Abstract
Diacylglycerol kinases (DGKs) convert diacylglycerol (DAG) into phosphatidic acid (PA), acting as molecular switches between DAG- and PA-mediated signaling. We previously showed that Src-dependent activation and plasma membrane recruitment of DGKα are required for growth-factor-induced cell migration and ruffling, through the control of Rac small-GTPase activation and plasma membrane localization. Herein we unveil a signaling pathway through which DGKα coordinates the localization of Rac. We show that upon hepatocyte growth-factor stimulation, DGKα, by producing PA, provides a key signal to recruit atypical PKCζ/ι (aPKCζ/ι) in complex with RhoGDI and Rac at ruffling sites of colony-growing epithelial cells. Then, DGKα-dependent activation of aPKCζ/ι mediates the release of Rac from the inhibitory complex with RhoGDI, allowing its activation and leading to formation of membrane ruffles, which constitute essential requirements for cell migration. These findings highlight DGKα as the central element of a lipid signaling pathway linking tyrosine kinase growth-factor receptors to regulation of aPKCs and RhoGDI, and providing a positional signal regulating Rac association to the plasma membrane.
Keywords: cell migration, growth factors, phosphatidic acid
Cell migration, central to many biological and pathological processes such as cancer metastatic progression, is a multistep cycle involving extension of protrusions and formation of stable attachments near the leading edge, followed by translocation of the cell body forward (1). The protrusive activity occurring at the leading edge depends on the spatial and temporal coordination between cell substrate adhesion and actin reorganization. Rho-family small GTPases coordinate the recruitment at the leading edge of downstream effectors, thereby mediating the formation of ruffles and lamellipodia. Their GTP-bound state is tightly regulated by both guanine nucleotide exchange factors (GEFs), which stimulate GTP loading, and GTPase activating proteins (GAPs), which catalyze GTP hydrolysis. Moreover, Rho-family GTPases are regulated by guanine nucleotide dissociation inhibitors (GDIs), which antagonize both GEFs and GAPs and mediate the cycling of Rho proteins between the cytosol and the membrane (2, 3).
Atypical protein kinase C ζ and ι (aPKCζ/ι), unlike classical and novel PKCs, feature a C1-like domain which does not bind to either diacylglycerol or phorbol esters, and have recently been proposed as key transducers for establishment of cell polarity and migration (4).
Diacylglycerol kinases (DGKs), which convert diacylglycerol (DAG) to phosphatidic acid (PA), comprise a family of 10 distinct enzymes grouped into five classes, each featuring distinct regulatory domains and a highly conserved catalytic domain preceded by two cysteine-rich C1-like domains. An increasing body of evidence indicates that DGKs, by acting as terminators of diacylglycerol-triggered signaling, contribute to regulating C1 domain-containing proteins, such as classical and novel PKCs and the Rac-GAP β-chimaerin (5). Conversely, by generating PA, DGKs regulate several signaling proteins, including serine kinases, small-GTPase-regulating proteins, and lipid-metabolizing enzymes (reviewed in refs. 6 and 7). Thus, by regulating in a reciprocal manner the level of both DAG and PA lipid second messengers, DGK enzymes may act as terminators of DAG-mediated signals as well as activators of PA-mediated ones. We previously showed that DGKα is activated by growth factors on recruitment to the plasma membrane through its Src-mediated phosphorylation on Tyr335. Activation of DGKα mediates growth-factor-induced cell migration and proliferation in epithelial, endothelial, and lymphoma cells (8–12). Moreover, we demonstrated that in epithelial cells, DGKα is required for hepatocyte growth factor (HGF) -induced membrane ruffling by regulating Rac membrane targeting and activation (13). However, the molecular mechanisms by which DGKα regulates Rac function still remain to be elucidated.
Here we unveil a previously undescribed signaling pathway linking HGF receptor to Rac activation and targeting at the plasma membrane of epithelial cells, where it drives the formation of membrane protrusions. Our data highlight that upon HGF-induced plasma membrane recruitment and activation of DGKα, PA recruits at the plasma membrane and activates aPKCζ/ι, in complex with RhoGDI and Rac. Then, Rac is released from the inhibitory complex with RhoGDI, allowing its activation and formation of membrane ruffles.
Results
DGKα Regulates Rac by Directing Its Recruitment to the Plasma Membrane.
MDCK epithelial cells grow in discrete colonies and on HGF stimulation undergo scatter, involving cell spreading, dissolution of intercellular adhesions, and migration of cells away from one another. At early time points from HGF stimulation, cells at the periphery of a colony reorganize the actin cytoskeleton and form dynamic protrusions of the outer plasma membrane known as ruffles, mediated by membrane targeting and activation of Rac (14, 15). We previously showed that down-regulation of DGKα affected both HGF-induced membrane ruffling and recruitment of Rac to the plasma membrane (13). Here, by live-cell imaging, we report that HGF-induced rapid and transient recruitment of EGFP-Rac at sites of intense ruffling was impaired upon DGK inhibition (Fig. S1 and Movies S1 and S2), achieved by cell treatment with 1 μM R59949. R59949 is a DGK-specific inhibitor interacting with the catalytic domain, endowed with a strong preference for α, β, and δ isoforms (16, 17). We previously showed that overexpression of DGKα reverted R59949-mediated inhibition of HGF signaling (8).
