Abstract
The peptide substance P (SP) has been implicated in inflammatory conditions, such as psoriasis, where mast cells and VEGF are increased. A relationship between SP and VEGF has not been well studied, nor has any interaction with the proinflammatory cytokines, especially IL-33. Here we report that SP (0.1–10 μM) induces gene expression and secretion of VEGF from human LAD2 mast cells and human umbilical core blood-derived cultured mast cells (hCBMCs). This effect is significantly increased by coadministration of IL-33 (5–100 ng/mL) in both cell types. The effect of SP on VEGF release is inhibited by treatment with the NK-1 receptor antagonist 733,060. SP rapidly increases cytosolic calcium, and so does IL-33 to a smaller extent; the addition of IL-33 augments the calcium increase. SP-induced VEGF production involves calcium-dependent PKC isoforms, as well as the ERK and JNK MAPKs. Gene expression of IL-33 and histidine decarboxylase (HDC), an indicator of mast cell presence/activation, is significantly increased in affected and unaffected (at least 15 cm away from the lesion) psoriatic skin, as compared with normal control skin. Immunohistochemistry indicates that IL-33 is associated with endothelial cells in both the unaffected and affected sites, but is stronger and also associated with immune cells in the affected site. These results imply that functional interactions among SP, IL-33, and mast cells leading to VEGF release contribute to inflammatory conditions, such as the psoriasis, a nonallergic hyperproliferative skin inflammatory disorder with a neurogenic component.
Keywords: inflammation, cytokines, IL-1, innate immunity, stress
Substance P (SP) is an 11–amino acid peptide that mediates inflammation (1, 2), partially through mast cell activation (3, 4). Neuropeptides (5), especially SP, could be involved in the pathogenesis of inflammatory skin disorders, such as psoriasis (6, 7), characterized by increased epidermal vascularization, keratinocyte hyperproliferation, and inflammation (8). SP-positive nerve fibers are more dense in psoriatic lesions and have an increased number of mast cell contacts compared with normal skin (9–12). Mast cells are also increased in lesional psoriatic skin (13) and there appears to be an association among sensory nerves, mast cell numbers, and stress (13, 14). SP-positive nerve fibers and mast cell contacts are also increased by acute stress in mice, leading to dermal mast cell degranulation (3, 15, 16). It also is interesting that psoriasis is worsened by acute stress (15, 17).
Psoriatic plaques contain increased levels of VEGF compared with normal skin (18–20). VEGF is a major proangiogenic factor involved in many inflammatory diseases (21). The VEGF 121 isoform is particularly increased in psoriatic plaques (22) and VEGF is also increased systematically in severe psoriasis (22, 23). Genetic studies have shown that several different VEGF polymorphisms are associated with an increased risk of developing psoriasis (24, 25). Mast cells can secrete VEGF in response to IgE (26, 27), and to corticotropin-releasing hormone (CRH) (28), secreted under stress. Epidermal overexpression of VEGF in transgenic mice leads to a phenotype nearly identical to that of psoriasis (29).
Given that psoriasis involves skin inflammation and is often present with arthritis (psoriatic arthritis) (30), we were intrigued by the finding that IL-33 exacerbates antigen-induced arthritis in mice by activating mast cells (31). IL-33 is one of the newest members of the IL-1 family of inflammatory cytokines (32), and was recently shown to mediate IgE-induced anaphylaxis in mice (33). IL-33 also induces release of IL-6 from mouse bone marrow–derived cultured mast cells (BMCMCs) (34), and IL-8 from human umbilical cord blood–derived cultured mast cells (hCBMCs) (35).
Mast cells are found in large numbers around blood vessels in the skin, where they participate in allergic and inflammatory reactions through release of multiple mediators with potent vasodilatory, inflammatory, and nociceptive properties (36, 37). For example, CRH increases vascular permeability through release of histamine (38), which also stimulates cutaneous sensory nerves (39), contributing to pruritus. Skin mast cells may have important functions as “sensors” of environmental and emotional stress (40).
In the present study, we show that SP stimulates human mast cells to secrete VEGF and that this action is augmented by IL-33. Furthermore, we show that IL-33 mRNA expression is increased along with histidine decarboxylase (HDC), an indicator of mast cell presence/activation, in psoriatic skin.
Results
SP Stimulates VEGF mRNA Expression and Protein Production in Human Mast Cells.
