Abstract
Autophagy is a cellular process for the disposal of damaged organelles or denatured proteins through a lysosomal degradation pathway. By reducing endogenous macromolecules to their basic components (i.e., amino acids, lipids), autophagy serves a homeostatic function by ensuring cell survival during starvation. Increased autophagy can be found in dying cells, although the relationships between autophagy and programmed cell death remain unclear. To date, few studies have examined the regulation and functional significance of autophagy in human lung disease. The lung, a complex organ that functions primarily in gas exchange, consists of diverse cell types (i.e., endothelial, epithelial, mesenchymal, inflammatory). In lung cells, autophagy may represent a general inducible adaptive response to injury resulting from exposure to stress agents, including hypoxia, oxidants, inflammation, ischemia–reperfusion, endoplasmic reticulum stress, pharmaceuticals, or inhaled xenobiotics (i.e., air pollution, cigarette smoke). In recent studies, we have observed increased autophagy in mouse lungs subjected to chronic cigarette smoke exposure, and in pulmonary epithelial cells exposed to cigarette smoke extract. Knockdown of autophagic proteins inhibited apoptosis in response to cigarette smoke exposure in vitro, suggesting that increased autophagy was associated with epithelial cell death. We have also observed increased morphological and biochemical markers of autophagy in human lung specimens from patients with chronic obstructive pulmonary disease (COPD). We hypothesize that increased autophagy contributes to COPD pathogenesis by promoting epithelial cell death. Further research will examine whether autophagy plays a homeostatic or maladaptive role in COPD and other human lung diseases.
Keywords: autophagy, apoptosis, pulmonary disease
The lung functions primarily in gas exchange. The bifurcating airways of this complex organ gradually diminish in size to terminate in alveolar sacs, the principle site where gas exchange occurs. The alveolae provide a portal by which inspired molecular oxygen (O2) diffuses to the bloodstream for delivery to the systemic tissues as a metabolic substrate, and by which waste gases (i.e., mono- and dioxides of carbon) are eliminated. To perform its function, the lung consists of a variety of specialized cell types (1). Pulmonary epithelial cells line the airways, bronchi, and alveolar wall, whereas mesenchymal cells (i.e., interstitial fibroblasts) produce extracellular matrix and provide structural support (2). The pulmonary vasculature consists of endothelial, smooth muscle, and adventitial fibroblast components (3). The lung also contains resident phagocytic cells (i.e., alveolar macrophages), as well as inflammatory cells that may be recruited from distal sites during injury or inflammation (4). The lung and its resident cells—in particular, epithelial cells—represent a primary target for injury arising from inhalation exposure to harmful environmental agents (e.g., particles, xenobiotics, noxious gases, cigarette smoke, and pathogens) (1). Epithelial cell injury can also arise during critical care procedures (e.g., mechanical ventilation, hyperoxia) (5). Pulmonary vascular cell injury may occur as the result of chronic hypoxia, or ischemia–reperfusion episodes (6, 7).
The fundamental processes governing the proliferation, death, and homeostasis of resident lung cells are, in principle, similar to those operating in the other soft tissues of the body. The modes in which lung cells may die include programmed (type I, apoptosis) and traumatic (necrotic) forms of cell death (8). Apoptosis, a form of regulated cell death characterized by activation of caspases, can be considered an injurious process in tissues, leading to loss of critical cell populations. Conversely, apoptosis can represent an “adaptive” or homeostatic process by providing an alternative to inflammatory necrotic cell death in injured tissue (9).
Macroautophagy (referred to hereafter as autophagy) is another fundamental homeostatic process operating in most mammalian cell types that can function as a cell survival mechanism. Autophagy provides a pathway for the turnover of cytoplasmic organelles and proteins, through a lysosome-dependent degradation process (10–14). During this process, nascent double-membraned autophagosomes or autophagic vacuoles (AV) surround and sequester a cargo, such as damaged organelles (e.g., mitochondria, peroxisomes, endoplasmic reticulum [ER] membrane), protein aggregates, or pathogens. Late-stage autophagosomes fuse with lysosomes to form single membrane-bound autolysosomes, where the cargo is degraded by lysosomal enzymes (i.e., cathepsins). This degradation process regenerates metabolic precursor molecules (e.g., amino acids, fatty acids) that can be reutilized for de novo synthesis of macromolecules and energy generation (10–14).
Autophagy represents an inducible response to stress in lung cells. Agents that trigger autophagy that are particularly relevant to lung cell biology include hypoxia, particle and cigarette smoke exposure, proinflammatory states, and conditions that promote ER stress or oxidative stress. Because relatively few studies have been done in the lung, the functional significance of autophagy in human lung disease remains an enigma. This review focuses on the regulation and function of autophagy as they may relate to lung injury and the pathogenesis of lung disease, with an emphasis on cigarette smoke exposure and chronic obstructive pulmonary disease (COPD).
