Abstract
Regional cerebral blood flow (CBF) and oxygen metabolism can be measured by positron emission tomography (PET) with 15O-labeled compounds. Hemoglobin (Hb) concentration of blood, a primary determinant of arterial oxygen content (CaO2), influences cerebral circulation. We investigated interindividual variations of CBF, cerebral blood volume (CBV), oxygen extraction fraction (OEF), and cerebral metabolic rate of oxygen (CMRO2) in relation to Hb concentration in healthy human volunteers (n=17) and in patients with unilateral steno-occlusive disease (n=44). For the patients, data obtained only from the contralateral hemisphere (normal side) were analyzed. The CBF and OEF were inversely correlated with Hb concentration, but CMRO2 was independent of Hb concentration. Oxygen delivery defined as a product of CaO2 and CBF (CaO2 CBF) increased with a rise of Hb concentration. The analysis with a simple oxygen model showed that oxygen diffusion parameter (L) was constant over the range of Hb concentration, indicating that a homeostatic mechanism controlling CBF is necessary to maintain CMRO2. The current findings provide important knowledge to understand the control mechanism of cerebral circulation and to interpret the 15O PET data in clinical practice.
Keywords: 15O PET, CBF, CMRO2, hemoglobin, OEF
Introduction
The energy required for normal brain function is mostly supplied by oxidation of glucose. Therefore, oxygen metabolism is a direct indicator of energy consumption by the brain. Positron emission tomography (PET) with oxygen-15 (15O) is virtually the only way to quantitatively measure regional oxygen consumption in human brains (Frackowiak et al, 1980; Mintun et al, 1984; Raichle et al, 1983). The PET with 15O can derive the cerebral metabolic rate of oxygen (CMRO2) as a product of cerebral blood flow (CBF), oxygen extraction fraction (OEF), and arterial oxygen content (CaO2):
These PET variables are used for assessing the hemodynamic status of patients with cerebrovascular disease. For patients with steno-occlusive disease, CBF and OEF are the primary parameters for estimating the degree of flow reduction and for detecting the brain tissues with misery perfusion, respectively (Okazawa and Kudo, 2009). Understanding physiologic factors that influence hemodynamic parameters is crucial to interpret the results of a 15O PET study.
Hemoglobin (Hb) concentration of blood, a primary determinant of CaO2, is one of these factors. Several human studies that used the 133Xe method showed that CBF is inversely correlated with Hb concentration (Brown and Marshall, 1985; Brown et al, 1985; Henriksen et al, 1981; Kusunoki et al, 1981; Thomas et al, 1977; Vorstrup et al, 1992). This inverse relationship of CBF-Hb has been interpreted as a homeostatic CBF response to changes in CaO2 for maintaining oxygen supply. In contrast to CBF, the relationship between Hb concentration and oxygen metabolism has been rarely reported in humans (Herold et al, 1986; Kuwabara et al, 2002). Although changes in Hb concentration may induce a biological response to keep CMRO2 constant, details of the regulation mechanism are not fully understood. Given the well-known relationship between CBF and Hb concentration, two scenarios are possible for keeping CMRO2 at a constant level: (1) in response to the variations in Hb concentration, CBF changes to maintain a constant oxygen delivery (DO2), defined as a product of CaO2 and CBF (CaO2 CBF), resulting in a constant CMRO2. In this instance, OEF remains at a constant level. (2) Both CBF and OEF change to keep CMRO2 at a constant level in response to the changes in Hb concentration. In this case, DO2 varies with the changes in Hb concentration. Simultaneous measurements of CBF, OEF, CMRO2, and Hb concentration can determine which of these scenarios is more plausible.
Several oxygen transport models that describe the coupling between CBF and CMRO2 have been proposed (Buxton and Frank, 1997; Gjedde, 2002; Hayashi et al, 2003; Hyder et al, 1998; Vafaee and Gjedde, 2000; Valabregue et al, 2003). Among them, the models incorporating a nonlinear binding of oxygen to Hb, that is, Hill equation, can be applied for understanding the relationship between Hb concentration and oxygen metabolism (Gjedde, 2002; Hayashi et al, 2003; Vafaee and Gjedde, 2000; Valabregue et al, 2003). Under the condition of a constant oxygen diffusion property and a constant CBF, these models predict that decreases in Hb concentration result in increase in OEF and slight decrease in CMRO2 in a nonlinear manner (Hayashi et al, 2003). However, actual human PET data validating the simple oxygen models are not currently available.
The aim of this study was to determine the effect of Hb concentration on cerebral circulation in humans. We retrospectively analyzed 15O PET data from two subject groups. One was a healthy volunteer group (n=17) and the other was a group of patients with unilateral major arterial steno-occlusive disease (n=44). For these subjects, interindividual variations of CBF, cerebral blood volume (CBV), OEF, CMRO2, and oxygen diffusion parameter derived from the simple oxygen model were investigated in relation to Hb concentration. As this study focused on the effect of Hb concentration on normal brain function, patients' data only from the contralateral hemisphere (normal side) were used. To examine the relationship between Hb concentration and the PET parameters while controlling for the effects of age, sex, and subject group (volunteer versus patient), multiple linear regression analysis was applied. The present analysis provides basic data for understanding the control mechanism of cerebral circulation with respect to changes in Hb concentration.
Materials and methods
Subjects
The PET data were retrospectively analyzed from the following two subject groups.