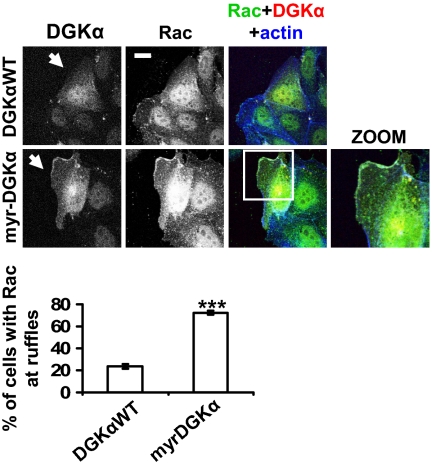
Thus, we investigated the mechanisms by which DGKα might regulate Rac-specific localization. In the absence of growth-factor stimulation, constitutively active RacV12 mutant localized mainly in the cytoplasm and at cell-cell adhesions, whereas its expression was not sufficient to induce ruffling at the leading edge of cells at the periphery of a colony, according to previous observations (15). HGF cell treatment increased the percentage of cells featuring concomitant membrane ruffles and RacV12 at the outer plasma membrane in a DGK-dependent manner (Fig. S2). In addition, we compared endogenous Rac localization in MDCK cells transiently transfected with either DGKα wild type (DGKαWT) or a constitutively active membrane-bound DGKα mutant (myr-DGKα). Indeed, the expression of myr-DGKα, but not of DGKαWT, promoted a 2.5-fold increase of the percentage of cells displaying membrane ruffles, along with Rac recruitment to the plasma membrane at ruffling sites (Fig. 1). Overall, these data indicate that DGKα activation provides a crucial signal which is both necessary and sufficient to direct Rac membrane localization at the leading edge, regardless of its GTP loading, thereby promoting ruffle formation.
Fig. 1.
DGKα provides the signal directing Rac to the nascent ruffle. MDCK cells, transfected with either DGKαWT or myr-DGKα, were cultured overnight in the absence of serum, fixed, and stained for Rac (green), myc tag (red), and actin (blue). Arrows indicate DGKαWT- or myr-DGKα-transfected cells. (Scale bar, 10 μm.) C, 30 transfected cells, scored for the presence of Rac at ruffling sites. n = 6, with SEM; ***P = 0.0002.
DGKα Regulates RhoGDI Membrane Recruitment.
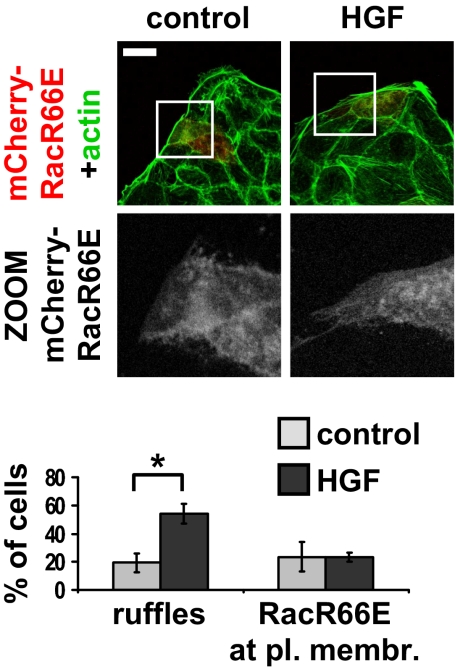
An increasing body of evidence indicates that RhoGDI, which in unstimulated cells associates with GDP-bound Rac in a cytosolic complex, regulates Rac targeting to specific adhesion sites at the plasma membrane through largely unidentified mechanisms (2). Once at the plasma membrane, Rac is released from the inhibitory complex with RhoGDI and is eventually activated by a Rac-GEF (2, 18). To verify whether growth factors trigger Rac localization at the plasma membrane through interaction with RhoGDI, we transiently transfected MDCK cells with a Rac mutant, RacR66E, unable to bind to RhoGDI (19). Indeed, RacR66E failed to be recruited at ruffling sites upon HGF cell treatment whereas HGF-induced membrane ruffle formation was not impaired, indicating that endogenous Rac is properly activated (Fig. 2). These data demonstrate that RhoGDI physical interaction with Rac mediates growth-factor-induced Rac targeting at ruffling sites.
Fig. 2.
Rac plasma membrane targeting requires Rac/RhoGDI interaction. MDCK cells were transiently transfected with mCherry-RacR66E, treated with 10 ng/mL HGF for 15 min, fixed, and stained for actin (green). (Scale bar, 24 μm.) C, 30 transfected cells, scored for the presence of ruffles or RacR66E plasma membrane localization. n = 3, with SEM; *P = 0.0057.