AUTOPHAGIC PATHWAY
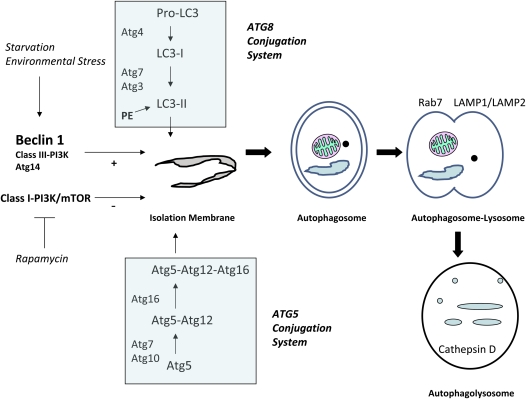
The molecular machinery of autophagic regulation has been extensively studied in yeast, followed by the identification of homologous systems in mammals. The reader is referred to excellent reviews on this subject (14, 15). In brief, the autophagic pathway consists of several distinct steps: (1) the formation of an isolation membrane; (2) the formation of an autophagosome with encapsulated cargo; (3) the fusion of the autophagosome to the lysosome; and (4) and the degradative phase with the digestion of lysosomal contents (Figure 1). A series of critical autophagy-related (Atg) genes have been identified, the gene products of which regulate discrete steps in the induction or progression of the autophagic pathway (14, 15). The Bcl-2–interacting protein, beclin 1 (homolog of yeast Atg6), forms a macromolecular complex with class III phosphatidylinositol 3-kinase. Upon cellular stimulation, the increased production of phosphatidylinositol-3-phosphate by this complex regulates the formation of nascent autophagosomes (16). Additionally, autophagosome formation also requires the action of two ubiquitin-like conjugation systems: the Atg5–Atg12 conjugation system, and the microtubule-associated protein-1 light chain (LC) 3 (Atg8) conjugation system, by which LC3 is conjugated to phosphatidyl-ethanolamine (17, 18). In mammals, the conversion of LC3 from LC3-I (free form) to LC3-II (phosphatidyl-ethanolamine–conjugated form) is regarded as a critical step in autophagosome formation (19). The appearance of punctate staining in green fluorescence protein (GFP)-LC3–expressing cells and tissues is widely used as a “gold standard” index of autophagosome formation (20). The autophagosome–lysosome fusion step requires additional cytosolic and lysosomal proteins—namely, the GTPase, Rab7, and lysosome-associated membrane protein–1/–2, respectively. Autophagy falls under negative regulation by a signaling pathway involving class I phosphatidylinositol 3-kinase and the mammalian target of rapamycin (21, 22).
Figure 1.
Autophagic pathway. The activation of autophagy is a multistep process that involves: (1) vessel nucleation; (2) vessel elongation and autophagosome formation, with assimilation of organelles or proteins to be degraded; (3) autophagosome–lysosome fusion; and (4) autophagolysosomal digestion of encapsulated material. Beclin 1, in complex with class III phosphatidylinositol 3-kinase (PI3K) and Atg14, acts as a major positive regulator of autophagy. The rapamycin-sensitive mammalian target of rapamycin (mTOR)/class I PI3K pathway acts as a major negative regulator of autophagy. Autophagosome formation requires two ubiquitin-like conjugation systems: the Atg8 (microtubule-associated protein-1 light chain [LC] 3) conjugation system, and the Atg5–Atg12 conjugation system. Autophagosome–lysosome fusion requires Rab7 and the lysosomal proteins, lysosome-associated membrane protein (LAMP)-1 and LAMP-2. PE = phosphatidyl-ethanolamine.
FUNCTIONAL SIGNIFICANCE OF AUTOPHAGY
Autophagy has been implicated in a number of fundamental biological processes, including aging, immunity, development, and differentiation (10, 23–25). Through cellular recycling, autophagy provides an endogenous mechanism for prolonging survival during starvation (10). A number of experimental studies have confirmed the activation of autophagy during starvation, and suggest a protective role for autophagy in this context (26–28). For example, mice in which Atg5 was genetically deleted (Atg5−/−), which display impaired autophagy in response to starvation, were more susceptible to cardiac dysfunction following starvation (28). Suppression of autophagy by small intefering RNA (siRNA)-directed knockdown of beclin 1 or LC3, or by chemical inhibitors of autophagy, such as 3-methyladenine (3MA), was shown to promote apoptosis and caspase-3 activation in starved HeLa cells. These studies generally support a role for autophagy as a means for prolonging cell survival, particularly during starvation (29).
The functional significance of autophagy in pathogenic states remains incompletely understood. Due to its homeostatic role in basal conditions, and its rapid induction by stress, autophagy likely provides a protective mechanism to maintain cell function during severe toxicant challenge or during the early stages of disease progression (30–32). The occurrence of autophagy during advanced stages of disease progression may represent either a continued adaptive response to cellular stress or an insufficient defense against the pathogenic process. On the other hand, autophagy may contribute to the pathogenic mechanism in certain disease contexts. In this case, dysregulation or excessive activation of autophagy has been proposed to trigger nonapoptotic cell death (33–36). Autophagic cell death (type II programmed cell death) is characterized by increased AV accumulation and absence of caspase activation, which distinguishes it from apoptosis (type 1 programmed cell death) (33). Cells with activated autophagy present increased numbers of AV (33–36). Although apoptosis is characterized by cytoskeletal collapse and nuclear condensation, autophagic cell death involves loss of organelles, with preservation of the cytoskeleton and chromatin (37, 38). Evidence in support of autophagic cell death includes increased AV formation and cell death in apoptosis-compromised (caspase-inhibited) cells (39–41). In recent studies, it has become evident that a potential cross-talk exists between the underlying mechanisms that regulate autophagy and apoptosis (34, 35). For example, Atg5 can interact with the proapoptotic protein, FADD, a component of the death-inducing signal complex (DISC), which implies a role for Atg5 in the regulation of the extrinsic apoptotic pathway (42). Furthermore, beclin 1 forms complexes with Bcl-2, an inhibitor of apoptosis (43). Overexpression of beclin 1 increases cell survival through inhibition of apoptosis, whereas suppression of apoptosis through Bcl-2 inhibition can increase autophagic death (43, 44).
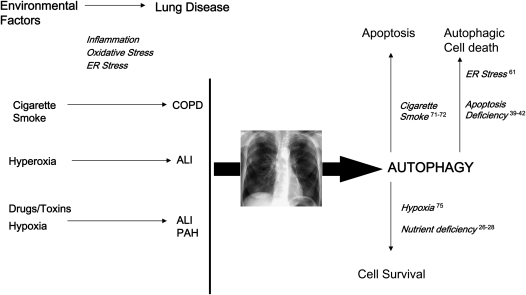
From these and similar studies, the current consensus is that autophagy can promote either cell survival or cell death, depending on the specific stimuli, environmental conditions, and cell type. The functional significance of autophagy in disease pathogenesis, therefore, may involve both adaptive and maladaptive outcomes, depending on the disease state, degree of activation, nature and duration of injurious stimuli, and other considerations (30, 31) (Figure 2).
