experiments were performed.
Matrigel plug assay
Matrigel plug assay was performed as described previously (26). Briefly, we subcutaneously injected 0.5 mL of Matrigel containing 100 ng VEGF, 20 units of heparin with or without 10 μg of Celastrol into the ventral area of C57/BL/6 mice (NIH). Five mice were used for each group. After 6 d, the mice were killed and intact Matrigel plugs with different treatments were removed. Hematoxylin and eosin staining was performed to identify the formation and infiltration of new microvessels. Functional microvessels with intact red blood cells were quantified manually using a microscope (Magnification, ×200).
Western immunoblot analysis
To determine the effects of Celastrol on the VEGFR2–dependent mTOR signaling pathway, HUVECs were first starved in serum-free ECGM for 6 h, and then pretreated with or without Celastrol for 30 min, followed by the stimulation with 50 ng/mL of VEGF for 2 min (for VEGFR2 activation) or 15-20 min (for mTOR pathway kinase activation). To examine mTOR pathway in prostate tumor cells, normal cultured PC-3 cells were directly treated with indicated dilutions of Celastrol for 4 h. The whole-cell extracts were prepared in radioimmunoprecipitation assay (RIPA) buffer (20 mmol/L Tris, 2.5 mmol/L EDTA, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 40 mmol/L NaF, 10 mmol/L Na4P2O7, and 1 mmol/L PMSF) supplemented with a proteinase inhibitor cocktail (Calbiochem, San Diego, CA). About 40 μg cellular protein of each sample was applied to immunoblot using 6%–12% SDS-polycrylamide gel and probed with specific antibodies. The intensity of immunoreactivity was measured using densitometer and Image J software (Version 1.34e; NIH).
Statistical analysis
Data were presented as mean ± standard deviations (SD), and statistical comparisons between treated group and untreated group were performed using one-way analysis of variance followed by Dunnet’s test. P values ≤0.05 were considered statistically significant.
Results
The goal of this study was to investigate whether Celastrol, a triterpene from traditional Chinese medicine, can suppress angiogenesis and if so through what mechanism. For this, we employed a wide variety of in vitro and in vivo assays. These included tumor angiogenesis in vivo, cell proliferation, invasion and migration of VEGF–induced endothelial cells in vitro, VEGF microvessel tubule formation, VEGF-induced microvessel sprouting, and VEGF-induced blood vessel formation ex vivo. We also investigated the effect of Celastrol on the VEGF-induced cell signaling pathways and on the survival of prostate cancer cells.
Celastrol inhibits tumor growth and tumor angiogenesis in a xenograft mouse model
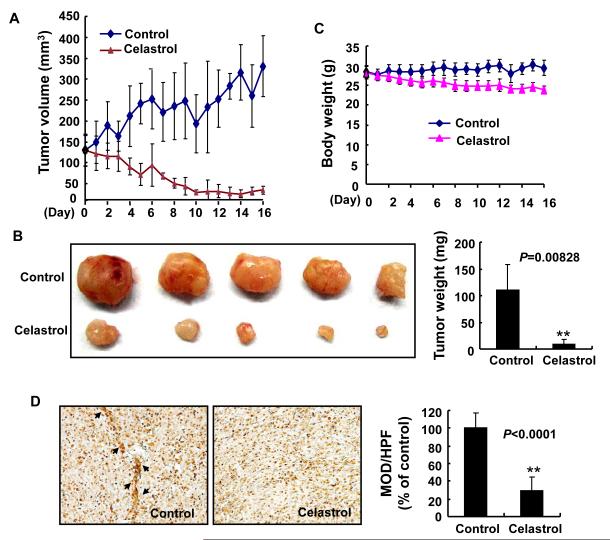
To investigate the effect of Celastrol on tumor growth and tumor angiogenesis in vivo, we applied different concentrations of Celastrol in a prostate tumor (PC-3) xenograft model. We found that administration of 2 mg/kg/d Celastrol for 16 d substantially suppressed tumor volume (Fig. 1A) and reduced tumor weight (Fig. 1B). The average tumor volume in the control mice increased from 130.63 ± 37.62 mm3 to 330.74 ± 72.20 mm3 after 16 d, whereas the average tumor volume in the Celastrol -treated mice decreased from 129.36 ± 37.35 mm3 to 35.49 ± 9.71 mm3. Additionally, the average tumor weight in the control group was 110.16 ± 47.73 mg, whereas the average tumor weight in Celastrol -treated group was only 9.46 ± 8.46 mg (Fig. 1B), a significant inhibition in tumor growth. However, the same dose of Celastrol had no significant effect on the body weight of mice (Fig. 1C), suggesting low toxicity of Celastrol at the test dosage and conditions. A lower dose of Celastrol (0.5 mg/kg/d) had little effect on the tumor growth (data not shown).