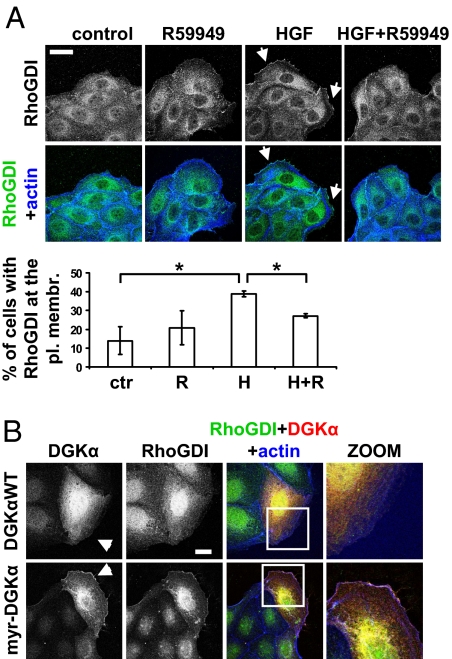
Thus, we investigated whether HGF, although inducing membrane ruffles, was able to recruit RhoGDI to the leading edge and whether this process required DGKα activity. We show that HGF increased the percentage of cells displaying RhoGDI at ruffling sites, which was impaired by both pharmacological inhibition (Fig. 3A) and siRNA-mediated silencing of DGKα (Fig. S3A). In addition, in time-lapse experiments, HGF-induced rapid and transient recruitment of RhoGDI to ruffling sites required DGK activity (Fig. S4 and Movies S3 and S4).
Fig. 3.
DGKα regulates RhoGDI targeting to the plasma membrane. (A) MDCK cells were stimulated with 10 ng/mL HGF for 5 min in the presence or absence of 1 μM R59949, fixed, and stained for RhoGDI (green) and actin (blue). Arrows indicate RhoGDI at membrane ruffles. (Scale bar, 24 μm.) C, 70 cells, scored for RhoGDI localization at ruffling sites. n = 3, with SEM; *P < 0.05. (B) MDCK cells, transfected with either DGKαWT or myr-DGKα, were cultured overnight in the absence of serum, fixed, and stained for RhoGDI (green), myc tag (red), and actin (blue). Arrowheads indicate transfected cells. (Scale bar, 10 μm.)
Moreover, we investigated whether DGKα constitutive activation at the plasma membrane was sufficient to recruit RhoGDI. Thus, the expression of myr-DGKα, but not of DGKαWT, was sufficient to recruit RhoGDI at ruffling sites in almost 80% of transfected cells (Fig. 3B and Fig. S3B). Altogether, these findings demonstrate that stimulation of DGKα enzymatic activity provides a crucial signal which is necessary and sufficient to direct the recruitment of RhoGDI, which in turn is required for Rac targeting at protrusion sites and ruffle formation.
DGKα Regulates Membrane Ruffling by Modulating aPKCζ/ι Function.
The molecular mechanisms regulating RhoGDI localization are still largely unknown, as it lacks membrane-interacting domains. PKCζ, reported to be regulated by direct binding to PA but not to DAG (20), associates with and phosphorylates RhoGDI, thereby allowing its dissociation from Rac (21). Indeed, in MDCK cells, a small amount of the whole cellular aPKCζ/ι was constitutively associated with RhoGDI (see below, Fig. 6). Thus, we raised the hypothesis that DGKα might control RhoGDI by providing the lipid-regulating aPKCζ/ι.
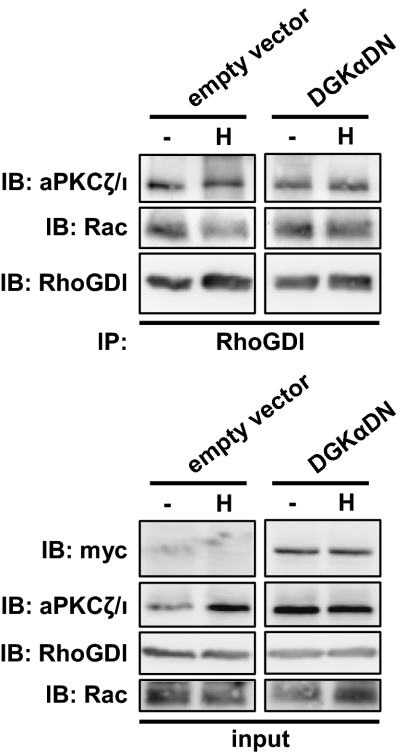
Fig. 6.
DGKα regulates Rac/RhoGDI complex dissociation. MDCK/empty vector or MDCK/DGKα-DN cells were stimulated with 50 ng/mL HGF for 15 min. Cell lysates were immunoprecipitated for RhoGDI and analyzed by western blot.
