Abstract
Vascular hyperpermeability is a clinical complication associated with hemorrhagic shock (HS) and occurs mainly due to the disruption of the adherens junctional complex. The objective of this study was to understand the role of 17β-estradiol in HS-induced hyperpermeability particularly focusing on estrogen receptors. In male Sprague-Dawley rats, HS was induced by withdrawing blood to reduce the mean arterial pressure (MAP) to 40 mmHg for 1 hour followed by 1 hour of resuscitation. to 90 mmHg. The study groups were; 17β-estradiol, tamoxifen, fulvestrant plus 17β-estradiol, propyl pyrazole triol plus 17β-estradiol, and diarylpropionitrite plus 17β-estradiol. Intravital microscopy was utilized to study changes in mesenteric post-capillary venules. Mitochondrial reactive oxygen species formation was studied in vivo utilizing dihydrorhodamine 123. The mitochondrial transmembrane potential (MTP) was studied using the fluorescent cationic probe JC- 1. The mesenteric microvasculature was analyzed for cytochrome c levels by ELISA and caspase-3 activity by a fluorometric assay. Our results demonstrated that 17β-estradiol attenuated HS-induced hyperpermeability. Fulvestrant reversed this protective effect (p<0.05). Tamoxifen 5 mg/kg attenuated HS-induced hyperpermeability where as 10 mg/kg induced permeability (p<0.05). Both α and β estrogen receptor agonists inhibited HS-induced hyperpermeability (p<0.05). 17β-Estradiol decreased HS-induced ROS formation and restored MTP. 17β-Estradiol decreased both cytosolic cytochrome c level and activation of caspase-3 (p<0.05). These findings suggest that 17β-estradiol protects the microvasculature following HS and that this protection may be mediated through the α and β estrogen receptors.
Keywords: ischemia-reperfusion, vascular leak, mitochondrial membrane potential, caspase-3, ROS
INTRODUCTION
Hemorrhagic shock results in the disruption of the microvascular endothelial cell barrier leading to vascular leak (1-3). Hemorrhagic shock is associated with the formation of mitochondrial reactive oxygen species (ROS) and mitochondrial mediated ‘intrinsic’ apoptotic signaling (3-5). Studies from our laboratory have shown that ROS formation and the activation of the “intrinsic” apoptotic signaling cascade are major mediators of vascular leak following hemorrhagic shock (3, 6, 7). The ‘intrinsic’ apoptotic signaling pathway is mediated through the release of cytochrome c, smac (second mitochondrial-derived activator of caspases) and AIF (apoptosis inducing factor) from the mitochondria, all of which are regulated by proapoptotic and antiapoptotic Bcl-2 family proteins, such as Bax/Bak and Bcl-2/xL, respectively (8). Hemorrhagic shock activates the “intrinsic” apoptotic cascade by decreasing the mitochondrial transmembrane potential resulting in the release of cytochrome c into the cytosol which eventually activates caspase-3 (6, 7).
Sex dimorphism in the clinical manifestation of conditions such as trauma, hemorrhage and sepsis have been subjects of recent investigation (9). Studies on the non-reproductive functions of estrogens have rapidly expanded over the last few years supporting the protective effect of estrogen in various organ systems (10-12). Pulmonary injuries following trauma have been shown to be estrus cycle specific with female rats more resistant to injury (13). 17β-Estradiol l has been intensely studied for its protective effects in tissues including the cardiovascular system, central nervous system, immune system and bone (14, 15). Administration of 17β-estradiol following trauma hemorrhage has been shown to restore depressed cardiac, hepatocellular and immune functions (16). It has also been shown that the administration of 17β-estradiol after trauma/hemorrhage improves cardiovascular and hepatocellular functions in male animals (14). 17β-Estradiol is thought to exert its physiological effects via estrogen receptor-α (ER α) and estrogen receptor β (ER β). Functional estrogen receptors are present in endothelial cells (17-19).
We hypothesized that 17β-estradiol attenuates vascular leak following hemorrhagic shock by inhibiting apoptotic signaling. Inhibition of endothelial cell apoptosis by 17βestradiol has been demonstrated by several other investigators (20, 21). We have used a hemorrhagic shock rat model to investigate the role of 17β-estradiol and its interaction with estrogen receptors to regulate vascular leak in mesenteric post-capillary venules. We have also investigated the effect of 17β-estradiol on mitochondrial ROS formation, mitochondrial transmembrane potential and mitochondrial release of cytochrome c and caspase-3 activation.
MATERIALS AND METHODS
Reagents
17β-Estradiol, propyl pyrazole triol, diarylpropionitrite, FITC-albumin, dihydrorhodamine 123, fulvestrant and tamoxifen were obtained from Sigma (St. Louis, MO). FITC-albumin solution was prepared by dissolving FITC-albumin into saline (50 mg/kg). JC- 1 (5,5′, 6,6′ tetrachloro- 1,1′, 3, 3′ tetraethylbenzimidazolyl carbocyanine iodide) was obtained from Cell Technology Inc. (Mountain View, CA). The JC-1 reagent was prepared by reconstituting the lyophilized reagent with 500μl of dimethyl sulfoxide (DMSO) to obtain a 100X stock solution. Immediately prior to the experiments the 100X solution was diluted 1:100 in 1X assay buffer. DMSO at this dilution does not have significant effect on vascular leak.
Animals
Adult male Sprague-Dawley rats (275-325 g) obtained from Charles River Laboratories (Wilmington, MA) were housed in the institutional animal facility at Texas A&M Health Science Center College of Medicine and Scott and White Memorial Hospital, Temple, Texas, USA. The rats were maintained on a 12:12 hour dark/light cycle, with free access to food and water. The care and use of all animals and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the Institutional Animal Care and Use Committee.