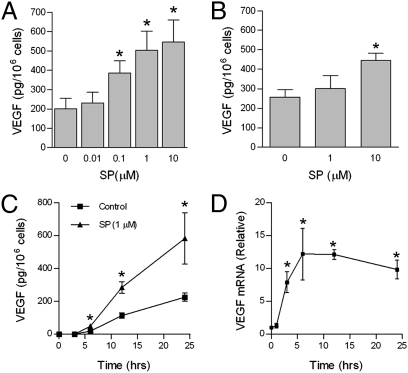
To examine the effect of SP on VEGF secretion, LAD2 cells were treated with SP (0.01–10 μM) for 24 h. Stimulation with SP caused a concentration-dependent production and release of VEGF with a maximum of 546 pg/106 cells at 10 μM, a 2.7-fold induction (Fig. 1A). To investigate whether this effect was limited to the leukemic human LAD2 mast cells, we repeated the experiments using hCBMCs (Fig. 1B). SP stimulation caused a maximum release from hCBMCs of 445 pg/106 cells at 10 μM. VEGF release in response to 1 μM SP was time dependent over 24 h (Fig. 1C). This slow VEGF secretion suggests de novo synthesis. We next examined whether the VEGF mRNA transcript is induced by SP using quantitative PCR. VEGF mRNA was increased after SP (1 μM) stimulation with a maximum ∼12-fold increase occurring at 6 h (Fig. 1D). Elevation of VEGF mRNA was sustained for at least 24 h. Together, these data show that SP induces both VEGF mRNA and protein synthesis in human mast cells.
Fig. 1.
SP stimulates VEGF production in human mast cells. LAD2 cells (A) and hCBMCs (B) were stimulated with the indicated concentration of SP (0–10 μM) for 24 h, and supernatant VEGF was measured by ELISA. (C) Time-dependent secretion of VEGF. Cells were stimulated with SP (1 μM) or vehicle for the indicated times and supernatant VEGF was measured by ELISA. (D) SP induces VEGF mRNA. LAD2 cells were stimulated with SP (1 μM) for the indicated times, RNA was extracted, and relative VEGF mRNA levels were determined by real-time PCR. Data are mean ± SD of three separate experiments performed in triplicate (*P < 0.05 vs. unstimulated cells).
IL-33 Augments Effect of SP on VEGF Release from Human Mast Cells.
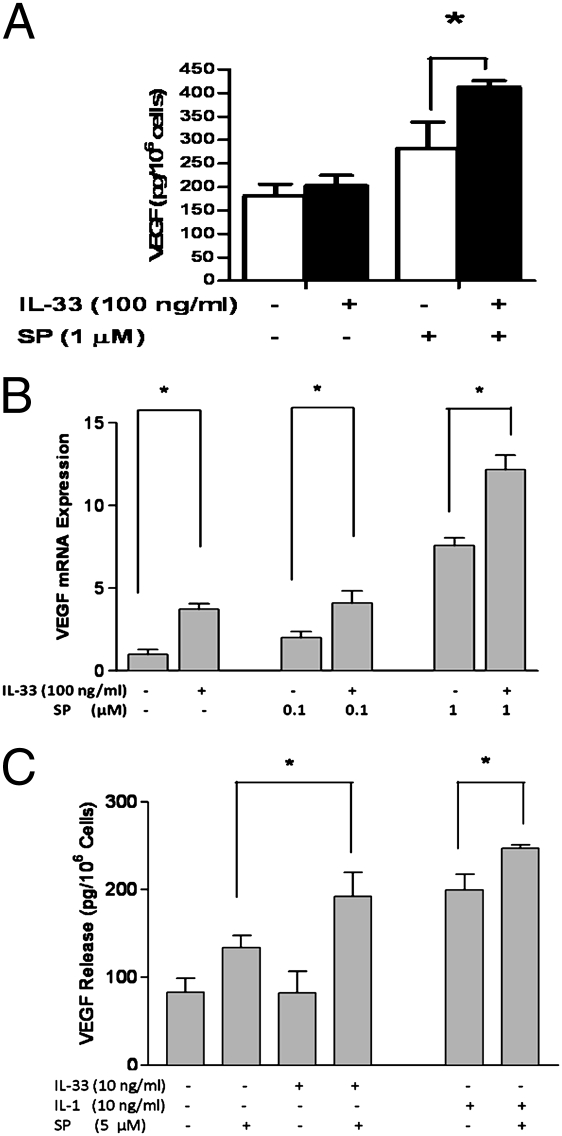
We next examined whether IL-33 could induce VEGF release from LAD2 mast cells. IL-33 alone (5–100 ng/mL) did not induce VEGF release (Fig. 2A). However, the addition of IL-33 (100 ng/mL) to SP (1 μM) augmented VEGF production by 1.5-fold as compared with that induced by SP alone (Fig. 2A). IL-33 alone (5–100 ng/mL) over 6 h increased VEGF gene expression up to 4.5-fold (Fig. 2B). The addition of IL-33 (100 ng/mL) to SP (0.1 or 1 μM) further increased VEGF mRNA expression (Fig. 2B). To ensure that these findings were not limited to the use of the LAD2 leukemic human mast cells, we repeated the experiments unsing hCBMCs, except that these cells require a higher amount of SP for stimulation. IL-33 (100 ng/mL) again did not induce any VEGF release on its own, but augmented (Fig. 2C) VEGF release induced by SP (5 μM).
Fig. 2.
IL-33 augments SP in inducing (A) VEGF protein secretion from LAD2 cells, and (B) VEGF mRNA expression from LAD2 cells, or (C) VEGF protein secretion from hCBMCs. Cells were treated for 6 h with IL-33 (100 ng/mL) or IL-1 (10 ng/mL) alone or together with SP as shown (n = 3). *P < 0.05.