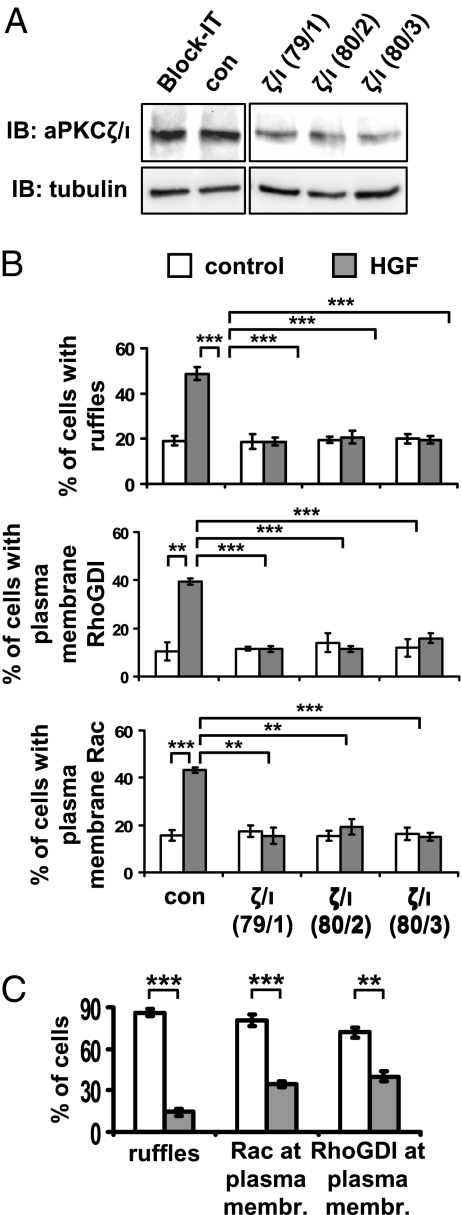
To obtain an appreciable down-regulation of cellular aPKCs, we silenced both PKCζ and PKCι by different combinations of their respective specific siRNAs (Fig. 4A). We observed that aPKCζ/ι silencing impaired HGF-induced membrane ruffle formation, as well as both RhoGDI and Rac recruitment to the outer plasma membrane (Fig. 4B). The aPKCζ/ι catalytic activity was actually required, as aPKCζ/ι inhibition by cell treatment with PKCζ/ι pseudosubstrate peptide impaired HGF-induced membrane ruffle formation and Rac plasma membrane translocation (Fig. S5A). Similarly, inhibition of PKCζ by expression of PKCζ dominant-negative mutant (PKCζKW) impaired HGF-induced RhoGDI translocation to the outer plasma membrane (Fig. S5B). Moreover, aPKCζ/ι was also required for myr-DGKα-induced membrane ruffle formation and Rac and RhoGDI targeting to the outer plasma membrane (Fig. 4C and Fig. S5C). Together these data indicate that, upon HGF stimulation or myr-DGKα expression, aPKCζ/ι mediates membrane ruffle formation and recruitment of the molecular machinery necessary for polarized extension of plasma membrane protrusions.
Fig. 4.
aPKCζ/ι mediates both HGF- and myr-DGKα-induced extension of membrane protrusions. (A) MDCK cells were transfected with three combinations of PKCζ (79, 80) and PKCι (1–3) specific siRNAs. Whole-cell lysates were analyzed for levels of aPKCζ/ι expression by western blot. (B) MDCK cells were transfected as in A, treated with 10 ng/mL HGF for 15 min, fixed, and stained for Rac or RhoGDI and actin. C, 110 cells, analyzed for the presence of ruffles and Rac or RhoGDI at the plasma membrane. n = 4 (Rac and RhoGDI), n = 8 (ruffles), with SEM; **P < 0.005, ***P < 0.0005. (C) MDCK cells were transiently transfected either with myr-DGKα alone or cotransfected with myr-DGKα and PKCζKW, cultured overnight in the absence of serum, fixed, and stained for myc and flag tags, actin, and Rac or RhoGDI. C, 20 transfected cells, scored for the presence of ruffles and Rac or RhoGDI at protrusion sites. n = 4, with SEM; **P < 0.001, ***P < 0.0001.
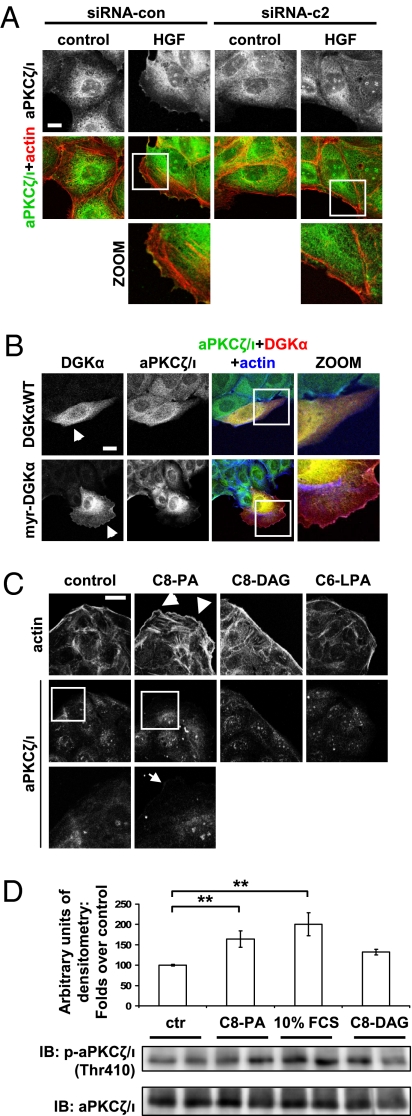
We then investigated whether DGKα was involved in the regulation of aPKCζ/ι downstream of HGF signaling. Indeed, we observed that HGF induced the recruitment of aPKCζ/ι to membrane ruffles in a DGKα-dependent manner. In fact, both specific down-regulation of DGKα by two different siRNAs (Fig. 5A and Fig. S6A) and its pharmacological inhibition by R59949 cell treatment (Fig. S6B) completely abolished HGF-induced recruitment of aPKCζ/ι to the outer plasma membrane, indicating that enzymatic activity of DGKa was required.