Hemorrhagic shock and intravital microscopy
Rats were fasted for 18 hours prior to each experiment and were given ad libitum water. The animals were anesthetized by a single intramuscular injection of 50% urethane (1.5g/kg). Polyethylene tubing (PE-50, 0.58 mm ID) was placed in the right internal jugular vein for drug and fluid resuscitation (normal saline; 3 ml/hr) by continuous infusion pump (Harvard Apparatus, South Natick, MA) and into the right carotid artery for blood withdrawal. The mean arterial pressure (MAP) was monitored continuously using a PE-50 (0.58 mm ID) cannula placed in the left femoral artery connected to a blood pressure analyzer (Dig-Med, BPA 400A, Micromed, Louisville, KY). A midline laparotomy incision was performed. The rats were placed in a lateral decubitus position on a Plexiglas platform mounted to an intravital upright microscope (Nikon E600, Tokyo, Japan). A section of the mesentery from the proximal ileum was exposed. The mesenteric vasculature was maintained at 37 °C. The mesentery was superfused with normal saline at 2 ml/min via cannula while covered with plastic wrap to reduce evaporation. Postcapillary venules with diameters of 20 to 35 μm were selected for study with a Nikon 20× objective, 0.45 to 2.16 mm working distance (Nikon Instruments, Inc., Natick, MA). The same venule was studied throughout each experiment. Images were obtained with a Photometric Cascade Camera (Roper Scientific, Tucson, AZ), projected onto a computer monitor and captured digitally on computer disc. The data analysis was performed using MetaMorph 4.5/4.6 (Universal Imaging Corp., Downingtown, PA) (6, 7). An equilibration period of 30 minutes was given prior to each experiment to allow the animals to recover from surgical manipulation. During this period, the animals were given FITC-albumin (50 mg/kg) and baseline-integrated optical intensities were obtained intra- and extravascularly (two sites, same computed areas, the mean values were used). To produce hemorrhagic shock, the MAP was decreased to 40 mmHg by withdrawing blood from the right carotid artery into a syringe containing 100 units of heparin. Blood was withdrawn in approximately five minutes. To obtain level IV hemorrhagic shock, removal of approximately 50-60% of the animal's blood volume is required. After the shock period, the shed blood plus normal saline was reinfused within 5 minutes to maintain a MAP at or above 90 mmHg. The infusion was done at the rate of approximately 1 ml/minute. Mesenteric postcapillary venules in a transilluminated segment of small intestine were examined to quantitate changes in albumin flux. Parameters were recorded immediately after the shock period (post shock) at 10 minute intervals for 60 minutes.
Vascular hyperpermeability in vivo
The rats were divided into the following groups: sham-control (n=5), hemorrhagic shock (HS; n=5), HS group plus 17β-estradiol (2 mg/kg; n=5), HS group plus the estrogen receptor antagonist fulvestrant (1 mg/kg; n=5) and 17β-estradiol, HS group that received selective estrogen receptor modulator tamoxifen (5 mg/kg; n=4), a group treated with tamoxifen alone (10 mg/kg; n=4) and two groups of rats that received the estrogen receptor α agonist propyl pyrazole triol (PPT; 5 mg/kg; n=5) or the β receptor agonist diarylpropionitrite (DPN; 5 mg/kg; n=5). The agonists/antagonists were injected intravenously 10 minutes prior to the shock period. The FITC-albumin flux was measured by determining the changes in integrated optical intensity. The changes in optical intensity were determined in the extravascular space relative to the intensity inside the lumen. The following formula was used for calculating change in optical intensity; AI=1-(Ii-Io/Ii) where AI is change in fluorescent intensity, Ii is fluorescent intensity inside the vessel, and Io is fluorescent intensity outside the vessel (6, 7).
Additional experiments were performed to determine if the attenuation of vascular leak with 17β-estradiol correlated with a reduction in volume necessary to maintain a MAP at or above 90 mmHg.
Mitochondrial ROS formation in vivo
The rats were divided into the following groups: sham-control (n=4), hemorrhagic shock (HS) (n=4), HS plus 17β-estradiol (2 mg/kg) (n=4). 17β-Estradiol was given 10 minutes prior to the shock period. HS was induced as described above. Visualization and quantification of ROS from the mesenteric postcapillary venules was performed using dihydrorhodamine 123 (DHR 123) (50 mg/kg) by intravital microscopy. Images of the mesenteric venules were obtained prior to the shock period and at 10, 20, 30, 40, 50 and 60 minutes into resuscitation. To determine the relative expression of ROS, the fluorescent intensity was measured in two adjacent areas along the vessel using MetaMorph 4.5/4.6 (Universal Imaging Corp., Downingtown, PA). Values of DHR 123 fluorescence were expressed as change in intensity over time versus baseline values.
Mitochondrial transmembrane potential in vivo
The rats were divided into the following groups: sham-control (n=4), hemorrhagic shock (n=4) and HS group that received 17β-estradiol (2 mg/kg( (n=4). 17β-Estradiol was given 10 minutes prior to the shock period. The mesenteric vasculature was superfused with JC-1 reagent (1:100) to measure changes in mitochondrial transmembrane potential utilizing intravital microscopy. The JC-1 reagent was superfused over the exposed mesenteric vessels at a volume of 300μl into a bath of 2 mls of saline. The JC-1 rapidly diffused into the vasculature and was detected in the endothelial cells. The use of JC-1 for determination of the changes mitochondrial transmembrane potential of rats in vivo was previously published from our laboratory (6, 22).
Cytochrome c release
The rats were divided into the following groups: sham-control group (n=5), HS group (n=5), HS group plus 17β-estradiol (2 mg/kg), and 17β-estradiol alone group. 17βEstradiol was given 10 minutes prior to the shock period. Cytochrome c assay from the cytosolic fraction of the rat mesenteric tissue was performed by using an ELISA kit (R&D Systems, Minneapolis, MN, USA). The mesenteric vessels were dissected from the rat, weighed, and homogenized in a cold preparation buffer (10 mM Tris _HCl pH 7.5, 0.3 M sucrose, 10 μM apoptinin, 10 μM pepstatin, 10 μM leupeptin, 1 mM PMSF). The vasculature was carefully dissected to remove the adipose tissue. The tissue homogenates were centrifuged (10,000 g for 60 minutes at 4°C), and the supernatant (cytosol fraction) was collected and subjected to protein estimation. The samples were then treated with a conjugate reagent, transferred to microwell strips coated with anti-cytochrome c antibody, and incubated for 60 minutes at room temperature. The samples were treated with a peroxidase substrate reagent and incubated for 15 minutes at room temperature. After the addition of a stop solution (0.5 M H2SO4), the optical density of each well was measured at 450 nm. The concentration of cytochrome c was calibrated from the standard curve.
