Abstract
An optimized total synthesis of the 2-amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core structure of a new fluoroquinolone-like class of antibacterial agents is described. This synthesis is highlighted by a nearly quantitative ring-closing reaction to form the pyrido[1,2-c]pyrimidine core. This bicyclic ring system serves as a scaffold for a family of biologically active compounds.
Fluoroquinolones are a ubiquitous class of broad-spectrum antibacterial agents,1 typically inhibiting DNA gyrase in Gram-negative bacteria and topoisomerase IV (TopoIV) in Gram-positive bacteria.2 Newer 8-methoxy fluoroquinolones more effectively inhibit both DNA gyrase and TopoIV, thus retaining activity against many fluoroquinolone-resistant mutants.3 Due to the need for improved spectrum antibacterial agents, and to combat antibiotic-resistance, several generations of quinolone-class antibacterial agents with varied core structures have been introduced (Figure 1).4
Figure 1.
Quinolone-class antibacterial agents. (A) Representative structures of naphthyridone, fluoroquinolone and 2-pyridone type cores. (B) Representative structures of newer quinazoline-dione type cores (R4 = H or NH2)
Since the initial discovery of nalidixic acid, the quinolone class of antibacterial agents evolved to more potent fluoroquinolones.5 Subsequent generations of the quinolone-class of antibacterial agents have focused on various substitutions at the N-1, C-7, and C-8 positions (corresponding to R1, R3, and R2 respectively, Figure 1).5,6
Although a number of quinolone-like core ring structures have been pursued, because of the inherent requirement of a 3-carboxylate moiety for quinolone-class agents to retain activity,6 there is limited precedent for modification of this portion of these structures. Recently, quinazoline-2,4-diones, fluoroquinolone-like agents lacking a cognate 3-carboxylate group (figure 1),7 were reported to be potent broad spectrum antibacterial agents.8 The quinazoline-2,4-diones are also active against many fluoroquinolone resistant gyrase mutants.8d A single patent has shown quinazoline 1,3-diones ((pyrido[1,2-c]pyrimidine-1,3-diones) possess similar antibacterial activity.9
We have recently begun working to understand the structural requirements for select 8-methoxy fluoroquinolones to promote chromosome fragmentation and to rapidly kill cells.10 To this end, we initiated a program to synthesize 8-methoxy quinazoline diones in order to evaluate these unique quinolone-like gyrase inhibitors for anti-mutant activity and rapid lethality.8d Described here is the first report of an optimized synthesis of the 2-amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core structure, and elaboration at C-7 to give a 1,3-dione based fluoroquinolone analog.
In the previously documented 1,3-dione synthesis,9 good overall yields (~85%) of 5-methyl-pyrido[1,2-c]pyrimidine-1,3-diones and poor overall yields (~15%) of 5-methoxy-pyrido[1,2-c]pyrimidine-1,3-diones proceeded through the corresponding 3-methyl and 3-methoxy cyanomethyl-substituted pyridine intermediates, which are known intermediates in the synthesis of 2-pyridone analogs of fluoroquinolones.11 Guided by these reports, we initially undertook the synthesis of 3-methoxy pyridine derivative 7 (Scheme 1).
Scheme 1.
Starting with commercially available 3-chloro-2,4,5,6-tetrafluoropyridine (2), reaction with lithium t-butoxide afforded desired tert-butoxy substituted derivative 3 and the C-6 regioisomer in a 4:1 ratio, respectively. In the published report,11b conversion of chloride 3 to hydroxypyridine 4 was accomplished in two steps: hydrogenation to remove the chlorine followed by a hydroboration/oxidation sequence. We observed inconsistent results in the hydrogenation step, resulting in low conversion and incomplete reactions even at high catalyst loading. To avoid this problem, lithium/halogen exchange directly followed by hydroboration-oxidation was employed to consistently provide hydroxypyridine 4 from 3 in over 80% yield. Methylation of the resulting hydroxyl group was achieved via Mitsunobu reaction, as reported,11 to give methoxypyridine 5 in excellent yield.