Given that IL-33 belongs to the IL-1 cytokine family, we also tested whether IL-1 could induce VEGF release and whether it could augment the release due to SP. Although IL-33 (100 ng/mL) had no effect, IL-1 (10 ng/mL) induced significant VEGF release (Fig. 2C); the addition of IL-1 (10 ng/mL) to SP (5 μM) augmented the effect of SP (Fig. 2C, P < 0.05).
NK-1 Receptor Antagonist Inhibits SP-Induced VEGF Release.
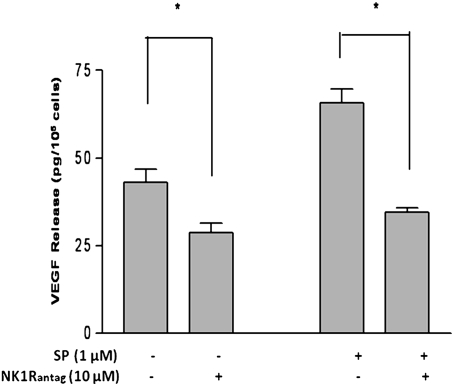
To determine whether SP-induced VEGF release is mediated through the NK-1 receptor, we preincubated LAD2 cells with the NK-1 receptor antagonist L-733,060 (10 μM) for 30 min and then during stimulation with the SP (1 μM). Treatment of LAD2 cells with L-733,060 (10 μM) completely blocked SP-induced VEGF release (Fig. 3). In fact, this antagonist also significantly reduced basal VEGF secretion (Fig. 3).
Fig. 3.
NK-1 receptor antagonist inhibits SP-induced VEGF release from LAD2 cells. LAD2 cells were pretreated with NK-1 receptor antagonist (NK1RAntag) L-733,060 (10 μM) for 30 min and were then retained throughout stimulation with SP (1 μM) for 24 h. VEGF was measured in the supernatant fluid by ELISA (n = 3). *P < 0.05.
IL-33 Augments Cytosolic Calcium Ion Levels Increased by SP.
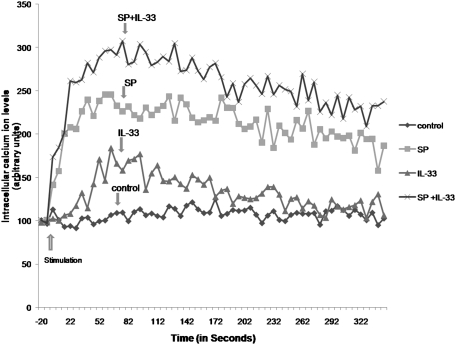
To examine the possible mechanism of action through which IL-33 enhances the ability of SP to increase VEGF release, we measured their effects on intracellular calcium ion levels. SP (1 μM) significantly increased cytosolic calcium, whereas IL-33 (100 ng/mL) produced a similar but smaller increase (Fig. 4). The addition of IL-33 to SP augmented the cytosolic calcium increase due to SP (Fig. 4).
Fig. 4.
Effect of SP and IL-33 on LAD2 cytosolic calcium levels. Cytosolic calcium was measured in LAD2 cells using Fura-2 AM (1 mM; Invitrogen). Cells were stimulated with IL-33 (100 ng/mL) or SP (1 μM) or both for the time indicated. Results were processed according to the Invitrogen Fura-2 protocol. Stimulation was carried out in the presence of extracellular calcium (1 mM). Results of one experiment, representative of three equivalent experiments, are shown.
PKC Isoforms Are Involved in SP-Induced VEGF Release.
We investigated whether PKC plays a role in VEGF release from LAD2 cells using two PKC inhibitors: bisindolylmaleimide I, a nonselective inhibitor of PKC, and Gö6976, an inhibitor selective for the calcium-dependent PKCα and βI isoforms. Both bisindolylmaleimide I (Fig. S1A) and Gö6976 (Fig. S1B) decreased (∼30%) SP-induced VEGF secretion at 1 μM without affecting basal release. These data show that the calcium/PKC pathway is important but not mandatory for maximum induction of VEGF by SP.
ERK and JNK Pathways Are Involved in SP-Induced VEGF Release.
We then examined MAPK activation by performing Western blots of the active form of the MAPKs using phospho-specific antibodies. Upon stimulation with SP (1 μM), increased ERK phosphorylation was detected within 5 min, and sustained levels of phospho-ERK lasted for at least 120 min (Fig. S2A). Increased phospho-JNK was detected, but with kinetics different from those of phospho-ERK (Fig. S2B); JNK phosphorylation was more delayed, with peak activation at 45 min. SP did not cause any discernible changes in the level of p38 phosphorylation (Fig. S1C).