Casapse-3 activity
The rats were divided into the following groups: sham-control group (n=5), HS group (n=5), HS group plus 17β-estradiol (2 mg/kg) (n=5), and sham group treated with 17β-estradiol alone (n=5). 17β-Estradiol treatment was performed 10 minutes prior to the shock period. Caspase-3 activity was determined using a fluorometric assay (Calbiochem, La Jolla, CA). The mesenteric micro-vessels harvested from the animals were homogenized in caspase-3 sample lysis buffer provided in the assay kit. The homogenates were centrifuged at 10,000 rpm and the resulting supernatant was used for protein estimation and caspase-3 assays. Active caspase-3 cleaves after aspartate residues in a particular peptide sequence (DEVD). The DEVD substrate was labeled with a fluorescent molecule, 7-amino-4-trifluoromethyl coumarin (AFC). The lysates were treated with the substrate conjugate and the resulting fluorescence was measured in a fluorescent plate reader at 400 nm/505 nm.
Statistics
All data are expressed as mean ± SE. Statistical analysis was performed utilizing analysis of variance (ANOVA) followed by the Bonferroni's post-test for multiple comparisons. A ‘p’ value of < 0.05 was considered to indicate a significant difference. In vascular leak studies, each experimental value was compared to the initial baseline value and expressed as percentage-change. This method decreases bias between animals due to hematocrit levels and changes in room lighting.
RESULTS
1 7β-Estradiol attenuated vascular hyperpermeabiity following hemorrhagic shock
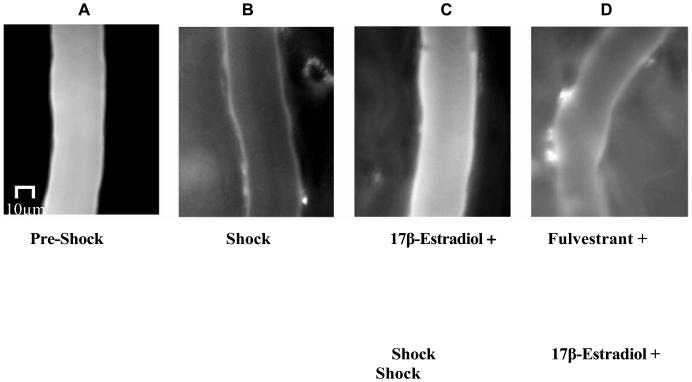
Hemorrhagic shock-induced vascular leak was evidenced by a significant increase in extravasation of FITC-albumin into the extravascular space versus the sham-operated group (p< 0.05; n=5; Figures 1 and 2). 17β-Estradiol (2 mg/kg) significantly attenuated HS-induced vascular leak compared to the hemorrhagic shock alone group (p< 0.05; n=5; Figures 1 and 2). Estrogen receptor antagonist fulvestrant inhibited the protective effect of 17β-estradiol (p< 0.05; n=5; Figures 1 and 2). Figure 1 (A) is a composite image of the rat's mesenteric postcapillary venule prior to HS (pre-shock) demonstrating minimal extravasation of FITC -albumin into the extravascular space; (B) taken following 60 minutes of HS and 60 minutes of resuscitation, demonstrates marked extravasation of FITC-albumin into the extravascular space; (C) corresponds to the rat's vasculature pre-treated with 17β-estradiol (10 minutes prior to the hemorrhagic shock period). 17βEstradiol significantly attenuated the extravasation of FITC albumin into the extravascular space; (D) corresponds to a rat that received estrogen receptor antagonist fulvestrant which inhibited the protective effect of 1 7β-estradiol.
Figure-1.
17β-Estradiol inhibits hemorrhagic shock (HS)-induced vascular leak in rat mesentery post-capillary venules. Intravital microscopy images show FITC -albumin extravasation in shock but not in pre-shock condition (Panels A and B). 17β-Estradiol treatment leads to a decrease in FITC-albumin extravasation in shock (Panel C). The estrogen receptor antagonist fulvestrant inhibits the protective effect of 17β-estradiol (Panel D). The treatments were given 10 minutes prior to the hemorrhagic shock period and image taken after 60 minutes of HS and 60 minutes of resuscitation.
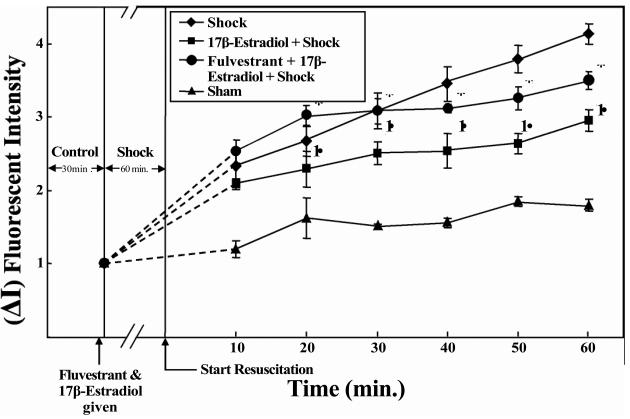
Figure-2.
17β-Estradiol inhibits hemorrhagic shock-induced vascular leak in rat mesentery post-capillary venules. Hemorrhagic shock induces significant increase in FITC-albumin extravasation. 17β-Estradiol treatment significantly decreases this effect. † Significant difference vs. shock group (p < 0.05). Estrogen receptor antagonist fulvestrant inhibits the protective effect of 17β-estradiol. * Significant difference vs. 117β-estradiol + hemorrhagic shock group (p < 0.05).