Figure 2.
Potential factors leading to autophagy in the lung. A number of environmental factors may trigger the activation of autophagy in the lung, some of which may be associated with the pathogenesis of disease. These include cigarette smoke, particle inhalation, adverse oxygen environments, pharmaceuticals, and xenobiotics. Exposure to these agents may trigger adverse stress responses, including enhanced inflammation, oxidative stress, and perturbation of cellular organelle (i.e., endoplasmic reticulum [ER], mitochondria) function. The activation of autophagy, as an endogenous inducible response to cellular stress, may have both adaptive and maladaptive consequences, depending on the experimental model. The beneficial roles of autophagy are associated with the homeostatic turnover of damaged cellular organelles and protein, thus promoting the recycling of vital metabolic building blocks and the regeneration of energy charge. In this regard, autophagy is regarded as a survival mechanism. On the other hand, excessive autophagy may be associated with aberrant degradation of intracellular constituents, leading to autophagic cell death, or, alternatively, to apoptotic cell death, depending on the cell model, both of which may potentially play a contributory role in disease pathogenesis. ALI = acute lung injury; COPD = chronic obstructive pulmonary disease; PAH = pulmonary arterial hypertension.
FACTORS POTENTIALLY INFLUENCING AUTOPHAGY IN THE LUNG
ER Stress Response
The ER is a vital organelle that performs important cellular functions, including the post-translational processing, folding, and trafficking of newly synthesized proteins, and the regulation of the intracellular calcium flux. A variety of chemicals and adverse environmental conditions can disturb the normal function of this organelle, causing ER stress associated with the accumulation of malfolded protein aggregates (45, 46). Such agents include calcium ionophores, oxidative stress, and inhibitors of protein glycosylation (47). The unfolded protein response (UPR) occurs as an adaptive compensatory reaction to ER stress, characterized by the up-regulated synthesis of ER chaperone proteins (i.e., Bip/GRP78), the attenuation of protein translation, and the activation of an ER-associated degradation system (45). In the ER-associated degradation pathway, malfolded or denatured proteins are exported from the ER lumen to the cytosol, and are then degraded by the proteosome (48). The UPR is mediated by several regulatory molecules that can complex with Bip/GRP78 under basal conditions, including inositol-requiring kinase (IRE)-1, activating transcription factor-6, and protein kinase R–like ER kinase, which are subsequently released under stress conditions to activate a signal transduction cascade (45, 49, 50). Bip/GRP78 functions as a chaperone for misfolded proteins in the ER that prevents their aggregation. Thus, induction of the UPR provides a homeostatic function that protects cells from ER stress (47). However, conditions of excessive ER stress may lead to apoptotic cell death through activation of specific caspases (i.e., caspase-12, -9, -8), and the transcription factor, C/EBP homologous protein/growth arrest DNA damage–inducible gene 153 (51–54).
Several studies have indicated that the ER stress response can be activated in normal or transformed lung cells or in fibroblasts as a general response to cigarette smoke (55, 56), or agents associated with acute lung injury (ALI), such as hyperoxia (57), and bacterial LPS-induced inflammation in mice (58).
The relationship between ER stress and the activation of autophagy has been recently demonstrated in several cell types. For example, in neuroblastoma cells, ER stress increased autophagy (as evidenced by increased AV formation) and accumulation of LC3-II. The induction of autophagy in these cells by ER stress was associated with increased cell survival, and required IRE1 and the c-Jun NH2-terminal kinase signaling pathways (59). In yeast, induction of ER stress led to activation of the autophagic initiator protein, Atg1 (60). Activation of autophagy by chemical inducers of the ER stress response promoted cell survival in transformed cell lines, but promoted cell death in the corresponding untransformed lines (61). In the latter study, the mechanisms governing cell-type specificity in the functional significance of autophagy were not entirely clear, and were potentially related to differential capacity of the autophagy to resolve the stress-induced state depending on the cell type (61).
The activation of autophagy by ER stress, specifically in lung cells, has been demonstrated by Oh and Lim (62). In these studies, WI38 lung epithelial fibroblast cells subjected to ER stress–inducing chemicals (e.g., thapsigargin, MG132) displayed increased accumulation of LC3-II in parallel with activation of the UPR. The capsaicin analog, dihydrocapsaicin (DHC), inhibited WI38 lung cell proliferation, and induced ER stress associated with the expression of Bip/GRP78, and activation of IRE1, C/EBP homologous protein, and caspase-4. DHC induced an autophagic response in these cells characterized by AV formation, which was blocked by 3MA and accumulated by bafilomycin A1. Blocking of DHC-induced autophagy by 3MA enhanced caspase-dependent apoptotic cell death. The siRNA-directed knockdown of IRE1, which compromises ER-stress signaling, attenuated the accumulation of LC3-II in response to DHC treatment. The accumulation of LC3-II in response to DHC also involved the c-Jun NH2-terminal kinase and extracellular signal–regulated kinase–mitogen-activated protein kinase pathways (62). These studies, taken together, indicate that the ER stress response can influence autophagy in transformed lung cells. Autophagic activation during ER stress is currently thought to represent an overflow pathway for the degradation of a toxic accumulation of aggregated protein. Further studies will be required to better define the relationship between the ER pathway and autophagy in the pulmonary context, including studies in normal or primary lung cells. Additional work is also needed to determine the variable environmental and cell-type–specific factors that determine the functional significance of autophagy in lung injury models.