Fig. 5.
DGKα regulates aPKCζ/ι function. (A) MDCK cells were transfected either with control siRNA or DGKα siRNA c1 and c2, treated with 10 ng/mL HGF for 15 min, fixed, and stained for aPKCζ/ι (green) and actin (red). (Scale bar, 10 μm.) (B) MDCK cells, transfected either with DGKαWT or myr-DGKα, were cultured overnight in the absence of serum, fixed, and stained for aPKCζ/ι (green), myc tag (red), and actin (blue). Arrowheads indicate transfected cells. (Scale bar, 10 μm.) (C) MDCK cells were stimulated with either 250 μM C8-PA, C8-DAG, or C6-LPA or left untreated, and fixed and stained for actin (red) and aPKCζ/ι (green). Arrowheads indicate cortical actin rearrangements, while the arrow indicates aPKCζ/ι membrane localization. (Scale bar, 19 μm.) (D) MDCK cells were stimulated with either 250 μM C8-PA, 10% FCS medium (as positive control), 250 μM C8-DAG, or left untreated. Whole-cell lysates were analyzed by western blot and the intensity of phospho-aPKCζ/ι bands was quantified by densitometry. For each condition, nine replicate points were performed in four independent experiments. The densitometry of each band was normalized as the percentage of the densitometry mean of control points in the same experiment, and is shown in the histogram, with SEM; **P < 0.005. A representative picture is shown.
Moreover, expression of myr-DGKα was sufficient to recruit aPKCζ/ι to ruffling sites, as the percentage of cells displaying this localization of aPKCζ/ι increased 3-fold in myr-DGKα-expressing cells compared to DGKαWT-expressing ones (Fig. 5B and Fig. S6C). Accordingly, cell treatment with PA but not with lysophosphatidic acid (LPA) or DAG was sufficient to promote concurrent cortical actin rearrangements and plasma membrane translocation of aPKCζ/ι, Rac, and RhoGDI (Fig. 5C and Fig. S7). Moreover, cell treatment with exogenous PA, but not with DAG, induced aPKCζ/ι activation, as revealed by the increase in phosphorylation of the catalytic domain Thr410 (Fig. 5D). Together, these data strongly support the idea that the generation of PA by DGKα is a crucial signal driving the recruitment and activation of the molecular machinery necessary for extension of membrane protrusions.
As accumulating evidence suggests that the small GTPase Cdc42 acts upstream of PKCζ and regulates it through the Par3/Par6 complex, thus driving cell polarity and directional migration (22), we further verified whether DGKα might regulate aPKCζ/ι via Cdc42. Indeed, the expression of Cdc42N17, although impairing membrane ruffles induced by myr-DGKα, did not affect aPKCζ/ι plasma membrane recruitment (Fig. S8). Thus, although Cdc42 is required for ruffle formation induced by constitutive generation of PA at the plasma membrane, it is not required for the recruitment of aPKCζ/ι. Overall, these data indicate that DGKα acts upstream of aPKCζ/ι, in a Cdc42-independent manner, by regulating its recruitment to ruffling sites through production of PA.
We then investigated whether, by regulating the function of aPKCζ/ι, DGKα might control the dissociation of Rac/RhoGDI complex. We immunoprecipitated RhoGDI from MDCK cells stably expressing either the control vector (MDCK/empty vector) or a kinase-defective dominant-negative mutant of DGKα (DGKαDN). Rac coimmunoprecipitated with RhoGDI both in MDCK/empty vector and in MDCK/DGKαDN unstimulated cells. Upon HGF stimulation, Rac was released from the complex with RhoGDI in MDCK/empty vector cells, whereas expression of DGKαDN prevented both dissociation of the complex (Fig. 6 and Fig. S9) and Rac activation (13). Collectively, the data presented above identify DGKα as an upstream regulator of aPKCζ/ι. DGKα, by producing PA, provides the crucial signal for the recruitment at the plasma membrane and activation of aPKCζ/ι, in complex with RhoGDI and Rac, which finally dissociates in a DGKα-dependent manner, thereby allowing Rac activation.
