Abstract
The fruit fly, Drosophila melanogaster, has been extensively used as a model organism in genetics research and has significantly contributed to understanding molecular, cellular and evolutionary aspects of human behavior. Recently, research has focused on developing analytical methods to obtain highly sensitive chemical quantification along with spatiotemporal information from Drosophila melanogaster. We review a number of these advances in capillary electrophoresis, high-performance liquid chromatography, mass spectrometry and technologies involving intact organisms, including in vivo electrochemistry.
Keywords: Capillary electrophoresis, Embryo, Drosophila, High-performance liquid chromatography, in vivo electrochemistry, Larva, Mass spectrometry, MEMS-based system, Micellar electrokinetic chromatography, Microfluidics
1. Introduction
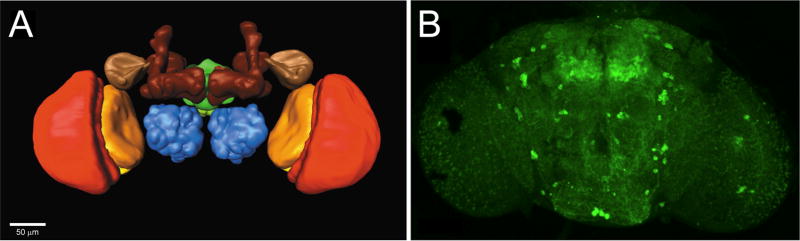
Drosophila melanogaster, the fruit fly, is an attractive model system with experimental and genetic qualities that have made it a prominent tool in developmental biology and genetics research. Originally pioneered by Thomas H. Morgan at the beginning of the last century, research using Drosophila has led to important insights into the mechanisms of human developmental and physiological processes. The fly matures relatively quickly, developing from embryo, to larva (divided into 1st, 2nd, and 3rd instar larva stages), to pupa, to sexually-mature adult in a span of ~12 days. An adult-fly brain is approximately 5 nL in volume and comprises several distinct structures that control specific tasks (Fig. 1A) [1]. These very small dimensions are a challenge for researchers attempting chemical quantification in the fly and necessitate the use of techniques capable of handling mass-limited samples. Although the adult fly has a simpler nervous system than those of vertebrates, it is capable of higher-order brain functions, including aversive and appetitive learning, and recalling learned information from prior experiences [2,3]. In addition, Drosophila larvae can be used as a model for investigating basic neurotransmission and chemosensory pathways [4], as the conservation between the Drosophila and mammalian proteomes is high with approximately half the protein sequences in the fly having similar counterparts in the human [5]. The evolutionary conservation between the two species has made the fly a successful model organism.
Figure 1.
Drosophila brain regions. (A) A polygonal model of the Drosophila melanogaster brain. Major neuropil regions are highlighted in color (brown = mushroom body; beige = lateral horn; blue = antennal lobe; green = central complex; red = medulla; orange = lobula; yellow = lobula plate). Scale bar, 50 μm. (B) Tyrosine hydroxylase immunolabeling showing dopaminergic neuron patterns in multifocal confocal views of adult-fly brain. (Reprinted from [63,64], with permission from Elsevier and the Society for Neuroscience).
1.1. Neurochemical basis for observed behaviors
Studies of neurotransmitters are under way in Drosophila to elucidate the roles of neurochemicals in human behavior. Biogenic amines, namely dopamine, serotonin and tyramine, are known to be involved in physiological processes found in both mammalian and Drosophila systems [3,6–9]. For example, dopamine has been implicated in human and fly behaviors, such as reward and motivation, sleep cycles, alcohol tolerance, and sensitivity to addictive drugs [6,7,10]. Tools that fluorescently label specific neuron clusters in the brain are available (Fig. 1B) and have made possible recent real-time, sensitive in vivo measurements of dopamine uptake by dopaminergic neurons in Drosophila [11,12]. In addition, neurotransmitter octopamine is thought to control many behaviors in the fly that norepinephrine regulates in mammals [13]. This evidence suggests that many of the neurotransmitter systems that regulate behavior are comparable between mammals and Drosophila.