We also used inhibitors of the ERK, JNK, and p38 pathways to investigate their role in SP-induced VEGF release from LAD2 cells. MEK is the upstream kinase responsible for ERK phosphorylation. The MEK inhibitor PD98059 (1–50 μM) attenuated SP-induced VEGF release in a concentration-dependent fashion (Fig. S3A). PD98059 (50 μM) also significantly inhibited basal VEGF release, implicating the ERK pathway in both basal and SP-stimulated VEGF induction. SP-induced VEGF release was also inhibited in a similar manner by the JNK inhibitor SP600125 (Fig. S3B). Together, these data demonstrate that SP induces phosphorylation of both ERK and JNK in mast cells and that activation of both is necessary for maximum VEGF production.
IL-33 and HDC mRNA Expression Is Increased in Psoriatic Skin.
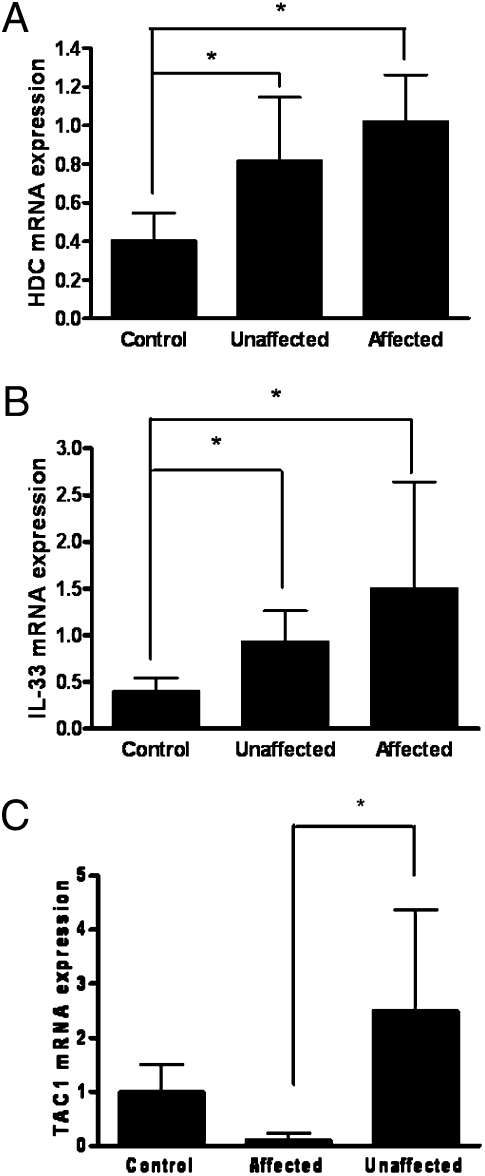
IL-33 and HDC, an indicator of mast cell activation, mRNA expression was studied in psoriatic affected skin and unaffected skin obtained from at least 15 cm away from the lesion, as well as normal skin from healthy controls.
Analysis of skin biopsy samples from psoriatic lesional, unaffected, and control normal skin revealed that IL-33 gene expression is significantly higher in lesional psoriatic skin than in normal control skin (Fig. 5A). Expression of HDC mRNA, investigated as an index of mast cell presence/activation, was also significantly increased in lesional psoriatic skin (Fig. 5B). Interestingly, both IL-33 and HDC gene expression were also significantly increased in unaffected psoriatic skin as compared with control skin (Fig. 5). These results suggest that IL-33 and HDC gene expression is not associated with lymphocyte infiltration or keratinocyte proliferation in the psoriatic plaque. SP (TAC1) mRNA expression was increased in the unaffected skin but was lower in the affected skin (Fig. 5C).
Fig. 5.
Increased gene expression in psoriatic affected (lesional) skin, psoriatic unaffected (at least 15 cm away from the lesion) skin, and normal skin from healthy controls. (A) IL-33 (control n = 7; unaffected, n = 6; affected n = 9); (B) HDC (control n = 5; unaffected n = 6; affected n = 5); and (C) TACI. Relative quantities of mRNA expression were measured by quantitative RT-PCR and normalized to 18S. TaqMan was performed with cDNA reverse transcribed from 100 ng RNA from each sample. The number of samples was less for HDC because the amount of cDNA from some samples had been exhausted (*P < 0.05 vs. control).
IL-33 Immunohistochemistry.
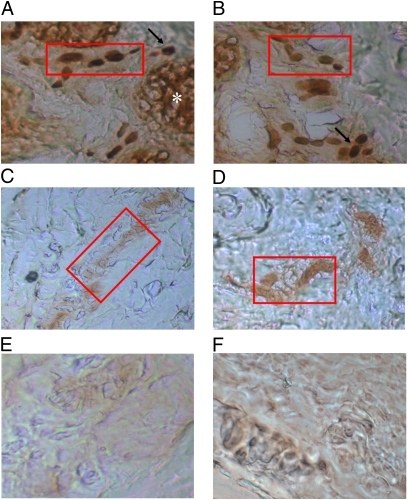
Investigation of IL-33 protein expression by immunohistochemistry showed that IL-33 was strongly associated with blood vessels, infiltrating inflammatory cells, and sweat glands in the affected psoriatic skin areas (Fig. 6A). On the contrary, IL-33 was weakly associated with blood vessels and sweat glands in unaffected psoriatic areas (Fig. 6B). There was no apparent association with mast cells.