Selective defluorination of 5 to give 6 was anticipated to be problematic due to similar reactivity at the C-6 and C-2 positions. Initial attempts at hydride displacement with Red-Al gave poor selectivity and consistently provided 6 in less than 25% yield. Previously, 3-methyl derivatives of 5 were reported to undergo selective displacement of fluorine by hydrazine followed by oxidation of the hydrazine intermediate to provide the 3-methyl version of 6 in good yields (47–91%).11b Here, reaction of 3-methoxy pyridine derivative 5 with hydrazine gave a 3:1 mixture of adducts at the desired and C-2 position, respectively. Reaction of the crude mixture with atmospheric oxygen and base over three days gave 6 and the corresponding 5,6-difluoro isomer, which were separated chromatographically. Installation of the cyclopropyl moiety was achieved as reported,11 whereby reaction of 6 with the lithium anion of cyclopropylacetonitrile provided 7 in excellent yield.
Having improved yields in preparing methoxypyridine intermediate 7, conversion to the requisite precursor for cyclization was undertaken (amide 9, Scheme 2). Trifluoroacetic acid mediated deprotection of the tert-butyl group followed by treatment of the resulting phenol with POCl3 afforded chloropyridine 8 in reasonable yield (Scheme 2). Sulfuric acid catalyzed hydrolysis of the nitrile provided amide 9, however it was observed that extended reaction times at 100°C led to over-hydrolysis, resulting in formation of the corresponding carboxylic acid. Lowering the reaction temperature to 70°C combined with careful monitoring of the reaction negated this problem.
Scheme 2.
Treatment of amide 9 with phosgene and potassium tert-butoxide was previously reported to effect cyclization and give the 5-methoxy-pyrido[1,2-c]pyrimidine ring system (10) in 15% yield.9 The 3-methyl version of 9 was reported to give 85% yield of the corresponding 5-methyl-pyrido[1,2-c]pyrimidine ring system under the same conditions. Following this precedent, amide 9 was treated with phosgene and potassium tert-butoxide as reported. At cryogenic temperatures no reaction was observed. Somewhat surprisingly, upon warming to 0°C clean formation of nitrile 8 was observed (Scheme 3). While there is precedent for this type of dehydration using COCl2,12 the observation that no desired product was formed was unexpected. Varying combinations of temperature, phosgene stoichiometry, and the base used gave either no reaction or provided nitrile 8.
Scheme 3.

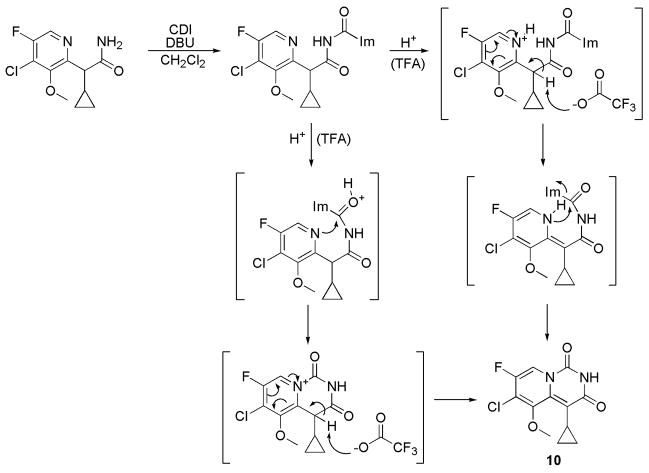
Fearing that phosgene was too reactive as the carbonyl source for efficient formation of the bicyclic ring system, attempts were made to use the less reactive carbonyl equivalent, carbonyldiimidazole (CDI). Using CDI in place of phosgene under the aforementioned conditions afforded no cyclization product at −78°C, and dehydration to nitrile 8 proceeded slowly at room temperature. However, replacing potassium tert-butoxide with DBU and employing CDI led to conversion of 9 to cyclized product 10 in ~ 20% yield, as determined by analytical HPLC. All attempts to drive the reaction to further increase cyclization resulted in dehydration back to the nitrile (using forcing conditions) or no improvement in percent conversion (HPLC-based yields following modification of stoichiometry, solvent, reaction times, etc.). Recognizing that there was a reported difference in yield of 70% between the formation of the 5-methoxy 10 and the corresponding 5-methyl derivative,9 it seemed likely that steric interactions between the methoxy and cyclopropyl groups here were interfering with ring closure. The reason for the reaction stalling at 15–20% conversion in both the previous report and in our hands was unclear.