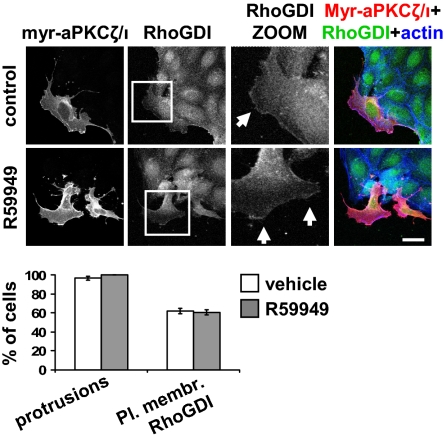
Finally, we tested the hypothesis that DGKα mediates the formation of membrane ruffles by regulating aPKCζ/ι. Thus, we verified whether transient expression of membrane-bound constitutively active myr-PKCζ could overcome DGKα inhibition, as expected for a downstream effector. Indeed, myr-PKCζ-expressing cells featured an altered morphology with membrane protrusions and ruffles, even in the absence of stimulation, and in 60% of them RhoGDI was localized at the outer plasma membrane. Prolonged (1-h) pharmacological inhibition of DGK activity by R59949 did not affect either formation of membrane protrusions or RhoGDI localization to the outer plasma membrane (Fig. 7), confirming that aPKCζ/ι is responsible, downstream of DGKα, for the recruitment of RhoGDI at the leading edge and consequent activation of the molecular machinery necessary for extension of membrane ruffles.
Fig. 7.
DGKα does not affect myr-PKCζ-induced events. MDCK cells were transiently transfected with myr-PKCζ, grown overnight in the absence of serum, and treated with 1 μM R59949 for 1 h. Cells were fixed and stained for flag tag (red), RhoGDI (green), and actin (blue). Arrows indicate RhoGDI staining at protrusion sites. (Scale bar, 24 μm.) C, 30 transfected cells, scored for the presence of protrusions and RhoGDI at protrusion sites. n = 3, with SEM.
Overall, our findings highlight that DGKα, by producing PA, provides a crucial signal to recruit aPKCζ/ι and RhoGDI/Rac complex and to activate aPKCζ/ι, thereby allowing Rac membrane targeting and activation and consequent actin cytoskeleton remodeling preliminary to cell migration.
Discussion
Epithelial cells at the periphery of a colony initiate migration by extending membrane protrusions whose formation relies on recruitment and activation of Rac to their tips, to harness and localize actin polymerization (23). Spatially restricted activation of Rac at nascent ruffles is triggered by coordinated signals provided by both growth factors and adhesion receptors. Moreover, the observation that in epithelial cells the expression of a constitutively active form of Rac is not per se sufficient to promote ruffle formation (15, 24) strongly indicates that a further localization signal, provided by growth factors, is required to generate RacGTP accumulation at protrusion sites. Starting from our initial observation that DGKα is required for HGF-induced RacV12 membrane targeting, we unveil a signaling pathway by which HGF regulates Rac localization to nascent ruffles through membrane recruitment of DGKα, aPKCζ/ι, and RhoGDI.
We previously showed that, upon HGF stimulation, activation and membrane recruitment of Rac, as well as ruffle formation, are fully dependent on DGKα (13). Our finding that expression of a myristoylated mutant of DGKα promoted ruffle formation and Rac recruitment at protrusion sites, in the absence of growth factor, strongly suggests that activation of DGKα at the plasma membrane provides a crucial signal to regulate Rac function.
Previous evidence underscored the role of RhoGDI in regulating the dynamics of Rac targeting to the plasma membrane, where Rac dissociates from the inhibitory complex with RhoGDI to interact with its downstream effectors (2, 18). Our observation that in HGF-treated cells RhoGDI is recruited to nascent ruffles, and that a Rac mutant unable to bind to RhoGDI is not, provides further support to the claim that the interaction between Rac and RhoGDI is necessary for Rac membrane targeting. Moreover, the present data demonstrate that growth factors promote Rac membrane localization by regulating RhoGDI targeting. We show that DGKα at the plasma membrane provides a key lipid signal necessary and sufficient to recruit RhoGDI. Furthermore, the failure to detect DGKα in a complex with RhoGDI and Rac suggests that DGKα, rather than acting as a scaffolding protein, may recruit RhoGDI through its enzymatic activity. However, RhoGDI does not feature any clear domain responsible for membrane binding, suggesting that the lipid signal generated by DGKα may recruit RhoGDI through an interacting lipid-binding protein.
Recent evidence indicates that PKCζ associates with RhoGDI and regulates the dissociation of Rac/RhoGDI complex, thereby allowing Rac activation (21). Moreover, PKCι regulates invasive behavior by activating Rac in lung cancer cells (25). Interestingly, PA, the lipid product of DGKα activity, has been reported to bind directly to PKCζ and to stimulate its enzymatic activity (20), whereas it was recently shown that DGKα enhances PKCζ-mediated phosphorylation of p65/Rel (26). Here we show that upon HGF stimulation, DGKα-generated PA is a necessary and sufficient signal to recruit aPKCζ/ι at protrusion sites and activate it, thereby promoting Rac and RhoGDI membrane targeting.