Healthy Volunteer Group
Seventeen healthy volunteers comprised of 10 males and 7 females aged 50 to 67 years were recruited and provided written informed consent. They were the same subjects in an earlier PET study (Ibaraki et al, 2009). All volunteers were determined to be healthy based on medical history, blood screening tests, anatomic MR imaging (T1 and T2-weighted images), and MR angiography of the brain. The PET study protocol was approved by the Ethics Committee of the Akita Research Institute of Brain and Blood Vessels.
Patient Group
Forty-four patients (33 males and 11 females aged 45 to 81 years) with unilateral major arterial steno-occlusive disease were involved in the study. All subjects were retrospectively selected from patients, who had a 15O PET study in our hospital between July 2007 and November 2008 as a routine clinical examination. Inclusion criteria were (1) unilateral occlusion or stenosis of internal carotid artery (ICA) or middle cerebral artery (MCA), and confirmed by MR angiography or 3D computed tomography angiography and (2) no evidence of large cortical infarctions in the contralateral hemisphere on T1- and T2-weighted images. Postoperative PET examinations, for example, extracranial–intracranial bypass surgery, carotid artery stenting, and carotid endarterectomy, were excluded. Among 44 patients, 13 had occlusion in ICA, 13 had stenosis in ICA, 12 had occlusion in MCA, and 7 had stenosis in MCA. One patient had both right ICA occlusion and right MCA stenosis. Sixteen patients had no symptoms, apart from minor signs, for example headache and vertigo. In symptomatic patients with cerebral infarction (n=28), the intervals between the onset of the symptom and PET study ranged from 7 to 2854 days (median, 42 days). According to visual assessment of PET imaging, the affected hemisphere showed CBF decrease in the majority of the patients (n=33) and OEF increase in half of the patients (n=23). Average values of affected-to-nonaffected hemispheric ratios (n=44) were 0.87±0.12, 1.06±0.07, 0.97±0.12, and 1.09±0.22 for CBF, OEF, CMRO2, and CBV, respectively.
Positron Emission Tomography Procedure
A SET-3000GCT/M (PET/CT; Shimadzu Corp, Kyoto, Japan) dedicated to the 3D-acquisition mode was used (Matsumoto et al, 2006). It uses gadolinium oxyorthosilicate detectors and provides 59 sections with a center-to-center distance of 2.6 mm. The axial FOV was 156 mm. The intrinsic spatial resolution was 3.5 mm FWHM in-plane and 4.2 mm FWHM axially. Before image reconstruction, 3D sinogram data were converted to 2D sinograms by a Fourier rebinning algorithm, and then processed with a hybrid scatter correction method based on acquisition in the dual-energy window (Ibaraki et al, 2008). FBP reconstruction followed by 3D Gaussian smoothing with 6-mm FWHM resulted in an effective in-plane resolution of ∼7 mm FWHM. All reconstructed images consisted of 59 slices of 128 × 128 voxels with a voxel size of 2.0 × 2.0 × 2.6 mm3.
Each PET study included a transmission scan for attenuation correction and three static emission scans with the inhalation of C15O, the inhalation of 15O2, and the injection of H215O (Hatazawa et al, 1995; Ibaraki et al, 2008). The interval between scans was 15 mins. Before the emission scans, a transmission scan (3 mins) with a 137Cs point source was performed with a BGO transmission detector ring coaxially attached to the gadolinium oxyorthosilicate emission detector ring (Matsumoto et al, 2006).
H215O PET studies were performed to measure CBF. The protocol consisted of 3 mins of static scanning initiated simultaneously with 2 mins of intravenous infusion of H215O (0.4 GBq at the start of scanning). An arterial input function was determined with a β detector system that continuously measured radioactivity in arterial whole blood taken from the radial artery. Delay and dispersion occurring in the β detector system and the internal arterial line were corrected as described earlier (Iida et al, 1988). The CBF was calculated by the autoradiographic method (Kanno et al, 1987; Raichle et al, 1983).
15O2 PET studies were performed to measure OEF and CMRO2 (Mintun et al, 1984). The protocol consisted of 3 mins of static scanning initiated simultaneously with 1.5 mins of inhalation of 15O2 (a total of 3.0 GBq supplied by mouth). The arterial input function was determined in the same way as for H215O PET scan. The contribution of 15O-labeled metabolic water was estimated according to the method by Iida et al (1993).
C15O PET studies were performed to measure CBV (Martin et al, 1987). Static PET scanning for 4 mins was initiated 3 mins after 1 mins of continuous inhalation of C15O gas (a total of 1.3 GBq supplied by mouth). Three arterial blood samples were taken during scanning to measure whole-blood radioactivity. The cerebral-to-large vessel hematocrit ratio was assumed to be 0.85.
Before the transmission scanning, an arterial blood sample was taken for measurement of Hb concentration. CaO2 was calculated as a sum of two components: oxygen combined with Hb and that physically dissolved in blood plasma,
where [Hb] is the Hb concentration (g/dL), SaO2 is arterial O2 saturation, PaO2 is arterial oxygen partial pressure (mm Hg), and α is the oxygen solubility (0.00315 mL/100 mL/mm Hg). The last term gives the dissolved component and is negligibly small (1% to 2% of total CaO2). SaO2 was calculated from pH-corrected PaO2 value based on oxygen–Hb dissociation curve with Hb half saturation oxygen tension P50=26 mm Hg and Hill constant h=2.8. For each PET scanning, two arterial blood samples, one at the beginning and one at the end of the scanning, were taken for measurements of PaCO2, PaO2, and Ph. The blood samples were analyzed with ABL 555 (Radiometer, Copenhagen, Denmark). Mean blood pressure and heart rate were monitored during the course of the PET study.