Oxidative Stress
As the route of entry for inhaled O2, the lung, especially the epithelial lining of the alveolae, is particularly susceptible to oxygen toxicity (5). To defend itself against oxygen-related stress, the lung possesses endogenous primary and secondary defenses against oxidative injury. The first-line defenses of the lung against such injury include enzymatic and chemical antioxidant systems that scavenge or detoxify reactive oxygen species (ROS), which may arise through metabolic stress or toxicant exposure. Antioxidant compounds include water and lipid-soluble compounds (e.g., glutathione, ascorbate, vitamin E, uric acid, β-caroteine). The enzymatic antioxidant activities of the lung include superoxide dismutases, catalases, and peroxidases that catalyze the conversion of ROS to derivative forms (63). As a secondary defense, lung cells can respond to oxidative or other forms of stress by up-regulating the synthesis of stress proteins that limit the propagation of injury. Among these, the low–molecular weight stress protein, heme oxygenase (HO)-1, confers cytoprotection against oxidative lung injury (64, 65).
Recent studies implicate oxidative stress or elevated ROS formation in promoting the activation of autophagy (66–68). Chen and colleagues (67) demonstrated that treatment of transformed or cancer cell lines with hydrogen peroxide (H2O2) promoted caspase-independent cell death associated with autophagic activation. The autophagic inhibitor, 3MA, or knockdown of specific autophagy genes (i.e., beclin 1, Atg5, Atg7) with siRNA, prevented H2O2-induced autophagic cell death (67). Similarly, treatment of transformed cell lines with inhibitors of mitochondrial respiration, such as rotenone (complex 1) and TTFA (complex II) increased intracellular ROS production, induced autophagy, and produced a caspase-independent form of cell death (68).
Although activation of autophagy can occur in response to supraphysiological levels of ROS, such as exposure to bolus H2O2, low levels of endogenous ROS may participate in the signaling pathways leading to autophagy. For example, starvation, a classical activator of autophagy, was associated with increased intracellular ROS production in CHO and HeLa cells (69). Treatment of CHO cells with antioxidant (i.e., N-acetyl-L-cysteine) or catalase reduced starvation-induced autophagosome formation. The authors identified Atg4 as a potential target for ROS, and proposed a redox regulation mechanism for the activity of this protein. Both Atg4A (Cys81) and Atg4B (Cys78) contained a critical cysteine residue implicated in redox regulation of the protein (69). Mutations in this residue abolished regulation of autophagy by exogenous H2O2 (69). The recent studies of Chen and colleagues (70), using a panel of inhibitors, suggest that starvation-induced autophagy is regulated primarily by the intracellular production of superoxide anion (O2−).
Relatively few studies have examined the relationship between oxidative stress and autophagy specifically in lung cells. In our recent studies, we found that treatment of human bronchial epithelial cells (Beas-2B) with aqueous cigarette smoke extract (CSE) increased intracellular ROS production (71). CSE treatment also induced autophagy in lung epithelial cells in vitro, as evidenced by increased AV formation by electron microscopic analysis. CSE triggered the accumulation of LC3-II, which was further increased by bafilomcyin A1 and protease inhibitors, indicative of autophagic flux (71, 72). In this context, treatment of Beas-2B cells with N-acetyl-L-cysteine and apopcynin, an inhibitor of reduced nicotinamide adenine dinucleotide phosphate:oxidase–dependent O2− production, impaired CSE-inducible LC3B activation (72). Furthermore, manipulation of the antioxidative stress protein, HO-1, modulated autophagic activation (71). Overexpression of HO-1 suppressed LC3-II accumulation in response to CSE, and also suppressed basal beclin 1 expression. Overexpression of HO-1 in this model was associated with reduction of cell death, in particular with attenuation of the extrinsic apoptotic program. Accordingly, HO-1 siRNA treatments augmented LC3 activation during CSE exposure, and promoted caspase-8–dependent cell death (71). Further experimentation is needed to elucidate the mechanisms by which HO-1 modulates autophagy and the expression of autophagic proteins in the context of its predominately cytoprotective function. Although activation of autophagy promotes epithelial cell death in the specific case of cigarette smoke exposure in vitro (71), further experiments are needed to address the functional significance of autophagy in models involving other forms of oxidative lung cell injury.
Hypoxia
In addition to oxidative stress, autophagy is particularly sensitive to hypoxic activation. Hypoxia has been implicated as a pro-oxidant state, due to impaired respiration and increased mitochondrial production of O2− (73). The hypoxia-inducible factor (HIF)-1 is a major regulator of the hypoxic response in mammals. Recent studies implicate HIF-1 as a major regulator of autophagy during hypoxia (74). Using mouse embryo fibroblasts genetically deleted for Hif-1α, Zhang and colleagues (74) demonstrate a major role for HIFα in the regulation of hypoxia-inducible autophagy and the turnover of damaged mitochondria. These studies also implicated the HIF-1 target gene, Bcl-2 family member Bcl-2/adenovirus E1B 19-kD–interacting protein-3 (BNIP3) in the hypoxic regulation of autophagy (74).
Autophagy has also been shown to be up-regulated in various cultured tumor cell lines by hypoxia. Overexpression or knockdown of BNIP3 modulates hypoxia-induced autophagy in tumor cell lines. Recently, Bellot and colleagues (75) demonstrated that siRNA-dependent knockdown of BNIP3 and BNIP3L caused inhibition of hypoxia-inducible autophagy, whereas overexpression of BNIP3 promoted autophagy under normoxia. These effects were found to depend entirely on the BH3 domain of these proteins. These studies, taken together, suggest that the HIF-1/BNIP pathway functions as a survival mechanism during hypoxia. The studies of Azad and colleagues (76) also implicated BNIP3 in the regulation of autophagy, although they concluded that the pathway results in hypoxic autophagic cell death with prolonged hypoxia. The reasons for these contrasting observations are not completely clear, but underscore the potential dual nature of autophagy in cell survival or cell death, depending on experimental conditions and duration of stimuli. Recent studies also specify a novel role for protein-kinase-C (PKC)δ-dependent signaling in the hypoxic regulation of autophagy (77, 78). Despite the fact that the lung and lung vasculature are major targets of hypoxia, no published reports to date have explored the role of autophagy in acute or chronic pulmonary hypoxia.