Figure 2 is a graphic representation of the changes in vascular leak. The change in vascular leak is expressed as change in fluorescent intensity inside the vessel versus fluorescent intensity outside the vessel. Hemorrhagic shock-induced a significant increase in FITC-albumin extravasation (p < 0.05; n = 5). 17β-Estradiol pre-treatment significantly decreased vascular leak (p <0.05; n = 5). The estrogen receptor antagonist fulvestrant inhibited the protective effect of 17β-estradiol (p <0.05; n = 5) suggesting a receptor- mediated event.
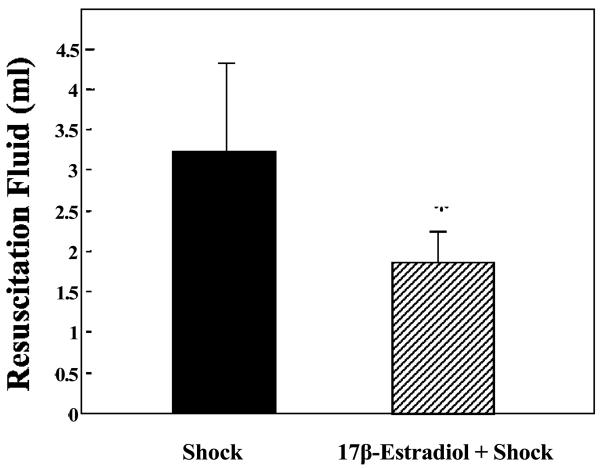
To better understand the significance of 17β-estradiol attenuation of HS-induced vascular leak, we evaluated the volume of saline necessary to resuscitate our animals following HS. 17β-Estradiol (2 mg/kg) significantly reduced the fluid requirement compared with the hemorrhagic shock alone group (p <0.05, Figure-3).
Figure-3.
17β-Estradiol treatment reduced the amount of resuscitation fluid required to achieve a MAP of 40 mmHg. * Significant difference vs. hemorrhagic shock group and 17β-estradiol + hemorrhagic shock groups (p <0.05).
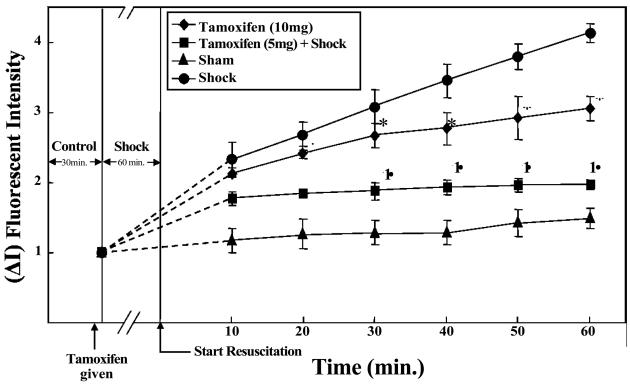
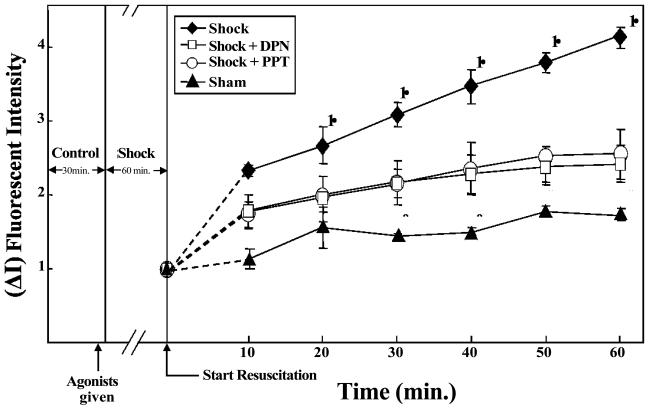
The estrogen receptor modulator tamoxifen showed variation in its response; at a dose of 5 mg/kg tamoxifen attenuated HS-induced vascular leak compared with tamoxifen at 10 mg/kg which induced vascular leak (p<0.05, n = 4; Figure-4). Tamoxifen has previously shown a dose dependent variation in response, with lower doses acting like estradiol and higher doses acting as an estrogen receptor antagonist.
Figure-4.
Estrogen receptor modulator tamoxifen induces differential effects on vascular leak in rat mesentery post capillary venules. Hemorrhagic shock induces significant increase in FITC-albumin extravasation. Tamoxifen 5 mg/kg inhibits HS-induced vascular leak whereas 10 mg/kg induces leak. † Significant difference vs. shock group (p <0.05). * Significant difference vs. sham group (p < 0.05).
Treatment with both estrogen receptor α agonist PPT (5 mg/kg) and β agonist DPN decreased HS-induced vascular leak. HS groups that received PPT and DPN showed significantly lower FITC-albumin extravasation compared to the HS alone group (p<0.05, n = 5; Figure-5).
Figure-5.
Estrogen receptor alpha agonist PPT and beta agonist DPN attenuate vascular leak following hemorrhagic shock in rat mesentery post capillary venules. Hemorrhagic shock induces significant increase in FITC -albumin extravasation. PPT and DPN DPN groups (p <0.05). * Significant difference vs. shock group (p <0.05).