Region of Interest Analysis
For CBF, CMRO2, OEF, and CBV maps, elliptical regions of interest with 16 × 48 mm were defined on the cerebral cortical region of MCA territory on a slice at the centrum semiovale level (Ito et al, 2004). The regions of interest were placed on both hemispheres for the healthy volunteers and on the contralateral hemispheres of steno-occlusive lesions for the patients. For the healthy volunteers, average values of both hemispheres were used in the analysis.
Simple Oxygen Model
Vafaee and Gjedde presented the following analytic expression of nonlinear coupling between CBF and CMRO2:
where L is the average oxygen diffusion capacity between capillary and brain tissue, P50 is the Hb half saturation oxygen tension, and h is the Hill constant (Vafaee and Gjedde, 2000). This simplified equation is practically identical to a more rigorous calculation of the oxygen transport (Hayashi et al, 2003). L value for each subject was calculated from the individual PET data based on equation (3) with fixed P50 (26 mm Hg) and h (2.8).
Statistical Analysis
Multiple linear regression analysis was performed to examine the relationship between Hb concentration and the PET parameters, that is, CBF, CMRO2, OEF, CBV, and L. Independent variables included Hb concentration, sex, age, and subject group (volunteer or patient). Statistical significance for linear regression model and regression coefficient for each variable was examined with F-statistics and t-statistics, respectively (P<0.05). The linear model for each PET parameter was optimized by a backward selection method based on the minimization of Akaike information criterion (AIC), calculated as n ln(RSS/n) + 2 p, where n is the number of data (=61), RSS is the residual sum of squares, and p is the number of parameters. For OEF model, CBF was also entered as the independent variable because of the physiologic correlation between OEF and CBF (Leenders et al, 1990; Marchal et al, 1992; Yamaguchi et al, 1986).
In exploratory analysis, Hb concentration was not significantly correlated with PaCO2 (P=0.61), PaO2 (P=0.39), pH (P=0.18), mean blood pressure (P=0.29), and heart rate (P=0.70) (Supplementary Figure 1). These variables were deemed to have insignificant effects on the investigation of Hb concentration–PET parameter relationship, and therefore not included in the regression analysis. Nevertheless, PaCO2, which is a strong influencing factor for cerebral circulation, may affect on the result of CBF analysis. However, the present data showed no statistical correlation between CBF and PaCO2 (P=0.23), consistent with our earlier study (Ito et al, 2008).
In addition to the multiple linear regression analysis, bivariate correlation analyses between Hb concentration, age, and PET parameters were performed. Sex difference and subject group difference (volunteer versus patient) in Hb concentration, age, and PET parameters were examined by analysis of variance.
R software version 2.8.0 (a free software environment for statistical computing and graphics available via http://www.R-project.org) was used for the statistical analysis.
Results
The mean values of age, Hb concentration, and PET parameters are shown in Table 1. The mean Hb concentration of the subjects was 12.9±1.6 (g/dL). It was significantly lower in females than in males (P<0.001) and tended to be lower in the patient group than the volunteer group (P=0.051). Age was significantly higher in the patient group than the volunteer group (P<0.001). The PET parameters presented were average values of both hemispheres for the healthy volunteers and values from the contralateral hemisphere for the patients. The CBF was significantly higher in females than in males (P=0.023). The OEF was significantly higher in the patient group than the volunteer group (P=0.032). In CMRO2, CBV, and L, no differences were noted for either sex or subject group. Average L value was 0.0758±0.0134 (mL/100 mL/min/mm Hg) or 3.38±0.60 (μmol/100 mL/min/mm Hg), similar to the results of Vafaee and Gjedde (2000). Correlation analysis showed that Hb concentration significantly correlated with age (Pearson correlation coefficient r=−0.26, P=0.047, Supplementary Figure 1).
Table 1. Average PET parameters, age, and Hb concentration.
| Total | Volunteer | Patient | ANOVA P-value | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male versus female | Volunteer versus patient | ||
| n | 61 | 10 | 7 | 33 | 11 | ||
| Age (years) | 64.3±9.2 | 55.5±3.3 | 59.4±5.9 | 67.5±9.0 | 65.7±9.5 | <0.001 | |
| Hb (g/dL) | 12.9±1.6 | 14.0±1.0 | 12.2±0.9 | 13.2±1.6 | 11.4±1.3 | <0.001 | 0.051 |
| CBF (mL/100 mL/min) | 39.8±5.7 | 39.5±3.7 | 43.6±3.3 | 38.4±6.5 | 41.8±4.3 | 0.023 | |
| OEF (%) | 45.3±5.5 | 41.6±3.6 | 45.2±4.7 | 45.8±6.1 | 47.3±4.6 | 0.032 | |
| CMRO2 (mL/100 mL/min) | 3.05±0.45 | 3.06±0.20 | 3.20±0.40 | 3.04±0.56 | 2.98±0.27 | ||
| CBV (mL/100 mL) | 3.19±0.53 | 3.44±0.50 | 3.16±0.49 | 3.16±0.60 | 3.07±0.27 | ||
| L (10−2 mL/100 mL/min/mm Hg) | 7.58±1.34 | 7.30±0.58 | 7.97±1.37 | 7.60±1.63 | 7.54±0.84 | ||
ANOVA, analysis of variance; CBF, cerebral blood flow; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate of oxygen; Hb, hemoglobin; L, oxygen diffusivity calculated from the simple oxygen model (Equation (3)); OEF, oxygen extraction fraction; PET, positron emission tomography.