Figure 1. Celastrol inhibits tumor growth and tumor angiogenesis in xenograft mice.
PC-3 cells were injected into 6-week-old BALB/cA nude mice (5×106 cells per mouse). After solid tumors grew to about 130 mm3, the mice were subcutaneously administrated with or without Celastrol (2 mg/kg/d). A, Celastrol inhibited tumor growth as measured by tumor volume. B, as shown by the body weight change in mice, Celastrol had little toxicity in the amount tested. C, solid tumors in the Celastrol-treated mice were significantly smaller than those untreated mice. D, blood vessel staining revealed that Celastrol inhibited tumor angiogenesis. The arrows indicate blood vessels. Columns, mean; bars, standard deviation; **, P < 0.01 vs. control.
To investigate whether Celastrol inhibited tumor growth through the inhibition of tumor angiogenesis, we used a blood vessel staining kit to stain solid tumor sections in the xenograft mouse model. We found that the mean optical density of blood vessels in Celastrol–treated group was 29.96±15.29/HPF compared to 100±17.87/HPF in the control group (Fig. 1D), indicating that Celastrol significantly inhibited tumor angiogenesis.
Celastrol inhibits VEGF-induced chemotactic migration, capillary structure formation, and viability of HUVECs in vitro
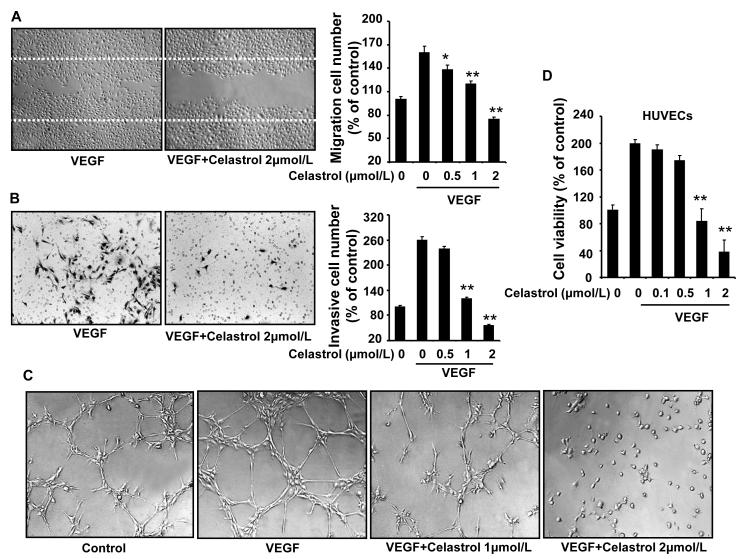
To assess the antiangiogenic property of Celastrol in vitro, we examined its inhibitory effects on the chemotactic motility of endothelial cells using the wound-healing migration assay and Transwell assay, respectively. We demonstrated that Celastrol at 1 μmol/L significantly inhibited VEGF-induced HUVEC migration in the wound-healing migration assay (Fig. 2A). Using the Transwell assays, we further showed that Celasrol dramatically reduced cell invasion (Fig. 2B). Both effects were dose-dependent, with significant inhibition first occurring at about 1 μmol/L of Celastrol.