Fig. 6.
Photomicrographs of skin biopsy samples from patients with psoriasis (A and B) lesional, affected skin; (C and D) unaffected skin; and (E and F) control without primary antibody. Immunohistochemical staining was performed using the LSAB+ system kit (DAKO). Incubation with the primary antibody (mouse monoclonal anti–human-IL-33 antibody, at 1:100 dilution; Abcam) was performed for 30 min; secondary antibody was provided in the DAKO kit and was also used for 30 min, followed by appropriate washes. Magnification, ×200; Red rectangle indicates blood vessel; asterisk indicates sweat gland; solid arrow indicates inflammatory cells.
Discussion
We report here that IL-33 augments the SP-induced VEGF mRNA expression and VEGF protein secretion both from leukemic and normal human mast cells. IL-33 cannot induce VEGF secretion on its own. IL-33 is the newest inflammatory member of the IL-1 cytokine family (32), and we show here that IL-1 can also induce VEGF secretion from mast cells as well as augment the effect of SP. IL-1 had previously been shown to induce VEGF secretion from inflammatory cells (41).
Here we also show that gene expression of IL-33 is increased in both affected and unaffected psoriatic skin. Gene expression of HDC, indicating increased mast cell presence/activation, is also increased in both affected and unaffected psoriatic skin, as reported previously (42). Moreover, IL-33 in unaffected skin is weakly associated with blood vessels, whereas it is localized strongly with blood vessels and infiltrating inflammatory cells in the lesional affected skin. IL-33 had previously been reported to be expressed by endothelial cells (43). These results indicate that the inflammatory process may be initiated in “unaffected” skin areas where IL-33 is initially secreted by endothelial cells and augments other nonallergic triggers, such as SP, to stimulate the mast cells. In this context, any participation of IgE is not relevant, because psoriasis is not an allergic condition, unlike atopic dermatitis, which involves allergic inflammation, and where IL-33 expression was recently reported to be increased in lesional areas (33).
The receptor for IL-33 is mostly expressed on mast cells and Th2 cells, for which it acts as a chemoattractant and trigger (44). It was recently shown that IL-33-mediated mouse anaphylaxis occurred only in the presence of IgE (33). In contrast, IL-33 induced release of proinflammatory cytokines from murine mast cells (45), especially IL-6 without degranulation from BMCMCs (34). It also enhanced IL-8 production from hCBMCs by IgE/anti-IgE stimulation, but without histamine release (35). IL-33 was also shown to augment the effect of IgE and stem cell factor (SCF) on activating mast cells and basophils (44).
The nonpeptide NK-1 receptor antagonist L-733,060 (46) blocked VEGF secretion from LAD2 cells by 100%, and also reduced basal VEGF release implying some autocrine activation. LAD2 mast cells (47) and skin mast cells (46) had previously been reported to express NK-1 receptors. The NK-1 receptor is also expressed on rat basophilic leukemia cells (47), activation of which by neurites occurred via SP (48). In contrast, murine bone marrow–derived mast cells did not release histamine in response to SP, but they did produce prostaglandin D2 and leukotrience C4 (49). Degranulation, as compared with de novo synthesis of selected mediators, may involve direct activation of G proteins (50, 51), as shown for SP (52) and the bee venom peptide mastoparan (53, 54). NK-1 receptor–independent activation of mast cells may involve activation of the MrgX2 receptor (55).
SP induces rapid cytosolic calcium increase in LAD2 cells; the addition of IL-33 further increases these levels, but to a lesser extent than what was recently reported for IL-33 addition to IgE-sensitized murine mast cells (33). Nevertheless, this augmentation of cytosolic calcium ion levels may be sufficient to lead to synergistic VEGF release. IL-33 may also induce downstream signaling steps, such as p38 activation, which was not apparent in our studies. For instance, IL-1 (from the same cytokine family as IL-33) increased p38 activation and VEGF release from human vascular smooth muscle cells (56). Moreover, SP induced p38 phosphorylation independent of ERK and JNK associated with IL-6 release from human dental pulp fibroblasts (57).
SP-induced calcium increase in human skin mast cells subsequently activates calcium PKC isoforms (58). In this report, PKC is involved, but is not mandatory for VEGF induction. We also show that SP stimulates phosphorylation of both ERK and JNK MAP kinases, which can be activated by PKC-dependent and PKC-independent mechanisms (59, 60). Activation of these MAP kinases leads to activation of the AP-1 transcription factor, a heterodimer of c-Fos and c-Jun (61–63). The VEGF promoter has several AP-1 binding sites that increase transcription (64), a possible explanation for the increased VEGF mRNA abundance in SP-stimulated cells. Induction of VEGF by hyperbaric oxygen in human umbilical vein endothelial cells also depended on AP-1 activation by ERK and JNK (65).