Values are shown as mean±s.d.
Sex difference and subject group difference (volunteer or patient) were examined by ANOVA.
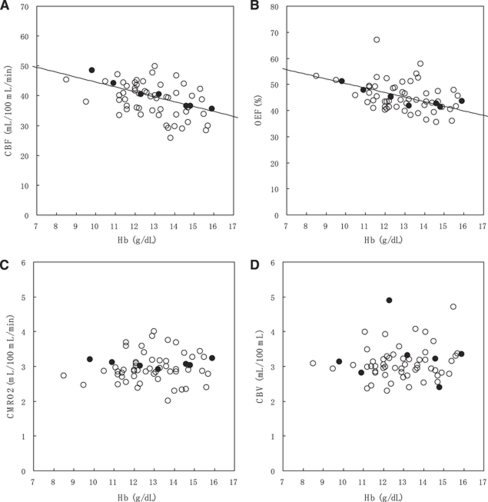
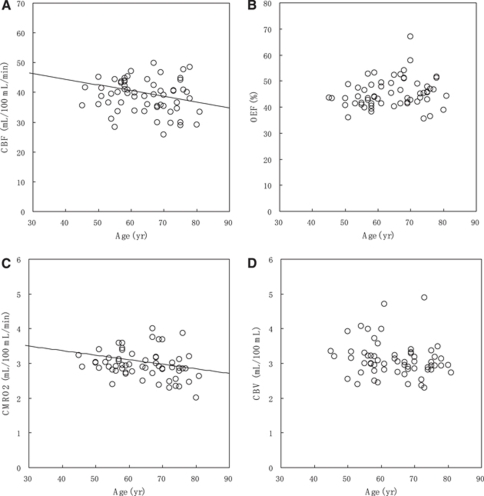
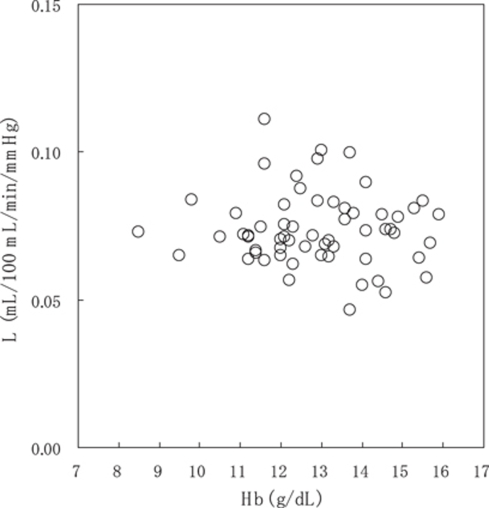
The relationships between PET parameters and Hb concentration are shown in Figure 1 (Supplementary Figure 2 is the same as Figure 1, but the subjects were divided into six groups depending on Hb concentration, and only group averages were plotted.). Figure 2 shows the relationships between PET parameters and age. The relationship between oxygen diffusivity (L) and Hb concentration is shown in Figure 3.
Figure 1.
Relationship between PET parameters and Hb concentration: (A) CBF, (B) OEF, (C) CMRO2, and (D) CBV. Filled circles indicate the subjects (n=7) illustrated in Figure 5. Solid lines in CBF and OEF represent the optimized models: CBF [mL/100 mL/min]=73.3−0.191 Age [years]—1.65 Hb [g/dL] (age was fixed to the average value, 64.3 years) and OEF [%]=80.5−1.75 Hb [g/dL]—0.317 CBF [mL/100 mL/min] (CBF was fixed to the average value, 39.8 mL/100 mL/min).
Figure 2.
Relationship between PET parameters and age: (A) CBF, (B) OEF, (C) CMRO2, and (D) CBV. Solid lines in CBF and CMRO2 represent the optimized models: CBF [mL/100 mL/min]=73.3−0.191 Age [years]—1.65 Hb [g/dL] (Hb concentration was fixed to the average value, 12.9 g/dL) and CMRO2 [mL/100 mL/min]=3.89−0.0131 Age [years].
Figure 3.
Relationship between L derived from the simple oxygen model and Hb concentration. Multiple regression analysis provided no valid linear model, indicating that the oxygen diffusion parameter L is independent of Hb concentration.
Cerebral Blood Flow Analysis
Multiple regression analysis showed that CBF was inversely correlated with Hb concentration and age (Supplementary Table 1). The AIC-based optimization removed sex and subject group from the linear model, and provided the final model:
The regression line as a function of Hb concentration is presented in Figure 1A, in which age is fixed to the average value (64.3 years). The regression line as a function of age is presented in Figure 2A, in which Hb concentration is fixed to the average value (12.9 g/dL). In bivariate correlation analysis, CBF was inversely correlated with Hb concentration (Pearson correlation coefficient r=−0.390, P=0.002), but not with age (P=0.140).
Oxygen Extraction Fraction Analysis
Multiple regression analysis showed that OEF was inversely correlated with Hb concentration and CBF (Supplementary Table 1). The AIC-based optimization removed age, sex, and subject group from the linear model, and provided the final model:
The regression line as a function of Hb concentration is presented in Figure 1B (solid line), in which CBF is fixed to the average value (39.8 mL/100 mL/min). Even if the CBF variable was not included in the multiple regression analysis, Hb concentration remained as a significant variable (P=0.004). In bivariate correlation analysis, OEF was inversely correlated with Hb concentration (Pearson correlation coefficient r=−0.386, P=0.002), but not with age (P=0.154) and CBF (P=0.335).