Figure 2. Celastrol inhibits VEGF–induced chemotactic motility, capillary-structure formation, and cell viability of endothelial cells.
A, Celastrol inhibited HUVEC migration. HUVECs were scratched by pipette and treated with or without 10 ng/mL VEGF and Celastrol. The migrated cells were quantified by manual counting. B, Celastrol inhibited HUVEC invasion. Migrated cells through the membrane were quantified in the Transwell assays. C, Celastrol inhibited the VEGF-induced tube formation of endothelial cells in matrigel. After incubation, endothelial cells were fixed, and tubular structures were photographed (Magnification, ×100). D, Celastrol inhibited the VEGF-induced cell survival of HUVECs. MTS was used to quantify endothelial cell viability. Columns, mean from three different experiments; bars, standard deviation; *, P < 0.05; **, P < 0.01 vs. VEGF alone.
To further determine the effect of Celastrol on angiogenesis, we examined how Celastrol regulates capillary tubule formation of HUVECs. When HUVECs were seeded on growth factor–reduced two-dimensional Matrigel, robust tubular-like structures were formed in the presence of VEGF. However, treatment with 1 or 2 μmol/L Celastrol abolished the VEGF-induced tubule formation of HUVECs (Fig. 2C), suggesting that potential effect of Celastrol on angiogenesis. Angiogenesis requires the proliferation of the endothelial cells. We next examined whether Celastrol modulates VEGF-induced proliferation of endothelial cells by the MTS assay. As shown in figure 2D, 1 μmol/L Celastol significantly decreased the VEGF-induced proliferation of HUVEC (Fig. 2D). Together, these results indicated that Celastrol could block VEGF-induced angiogenesis in vitro by inhibiting cell motility, cell proliferation, and endothelial cell tubular structure formation.
Celastrol inhibits VEGF-induced microvessel sprouting ex vivo
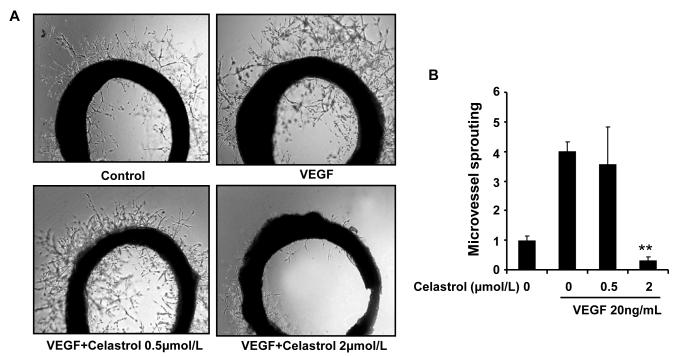
To study whether Celastrol affects VEGF-induced angiogenesis ex vivo, we examined the sprouting of vessels from aortic rings in the presence or absence of Celastrol. VEGF (20 ng/mL) significantly stimulated microvessel sprouting, leading to the formation of a network of vessels around the aortic rings (Fig. 3A). Addition of different concentrations of Celastrol antagonized the VEGF-induced sprouting in a dose-dependent manner, and 2 μmol/L Celastrol completely blocked microvessel sprouting (Fig. 3B).
Figure 3. Celastrol inhibits VEGF–induced microvessel sprouting ex vivo.
Aortic segments isolated from Sprague-Dawley rats were placed in the Matrigel-covered wells and treated with VEGF in the presence or absence of Celastrol. A, representative photographs of sprouts from the margins of aortic rings. B, sprouts were scored from 0 (least positive) to 5 (most positive) in a double-blinded manner. Columns, mean; bars, standard deviation; **, P < 0.01 vs. VEGF alone.