Resigned to a low yield in the cyclization step, the reaction to form 10 from 9 using DBU and CDI was performed on large scale. Upon quenching this reaction with water, as previously reported, no cyclized product (10) was observed despite the reaction displaying a typical HPLC profile of 20% conversion prior to quenching with water. Realizing that reaction aliquots analyzed by HPLC were diluted/quenched with mobile phase containing 0.1% TFA, we explored the effect of quenching this “base-catalyzed” cyclization reaction with aqueous acidic rather than water. Brief optimization of reaction conditions, in particular the acid used and the acid quench procedure, led to reproducible preparation of 10 in over 90% isolated yields.15 This near quantitative conversion of 9 to 10, as indicated by a HPLC analysis, and high isolated yield of 10 (92–95%) is a significant improvement over the previously reported 15% yield9 of 10 at this late stage of the synthesis.
Based on the necessity of acid for ring-closure in this system, the exact mechanism of the cyclization reaction remains unclear. However, it is postulated that after initial base catalyzed N-acylation of the amide by CDI, protonation of the pyridine ring upon addition of acid is the driving force behind dearomatization, affording formation of secondary amine, and thus the second, ring-closing, acylation (Scheme 4). Similarly, it is possible that acid is serving to simply activate the carbonylimidazole leaving group, driving cyclization. These hypotheses are supported both by the fact that no product formation is observed until after addition of acid to the reaction, which can be confirmed visually due to color change, and that there is an intermediate species that can be hydrolyzed back to starting material. Aqueous acid quenches gave varying yields based on acid concentration, suggesting a competing reaction between hydrolysis and ring-closure. In addition, quenching the reaction with anhydrous acidic resulted in varying yields based on time of reaction, indicating a slow buildup of intermediate species.
Scheme 4.
Having discovered that cyclization of amide 9 to give the pyrido[1,2-c]pyrimidine-1,3-dione core structure requires acid, it is now unclear if the previous report for forming 5-methoxy-and 5-methyl-pyrido[1,2-c]pyrimidine-1,3-dione ring systems using phosgene and potassium tert-butoxide to effect cyclization was fully base-mediated. It is possible that acid-mediated cyclization similarly occurred, as a result of HCl generated during quenching of the reaction (containing excess phosgene) with water. Further exploration and optimization of this reaction using variably substituted pyridine derivatives is planned.
With the penultimate 5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione derivative (10) in hand, the remainder of the synthesis to prepare 1,3-dione derivatives for future microbioligical studies was straightforward (Scheme 5). To this end, N-amination of 10 was achieved using O-(2,4-ditrophenyl)hydroxylamine (11), which was prepared via literature procedure,13 to supply 1,3-dione core structure 12 in high yield.15 Having completed an optimized synthesis of the title core ring system, installation of a C-6 side-chain was accomplished by simply heating a solution of (R)-3-N-Boc-aminomethyl pyrrolidine (13) and 12 to produce 14,15 demonstrating the ability of the 1,3-dione core to serve as a scaffold for a future library of quinolone-like compounds.
Scheme 5.
In summary, the studies here provide the first optimized synthetic route to the 5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core ring system.14 Further elaboration to give the C-6 substituted pyrimidine-1,3-dione as a representative analog of quinolone-like antimicrobial 1,3-diones is demonstrated. Synthesis of the 5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione core here, while originally guided by patent and literature precedent in similar syntheses, ultimately affords a significant improvement over previous reports. By simplifying early steps in the synthesis of intermediates that allows for more facile processing, and highlighted by a major improvement to the key ring-closing step, we have improved the overall yield of this synthesis from 1.2% to 8.0%.