Cerebral Metabolic Rate of Oxygen Analysis
Multiple regression analysis showed that CMRO2 was inversely correlated with age (Supplementary Table 1). The AIC-based optimization removed sex, subject group, and Hb concentration from the linear model, and provided the final model:
The regression line as a function of age is presented in Figure 2C. In bivariate correlation analysis, CMRO2 was inversely correlated with age (Pearson correlation coefficient r=−0.267, P=0.037), but not with Hb concentration (P=0.245).
Cerebral Blood Volume Analysis
Multiple regression analysis provided no valid linear model, but age tended to be correlated inversely (P=0.100) (Supplementary Table 1). In bivariate correlation analysis, there was no significant correlation between CBV and Hb concentration (P=0.162) and age (P=0.100).
Oxygen Diffusivity (L) Analysis
Multiple regression analysis provided no valid linear model (Supplementary Table 1). In bivariate correlation analysis, there was no significant correlation between L and Hb concentration (P=0.972) and age (P=0.234).
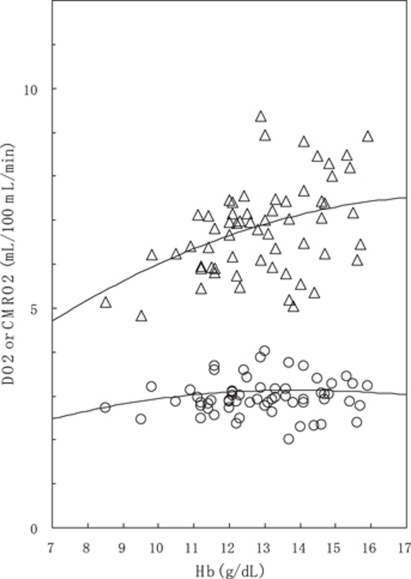
Figure 4 compares DO2 (CaO2 CBF) with CMRO2 (DO2 OEF) in respect to dependence on Hb concentration. Calculated values from the regression results (equations (4) and (5)) are also shown as solid lines. Over the range of Hb concentration in this study, DO2 increased with increasing Hb concentration, but CMRO2 maintained a relatively constant value.
Figure 4.
Relationship between DO2 defined as CaO2 × CBF (triangle) and CMRO2 (circle) and Hb concentration. Upper solid line represents DO2 as a function of Hb concentration, calculated from the regression line of CBF (equation (4)) and equation (2). CMRO2 (=DO2 × OEF) as a function of Hb concentration was also calculated from the DO2 and the regression line of OEF (equation (5)), in which CBF was varied as a function of Hb concentration according to equation (4). In these calculations, age, SaO2, and PaO2 were fixed to their average values of 64.3 years, 96.1%, and 84.1 mm Hg, respectively.
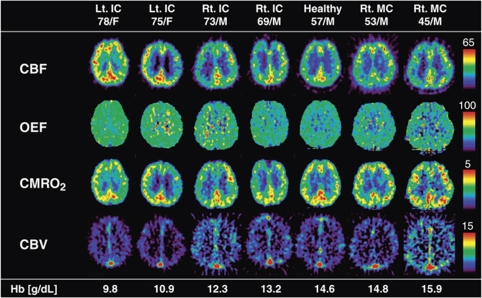
Representative PET parametric maps of seven subjects are shown in Figure 5. These subjects with various degrees of Hb concentration were selected based on the regression lines of CBF (equation (4)) and OEF (equation (5)), and indicated by filled circles in Figure 1. The CBF and OEF decreased with increasing Hb concentration. In contrast, CMRO2 stayed relatively constant.
Figure 5.
Representative PET parametric maps of seven subjects with various degrees of Hb concentrations. Selections were based in accordance with the regression lines of CBF and OEF. These subjects are indicated by the filled circles in Figure 1.
Discussion
This study was performed to determine the effect of Hb concentration on cerebral circulation by studying interindividual variations of CBF, CBV, OEF, and CMRO2 in relation to Hb concentration in a relatively large number of subjects (n=61).
Hemoglobin Concentration and Oxygen Extraction Fraction
The multiple regression analysis showed that OEF was inversely correlated with Hb concentration. This study is the first, to our knowledge, to show a clear relationship of OEF-Hb concentration in humans with normal physiologic variations in Hb concentration. The relationship between Hb concentration and oxygen metabolism has rarely been reported in humans because of the limited availability of 15O PET. In anemic hypoxia caused by sickle cell disease and chronic renal failure, CBF, CBV, OEF, and CMRO2 were measured by use of 15O PET (Herold et al, 1986; Kuwabara et al, 2002). Both studies showed significant increases in CBF in anemic patients compared with normal controls. The OEF and CMRO2 mildly increased and decreased in anemic patients, respectively, but the differences were not significant partly due to small number of subjects. In addition, pathologic factors complicated the interpretation of results.
Oxygen diffusivity L, derived from the simple oxygen model, was independent of Hb concentration. The nonlinear, inverse relationship of OEF-Hb concentration is merely a consequence of the diffusive nature of oxygen from a plasma space to brain tissues and the nonlinear binding of oxygen to Hb, rather than an active change in OEF against the variations of Hb concentration (Hayashi et al, 2003). It is worth noting that the simple oxygen model, originally developed for describing nonlinear coupling between CBF and CMRO2 in response to brain activation, can also describe the relationship of OEF-Hb concentration measured as interindividual variations in this study. The range of Hb concentration in our study, however, was too narrow to quantitatively evaluate the predictability of the model.