Celastrol inhibits VEGF-induced angiogenesis in vivo
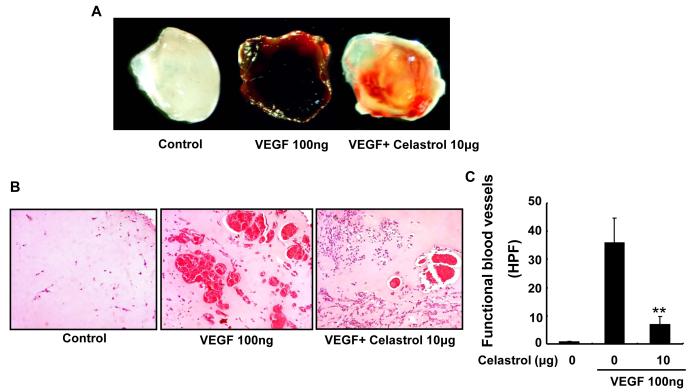
Matrigel plug assay has been used to evaluate the effects of Celastrol on VEGF-induced angiogenesis in vivo. As shown in figure 4A, Matrigel plugs containing VEGF alone appeared dark (Fig. 4A) and were filled with intact red blood cells, indicating that functional vasculatures had formed inside the Matrigel via angiogenesis triggered by VEGF. In contrast, addition of different concentrations of Celastrol dramatically inhibited vascular formation. As a result, the color of the Matrigel plugs of Celastrol-treated group became much lighter (Fig. 4A). Hematoxylin and eosin staining of the functional vasculature in Matrigel plugs (Fig. 4B) showed that Celastrol at a dose of 10 μg dramatically blocked VEGF-induced vasculature formation in vivo (Fig. 4C).
Figure 4. Celastrol inhibits VEGF–induced angiogenesis in vivo.
Six–week–old C57/BL/6 mice were injected with 0.5 mL of Matrigel containing 10 μg Celastrol, 100 ng of VEGF, and 20 units of heparin into the ventral area (n=5 per group). After 6 d, the skin of mice was pulled back to expose the intact Matrigel plugs. A, representative Matrigel plugs were photographed. B, Celastrol inhibited blood vessel formation. The Matrigel plugs were fixed, sectioned, and stained with hematoxylin and eosin (Magnification, ×200). C, infiltrating microvessels with intact red blood cells were quantified by manual counting. Columns, mean; bars, standard deviation; **, P < 0.01 vs. VEGF alone.
Celastrol inhibits the activation of AKT/mTOR/P70S6K pathway in endothelial cells
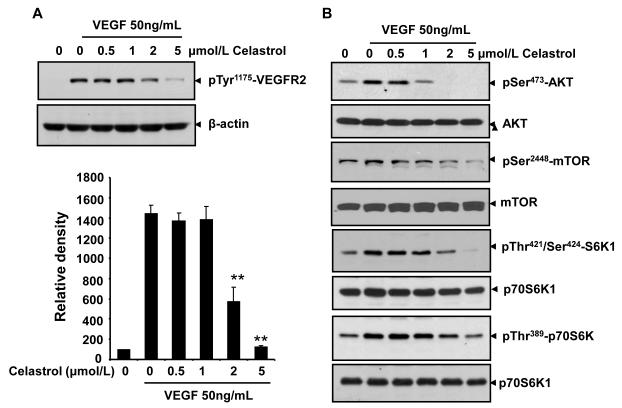
Interaction of VEGFR2 with VEGF led to the activation of various downstream signaling molecules responsible for endothelial cell migration, proliferation, and survival. To further delineate the mechanism that underlies the antiangiogenic effect of Celastrol, we examined the signaling molecules and pathways using Western blotting assays. As shown in figure 5A, the phosphorylation of VEGFR2 was suppressed by Celastrol in a dose-dependent manner (Fig. 5A). Thus the antiangiogenic property of Celastrol may be at least partially due to VEGR2 inhibition.
Figure 5. Celastrol inhibits the VEGF-triggered activation of VEGFR2 and mTOR pathway in endothelial cells.