Inflammation
ALI elicited by distinct agents, such as high oxygen stress (hyperoxia) or endotoxemia from bacterial LPS exposure are associated with a massive inflammatory response characterized by neutrophil influx into the lung, pulmonary edema, and production of proinflammatory cytokines (79). Although intriguing relationships between autophagy, inflammation, and the activation of Toll-like receptor (TLR) signaling pathways have been recently described (80), the role of autophagy in specific ALI models, such as endotoxin inhalation, remains largely unexplored. Activation of TLRs has been implicated in autophagy induction and autophagosome assembly (81–84). In macrophages, LPS was proposed to induce autophagy through TLR4 in macrophages involving the adaptor, TRIF, receptor-interacting protein 1, and the p38 mitogen-activated protein kinase signaling pathway (81). Autophagy was also shown to be activated by TLR3 and TLR7 ligands (82). The activation of autophagy in macrophages in response to TLR4 activation depends on ROS production from the activation of the phagocytic reduced nicotinamide adenine dinucleotide phosphate oxidase 2 (85). The induction of autophagy by LPS stimulation has recently also been demonstrated in cultured cardiomyocytes, dependent on increased ROS production (86). Because these systems are expected to operate in the lung, in particular in alveolar macrophages, further research will determine their functional significance in the lung or in the context of ALI models.
FUNCTIONAL ROLE OF AUTOPHAGY IN CIGARETTE SMOKE EXPOSURE: A SPECIFIC EXAMPLE OF CONCURRENT AUTOPHAGY AND APOPTOSIS
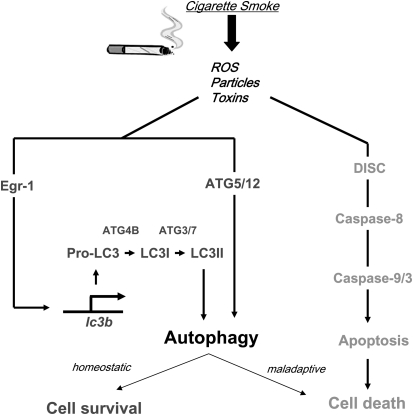
Autophagy is thought to promote survival in starvation and hypoxic states. However, autophagy and apoptosis can sometimes be activated simultaneously. In our recent studies (71, 72), we have observed evidence for concurrent up-regulation of both autophagy and apoptosis in lung epithelial cells subjected to CSE treatment (Figure 3). CSE initiated the extrinsic apoptosis pathway involving assembly of the Fas-associated DISC, activation of caspase-8, and ultimate activation of caspase-3 in epithelial cells. CSE also induced the accumulation of LC3-II, and increased intracellular AV formation in epithelial cells (71, 72). The interplay of the two processes is evident in experiments showing that siRNA-directed knockdown of autophagic regulator proteins inhibited CSE-induced apoptosis in cultured epithelial cells. Specifically, DISC formation was inhibited with siRNA-directed knockdown of beclin 1 or LC3B in CSE-treated epithelial cells (71, 72). The initiating events in apoptosis signaling, such as DISC formation, occurred within 1 hour of CSE treatment, and preceded the activation of LC3B. On the other hand, the activation of executioner caspases occurred despite the activation of autophagy. Thus, although autophagy and apoptosis were concurrently activated in CSE-treated epithelial cells, the relationship of these processes remains unclear (32). Activation of autophagy during CSE exposure may represent a specific case of “xenophagy,” whereby an autophagic response attempts to cope unsuccessfully with an intracellular accumulation of foreign matter. Thus, the climax of apoptosis, despite autophagic activation, is not an unexpected outcome under conditions whereby autophagy insufficiently protects cells against the inducing stimuli.
Figure 3.
Cigarette smoke exposure induces autophagy and apoptosis. Our recent studies indicate that exposure to cigarette smoke extract (CSE) in lung epithelial cells leads to the activation of autophagy. Specifically, CSE activated microtubule-associated protein-1 light chain (LC) 3B, a regulator of autophagosome formation, through an early growth response (Egr)-1–dependent transcriptional mechanism. Furthermore, CSE stimulated the increased expression of other molecules involved in activation of the autophagic pathway, including Atg4B, Atg5, and Atg7, in cultured epithelial cells. CSE exposure concurrently activated the extrinsic apoptotic pathway in lung epithelial cells, involving the activation of the death-inducing signal complex (DISC), and activation of downstream caspase-8, -9, and -3, leading to cell death by apoptosis. In the CSE model, we demonstrated that activation of the autophagic pathway led to increased apoptosis, as siRNA-directed knockdown of LC3B inhibited DISC formation and caspase activation in this model. Further studies are needed to define the relationship between autophagy and apoptosis in the CSE model. ROS = reactive oxygen species.
MOLECULAR REGULATION OF AUTOPHAGY BY CIGARETTE SMOKE: POTENTIAL ROLE FOR EARLY GROWTH RESPONSE–1
To date, little is known about the mechanisms that act to regulate the transcription of autophagy genes. Previously, the transcription factor, early growth response (Egr)-1, has been described as an immediate early response gene that is induced rapidly after various cellular stresses, and which regulates both pro- and antiapoptotic, inflammatory, and oxidant pathways (87). Sequence analysis of the LCB promoter revealed consensus binding sites for Egr-1 and E2F transcription factors. Both factors bound to the LC3B promoter in response to CSE exposure in epithelial cells, as determined by chromatin immunoprecipitation assays. Egr-1 formed a complex with E2F4 in response to CSE stimulation. These results imply E2F4 as a coregulator of the Egr-1–dependent activation of the LC3B gene. During CSE-induced oxidative stress, the activation of autophagy and Egr-1 were associated with the down-regulation of histone deacetylase activity (72). General inhibition of histone deactylase activities with tricostatin A also induced autophagy and Egr-1/E2F4 binding with the LC3B promoter. The role of Egr-1/E2F4 in CSE-induced autophagy was confirmed by observations of inhibited LC3B activation in response to CSE by siRNA-directed knockdown of Egr-1 or E2F4 (72).