1 7β-Estradiol decreased mitochondrial ROS formation
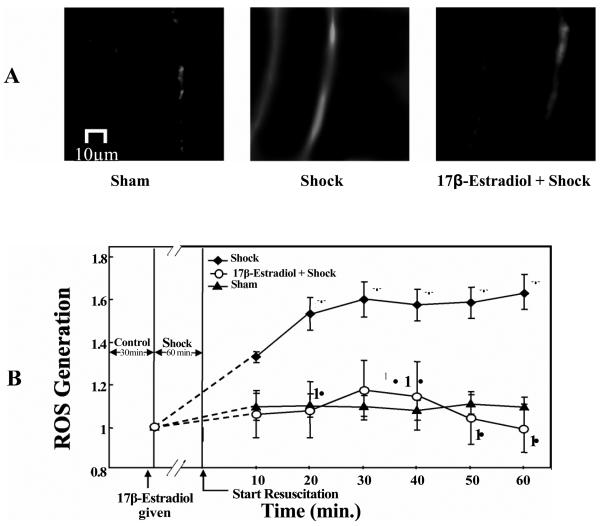
Hemorrhagic shock-induced animals showed a significant increase in ROS formation compared to the sham-control group (p< 0.05; n = 4; Figure 6 A, B). The hemorrhagic shock plus 17β-estradiol (2 mg/kg) group showed a significantly lower ROS formation compared to the hemorrhagic shock group alone (p< 0.05; n = 4; 6 A and B). In figure 6A, the first image is a composite image of a rat's mesenteric postcapillary venule from the pre-shock period demonstrating minimal ROS formation. The second image, taken from a rat after 60 minutes of HS and 60 minutes of resuscitation, demonstrates a marked increase in ROS formation. The third image in figure 6A represents attenuation of ROS formation by 17β-estradiol pre-treatment. Figure 6 B is a graphic representation of the changes in ROS formation in the sham-control, hemorrhagic shock, and hemorrhagic shock group plus 17β-estradiol (2mg/kg) groups.
Figure-6.
(A and B) 17β-Estradiol prevents hemorrhagic shock induced ROS formation in rat mesentery. The images shown are of mesenteric post-capillary venules of sham, hemorrhagic shock for 1 hour MAP 40 mm Hg followed by 60 minutes of resuscitation and estradiol treatment 10 minutes prior to hemorrhagic shock (17β-estradiol + Shock) are shown. Increased ROS formation is observed following hemorrhagic shock whereas 173-estradiol treatment decreased the ROS formation. (B) ROS formation is expressed based on the changes in fluorescent intensity. Hemorrhagic shock induces ROS formation compared to sham-control group. 17β-Estradiol treatment prevents HS-induced ROS formation compared with hemorrhagic shock group without 17β-estradiol. * Significant difference vs. sham group (p <0.05). Significant difference vs. shock group (p <0.05).
1 7β-Estradiol decreased mitochondrial transmembrane depolarization
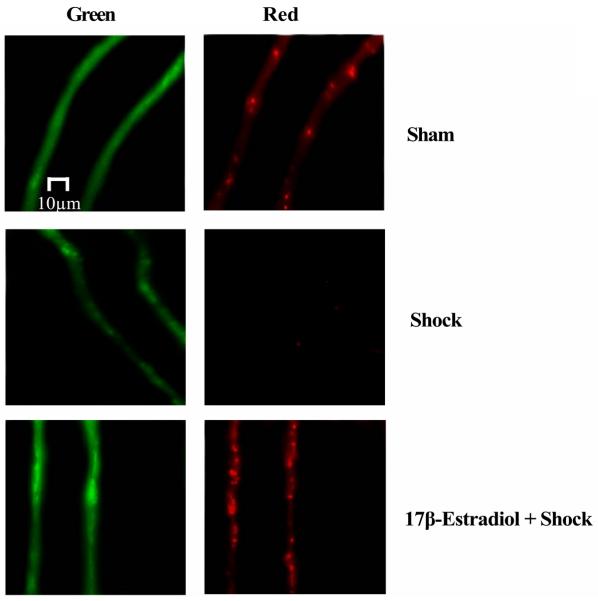
Opening of the mitochondrial permeability pores in response to an apoptotic stimuli results in immediate dissipation of the mitochondrial membrane potential and consequent loss of cytochrome c into the cytosol. We utilized a novel approach to monitor mitochondrial membrane potential in vivo utilizing JC- 1 (5,5′, 6,6′ tetrachloro- 1, 1′, 3,3′ tetraethylbenzimidazolyl-carbocyanine iodide) as described previously (11, 37). In nonapoptotic cells, JC-1 exists as a monomer in the cytosol (green) and accumulates as J-aggregates in the mitochondria, which fluoresce red. Upon the induction of apoptosis, JC1 exists only in a monomeric form (green). Following 60 minutes of hemorrhagic shock and 60 minutes of resuscitation, there was a significance decrease in red (mitochondrial) fluorescence indicating the loss of mitochondrial transmembrane potential versus the sham-control group where the red fluorescence remained stable (Figure 7; n = 4). Pre-treatment of rats with 1 7β-estradiol 10 minutes prior to hemorrhagic shock prevented the loss of mitochondrial transmembrane potential evidenced by the presence of a strong red fluorescence signal (Figure 7; n = 4).
Figure-7.
17β-Estradiol prevents hemorrhagic shock-induced decrease in mitochondrial transmembrane potential. In sham-control, the JC-1 fluoresced the mitochondria red (Cy3) and the cytoplasm green (FITC), indicating intact mitochondria. Hemorrhagic shock for 1 hour MAP 40 mm Hg followed by 60 minutes of resuscitation induced the collapse of membrane potential allowing diffusion of the red (Cy3) fluorescence into the cytoplasm. 17β-estradiol treatment 10 minutes prior to hemorrhagic shock prevents the decrease in membrane potential (leakage of JC- 1).
1 7β-Estradiol decreased mitochondrial cytochrome c release
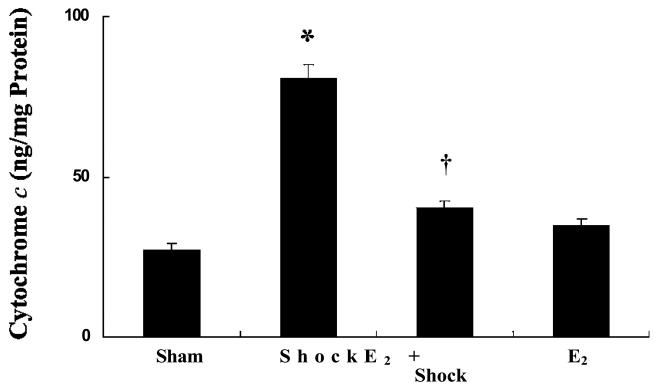
The release of cytochrome c from the mitochondria into the cytosol has been reported to be the key event in the intrinsic apoptosis cascade induced by various stimuli. Mesenteric vascular tissue levels of cytosolic cytochrome c were measured from sham-control, HS, and HS animals pre-treated with 17β-estradiol (Figure 8). The cytosolic cytochrome c level was elevated following hemorrhagic shock compared to the sham-control group (p < 0.05; n = 5). The hemorrhagic shock animals group pre-treated with 17β-estradiol showed a significantly lower level of cytosolic cytochrome c compared with the HS alone group (p< 0.05; n = 5; Figure 8).