A, Celastrol suppressed the activation of VEGFR2 triggered by VEGF in HUVECs. The activation of VEGFR2 from different treatments was tested by Western blotting and probed with anti-phospho-VEGFR2 antibody. The relative density of immunoreactivity was measured. Columns, mean from two independent experiments; bars, standard deviation, **, P < 0.01 vs. VEGF alone. B, Celastrol inhibited the activation of AKT/mTOR/S6K pathway in endothelial cells. Proteins from different treatments were probed with specific antibodies. Similar experiments were performed at least three times.
When examined for the key pathway components that regulate the endothelial cell function in angiogenesis, we found that Celastrol effectively suppressed VEGF-triggered activation of mTOR signaling cascade, including AKT, mTOR, and S6K kinase in HUVECs in a concentration-dependent manner (Fig. 5B), suggesting that Celastrol inhibit tumor angiogenesis through blocking the mTOR signaling pathway.
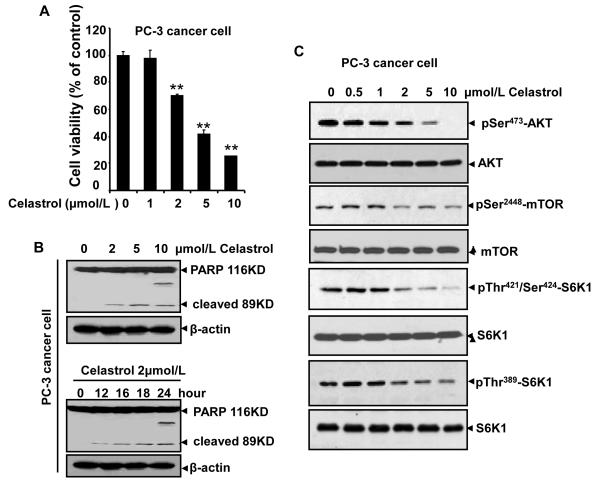
Celastrol induces cell apoptosis and inhibits AKT/mTOR/P70S6K pathway in PC-3 prostate cancer cells
As shown in our xenograft prostate cancer model, tumor growth was strongly suppressed by Celastrol administration, suggesting Celastrol also have direct cytotoxic effects on cancer cells besides its antiangiogenic effect on endothelial cells. To test this hypothesis, we examined tumor cell viability and the potential signaling pathways in prostate cancer cells. Our results demonstrate that Celastrol significantly decreased PC-3 cell viability (Fig. 6A) and induced tumor cell apoptosis by detecting full length PARP1 (116 kDa) and its large cleavage fragment (89 kDa) (Fig. 6B). Furthermore, Celastrol also inhibited the activation of AKT, mTOR, and P70S6K in a concentration-dependent manner in normal cultured PC-3 cancer cells (Fig. 6C), suggesting that the mTOR pathway is also a possible target of Celastrol in tumor cells.
Figure 6. Celastrol induces cell apoptosis and inhibits AKT/mTOR/P70S6K pathway in PC-3 cancer cells.
A. Celastrol inhibited cell viability of prostrate PC-3 cancer cells. Cell viability was quantified by MTS assay. Columns, mean from three different experiments; bars, standard deviation, **, P < 0.01 vs. VEGF alone. B. Celastrol induced PC-3 cancer cell apoptosis in concentration-dependent and time-dependent manners by the cleaved-PARP analysis. C, Celastrol suppressed the phosphorylation of mTOR signaling pathway kinases in PC-3 prostate cancer cell. To examine mTOR pathway in prostate tumor cells, normal cultured PC-3 cells (non-starving) were directly treated with indicated dilutions of Celastrol for 4 h. Proteins from different treatments were applied to Western blotting and probed with specific antibodies. Similar data were obtained from three independent experiments.
Discussion
In the present report, we have demonstrated that Celastrol, a component of traditional Chinese medicine, can inhibit the growth of human prostate cancer in a xenotransplant mouse model. One of the major mechanisms by which Celastrol mediates its effects against prostate cancer appears to be through suppression of angiogenesis. We found that Celastrol targeted AKT/mammalian target of rapamycin (mTOR)/P70S6 Kinase (S6K1) signaling pathway in a relatively specific manner both in endothelial cells and in prostate tumor cells. Celastrol effectively inhibited microvessel sprouting ex vivo and angiogenesis in vivo. Once angiogenesis was blocked by Celastrol, tumor growth was substantially suppressed, .