Mast cells are often located close to SP-positive neuronal processes (66–69). Mast cell–neuronal interactions might be involved in the pathophysiology of psoriasis and might participate in the exacerbation of symptoms by stress (7, 13, 70). The fact that SP mRNA is increased in the unaffected skin but not in the affected areas suggests that SP is synthesized in unaffected areas and secreted from nerve terminals in the affected site (Fig. S4). Increased HDC mRNA expression in the unaffected area indicates increased mast cell presence. These mast cells in the unaffected skin may be activated by IL-33 released from endothelial and epithelial cells (43), acting together with IgE (44). Activated mast cells would then release histamine or interleukins that could activate neurons to synthesize more SP (Fig. S4). In the affected psoriatic skin, other possible sources of IL-33 may include infiltrating lymphocytes, proliferating keratinocytes, as well as endothelial cells from new vessels (Fig. S4). IL-33 would augment the effect of SP on mast cells to release VEGF, thus increasing vascular permeability and contributing to inflammation.
SP-positive nerve fibers were shown to be denser in psoriatic skin (9–11) and to have increased numbers of mast cell contacts compared with normal skin (12, 67). Use of biotinylated SP suggested that NK-1 expression may be increased in keratinocytes from psoriatic plaques (71). Another study showed mast cells express the NK-1 receptor in both affected and unaffected psoriatic skin (6). NK-1 is also important in stress-induced murine skin mast cell activation (3, 16), and in the development of atopic dermatitis in mice (68). In fact, stress increases SP-positive nerve fibers and mast cell contacts in mice (69), whereas an NK-1 receptor antagonist inhibits stress-induced mast cell degranulation in mice (72). SP also induces mast cell-dependent leukocyte infiltration, thus amplifying the initial inflammatory response (73). SP may contribute to the pruritus associated with psoriasis (74). However, the effect of SP is apparently localized to the skin, as plasma SP levels did not differ between psoriasis patients and controls (75).
The ability of IL-33 to augment the effect of SP on inducing mast cell release of VEGF is certainly relevant, as angiogenesis (21) is at the core of psoriasis pathogenesis (18). VEGF levels are increased in psoriatic plaques compared with normal skin (19, 20), especially the VEGF 121 isoform, which causes vascular permeability (22, 23). Moreover, the higher VEGF expression correlates with the clinical severity of psoriasis (76, 77). Genetic studies have shown that several different VEGF polymorphisms are associated with an increased risk of developing psoriasis (24, 25). Moreover, transgenic delivery of VEGF in mouse skin can lead to an inflammatory state resembling psoriasis (29).
The present results indicate that interactions among SP, IL-33, and mast cells may be important in inflammatory diseases where there is excessive angiogenesis, such as psoriasis. SP, IL-33 and mast cells may also represent novel therapeutic targets.
Materials and Methods
Culture of Human Mast Cells.
LAD2 cells (kindly supplied by Dr. A.S. Kirshenbaum, National Institutes of Health) derived from a human mast cell leukemia (78) were cultured in StemPro-34 medium (Invitrogen) supplemented with 100 U/mL penicillin/streptomycin and 100 ng/mL recombinant human stem cell factor (rhSCF; kindly supplied by Amgen). Human umbilical cord blood was collected at Tufts Medical Center. Hematopoietic stem cells (CD34+) were isolated by positive selection of CD34+/AC133+ cells by magnetic cell sorting using an AC133+ cell isolation kit (Milltenyi Biotec) as previously reported (79).
Cytosolic Calcium Measurements.
Detailed methods are provided in SI Materials and Methods.
Patients and Biopsies.
Methods are described in SI Materials and Methods.
IL-33 Immunohistochemistry.
Methods are detailed in SI Materials and Methods.
Statistical Analysis.
Data are expressed as the mean ± SD. Statistical significance between experimental samples and controls was calculated using the Student’s t test. P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Amgen, Inc. (Thousand Oaks, CA) for the kind gift of rhSCF and Drs. Dean Metcalfe and A.S. Kirshenbaum, National Insitutes of Health (NIH) for the LAD2 mast cells. We also thank Jessica Christian for word processing skills. This work was supported in part by NIH Grant R01 AR47652 (to T.C.T.). Both K.-D.A. and A.A. are recipients of postgraduate scholarships from the Hellenic State Scholarships Foundation (Athens, Greece).
Footnotes
The authors declare no conflict of interest.
2B.Z., D.K., and M.T. contributed equally to this work.
3Present address: Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, TN 38105.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000803107/DCSupplemental.