Figure-8.
17β-Estradiol inhibits hemorrhagic shock-induced cytochrome c release in mesenteric vasculature. Cytosolic cytochrome c levels increased significantly 60 minutes after resuscitation compared with sham-control. 17β-Estradiol inhibited hemorrhagic shock- induced increase in cytochrome c levels. * Significant difference vs. sham group (p < 0.05). Significant difference vs. shock group (p < 0.05). 173-Estradiol treated group shows no significant change in vascular leak compared with sham group.
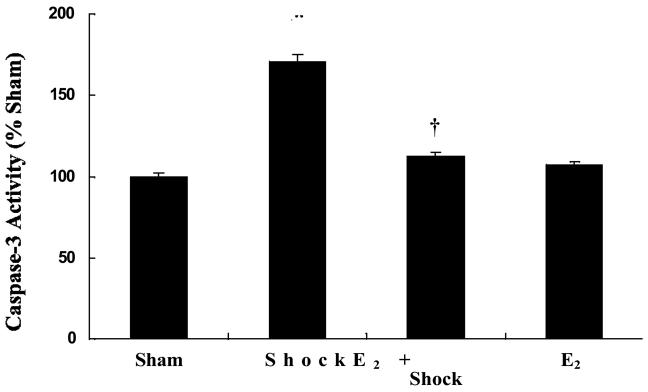
1 7β-Estradiol attenuated caspase-3 activation
Release of cytochrome c from the mitochondria into the cytosol results in the activation of caspase-3. Activation of caspase-3 was determined from the rat mesenteric vasculature. The hemorrhagic shock group showed a significant increase in caspase-3 activity compared to the sham-control group (p< 0.05; n = 5; Figure 9). The hemorrhagic shock group pre-treated with 17β-estradiol showed significantly lower caspase-3 activity compared with hemorrhagic shock alone group (p< 0.05; n = 5; Figure 9).
Figure-9.
17β-Estradiol inhibits hemorrhagic shock-induced caspase-3 activation in mesenteric vasculature. Caspase-3 activity increased significantly after shock at 60 minutes after resuscitation compared with sham-control. 17β-Estradiol inhibited hemorrhagic shock-induced caspase-3 activation. * Significant difference vs. sham group (p < 0.05; n = 5). Significant difference vs. shock group (p < 0.05). 17β-Estradiol treated group shows no significant change in permeability compared with sham group.
DISCUSSION
This study using a rat model demonstrated that 17β-estradiol provided protection against vascular leak following hemorrhagic shock. Further, our studies suggested involvement of both estrogen receptors (α and β). We also delineated that oxidative stress and apoptotic signaling are involved in mediating this protective effect. These findings support our original hypothesis that activation of the “intrinsic” apoptotic signaling cascade induces vascular leak following hemorrhagic shock.
In addition to its major role in the female reproductive system, estradiol has been shown to play important roles in non-reproductive systems such as the cardiovascular system, immune system and the central nervous system (10, 14). Our results in male rats suggest that 17β-estradiol has a protective role on the endothelial cell barrier that regulates vascular leak. It has been shown recently that 17β-estradiol has a protective function in hepatic microcirculation following ischemia reperfusion (9). The physiological effects of 17β-estradiol are mainly mediated through estrogen receptors. Estrogen receptors (α and β) are expressed in several tissues of male rats including the reproductive tract (23) and vascular endothelial cells (24). The results from our study show that the protective effect of 117β-estradiol against HS-induced vascular leak was reversed by the estrogen receptor antagonist fulvestrant, which blocks both α and β estrogen receptors. Fulvestrant, also known as ICI 182780, is the first in a new class of novel, ‘pure’ antiestrogens-estrogen receptor down-regulators (25). Lu et al (15) showed that fulvestrant prevented the protective effects of 17β-estradiol in H2O2 induced apoptosis in rat endothelial cells (15).
Results from our study showed that the dose dependent estrogen receptor modulator tamoxifen had a biphasic response to HS-induced vascular leak. At 5 mg/kg, tamoxifen attenuated HS-induced vascular leak whereas at 10 mg/kg, tamoxifen enhanced vascular leak. While the effect of the lower dose of tamoxifen is attributed to the estrogen agonistic property, the opposite effect observed by the higher dose is due to its antagonistic role at the estrogen receptors (25). In addition to its effect on estrogen receptors, tamoxifen is also known to have anti-oxidant properties with scavenging effects on ROS (26). It is possible that in our study the lower dose of tamoxifen acted as a receptor antagonist as well as an ROS scavenger.
We further demonstrate involvement of the estrogen receptors in mediating protection by inhibiting both α and β estrogen receptors with propyl pyrazole triol (PPT) and diaryl propionitrile (DPN), respectively. These drugs are selective estrogen receptor ligands. Estrogen receptors α and β are present on the mitochondria of cells and are shown to mediate many protective effects of estrogens (20, 15, 27). It has also been shown that the functional estrogen receptors are present on the mitochondria of endothelial cells (15, 17, 19). Recent studies showed the involvement of estrogen receptors in mediating the beneficial effects of 17β-estradiol following vascular injury (14, 10, 12). In this study we also show that both α and β are involved in facilitating the protective effects of 17β-estradiol l against HS-induced vascular leak.