We are aware that the present oxygen model analysis neglects the intersubject variations in oxygen-Hb affinity (P50), which shifts the oxygen–Hb dissociation curve. According to equation (3), an obtainable parameter from PET data is LP50, and therefore the contributions of L and P50 cannot be separated. In this analysis, by fixing P50 to 26 mm Hg, we showed that L was independent of Hb concentration. P50 is readily affected by changes in chemical environment, for example temperature, carbon dioxide, hydrogen ion, and 2,3-diphosphoglycerate (2,3-DPG). Anemia with reduced Hb concentration may induce upregulation of P50 via increases in 2,3-DPG. Although the possibility cannot be excluded that the both L and P50 changed to cancel out, it appears reasonable that both remained at constant levels, at least for the relatively narrow range of Hb concentration in this study. The assumption was made in using equation (3) to be at unity for arterial O2 saturation SaO2. Applications for arterial hypoxic state need to use a model including the SaO2 term (Gjedde, 2002; Hayashi et al, 2003). The simple oxygen model assumes that the oxygen transport from capillaries to brain tissues depends solely on mean capillary PO2, not on a PO2 gradient between the capillaries and the brain tissues. This assumption does not mean that the tissue oxygen is nonexistent. Rather, the tissue PO2 is negligible at the end of the diffusion path, that is, mitochondria; actually, average tissue PO2 was estimated to be around 20 mm Hg (Gjedde et al, 1999).
Hemoglobin Concentration and Cerebral Blood Flow Regulation
The multiple regression analysis showed a clear inverse relationship between resting CBF and Hb concentration, consistent with the preceding studies (Brown and Marshall, 1985; Brown et al, 1985; Henriksen et al, 1981; Herold et al, 1986; Kusunoki et al, 1981; Kuwabara et al, 2002; Thomas et al, 1977; Vorstrup et al, 1992). This inverse relationship of CBF-Hb has been interpreted as a homeostatic CBF response to changes in CaO2 for maintaining oxygen supply, and not a result of accompanying alteration of blood viscosity (Brown and Marshall, 1985; Brown et al, 1985; Henriksen et al, 1981). The assertion that the primary factor for CBF determination is the CaO2 rather than the blood viscosity was supported by fully prepared animal experiments (Rebel et al, 2001; Waschke et al, 1994). This study showed an inverse relationship even with physiologically normal ranges of Hb concentration. The observation of constant CMRO2 against the variation in Hb concentration strongly indicates that CBF regulates the maintenance of oxygen transport to brain tissues. In contrast to CMRO2, DO2 (CaO2 CBF) increased with increasing Hb concentration. These results, combined with the result that oxygen diffusivity L was constant over the range of Hb concentration, indicate that a homeostatic mechanism controlling CBF is necessary to maintain CMRO2. The observation that both CMRO2 and L were constant for the variations in Hb concentration is consistent with the idea that oxygen partial pressure in brain tissue (PtO2) is maintained with a narrow range during normal conditions (Masamoto and Tanishita, 2009). These PET data may support a concept, based on a macroscopic view, that CBF regulation is controlled by maintaining PtO2 constant via some mechanism that senses the changes in tissue oxygen content. There is general agreement that CBF is elevated during hypoxia with reduced PaO2 (Gjedde, 2002; Mintun et al, 2001). The mechanism regulating CBF to maintain CMRO2 may be similar for hypoxic hypoxia and anemic hypoxia, although the details on how CBF increase is triggered remain to be clarified.
The present results, obtained from the intersubject correlations, should be interpreted as long-term effects of Hb concentration on cerebral circulation. Acute changes in Hb concentration, for example, hemodilution, may induce compelling effects on cerebral circulation in a fundamentally different way from the long-term regulation (Hino et al, 1992; Yamauchi et al, 1993). Nevertheless, the present findings shed light on the control mechanism of cerebral circulation and provide valuable information for interpreting 15O PET results in clinical practice.
Clinical Implications
In patients with steno-occlusive disease, 15O PET is used for detecting hemodynamically compromised tissues that exhibit increased OEF, so-called ‘misery perfusion' (Okazawa and Kudo, 2009). The tight correlation between OEF and Hb concentration, as revealed in this study, indicates that OEF elevation in absolute measures does not purely correspond to the hemodynamic compromise: whole-brain OEF might be elevated because of the reduction in Hb concentration. On the basis of our present analysis, Hb concentrations under 10 g/dL correspond to absolute OEF values >50%, which lie beyond the upper limit of the normal range (defined as mean±2 s.d. of normal database) clinically used in our hospital (Ibaraki et al, 2009).
Aging and Sex Difference in Cerebral Blood Flow and Cerebral Metabolic Rate of Oxygen
This study focused on the intersubject correlation between Hb concentration and CBF or oxygen metabolism. To control for the effects of confounding factors, that is age, sex, and subject type (volunteer or patient), multiple linear regression analysis was applied. These analyses of CBF, CBV, OEF, and CMRO2 showed the effects of sex and subject group were not statistically significant. However, analysis of variance showed significance for CBF and OEF (Table 1). This indicated that, in the present population, sex and subject group differences were below detection limits when the effects of age and Hb concentration were included in the analysis.
Although the correlation between CBF and age did not reach statistical significance in bivariate analysis (P=0.140), multiple regression analysis showed a significant correlation of CBF with age (P=0.011). These results are because CBF, Hb concentration, and age were correlated inversely with each other, and therefore the correlation between CBF and age was hidden in the bivariate analysis. The analysis using only bivariate correlation can result in misinterpretations and therefore multiple regression analysis is useful under these circumstances.