Acknowledgments
This work was supported by NIH grant AI073491.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.(a) Drlica K, Malik M, Kerns RJ, Zhao X. Antimicrob Agents and Chemother. 2008;52:385–392. doi: 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kresse H, Belsey M, Rovini H. Nat Rev Drug Discov. 2007;6:19–20. doi: 10.1038/nrd2226. [DOI] [PubMed] [Google Scholar]
- 2.(a) Wang JC. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]; (b) Drlica K, Malik M. Curr Top Med Chem. 2003;3:249–282. doi: 10.2174/1568026033452537. [DOI] [PubMed] [Google Scholar]; (c) Gould KA, Pan XS, Kerns RJ, Fisher LM. Antimicrob Agents Chemother. 2004;48:2108–2115. doi: 10.1128/AAC.48.6.2108-2115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao X, Quinn B, Kerns RJ, Drlica K. J Antimicrob Chemother. 2006;58:1283–1286. doi: 10.1093/jac/dkl388. [DOI] [PubMed] [Google Scholar]
- 3.(a) Okumura R, Hirata T, Onodera Y, Hoshino K, Otani T, Yamamoto T. J Antimicrob Chemother. 2008;62:98–104. doi: 10.1093/jac/dkn136. [DOI] [PubMed] [Google Scholar]; (b) Pan XS, Fisher LM. Antimicrob Agents and Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ince D, Zhang X, Silver LC, Hooper DC. Antimicrob Agents Chemother. 2002;46:3370–3380. doi: 10.1128/AAC.46.11.3370-3380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim OK, Barrett JF, Ohemeng K. Exp Opin Invest Drugs. 2001;10:199–212. doi: 10.1517/13543784.10.2.199. [DOI] [PubMed] [Google Scholar]
- 5.(a) Emmerson AM, Jones AM. J Antimicrob Chemother. 2003;51(suppl S1):13–20. doi: 10.1093/jac/dkg208. [DOI] [PubMed] [Google Scholar]; (b) Gootz TD, Brightly KE. Med Res Rev. 1996;16:433–486. doi: 10.1002/(SICI)1098-1128(199609)16:5<433::AID-MED3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Domagala JM. J Antimicrob Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 7.For recent syntheses: Beylin V, Boyles DC, Curran TT, Macikenas D, Parlett RV, IV, Vrieze D. Org Proc Res & Dev. 2007;11:441–449.Nikpour F, Sheikh D. Chem Lett. 2007;36:858–859.Colotta V, Catarzi D, Varano F, Calabri FR, Filacchioni G, Costagli C, Galli A. Bioorg Med Chem Lett. 2004;14:2345–2349. doi: 10.1016/j.bmcl.2004.01.109.Tran TP, Ellsworth EL, Stier MA, Domagala JM, Showalter HDH, Gracheck SJ, Shapiro MA, Joannides TE, Singh R. Bioorg Med Chem Lett. 2004;14:4405–4409. doi: 10.1016/j.bmcl.2004.06.063.Rivero IA, Espinoza K, Somanathan R. Molecules. 2004;9:609–616. doi: 10.3390/90700609.