Kuribayashi et al. showed that PKCζ, associated in a complex with RhoGDI, mediates its phosphorylation on threonine, thereby allowing Rac release (21). Based on this observation and on our data, we expected that Rac release from the complex with RhoGDI depended on DGKα activity. Consistently, in the present report, we show that DGKα enzymatic activity mediated HGF-induced Rac release from RhoGDI, although we could not detect any threonine phosphorylation of endogenous RhoGDI. Finally, these results suggest the following working model: Upon HGF stimulation, Src-mediated activation of DGKα, once targeted to the plasma membrane, results in accumulation of PA, which directs the recruitment of aPKC/RhoGDI/Rac complex at protrusion sites, likely through the interaction of DGKα-produced PA with the C1-like domain of aPKCζ/ι, which is finally activated. Thus, aPKCζ/ι is identified as the direct downstream effector of DGKα. Once at the plasma membrane, aPKCζ/ι may allow Rac release from RhoGDI, likely through RhoGDI phosphorylation. This model is confirmed by the finding that expression of myr-PKCζ, in the absence of growth factors, recapitulates both RhoGDI recruitment and protrusion formation in a DGKα-independent manner, providing further support to the tenet that DGKα regulates RhoGDI and Rac by acting upstream of aPKCζ/ι (Fig. 8).
Fig. 8.
Model proposed for Rac-localized activation at the leading edge upon growth-factor stimulation. Upon growth-factor stimulation, DGKα is activated in a Src-dependent manner and recruited to the plasma membrane. The production of PA is the crucial signal to direct the recruitment of aPKCζ/ι, in complex with RhoGDI and Rac. PKCζ/ι, in turn, mediates the dissociation of Rac from the inhibitory complex with RhoGDI, which may become prone to activation by a RacGEF.
aPKC is recruited to the plasma membrane through binding to active Cdc42 (27) or by directed interaction with ceramide in a Cdc42-independent manner (28). Our finding that Cdc42 is dispensable for aPKCζ/ι recruitment induced by myr-DGKα suggests that Cdc42 function is not required for lipid-mediated aPKCζ/ι membrane targeting, albeit necessary for ruffle formation downstream of DGKα.
As RhoGDI also controls Cdc42, we may speculate that activation of DGKα provides a localization signal driving plasma membrane recruitment of aPKCs in complex with RhoGDI and Rac or Cdc42, eventually leading to the activation of Cdc42 and Rac, which may further recruit aPKCs in a Cdc42-dependent manner. This might establish a feed-forward mechanism that allows the full activation of the molecular machinery driving actin polymerization and consequent formation of membrane protrusions.
PAK1-mediated serine phosphorylation of RhoGDI promotes the selective release of Rac downstream of Cdc42 activation (29). Recently, DGKζ was shown to regulate PAK1-mediated phosphorylation of RhoGDI, leading to selective activation of Rac but not of Cdc42 (30). Conversely, PKCζ-dependent phosphorylation of RhoGDI promotes the release of both Rac and Cdc42 (21). Thus, it seems that DGKα and DGKζ, by regulating, respectively, aPKCζ/ι and PAK1, are involved in two distinct molecular mechanisms leading to the control of small-GTPase function.
In conclusion, our findings constitute a coherent demonstration that DGKα, upon Src-mediated activation, acts as a positive transducer of growth-factor signaling by producing PA rather than removing DAG. Here we unveil a PA-mediated signaling pathway linking tyrosine kinase receptors to Rac activation, membrane ruffling, and cell migration. In particular, we highlight a pivotal role for a DGKα-aPKCζ/ι-RhoGDI axis in the regulation of the initial events leading to activation of Rac at the leading edge of migrating cells.
Materials and Methods
Cell Stimulation.
Before any treatment, cells were cultured overnight in the absence of serum. In the case of R59949 cell treatment, a 15-min pretreatment with R59949, or vehicle alone, was performed.
Immunofluorescence.
Culturing, fixing, and staining detailed procedures are described in SI Materials and Methods and ref. 13. Representative pictures are shown in the figures.
Statistical Analysis.
In experiments involving counting of cells displaying ruffles, membrane protrusions, or localization of proteins at the outer plasma membrane (not at cell-cell contacts), only colony-edge cells were considered. The average number of cells scored for each condition in each experiment is indicated in figure legends by “C.” Statistical analysis was performed considering n independent experiments (indicated in the figure legends). The P values were calculated by one-tailed Standard t test.
Further details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro, Ministero dell’Istruzione, dell’Università e della Ricerca, PRIN Program 2007, and Regione Piemonte (Ricerca Sanitaria Finalizzata). F.C. is funded by Regione Piemonte (L.R. 4/2006 for brain drain containment, D.R. 392-2007). We thank Paola Chiarugi, Celine DerMardirossian, Louise Hodgson, Giorgio Scita, and Alex Toker for providing reagents.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908326107/DCSupplemental.