References
- 1.Leeman SE, Ferguson SL. Substance P: An historical perspective. Neuropeptides. 2000;34:249–254. doi: 10.1054/npep.2000.0826. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor TM, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 3.Kawana S, Liang Z, Nagano M, Suzuki H. Role of substance P in stress-derived degranulation of dermal mast cells in mice. J Dermatol Sci. 2006;42:47–54. doi: 10.1016/j.jdermsci.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kandere-Grzybowska K, et al. Stress-induced dura vascular permeability does not develop in mast cell-deficient and neurokinin-1 receptor knockout mice. Brain Res. 2003;980:213–220. doi: 10.1016/s0006-8993(03)02975-5. [DOI] [PubMed] [Google Scholar]
- 5.Saraceno R, Kleyn CE, Terenghi G, Griffiths CE. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876–882. doi: 10.1111/j.1365-2133.2006.07518.x. [DOI] [PubMed] [Google Scholar]
- 6.Remröd C, Lonne-Rahm S, Nordlind K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299:85–91. doi: 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- 7.Farber EM, Nickoloff BJ, Recht B, Fraki JE. Stress, symmetry, and psoriasis: Possible role of neuropeptides. J Am Acad Dermatol. 1986;14:305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- 8.Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 9.Jiang WY, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37:572–574. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan J, Smoller BR, Raychauduri SP, Jiang WY, Farber EM. Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Arch Dermatol Res. 1997;289:611–616. doi: 10.1007/s004030050249. [DOI] [PubMed] [Google Scholar]
- 11.Al’Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384–389. doi: 10.1111/j.1365-2230.1995.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 12.Naukkarinen A, et al. Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol. 1996;180:200–205. doi: 10.1002/(SICI)1096-9896(199610)180:2<200::AID-PATH632>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Özdamar SO, Seçkin D, Kandemir B, Turanli AY. Mast cells in psoriasis. Dermatology. 1996;192:190. doi: 10.1159/000246359. [DOI] [PubMed] [Google Scholar]
- 14.Harvima IT, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168–176. doi: 10.1159/000288690. [DOI] [PubMed] [Google Scholar]
- 15.Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: Consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Paus R, Heinzelmann T, Robicsek S, Czarnetzki BM, Maurer M. Substance P stimulates murine epidermal keratinocyte proliferation and dermal mast cell degranulation in situ. Arch Dermatol Res. 1995;287:500–502. doi: 10.1007/BF00373436. [DOI] [PubMed] [Google Scholar]
- 17.Harvima RJ, et al. Association of psychic stress with clinical severity and symptoms of psoriatic patients. Acta Derm Venereol. 1996;76:467–471. doi: 10.2340/0001555576467471. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232–248. doi: 10.1111/j.1365-2613.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yalçin B, Tezel GG, Arda N, Erman M, Alli N. Vascular endothelial growth factor, vascular endothelial growth factor receptor-3 and cyclooxygenase-2 expression in psoriasis. Anal Quant Cytol Histol. 2007;29:358–364. [PubMed] [Google Scholar]
- 20.Simonetti O, et al. VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis: Results of an immunohistochemical study. Int J Immunopathol Pharmacol. 2006;19:751–760. doi: 10.1177/039463200601900405. [DOI] [PubMed] [Google Scholar]
- 21.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creamer D, et al. Mediation of systemic vascular hyperpermeability in severe psoriasis by circulating vascular endothelial growth factor. Arch Dermatol. 2002;138:791–796. doi: 10.1001/archderm.138.6.791. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Matsuo H, Morita E. Vascular endothelial growth factor 121 is the predominant isoform in psoriatic scales. Exp Dermatol. 2005;14:758–764. doi: 10.1111/j.1600-0625.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 24.Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol. 2004;122:209–215. doi: 10.1046/j.0022-202X.2003.22140.x. [DOI] [PubMed] [Google Scholar]
- 25.Barile S, et al. Vascular endothelial growth factor gene polymorphisms increase the risk to develop psoriasis. Exp Dermatol. 2006;15:368–376. doi: 10.1111/j.0906-6705.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 26.Boesiger J, et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc ε receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grützkau A, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 29.Xia YP, et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 30.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 31.Xu D, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellani ML, et al. The latest interleukin: IL-33 the novel IL-1-family member is a potent mast cell activator. J Biol Regul Homeost Agents. 2009;23:11–14. [PubMed] [Google Scholar]
- 33.Pushparaj PN, Tay HK, H’ng SC, Pitman N, Xu D, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Ho LH, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 35.Iikura M, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 36.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 37.Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am. 2006;26:465–485. doi: 10.1016/j.iac.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Theoharides TC, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 39.Christian EP, Undem BJ, Weinreich D. Endogenous histamine excites neurones in the guinea-pig superior cervical ganglion in vitro. J Physiol. 1989;409:297–312. doi: 10.1113/jphysiol.1989.sp017498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1473. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 42.Michaëlsson G, et al. The skin and the gut in psoriasis: The number of mast cells and CD3+ lymphocytes is increased in non-involved skin and correlated to the number of intraepithelial lymphocytes and mast cells in the duodenum. Acta Derm Venereol. 1997;77:343–346. doi: 10.2340/0001555577343346. [DOI] [PubMed] [Google Scholar]