- 8.(a) Ellsworth E, Tran T, Showalter HH, Sanchez J, Watson B, Stier M, Domagala S, Gracheck E, Joannides E, Shapiro M, Dunham S, Hanna D, Huband M, Gage J, Bronstein J, Liu J, Nguyen D, Singh R. J Med Chem. 2006;49:6435–6438. doi: 10.1021/jm060505l. [DOI] [PubMed] [Google Scholar]; (b) Huband MD, Cohen MA, Zurack M, Hanna DL, Skerlos LA, Sulavik MC, Gibson GW, Gage JW, Ellsworth E, Stier MA, Gracheck SJ. Antimicrob Agents and Chemother. 2007;51:1191–1201. doi: 10.1128/AAC.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tran T, Ellsworth E, Sanchez J, Watson B, Stier M, Showalter H, Domagala J, Shapiro M, Joannides E, Gracheck S, Nguyen D, Bird P, Yip J, Sharadendu A, Ha C, Ramezani S, Wu X, Singh R. Bioorg Med Chem Lett. 2007;17:1312–1320. doi: 10.1016/j.bmcl.2006.12.005. [DOI] [PubMed] [Google Scholar]; (d) German N, Malik M, Rosen JD, Drlica K, Kerns RJ. Antimicrob Agents Chemother. 2008;52:3915–3921. doi: 10.1128/AAC.00330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.One patent describes, in part, the synthesis of some 2-amino-6-chloro-4-cyclopropyl-7-fluoro-pyrido[1,2-c]pyrimidine-1,3-dione core ring intermediates toward preparing C-6 variants as fluoroquinolone analogs. Ellsworth EL, Showalter HDH. U.S. Patent 0114458. 2003:A1.
- 10.(a) Malik M, Lu T, Zhao X, Singh A, Hattan C, Domagala J, Kerns R, Drlica K. Antimicrob Agents Chemother. 2005;49:2008–2014. doi: 10.1128/AAC.49.5.2008-2014.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Malik M, Zhao X, Drlica K. Mol Microbiol. 2006;61:810–825. doi: 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 11.(a) Li Q, Sowin T, Claiborne A, Lijewski L, Zhang X, Raye K, Mazdiyasni H, Arnold W, Melcher LM, Wang W, Hasvold L, Fung A, Chu DTW, Plattner JJ. Heterocycles. 1999;51:1345–1353. [Google Scholar]; (b) Li Q, Chu DTW, Claiborne A, Cooper CS, Lee CM, Raye K, Berst KB, Donner P, Wang W, Hasvold L, Fung A, Ma Z, Tufano M, Flamm R, Shen LL, Baranowski J, Nilius A, Alder J, Meulbroek J, Marsh K, Cowell D, Hui Y, Seif L, Melcher LM, Henry R, Spanton S, Faghih R, Klein LL, Tanaka SK, Plattner JJ. J Med Chem. 1996;39:3070–3088. doi: 10.1021/jm960207w. [DOI] [PubMed] [Google Scholar]
- 12.Ficken GE, France H, Linstead RP. J Chem Soc. 1954;10:3730–3734. [Google Scholar]
- 13.Boyle PH, Ali HDP, McDonald TJ. ARKIVOK. 2003;7:67–79. [Google Scholar]
- 14.One early report of an unrelated 1,3-dione core, having no 5-methoxy group and not suitable for requisite substitution purposes, was found: Hunger A, Hoffmann K. Helv Chim Acta. 1957;40:1319–1330.
- 15.Experimental details for final quinazoline dione products 10, 12, and 14.For 6-Chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione (10): To a solution of 9 (100 mg, 0.38 mmol) in dry dichloromethane (4 mL) was added 1,1′-carbonyldiimidazole (184 mg, 1.14 mmol) followed by 1,8-diazabicyclo[5.4.0]undec-7-ene (117 μL, 0.76 mmol). The resulting solution was stirred under argon for two hours at room temperature, cooled in a water/ice bath and quenched by drop-wise addition of a solution of TFA in dichloromethane (3 