Studies of in vivo cigarette smoke exposure have suggested that Egr-1−/− mice were resistant to the proapoptotic and proautophagic effects of environmental cigarette smoke exposure, as well as cigarette smoke–induced airspace enlargement. It should be noted that Egr-1−/− mice basally exhibited airspace enlargement relative to corresponding wild-type controls. Because chronic cigarette smoke exposure did not further increase airspace enlargement in Egr-1−/− mice, these mice were apparently resistant to smoke-induced injury. Whereas Egr-1 plays an important role in mediating cigarette smoke–induced autophagy and apoptosis in vitro, this factor apparently exerts a dominant role in lung homeostasis and growth in vivo. Thus, Egr-1−/− mice reach a critical threshold of airspace enlargement under basal conditions such that further histologic changes induced by cigarette smoke are not observed in vivo (72).
AUTOPHAGY IN COPD
COPD is a complex disease process involving adverse tissue responses to inhaled particles, (i.e., cigarette smoke), which involves tissue destruction (emphysema), bronchitis, and fibrogenesis (88). We have recently described increased autophagy in clinical specimens of the lung from patients with COPD relative to normal tissue, as evidenced by morphological and biochemical markers (72). This evidence included evaluation of lung tissue morphology by electron microscopy, as well as increased expression and activation of autophagic regulator proteins (i.e., LC3B, beclin 1, Atg5, Atg7). Similar evidence of increased autophagy was observed in the lungs of mice that were subjected to chronic inhalation of cigarette smoke (72). The initial screen of autophagic protein activation in human lung tissue from a series of clinical pulmonary conditions revealed the most pronounced activation in COPD. Interestingly, increased autophagy was also observed in the genetic variant of emphysema, α1-antitrypsin deficiency, the etiology of which is not necessarily related to particle inhalation. Unlike the COPD specimens, where comprehensive smoking histories were obtained, no smoking histories were available for the patients with α1-antitrypsin. Nevertheless, these observations might suggest that intrinsic factors, such as increased matrix proteolysis or altered signaling pathways, may contribute to the activation of autophagy in COPD, in addition to direct cellular responses to cigarette smoke. In contrast, activation of autophagy was not observed in specimens from other types of lung disease, including idiopathic pulmonary fibrosis, cystic fibrosis, sarcoidosis, and systemic sclerosis (72). These observations suggest that enhanced autophagy may represent a morphological marker of select pulmonary disease conditions. In clinical samples from patients with COPD, autophagic markers were elevated in early stages of disease progression, whereas caspase activation was detectable at late stages of disease progression. These findings imply that autophagy may precede apoptosis as a general response to chronic cigarette smoke stress in the lung (72).
AUTOPHAGY IN ACUTE PARTICLE INHALATION
Particle inhalation (i.e., silica, diesel fume, dusts) can cause ALI and/or fibrotic lung diseases. Other than those focusing on cigarette smoke inhalation, few studies to date have directly addressed the role of autophagy after particle inhalation. The advent of nanotechnology for biotechnical applications has created new classes of compounds with unclear inhalation toxicological characteristics. The increasing manufacturing output of these substances raises new concerns for industrial hygiene. In a recent report, Li and colleagues (89) demonstrated that nanoparticle formulations can cause ALI associated with the activation of pulmonary autophagy. Mice were subjected to intratracheal administration of the nanoparticle formulation, cationic Starburst polyamidoamine dendrimer (PAMAM; G3). Administration of the autophagy inhibitor, 3MA, to mice alleviated the ALI induced by PAMAM, as evidenced by reduced edema. PAMAM also caused induction of autophagy in cultured lung A549 cells, as evidenced by accumulation of LC3-II. This up-regulation was attributed to deregulation of the mammalian target of rapamycin pathway by PAMAM in A549 cells (89).
AUTOPHAGY IN LUNG CANCER
Autophagy has functional implications in the pathogenesis of cancer and in the pharmacological management of this disease (90, 91). The Bcl-2–interacting protein, beclin 1, a major regulator of autophagy, is frequently monoallelically deleted in many human cancers, including breast, ovarian, and prostate cancers. Beclin 1 functions as a haploinsufficient tumor suppressor gene (92–94). Beclin 1+/− mice display a high frequency of spontaneous malignancies, including lymphomas, hepatocellular carcinomas, and lung tumors (94).
In principle, tumor cells can potentially use autophagy as a survival mechanism in hypoxic states, which may also reduce the efficacy of chemotherapeutic regimens. On the other hand, excessive autophagy may also be associated with tumor cell death. In this regard, enhanced autophagy, leading to autophagic cell death, may be the basis for the efficacy of certain pharmacotherapeutic agents. Thus, manipulation of autophagy, either positively or negatively, could conceivably be applied for therapeutic gain in cancer. Although few studies have been performed specifically in lung cancer, a recent monograph lays the foundation for studying autophagy in human lung cancer (95). Several studies have evaluated the role of autophagy in response to chemotherapeutic agents using cultured human lung A549 adenocarcinoma cells (96, 97). Interestingly, nelfinavir, an HIV protease inhibitor, was recently demonstrated to exhibit antiproliferative activity in lung cancer cells (98). This compound produced a strong ER stress response, which was associated with activation of autophagy (98).