Age-dependent declines in CBF and CMRO2 are well known and have been reported with PET studies (Leenders et al, 1990; Lenzi et al, 1981; Pantano et al, 1984). At the same time, a lack of or less age-dependent CBF decline was reported by others in contrast to the significant decline in CMRO2 (Marchal et al, 1992; Yamaguchi et al, 1986). Yamaguchi et al (1986) used correlation analysis and reported that gray matter CMRO2 decreased significantly with aging, whereas there was no significant correlation between CBF and age, and concluded that CMRO2 was the best indicator of healthy brain aging. Their findings are identical to our present results obtained with the bivariate correlation analysis. Therefore, if the multiple regression analysis including Hb concentration was used, an age-dependent decline of CBF might have emerged in their study.
Conclusion
To investigate the effect of Hb concentration on cerebral circulation, interindividual variations of CBF, CBV, OEF, and CMRO2 were examined in relation to Hb concentration. The CBF and OEF were inversely correlated with Hb concentration, but CMRO2 was independent of Hb concentration. In contrast to CMRO2, DO2 (CaO2 CBF) increased with increasing Hb concentration. The analysis with the simple oxygen model showed that the oxygen diffusivity L was constant over the range of Hb concentration, indicating that a homeostatic mechanism controlling CBF is necessary to maintain CMRO2.
Acknowledgments
We are grateful to the technical staff of the Akita Research Institute of Brain and Blood Vessels for their help in performing PET studies. We thank Drs T Hayashi, H Watabe, K Masamoto, and E Shimosegawa for helpful discussions on oxygen transport models. We also thank Dr H Ito for constructive comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Brown MM, Marshall J. Regulation of cerebral blood flow in response to changes in blood viscosity. Lancet. 1985;1:604–609. doi: 10.1016/s0140-6736(85)92145-2. [DOI] [PubMed] [Google Scholar]
- Brown MM, Wade JP, Marshall J. Fundamental importance of arterial oxygen content in the regulation of cerebral blood flow in man. Brain. 1985;108 (Pt 1:81–93. doi: 10.1093/brain/108.1.81. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Frackowiak RS, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Poulsen PH, Ostergaard L. On the oxygenation of hemoglobin in the human brain. Adv Exp Med Biol. 1999;471:67–81. doi: 10.1007/978-1-4615-4717-4_9. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Cerebral blood flow change in arterial hypoxemia is consistent with negligible oxygen tension in brain mitochondria. Neuroimage. 2002;17:1876–1881. doi: 10.1006/nimg.2002.1272. [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Fujita H, Kanno I, Satoh T, Iida H, Miura S, Murakami M, Okudera T, Inugami A, Ogawa T, et al. Regional cerebral blood flow, blood volume, oxygen extraction fraction, and oxygen utilization rate in normal volunteers measured by the autoradiographic technique and the single breath inhalation method. Ann Nucl Med. 1995;9:15–21. doi: 10.1007/BF03165003. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Watabe H, Kudomi N, Kim KM, Enmi J, Hayashida K, Iida H. A theoretical model of oxygen delivery and metabolism for physiologic interpretation of quantitative cerebral blood flow and metabolic rate of oxygen. J Cereb Blood Flow Metab. 2003;23:1314–1323. doi: 10.1097/01.WCB.0000090506.76664.00. [DOI] [PubMed] [Google Scholar]
- Henriksen L, Paulson OB, Smith RJ. Cerebral blood flow following normovolemic hemodilution in patients with high hematocrit. Ann Neurol. 1981;9:454–457. doi: 10.1002/ana.410090507. [DOI] [PubMed] [Google Scholar]
- Herold S, Brozovic M, Gibbs J, Lammertsma AA, Leenders KL, Carr D, Fleming JS, Jones T. Measurement of regional cerebral blood flow, blood volume and oxygen metabolism in patients with sickle cell disease using positron emission tomography. Stroke. 1986;17:692–698. doi: 10.1161/01.str.17.4.692. [DOI] [PubMed] [Google Scholar]
- Hino A, Ueda S, Mizukawa N, Imahori Y, Tenjin H. Effect of hemodilution on cerebral hemodynamics and oxygen metabolism. Stroke. 1992;23:423–426. doi: 10.1161/01.str.23.3.423. [DOI] [PubMed] [Google Scholar]
- Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- Ibaraki M, Miura S, Shimosegawa E, Sugawara S, Mizuta T, Ishikawa A, Amano M. Quantification of cerebral blood flow and oxygen metabolism with 3-dimensional PET and 15O: validation by comparison with 2-dimensional PET. J Nucl Med. 2008;49:50–59. doi: 10.2967/jnumed.107.044008. [DOI] [PubMed] [Google Scholar]
- Ibaraki M, Sato K, Mizuta T, Kitamura K, Miura S, Sugawara S, Shinohara Y, Kinoshita T. Evaluation of dynamic row-action maximum likelihood algorithm reconstruction for quantitative 15O brain PET. Ann Nucl Med. 2009;23:627–638. doi: 10.1007/s12149-009-0280-2. [DOI] [PubMed] [Google Scholar]
- Iida H, Higano S, Tomura N, Shishido F, Kanno I, Miura S, Murakami M, Takahashi K, Sasaki H, Uemura K. Evaluation of regional differences of tracer appearance time in cerebral tissues using [15O] water and dynamic positron emission tomography. J Cereb Blood Flow Metab. 1988;8:285–288. doi: 10.1038/jcbfm.1988.60. [DOI] [PubMed] [Google Scholar]
- Iida H, Jones T, Miura S. Modeling approach to eliminate the need to separate arterial plasma in oxygen-15 inhalation positron emission tomography. J Nucl Med. 1993;34:1333–1340. [PubMed] [Google Scholar]