Previous studies have shown that Celastrol suppresses the growth of human tumors in nude mouse models. Yang’s group showed that Celastrol could inhibit the growth of human prostate cancer through suppression of proteasomal pathway (9). Zhang and colleague, however, showed the inhibition of human pancreatic cancer growth and metastasis by Celastrol through the suppression of heat shock protein Hsp90 (12). These studies differ from that of Huang’s group which showed the suppression of human gliomas by Celastrol through the downregulation of expression of mRNA and protein for VEGFR1 and VEGFR2 (14). The effect on VEGFR2 is in agreement with our studies, as shown in Fig.5A, that Celastrol inhibits VEGFR2 activation in endothelial cells. However, in the present study, we further found that the triterpenoid mediates antitumor effects through suppression of both angiogenesis and tumor growth. Additionally, our results suggested that VEGF receptor is not the principle target of Celastrol in suppression of tumor angogenesis and tumor growth, since PC-3 prostate cancer cells express little Flt-1 (VEGFR1) and KDR (VEGFR2), as reported previously (27).
Importantly, our study is the first to demonstrate the effect of Celastrol on AKT/mTOR/S6 kinase pathway. We showed Celastrol similarly and consistently reduces signaling from AKT pS473 in a relative specific manner in both prostate tumor cells and endothelial cells, which suggests the possibility that Celastrol is competing directly with TORC2 (the mTOR-rictor complex that phosphorylates Akt at Ser473). Other potential targets of Celastrol include HSP90-cdc (28), topoisomerase II (10), Kir 2.1 and hERG potassium channels (29), downregulation of expression of adhesion molecules (30), and suppression of TGF-b1 expression (31). A contribution of these targets to the antitumor effects of Celastrol can not be ruled out in our studies. Additionally, we have shown that Celastrol is a potent inhibitor of NF-κB activation (8). The latter is known to regulate the expression of antiapoptotic and angiogenic gene products. Thus, the suppression of AKT/mTOR/S6 kinase pathway may also contribute to the antitumor effects of the tripterine. Probably, all the above mechanisms might lead to synergic effect on angiogenesis in vitro and ex vivo.
Dysregulation of mTOR signaling frequently leads to tumorigenesis (16, 19, 32). mTOR inhibitors are currently being applied to malignant tumors in clinical trials (33), suggesting that agents targeting mTOR signaling are powerful and potential agents in cancer therapy. Recently, several studies have demonstrated the role of p70S6K1, a direct downstream target of the mTORC1 (mTOR, GβL, and raptor), in modulating cell migration (34, 35). Phosphorylation of p70S6K1 is sufficient to induce actin filament remodeling, form lamellipodia and filopodia structures, and decrease actin stress fibers (36). In accord with this evidence, our results in the VEGF-stimulation models in vitro demonstrated that Celastrol at lower concentration of 1 μmol/L could effectively inhibit endothelial cell migration, invasion and capillary-structure formation.
In conclusion, our results have shown that Celastrol can suppress tumor growth through inhibition of tumor angiogenesis in vitro and in vivo via targeting AKT/mTOR/S6K kinase signaling pathway. Our discovery of this novel mechanism of Celastrol in anticancer actions not only confirms its ethno-pharmacological value, but also contributes to modern drug developments.
Acknowledgments
This work is partially supported by the Science and Technology Commission of Shanghai Municipality, Research Platform for Cell Signaling Networks (06DZ22923), by Shanghai Pujiang Program (09PJ1403900), and by a grant from National Institute of Health (NCI) (1R01CA106479). We thank Walter Pagel from M. D. Anderson’s Department of Scientific Publications for editing. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from M. D. Anderson’s Center for Targeted Therapy.