Results from our study show that 17β-estradiol l inhibited HS-induced mitochondrial ROS formation. Hemorrhagic shock has been demonstrated to induce oxidative stress and reactive oxygen species formation (3, 4,28,29). Ischemia-reperfusion selectively damages endothelial cells through a mechanism that involves oxygen-derived free radicals (30, 31). Studies from our laboratory have shown that oxidative stress due to ROS formation, particularly ROS from mitochondrial complex-III of the respiratory chain, is a major trigger for the “intrinsic” apoptotic signaling cascade. In addition, a close relationship between mitochondrial ROS formation and mitochondria mediated apoptotic signaling in the context of vascular leak has been well established in our laboratory (3, 22), although the specific role of ROS in activating various apoptotic-signaling pathways and their intracellular site of origin and/or their relationship to barrier dysfunction has not been well established. Mitochondrial oxidative stress caused by ROS generation following reperfusion injury has been shown to regulate the mitochondrial release of cytochrome c (21, 32). Studies by Morkuniene et al (33) have shown that 17β-estradiol prevented the ischemia-induced release of cytochrome c from mitochondria and caspase activation in isolated rat hearts. Mitochondrial release of cytochrome c is regulated by the change in mitochondrial permeability transition. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia (34).
17β-Estradiol has been shown to attenuate H2O2 induced rat endothelial cell apoptosis by inhibiting cytochrome c release and caspase-3 activation (15). Thus, the inhibition of apoptotic signaling particularly at the level of cytochrome c release and subsequent caspase-3 activation by 17β-estradiol seems to be a major mechanism by which it regulates vascular leak following hemorrhagic shock. The activation of caspase-3 leads to the cleavage of a variety of cell adhesion proteins (33). β-Catenin, a member of the Armadillo repeat protein family, functions as a regulator of the cadherin-mediated cell-cell adhesion in endothelial cells and in its absence vascular leak occurs (35). Proteolytic cleavage of β-catenin occurs following the activation of procaspase-3, -6 or -8 (33,36-38). Cleavage of β-catenin was found to be caspase-dependent (39). Previous studies from our laboratory have shown that caspase-3 inhibition attenuated HS-induced vascular leak and hemorrhagic shock mediates disruption of β-catenin (6, 7). We hypothesized that 17β-estradiol mediated inhibition of caspase-3 activation and subsequent prevention of microvascular endothelial cell-cell disruption, were possible mechanism(s) that prevents vascular leak following hemorrhagic shock. However, future studies showing direct involvement of 17β-estradiol at the adherens junctions are required to establish this hypothesis.
In summary, this study demonstrates that 17β-estradiol inhibits hemorrhagic shock-induced vascular leak. 17β-Estradiol mediates this protective effect through estrogen receptors. This protective effect of 17β-estradiol on the endothelial cell barrier is at least partially mediated through the inhibition of the mitochondrial-mediated “intrinsic” apoptotic signaling pathway.
Acknowledgments
GRANT SUPPORT:
This work was supported by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (K01-HL-07815-01A1).
REFERENCES
- 1.Baluk P, Hirata A, Thurston G, Fujiwara T, Neal CR, Michel CC, McDonald DM. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am J Physiol. 1997;272:155–170. doi: 10.1152/ajplung.1997.272.1.L155. [DOI] [PubMed] [Google Scholar]
- 2.McDonald DM. Endothelial Gaps: Plasma Leakage During Inflammation. News PhysiolSci. 1998;13:104–105. doi: 10.1152/physiologyonline.1998.13.2.104. [DOI] [PubMed] [Google Scholar]
- 3.Childs EW, Udobi KF, Wood JG, Hunter FA, Smalley DM, Cheung LY. In vivo visualization of reactive oxidants and leukocyte-endothelial adherence following hemorrhagic shock. Shock. 2002;18:423–427. doi: 10.1097/00024382-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Davidson MT, Deitch EA, Lu Q, Hasko G, Abungu B, Nemeth ZH, Zaets SB, Gaspers LD, Thomas AP, Xu DZ. Trauma-hemorrhagic shock mesenteric lymph induced endothelial apoptosis that involves both caspase-dependent and caspase-independent mechanisms. Ann Surg. 2004;240:123–131. doi: 10.1097/01.sla.0000129341.94219.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauriz JL, Gonzalez P, Jorquera F, Olcoz JL, González-Gallego J. Caspase inhibition does not protect against liver damage in hemorrhagic shock. Shock. 2003;19:33–37. doi: 10.1097/00024382-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am JPhysiol Heart Circ Physiol. 2007;292P:H 3 179–89. doi: 10.1152/ajpheart.01337.2006. [DOI] [PubMed] [Google Scholar]
- 7.Childs EW, Tharakan B, Byrge N, Tinsley JH, Hunter FA, Smythe WR. Angiopoietin-1 inhibits intrinsic apoptotic signaling and vascular hyperpermeability following hemorrhagic shock. Am JPhysiol Heart Circ Physiol. 2008;294:H2285–95. doi: 10.1152/ajpheart.01361.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. JBiol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt M, Slotta JE, Garcia P, Seekamp A, Menger MD, Pohlemann T. The effect of estrogen on hepatic microcirculation after ischemia/reperfusion. Int J Colorectal Dis. 2008;23:113–119. doi: 10.1007/s00384-007-0360-5. [DOI] [PubMed] [Google Scholar]