- Ito H, Kanno I, Kato C, Sasaki T, Ishii K, Ouchi Y, Iida A, Okazawa H, Hayashida K, Tsuyuguchi N, Ishii K, Kuwabara Y, Senda M. Database of normal human cerebral blood flow, cerebral blood volume, cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen measured by positron emission tomography with 15O-labelled carbon dioxide or water, carbon monoxide and oxygen: a multicentre study in Japan. Eur J Nucl Med Mol Imaging. 2004;31:635–643. doi: 10.1007/s00259-003-1430-8. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Ibaraki M, Suhara T, Miura S. Relationship between baseline cerebral blood flow and vascular responses to changes in PaCO2 measured by positron emission tomography in humans: implication of inter-individual variations of cerebral vascular tone. Acta Physiol (Oxf) 2008;193:325–330. doi: 10.1111/j.1748-1716.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- Kanno I, Iida H, Miura S, Murakami M, Takahashi K, Sasaki H, Inugami A, Shishido F, Uemura K. A system for cerebral blood flow measurement using an H215O autoradiographic method and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:143–153. doi: 10.1038/jcbfm.1987.37. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Kimura K, Nakamura M, Isaka Y, Yoneda S, Abe H. Effects of hematocrit variations on cerebral blood flow and oxygen transport in ischemic cerebrovascular disease. J Cereb Blood Flow Metab. 1981;1:413–417. doi: 10.1038/jcbfm.1981.45. [DOI] [PubMed] [Google Scholar]
- Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, Kaneko K, Masuda K, Fujishima M. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61:564–569. doi: 10.1046/j.1523-1755.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113 (Pt 1:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Lenzi GL, Frackowiak RS, Jones T, Heather JD, Lammertsma AA, Rhodes CG, Pozzilli C. CMRO2 and CBF by the oxygen-15 inhalation technique. Results in normal volunteers and cerebrovascular patients. Eur Neurol. 1981;20:285–290. doi: 10.1159/000115248. [DOI] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Petit-Taboue MC, Sette G, Travere JM, Le Poec C, Courtheoux P, Derlon JM, Baron JC. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. doi: 10.1001/archneur.1992.00530340029014. [DOI] [PubMed] [Google Scholar]
- Martin WR, Powers WJ, Raichle ME. Cerebral blood volume measured with inhaled C15O and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:421–426. doi: 10.1038/jcbfm.1987.85. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Tanishita K. Oxygen transport in brain tissue. J Biomech Eng. 2009;131:074002. doi: 10.1115/1.3184694. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kitamura K, Mizuta T, Tanaka K, Yamamoto S, Sakamoto S, Nakamoto Y, Amano M, Murase K, Senda M. Performance characteristics of a new 3-dimensional continuous-emission and spiral-transmission high-sensitivity and high-resolution PET camera evaluated with the NEMA NU 2-2001 standard. J Nucl Med. 2006;47:83–90. [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA. 2001;98:6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H, Kudo T. Clinical impact of hemodynamic parameter measurement for cerebrovascular disease using positron emission tomography and 15O-labeled tracers. Ann Nucl Med. 2009;23:217–227. doi: 10.1007/s12149-009-0235-7. [DOI] [PubMed] [Google Scholar]
- Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15:635–641. doi: 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- Rebel A, Lenz C, Krieter H, Waschke KF, Van Ackern K, Kuschinsky W. Oxygen delivery at high blood viscosity and decreased arterial oxygen content to brains of conscious rats. Am J Physiol Heart Circ Physiol. 2001;280:H2591–H2597. doi: 10.1152/ajpheart.2001.280.6.H2591. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, du Boulay GH, Marshall J, Pearson TC, Ross Russell RW, Symon L, Wetherley-Mein G, Zilkha E. Cerebral blood-flow in polycythaemia. Lancet. 1977;2:161–163. doi: 10.1016/s0140-6736(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Vafaee MS, Gjedde A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab. 2000;20:747–754. doi: 10.1097/00004647-200004000-00012. [DOI] [PubMed] [Google Scholar]
- Valabregue R, Aubert A, Burger J, Bittoun J, Costalat R. Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange. J Cereb Blood Flow Metab. 2003;23:536–545. doi: 10.1097/01.WCB.0000055178.31872.38. [DOI] [PubMed] [Google Scholar]
- Vorstrup S, Lass P, Waldemar G, Brandi L, Schmidt JF, Johnsen A, Paulson OB. Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab. 1992;12:745–749. doi: 10.1038/jcbfm.1992.105. [DOI] [PubMed] [Google Scholar]
- Waschke KF, Krieter H, Hagen G, Albrecht DM, Van Ackern K, Kuschinsky W. Lack of dependence of cerebral blood flow on blood viscosity after blood exchange with a Newtonian O2 carrier. J Cereb Blood Flow Metab. 1994;14:871–876. doi: 10.1038/jcbfm.1994.109. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke. 1986;17:1220–1228. doi: 10.1161/01.str.17.6.1220. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Ogawa M, Ouchi Y, Kimura J. Hemodilution improves cerebral hemodynamics in internal carotid artery occlusion. Stroke. 1993;24:1885–1890. doi: 10.1161/01.str.24.12.1885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.