Reference
- 1.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–74. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao X, Cush JJ, Garret M, Lipsky PE. A phase I study of ethyl acetate extract of the chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol. 2001;28:2160–7. [PubMed] [Google Scholar]
- 3.Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Ethnopharmacol. 2008;118:479–84. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Zhang YY, Huang XY, Sun YN, Jia YF, Li D. Beneficial effect of tripterine on systemic lupus erythematosus induced by active chromatin in BALB/c mice. Eur J Pharmacol. 2005;512:231–7. doi: 10.1016/j.ejphar.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–54. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 6.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–57. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Koo TH, Yoon H, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–21. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–35. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–65. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 10.Nagase M, Oto J, Sugiyama S, Yube K, Takaishi Y, Sakato N. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci Biotechnol Biochem. 2003;67:1883–7. doi: 10.1271/bbb.67.1883. [DOI] [PubMed] [Google Scholar]
- 11.Hieronymus H, Lamb J, Ross KN, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–30. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Hamza A, Cao X, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7:162–70. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 13.Trott A, West JD, Klaic L, et al. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–12. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Zhou Y, Fan Y, Zhou D. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008;264:101–6. doi: 10.1016/j.canlet.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8:647–65. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 16.Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev. 2009;35:148–59. doi: 10.1016/j.ctrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Lee DF, Kuo HP, Chen CT, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Liu LZ, Zheng JZ, Wang XR, Jiang BH. Endothelial p70 S6 kinase 1 in regulating tumor angiogenesis. Cancer Res. 2008;68:8183–8. doi: 10.1158/0008-5472.CAN-08-0819. [DOI] [PubMed] [Google Scholar]
- 19.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 20.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–8. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 23.Pang X, Yi T, Yi Z, et al. Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting rho GTPases and extracellular signal-regulated kinase signaling pathways. Cancer Res. 2009;69:518–25. doi: 10.1158/0008-5472.CAN-08-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi T, Yi Z, Cho SG, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–50. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang X, Yi Z, Zhang X, et al. Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. Cancer Res. 2009;69:5893–900. doi: 10.1158/0008-5472.CAN-09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyun BJ, Choi S, Lee Y, et al. Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res. 2008;68:227–35. doi: 10.1158/0008-5472.CAN-07-2799. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa Y, Dai J, Zhang J, et al. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res. 2005;65:10921–9. doi: 10.1158/0008-5472.CAN-05-1809. [DOI] [PubMed] [Google Scholar]
- 28.Westerheide SD, Bosman JD, Mbadugha BN, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–60. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 29.Sun H, Liu X, Xiong Q, Shikano S, Li M. Chronic inhibition of cardiac Kir2.1 and HERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J Biol Chem. 2006;281:5877–84. doi: 10.1074/jbc.M600072200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang DH, Marconi A, Xu LM, et al. Tripterine inhibits the expression of adhesion molecules in activated endothelial cells. J Leukoc Biol. 2006;80:309–19. doi: 10.1189/jlb.1005611. [DOI] [PubMed] [Google Scholar]
- 31.Xu LM, Zhang DH, Yang CX, Liu XH, Uzan G, Qin WZ. [Tripterine inhibits all-trans retinoic acid-caused adhesion between leukemia cells and endothelial cells] Zhong Xi Yi Jie He Xue Bao. 2007;5:282–6. doi: 10.3736/jcim20070311. [DOI] [PubMed] [Google Scholar]
- 32.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Campone M, Levy V, Bourbouloux E, et al. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer. 2009;100:315–21. doi: 10.1038/sj.bjc.6604851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Q, Yang M, Tsang BK, Gruslin A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004;10:677–84. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 35.Berven LA, Willard FS, Crouch MF. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp Cell Res. 2004;296:183–95. doi: 10.1016/j.yexcr.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Qian Y, Corum L, Meng Q, et al. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol. 2004;286:C153–63. doi: 10.1152/ajpcell.00142.2003. [DOI] [PubMed] [Google Scholar]