CONCLUSIONS
Autophagy is now recognized as a fundamental homeostatic process operating in most mammalian cell types that ensures basal homeostasis or survival under starvation and/or hypoxic states. Autophagy has previously been implicated in several human diseases, including cancer (90, 91), neurodegenerative disorders (99), and inflammatory bowel disease (100). A general conclusion has been drawn from many of these studies, that autophagy plays a dual role in cell fate, exerting both beneficial consequences as an adaptive response, and deleterious consequences during its dysregulation. The relationships between autophagy and cell death regulation remain incompletely understood, and further examination of these relationships may reveal mechanisms of disease pathogenesis (33–36). The functional outcome of autophagy in determining cell death or survival likely represents an important variable by which autophagy may impact disease pathogenesis in the lung, thereby conferring resistance to adverse stimuli in target cell populations (i.e., during hypoxia exposure) or depleting critical cell populations (as in cigarette smoke exposure).
Manipulation of autophagy or its component regulatory proteins may represent a novel therapeutic strategy in the prevention or treatment of human disease (30, 31). Despite demonstrated activation of autophagy by many agents that are capable of eliciting lung injury, such as oxidants, hypoxia, and proinflammatory states, as reviewed in this article, surprisingly few studies have been performed in lung cells or in the context of human lung disease. In the specific case of cigarette smoke exposure, we observe predominantly a deleterious role of autophagy in epithelial cell responses (71, 72). Studies attempting to correlate autophagy with human pulmonary disease pathogenesis are limited to our own observations of autophagic cells in human COPD (72). Further studies are needed to determine whether autophagy exerts a causative role in the pathogenesis of chronic lung disease.
Acknowledgments
The authors thank Drs. Seon-Jin Lee and Tamas Dolinay for assistance with figures, and Dr. Akaya Smith for proofreading the manuscript.
Supported in part by National Institutes of Health grants R01-HL60234, R01-HL55330, and R01-HL079904 (A.M.K.C.).
Conflict of Interest Statement: S.W.R. has received funding from the noncommercial entity, NIH (more than $100,001). A.M.K.C. has received funding from the noncommercial entity, the National Institutes of Health (NIH) (more than $100,001).
References
- 1.Menzel DB, Amdur MO. Toxic response of the respiratory system. In: Klaassen K, Amdur MO, Doull J, editors. Casarett and Doull's toxicology, the basic science of poisons, 3rd ed. New York, NY, MacMillan Publishing Company; 1986. pp. 330–358.
- 2.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 1982;126:332–337. [DOI] [PubMed] [Google Scholar]
- 3.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc 2008;5:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care 2003;7:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci 2003;1010:405–416. [DOI] [PubMed] [Google Scholar]
- 6.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 2007;30:364–372. [DOI] [PubMed] [Google Scholar]
- 7.Ng CS, Wan S, Yim AP. Pulmonary ischaemia–reperfusion injury: role of apoptosis. Eur Respir J 2005;25:356–363. [DOI] [PubMed] [Google Scholar]
- 8.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 2008;294:L632–L641. [DOI] [PubMed] [Google Scholar]
- 9.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol 2008;294:L601–L611. [DOI] [PubMed] [Google Scholar]
- 10.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004;6:463–477. [DOI] [PubMed] [Google Scholar]
- 11.Kelekar A. Autophagy Ann N Y Acad Sci 2006;1066:259–271. [DOI] [PubMed] [Google Scholar]
- 12.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000;290:1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005;12:1542–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci 2005;118:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 2008;19:5360–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsumi Y, Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol 2004;15:231–236. [DOI] [PubMed] [Google Scholar]
- 18.He H, Dang Y, Dai F, Guo Z, Wu J, She X, Pei Y, Chen Y, Ling W, Wu C, et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J Biol Chem 2003;278:29278–29287. [DOI] [PubMed] [Google Scholar]
- 19.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000;19:5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008;4:151–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 2004;117:2687–2697. [DOI] [PubMed] [Google Scholar]
- 22.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004;2004:4837–4848. [DOI] [PubMed] [Google Scholar]
- 23.Terman A, Gustafsson B, Brunk UT. Autophagy, organelles and ageing. J Pathol 2007;211:134–143. [DOI] [PubMed] [Google Scholar]
- 24.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 2008;15:344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009;5:527–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 2004;15:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanamori H, Takemura G, Maruyama R, Goto K, Tsujimoto A, Ogino A, Li L, Kawamura I, Takeyama T, Kawaguchi T, et al. Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. Am J Pathol 2009;174:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature 2004;432:1032–1036. [DOI] [PubMed] [Google Scholar]
- 29.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005;25:1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GR. Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci (Lond) 2009;116:697–712. [DOI] [PubMed] [Google Scholar]
- 32.Ryter SW, Chen ZH, Kim HP, Choi AM. Autophagy in chronic obstructive pulmonary disease: homeostatic or pathogenic mechanism? Autophagy 2009;5:235–237. [DOI] [PubMed] [Google Scholar]
- 33.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 2005;115:2679–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr Mol Med 2008;8:78–91. [DOI] [PubMed] [Google Scholar]
- 35.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007;8:741–752. [DOI] [PubMed] [Google Scholar]
- 36.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 2008;9:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bursch W, Hochegger K, Torok L, Marian B, Ellinger A, Hermann RS. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J Cell Sci 2000;113:1189–1198. [DOI] [PubMed] [Google Scholar]
- 38.Bursch W, Ellinger A, Gerner C, Fröhwein U, Schulte-Hermann R. Programmed cell death (PCD): apoptosis, autophagic PCD, or others? Ann N Y Acad Sci 2000;926:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem 2006;281:19179–19187. [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA 2006;103:4952–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7–beclin 1 program of autophagic cell death by caspase-8. Science 2004;304:1500–1502. [DOI] [PubMed] [Google Scholar]
- 42.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, Cho DH, Choi B, Lee H, Kim JH, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem 2005;280:20722–20729. [DOI] [PubMed] [Google Scholar]
- 43.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2–interacting protein. J Virol 1998;72:8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004;6:1221–1228. [DOI] [PubMed] [Google Scholar]