mL of 10% w/w) causing a brilliant yellow color to persist. The reaction was transferred to a separatory funnel, diluted with dichloromethane (8 mL) and washed with water (5 mL). The organic was collected and the aqueous back-extracted with water (5 mL). The combined organic was washed with water (5 mL) and dried en vacuo to a bright yellow solid. The solid was dissolved in EtOAc and separated by flash chromatography (SiO2, 3:1 EtOAc/hexanes) to give 102 mg (93%) of 10 as a crystalline yellow solid. 1H NMR (300 MHz, CDCl3, δ) 10.40 (s, 1H), 8.19 (d, J = 5.1 Hz, 1H), 3.83 (s, 3H), 1.83-1.74 (m, 1H), 1.02–0.96 (m, 2H), 0.64-0.58 (m, 2H); 13C NMR (75 MHz, CDCl3, δ) 162.2, 149.2 (d, J = 1.6 Hz), 147.1 (d, J = 2.2 Hz), 146.4 (d, J = 242.1 Hz), 142.6, 124.5 (d, J = 22.5 Hz), 110.5 (d, J = 42.8 Hz), 106.6, 61.0, 8.8, 7.6; HRMS (ESI+) calc’d for C12H10ClFN2O3, 284.0364; m/z found, 284.0360.For 2-Amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-pyrido[1,2-c]pyrimidine-1,3-dione (12): To a solution of 10 (35 mg, 0.12 mmol) in dry THF (1 mL) was added 11 (35 mg, 0.18 mmol), potassium carbonate (33 mg, 0.24 mmol), and dry DMF (250 μL). The suspension was heated to 80°C for two hours and judged complete by TLC. The reaction was cooled to room temperature and the slurry was filtered trough a glass wool plug. The residual solids were washed with THF (2 mL), the filtrates combined and concentrated en vacuo to a brown oil. Separation by flash chromatography (SiO2, 100% EtOAc) gave 32 mg (88%) of 12 as a bright yellow oil. 1H NMR (300 MHz, CDCl3, δ) 8.28 (d, J = 5.1 Hz, 1H), 5.56 (s, 2H), 3.84 (d, J = 0.3 Hz, 3H), 1.90-1.81 (m, 1H), 1.05–0.99 (m, 2H), 0.63-0.57 (m, 2H); 13C NMR (75 MHz, CDCl3, δ) 158.2, 149.1 (d, J = 1.6 Hz), 146.7 (d, J = 242.3 Hz), 144.7 (d, J = 2.2 Hz), 139.4, 123.7 (d, J = 22.2 Hz), 110.5 (d, J = 43.6 Hz), 105.0, 61.1, 8.8, 8.1; HRMS (ESI+) calc’d for C12H11ClFN3O3, 299.0473; m/z found, 299.0461.For [1-(2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-1,3-dioxo-2,3-dihydro-1H-pyrido[1,2-c]pyrimidin-6-yl)-pyrrolidin-3-ylmethyl]-carbamic acid tert-butyl ester (14): To a solution of 12 (30 mg, 0.10 mmol) in dry acetonitrile (1 mL) was added 13 (60 mg, 0.30 mmol). This solution was heated to 85°C and stirred for 24 hours. The solution was concentrated in vacuo to an oil and purified by flash chromatography (SiO2, 9:1 EtOAc/MeOH) to give 39 mg (83%) of 14 as a bright yellow oil. 1H NMR (300 MHz, CDCl3, δ) 8.19 (d, J = 10.0 Hz, 1H), 5.43 (s, 2H), 4.82-4.73 (m, 1H), 3.80-3.72 (m, 3H), 3.53-3.45 (m, 1H), 3.43 (s, 3H), 3.29-3.23 (m, 2H), 2.51-2.39 (m, 1H), 2.15-2.05 (m, 1H), 1.78-1.67 (m, 2H), 1.46 (s, 9H), 1.00-0.91 (m, 1H), 0.88-0.80 (m, 1H), 0.47-0.33 (m, 2H); 13C NMR (75 MHz, CDCl3, δ) 158.2, 156.1, 146.5 (d, J = 245.2 Hz), 145.0 (d, J = 1.6 Hz), 142.1, 136.8 (d, J = 12.6 Hz), 132.6 (d, J = 6.6 Hz), 111.8 (d, J = 46.3 Hz), 96.3, 79.6, 60.5, 55.4 (d, J = 7.1 Hz), 51.6 (d, J = 7.1 Hz), 42.6, 39.6, 29.2 (d, J = 1.5 Hz), 28.4, 8.94 (d, J = 5.9 Hz), 8.33; HRMS (ESI+) calc’d for C22H30FN5O5, 463.2231; m/z found, 463.2227.