- 43.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver MR, et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm Res. 2009 doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 45.Moulin D, et al. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Seabrook GR, et al. L-733,060, a novel tachykinin NK1 receptor antagonist; effects in [Ca2+]i mobilisation, cardiovascular and dural extravasation assays. Eur J Pharmacol. 1996;317:129–135. doi: 10.1016/s0014-2999(96)00706-6. [DOI] [PubMed] [Google Scholar]
- 47.Cooke HJ, Fox P, Alferes L, Fox CC, Wolfe SA., Jr Presence of NK1 receptors on a mucosal-like mast cell line, RBL-2H3 cells. Can J Physiol Pharmacol. 1998;76:188–193. [PubMed] [Google Scholar]
- 48.Suzuki R, et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 1999;163:2410–2415. [PubMed] [Google Scholar]
- 49.Karimi K, Kool M, Nijkamp FP, Redegeld FA. Substance P can stimulate prostaglandin D2 and leukotriene C4 generation without granule exocytosis in murine mast cells. Eur J Pharmacol. 2004;489:49–54. doi: 10.1016/j.ejphar.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Mousli M, Bueb J-L, Bronner C, Rouot B, Landry Y. G protein activation: A receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990;11:358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- 51.Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507–1515. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 52.Mousli M, Bronner C, Landry Y, Bockaert J, Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990;259:260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- 53.Mousli M, Bronner C, Bueb JL, Tschirhart E, Gies JP, Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J Pharmacol Exp Ther. 1989;250(1):329–335. [PubMed] [Google Scholar]
- 54.Higashijima T, Uzu S, Nakajima T, Ross EM. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins) J Biol Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- 55.Tatemoto K, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 56.Jung YD, et al. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 57.Tokuda M, Miyamoto R, Sakuta T, Nagaoka S, Torii M. Substance P activates p38 mitogen-activated protein kinase to promote IL-6 induction in human dental pulp fibroblasts. Connect Tissue Res. 2005;46:153–158. doi: 10.1080/03008200500182490. [DOI] [PubMed] [Google Scholar]
- 58.Columbo M, Botana LM, Horowitz EM, Lichtenstein LM, MacGlashan DW., Jr Studies of the intracellular Ca2+ levels in human adult skin mast cells activated by the ligand for the human c-kit receptor and anti-IgE. Biochem Pharmacol. 1994;47:2137–2145. doi: 10.1016/0006-2952(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Hirasawa N, Beaven MA. Antigen activation of mitogen-activated protein kinase in mast cells through protein kinase C-dependent and independent pathways. J Immunol. 1997;158:4968–4975. [PubMed] [Google Scholar]
- 60.Kawakami Y, Hartman SE, Holland PM, Cooper JA, Kawakami T. Multiple signaling pathways for the activation of JNK in mast cells: Involvement of Bruton’s tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J Immunol. 1998;161:1795–1802. [PubMed] [Google Scholar]
- 61.Dérijard B, et al. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 62.Kyriakis JM, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 63.Frost JA, Geppert TD, Cobb MH, Feramisco JR. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tischer E, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 65.Lee CC, et al. Hyperbaric oxygen induces VEGF expression through ERK, JNK and c-Jun/AP-1 activation in human umbilical vein endothelial cells. J Biomed Sci. 2006;13:143–156. doi: 10.1007/s11373-005-9037-7. [DOI] [PubMed] [Google Scholar]
- 66.Wiesner-Menzel L, Schulz B, Vakilzadeh F, Czarnetzki BM. Electron microscopical evidence for a direct contact between nerve fibres and mast cells. Acta Derm Venereol. 1981;61:465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]
- 67.Naukkarinen A, Harvima I, Paukkonen K, Aalto M-L, Horsmanheimo M. Immunohistochemical analysis of sensory nerves and neuropeptides, and their contacts with mast cells in developing and mature psoriatic lesions. Arch Dermatol Res. 1993;285:341–346. doi: 10.1007/BF00371834. [DOI] [PubMed] [Google Scholar]
- 68.Pavlovic S, et al. Further exploring the brain-skin connection: Stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 69.Peters EM, et al. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Al’Abadie MS, Kent GG, Gawkrodger DJ. The relationship between stress and the onset and exacerbation of psoriasis and other skin conditions. Br J Dermatol. 1994;130:199–203. doi: 10.1111/j.1365-2133.1994.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 71.Staniek V, Doutremepuich J, Schmitt D, Claudy A, Misery L. Expression of substance P receptors in normal and psoriatic skin. Pathobiology. 1999;67:51–54. doi: 10.1159/000028051. [DOI] [PubMed] [Google Scholar]
- 72.Erin N, Ersoy Y, Ercan F, Akici A, Oktay S. NK-1 antagonist CP99994 inhibits stress-induced mast cell degranulation in rats. Clin Exp Dermatol. 2004;29:644–648. doi: 10.1111/j.1365-2230.2004.01613.x. [DOI] [PubMed] [Google Scholar]
- 73.Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- 74.Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: Comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718–730. doi: 10.1046/j.1365-2133.2003.05586.x. [DOI] [PubMed] [Google Scholar]
- 75.Reich A, Orda A, Wiśnicka B, Szepietowski JC. Plasma concentration of selected neuropeptides in patients suffering from psoriasis. Exp Dermatol. 2007;16:421–428. doi: 10.1111/j.1600-0625.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 76.Detmar M, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999;141:1054–1060. doi: 10.1046/j.1365-2133.1999.03205.x. [DOI] [PubMed] [Google Scholar]
- 78.Kirshenbaum AS, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 79.Kempuraj D, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.