References
- 1.Webb D-J, Parsons J-T, Horwitz A-F. Adhesion assembly, disassembly and turnover in migrating cells: over and over and over again. Nat Cell Biol. 2002;4:97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 2.Del Pozo M-A, et al. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–239. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 3.Del Pozo M-A, Schwartz M-A. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. doi: 10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–27. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2006;32:27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: why so many of them? Biochim. Biophys Acta. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Mérida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 8.Cutrupi S, et al. Src-mediated activation of alpha-diacylglycerol kinase is required for hepatocyte growth factor-induced cell motility. EMBO J. 2000;19:4614–4622. doi: 10.1093/emboj/19.17.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldanzi G, et al. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene. 2004;23:4828–4838. doi: 10.1038/sj.onc.1207633. [DOI] [PubMed] [Google Scholar]
- 10.Bacchiocchi R, et al. Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood. 2005;106:2175–2182. doi: 10.1182/blood-2005-01-0316. [DOI] [PubMed] [Google Scholar]
- 11.Baldanzi G, et al. Diacylglycerol kinase-alpha phosphorylation by Src on Y335 is required for activation, membrane recruitment and HGF-induced cell motility. Oncogene. 2008;27:942–956. doi: 10.1038/sj.onc.1210717. [DOI] [PubMed] [Google Scholar]
- 12.Filigheddu N, et al. Diacylglycerol kinase is required for HGF-induced invasiveness and anchorage-independent growth of MDA-MB-231 breast cancer cells. Anticancer Research. 2007;27:1489–1492. [PubMed] [Google Scholar]
- 13.Chianale F, et al. Diacylglycerol kinase-alpha mediates hepatocyte growth factor-induced epithelial cell scatter by regulating Rac activation and membrane ruffling. Mol Biol Cell. 2007;18:4859–4871. doi: 10.1091/mbc.E07-02-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridley A-J, Comoglio P-M, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Sakane F, Kanoh H, Walsh JP. Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2,3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem Pharmacol. 2000;59:763–772. doi: 10.1016/s0006-2952(99)00395-0. [DOI] [PubMed] [Google Scholar]
- 17.Miele C, et al. Glucose regulates diacylglycerol intracellular levels and protein kinase C activity by modulating diacylglycerol kinase subcellular localization. J Biol Chem. 2007;282:31835–31843. doi: 10.1074/jbc.M702481200. [DOI] [PubMed] [Google Scholar]
- 18.Moissoglu K, Slepchenko B-M, Meller N, Horwitz A-F, Schwartz M-A. In vivo dynamics of Rac-membrane interactions. Mol Biol Cell. 2006;17:2770–2779. doi: 10.1091/mbc.E06-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi P-N, et al. An activating mutant of Rac1 that fails to interact with Rho GDP-dissociation inhibitor stimulates membrane ruffling in mammalian cells. Biochem J. 2004;378:409–419. doi: 10.1042/BJ20030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limatola C, Schaap D, Moolenaar W-H, van Blitterswijk W-J. Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem J. 1994;304:1001–1008. doi: 10.1042/bj3041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuribayashi K, et al. Essential role of protein kinase C zeta in transducing a motility signal induced by superoxide and a chemotactic peptide, fMLP. J Cell Biol. 2007;176:1049–1060. doi: 10.1083/jcb.200607019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrique D, Schweisguth F. Cell polarity: the ups and downs of the Par6/aPKC complex. Curr Opin Genet Dev. 2003;13:341–350. doi: 10.1016/s0959-437x(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 23.Small J-V, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa K, Itoh R-E, Yoshizaki H, Nakamura Y-O, Matsuda M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol Biol Cell. 2004;15:1003–1010. doi: 10.1091/mbc.E03-08-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frederick, et al. Matrix metalloproteinase-10 is a critical effector of protein kinase Ciota-Par6alpha-mediated lung cancer. Oncogene. 2008;27:4841–4853. doi: 10.1038/onc.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kai M, et al. Diacylglycerol kinase alpha enhances protein kinase Czeta-dependent phosphorylation at Ser311 of p65/RelA subunit of nuclear factor-kappaB. FEBS Lett. 2009;583:3265–3268. doi: 10.1016/j.febslet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy K, Wang G, Silva J, Condie B-G, Bieberich E. Ceramide regulates atypical PKCzeta/lambda-mediated cell polarity in primitive ectoderm cells. A novel function of sphingolipids in morphogenesis. J Biol Chem. 2007;282:3379–3390. doi: 10.1074/jbc.M607779200. [DOI] [PubMed] [Google Scholar]
- 29.DerMardirossian C, Schnelzer A, Bokoch G-M. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117–127. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Abramovici H, et al. Diacylglycerol Kinase {zeta} Regulates Actin Cytoskeleton Reorganization through Dissociation of Rac1 from RhoGDI. Mol Biol Cell. 2009 doi: 10.1091/mbc.E07-12-1248. 10.1091/mbc.E07-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.