- 45.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005;74:739–789. [DOI] [PubMed] [Google Scholar]
- 46.Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, et al. Endoplasmic reticulum stress. Ann N Y Acad Sci 2007;1113:58–71. [DOI] [PubMed] [Google Scholar]
- 47.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 2001;26:504–510. [DOI] [PubMed] [Google Scholar]
- 48.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol 2005;7:766–772. [DOI] [PubMed] [Google Scholar]
- 49.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–529. [DOI] [PubMed] [Google Scholar]
- 50.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000;2:326–332. [DOI] [PubMed] [Google Scholar]
- 51.Jimbo A, Fujita E, Kouroko Y, Ohnishi J, Inohara N, Kuida K, Sakamaki K, Yonehara S, Momoi T. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp Cell Res 2003;283:156–166. [DOI] [PubMed] [Google Scholar]
- 52.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program: an Apaf-1–independent intrinsic pathway. J Biol Chem 2002;277:21836–21842. [DOI] [PubMed] [Google Scholar]
- 53.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress–specific caspase cascade in apoptosis: cytochrome c–independent activation of caspase-9 by caspase-12. J Biol Chem 2002;277:34287–34294. [DOI] [PubMed] [Google Scholar]
- 54.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 2004;11:381–389. [DOI] [PubMed] [Google Scholar]
- 55.Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 2008;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hengstermann A, Müller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response–dependent PERK pathway of cell survival. Free Radic Biol Med 2008;44:1097–1107. [DOI] [PubMed] [Google Scholar]
- 57.Xu D, Perez RE, Rezaiekhaligh MH, Bourdi M, Truog WE. Knockdown of ERp57 increases BiP/GRP78 induction and protects against hyperoxia and tunicamycin-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 2009;297:L44–L51. [DOI] [PubMed] [Google Scholar]
- 58.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem 2005;138:501–507. [DOI] [PubMed] [Google Scholar]
- 59.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 2006;26:9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem 2006;281:30299–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress–induced autophagy on cell survival. J Biol Chem 2007;282:4702–4710. [DOI] [PubMed] [Google Scholar]
- 62.Oh SH, Lim SC. Endoplasmic reticulum stress–mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal–regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther 2009;329:112–122. [DOI] [PubMed] [Google Scholar]
- 63.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 2006;533:222–239. [DOI] [PubMed] [Google Scholar]
- 64.Choi AM, Alam J. Heme oxygenase–1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 1996;15:9–19. [DOI] [PubMed] [Google Scholar]
- 65.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest 1999;103:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal 2006;8:152–162. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 2008;15:171–182. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci 2007;120:4155–4166. [DOI] [PubMed] [Google Scholar]
- 69.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 2007;26:1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 2009;16:1040–1052. [DOI] [PubMed] [Google Scholar]
- 71.Kim HP, Wang X, Chen Z-H, Lee SJ, Huang M-H, Wang Y, Ryter SW, Choi AMK. Autophagic proteins regulate cigarette smoke induced apoptosis: protective role of heme oxygenase-1. Autophagy 2008;4:887–895. [DOI] [PubMed] [Google Scholar]
- 72.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 2008;3:e3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor–1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000;275:25130–25138. [DOI] [PubMed] [Google Scholar]
- 74.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1–dependent adaptive metabolic response to hypoxia. J Biol Chem 2008;283:10892–10903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 2009;29:2570–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 2008;4:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK. Novel roles for protein kinase Cdelta-dependent signaling pathways in acute hypoxic stress–induced autophagy. J Biol Chem 2008;283:34432–34444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen JL, Lin HH, Kim KJ, Lin A, Ou JH, Ann DK. PKC delta signaling: a dual role in regulating hypoxic stress–induced autophagy and apoptosis. Autophagy 2009;5:244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol 2009;10:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007;27:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J 2008;27:1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signaling in macrophages links the autophagy pathway to phagocytosis. Nature 2007;450:1253–1257. [DOI] [PubMed] [Google Scholar]
- 84.Shi CS, Kehrl JH. MyD88 and TRIF target beclin 1 to trigger autophagy in macrophages. J Biol Chem 2008;283:33175–33182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci USA 2009;106:6226–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol 2009;296:H470–H479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu C, Rangnekar VM, Adamson E, Mercola D. Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther 1998;5:3–28. [PubMed] [Google Scholar]
- 88.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 89.Li C, Liu H, Sun Y, Wang H, Guo F, Rao S, Deng J, Zhang Y, Miao Y, Guo C, et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J Mol Cell Biol 2009;1:37–45. [DOI] [PubMed] [Google Scholar]
- 90.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005;5:726–734. [DOI] [PubMed] [Google Scholar]
- 91.Levine B. Cell biology: autophagy and cancer. Nature 2007;446:745–747. [DOI] [PubMed] [Google Scholar]
- 92.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999;402:672–676. [DOI] [PubMed] [Google Scholar]
- 93.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 2003;100:15077–15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qu XYuJ, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 2003;112:1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaboin JJ, Hwang M, Lu B. Autophagy in lung cancer. Methods Enzymol 2009;453:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang JH, Fan CD, Zhao BX, Shin DS, Dong WL, Xie YS, Miao JY. Synthesis and preliminary biological evaluation of novel pyrazolo[1,5-a]pyrazin-4(5H)-one derivatives as potential agents against A549 lung cancer cells. Bioorg Med Chem 2008;16:10165–10171. [DOI] [PubMed] [Google Scholar]
- 97.He Q, Huang B, Zhao J, Zhang Y, Zhang S, Miao J. Knockdown of integrin beta4–induced autophagic cell death associated with P53 in A549 lung adenocarcinoma cells. FEBS J 2008;275:5725–5732. [DOI] [PubMed] [Google Scholar]
- 98.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, et al. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res 2007;13:5183–5194. [DOI] [PubMed] [Google Scholar]
- 99.Lee JA. Autophagy in neurodegeneration: two sides of the same coin. BMB Rep 2009;42:324–330. [DOI] [PubMed] [Google Scholar]
- 100.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 2007;39:207–211. [DOI] [PubMed] [Google Scholar]