- 10.Duckles SP, Krause DN, Stirone C, Procaccio V. Estrogen and mitochondria: a new paradigm for vascular protection? Mol Interv. 2006;6:26–35. doi: 10.1124/mi.6.1.6. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand F, Thobe BM, Hubbard WJ, Choudhry MA, Pape HC, Chaudry IH. Effects of 17beta-estradiol and flutamide on splenic macrophages and splenocytes after trauma-hemorrhage. Cytokine. 36:107–114. doi: 10.1016/j.cyto.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Chaudry IH. Salutary effects of 17beta-estradiol on T-cell signaling and cytokine production after trauma-hemorrhage are mediated primarily via estrogen receptor-alpha. Am J Physiol Cell Physiol. 2007;292:C2103–111. doi: 10.1152/ajpcell.00488.2006. [DOI] [PubMed] [Google Scholar]
- 13.Caruso JM, Xu DZ, Lu Q, Dayal SD, Deitch EA. The female gender protects against pulmonary injury after trauma hemorrhagic shock. Surg Infect (Larchmt) 2001;2:231–240. doi: 10.1089/109629601317202713. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann Surg. 2000;232:673–679. doi: 10.1097/00000658-200011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu A, Frink M, Choudhry MA, Schwacha MG, Hubbard WJ, Rue LW, 3rd, Bland KI, Chaudry IH. Mitochondria play an important role in 17beta-estradiol attenuation of H(2)O(2)-induced rat endothelial cell apoptosis. Am J Physiol Endocrinol Metab. 2007;292:E585–593. doi: 10.1152/ajpendo.00413.2006. [DOI] [PubMed] [Google Scholar]
- 16.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:8 1–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 17.Colburn P, Buonassisi V. Estrogen-binding sites in endothelial cell cultures. Science. 1978;201:817–819. doi: 10.1126/science.684408. [DOI] [PubMed] [Google Scholar]
- 18.Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation. 1996;94:727–733. doi: 10.1161/01.cir.94.4.727. [DOI] [PubMed] [Google Scholar]
- 19.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage (Review) Oncol Rep. 2005;14:595–599. [PubMed] [Google Scholar]
- 20.Alvarez RJ, Gips SJ, Moldovan N, Wilhide CC, Milliken EE, Hoang AT, Hruban RH, Silverman HS, Dang CV, Goldschmidt-Clermont PJ. 17beta-estradiol inhibits apoptosis of endothelial cells. Biochem Biophys Res Commun. 1997;237:372–381. doi: 10.1006/bbrc.1997.7085. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. JNeurosci. 2002;22:209–2 17. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tharakan B, Hunter FA, Smythe WR, Childs EW. Alpha-lipoic acid attenuates hemorrhagic shock-induced apoptotic signaling and vascular hyperpermeability. Shock. 2008;30:571–577. doi: 10.1097/SHK.0b013e31816a7308. [DOI] [PubMed] [Google Scholar]
- 23.Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. JAndrol. 1997;18:602–11. [PubMed] [Google Scholar]
- 24.Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res. 1998;83:224–9. doi: 10.1161/01.res.83.2.224. [DOI] [PubMed] [Google Scholar]
- 25.Robertson JF. Estrogen receptor downregulators: new antihormonal therapy for advanced breast cancer. Clin Ther. 2002:A17–30. doi: 10.1016/s0149-2918(02)85032-9. [DOI] [PubMed] [Google Scholar]
- 26.Yin Y, Huang WW, Lin C, Chen H, MacKenzie A, Ma L. Estrogen suppresses uterine epithelial apoptosis by inducing birc1 expression. Mol Endocrinol. 2008;22:113–25. doi: 10.1210/me.2007-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yager JD, Chen JQ. Mitochondrial estrogen receptors-new insights into specific functions. Trends Endocrinol Metab. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Childs EW, Udobi KF, Hunter FA, Dhevan V. Evidence of transcellular albumin transport after hemorrhagic shock. Shock. 2005;23:565–570. [PubMed] [Google Scholar]
- 29.Murao Y, Hata M, Ohnishi K, Okuchi K, Nakajima Y, Hiasa Y, Junger WG, Hoyt DB, Ohnishi T. Hypertonic saline resuscitation reduces apoptosis and tissue damage of the small intestine in a mouse model of hemorrhagic shock. Shock. 2003;20:23–28. doi: 10.1097/01.shk.0000078832.57685.6c. [DOI] [PubMed] [Google Scholar]
- 30.Savoye G, Tamion F, Richard V, Varin R, Thuillez C. Hemorrhagic shock resuscitation affects early and selective mesenteric artery endothelial function through a free radical-dependent mechanism. Shock. 2005;23:411–416. doi: 10.1097/01.shk.0000159928.49620.67. [DOI] [PubMed] [Google Scholar]
- 31.Therade-Matharan S, Laemmel E, Duranteau J, Vicaut E. Reoxygenation after hypoxia and glucose depletion causes reactive oxygen species production by mitochondria in HUVEC. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1037–1043. doi: 10.1152/ajpregu.00048.2004. [DOI] [PubMed] [Google Scholar]
- 32.Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 33.Morkuniene R, Arandarcikaite O, Borutaite V. Estradiol prevents release of cytochrome c from mitochondria and inhibits ischemia-induced apoptosis in perfused heart. Exp Gerontol. 2006;41:704–708. doi: 10.1016/j.exger.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. JMol Cell Cardiol. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 35.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervello M, Giannitrapani L, La Rosa M, Notarbartolo M, Labbozzetta M, Poma P, Montalto G, D'Alessandro N. Induction of apoptosis by the proteasome inhibitor MG1 32 in human HCC cells: Possible correlation with specific caspase-dependent cleavage of beta-catenin and inhibition of beta-catenin-mediated transactivation. Int JMol Med. 2004;13:741–748. [PubMed] [Google Scholar]
- 37.Van de Craen M, Berx G, Van den Brande I, Fiers W, Declercq W, Vandenabeele P. Proteolytic cleavage of beta-catenin by caspases: an in vitro analysis. FEBS Lett. 1999;458:167–170. doi: 10.1016/s0014-5793(99)01153-9. [DOI] [PubMed] [Google Scholar]
- 38.Nakamoto K, Kuratsu J, Ozawa M. β-cleavage in non-apoptotic cells with reduced cell adhesion activity. Int JMol Med. 2005;15:973–979. [PubMed] [Google Scholar]
- 39.Steinhusen U, Badock V, Bauer A, Behrens J, Wittman-Liebold B, Dörken B, Bommert K. Apoptosis-induced cleavage of beta-catenin by caspase-3 results in proteolytic fragments with reduced transactivation potential. J Biol Chem. 2000;275:16345–16353. doi: 10.1074/jbc.M001458200. [DOI] [PubMed] [Google Scholar]