1.2. Genetic manipulation for chemical analysis and behavioral studies
The Drosophila proteome has been a natural choice to study, as it was one of the first species with a fully sequenced genome [14]. The process of producing mutants to display a desired behavior via genetic manipulation is relatively straightforward with the fruit fly. The Drosophila genome contains little genetic redundancy, or multiple genes performing the same biochemical function, so that facilitates identification of individual genes and molecules that influence a particular behavior [1]. Many complex behavioral patterns found in mammalian systems with regards to learning and memory, courtship, alcohol tolerance, and circadian rhythms have been studied in the fruit fly using genetic mutants [15–18].
Drosophila mutants have been successfully used to model several human neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s, which are characterized by the late onset of progressive neurodegeneration and/or formation of abnormal neuronal inclusions or protein aggregates [19,20]. While these genetic mutants have helped link particular genes to a specific disease, little is known about the mechanisms leading to these pathologies. The ability to quantify all neuropeptides, amino acids, and neurotransmitters in Drosophila is a goal researchers are moving towards and will lead to greater understanding of these debilitating diseases.
The aim of this review is to highlight trends in recently developed analytical techniques for measuring chemicals in Drosophila. Methods that can obtain spatiotemporal information along with chemical quantification are at the forefront of this research. They provide a more analytical view of Drosophila and will likely improve understanding of the physiological mechanisms that underlie human behaviors, addictions and neurodegenerative diseases.
2. Detection methods for analysis of Drosophila homogenates
Many methods have been used to separate and to quantify mass-limited samples, including capillary electrophoresis (CE), high-performance liquid chromatography (HPLC), and mass spectrometry (MS). Indeed, these methods are sensitive and selective, making them capable of measuring and identifying multiple compounds in a complex biological sample. This ability allows determination of how different stimuli affect neurochemicals within the brain and is crucial to understanding changes throughout disease states.
Historically these analytical techniques have used homogenate methods to contend with the fly’s hard cuticle. Approximately 25–75 whole fly heads can be pulverized using small tissue grinders, or brains (~10) can be hand dissected using tungsten-carbide forceps, collected, and homogenized. While whole-fly-head samples allow for more flies in the homogenate, helping to reduce fly-to-fly variability, it leaves a significant matrix effect that can interfere with quantification. A sample of dissected brains can help to reduce these interfering signals; however, sample preparation is more time consuming, requires knowledge of dissection techniques, and limits the number of flies in a sample.
2.1. Capillary electrophoresis
CE separates ionic species according to their electrophoretic mobilities by applying a voltage over a narrow capillary filled with electrolytic solution. The small injection volumes associated with CE (nL to fL) make it an excellent method to study volume-limited samples, such as Drosophila [21,22]. Moreover, CE has high resolving power, due to its plug-like flow and minimal diffusion. Although CE uses electric fields to separate ions, neutral molecules can still be separated with CE by introducing a surfactant to carry out micellar electrokinetic chromatography (MEKC). In MEKC, a surfactant [e.g., sodium dodecyl sulfate (SDS)] is added to the running buffer at levels above the critical micelle concentration, resulting in the formation of micelles. The interaction of neutral molecules with the charged micelles results in retention and, when this interaction is differential, separation results.
Our laboratory has developed a procedure using MEKC to measure and to quantify biogenic amines, their metabolites and their precursors in Drosophila. End-column amperometry is used to detect electroactive species selectively, providing a simple, sensitive detection method without the need for derivatization. Using MEKC coupled to electrochemical detection (MEKC-EC), we have investigated different anatomical regions of Drosophila, including whole-body homogenates [23], whole-head homogenates [23], single-head homogenates [21] and, more recently, dissected brains.
Pioneering work by Ream et al. attempted to identify neurotransmitters in Drosophila using MEKC and resulted in identifying four important species: dopamine, tyramine, serotonin and dopamine precursor L-3,4-dihydroxyphenylalanine (L-DOPA) [23]. Migration times from standards obtained both before and after the fly sample were used for peak identification as well as normalization to the migration time of an internal standard, here dihydroxylbenzylamine (DHBA), to compensate for possible peak drifting. A collection of either heads or bodies (thoraces and abdomens) were homogenized and separated using TES (N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid)/SDS buffer [23]. A higher abundance of dopamine in samples from the body was observed when compared to the head only. This may be a consequence of dopamine being a main component in sclerotization (hardening) of the exterior cuticle of the fly body. The levels of L-DOPA remained unaltered between the two fly preparations. Furthermore, the levels of serotonin and tyramine were found to be higher in the head with tyramine levels close to the limit of detection in the body.
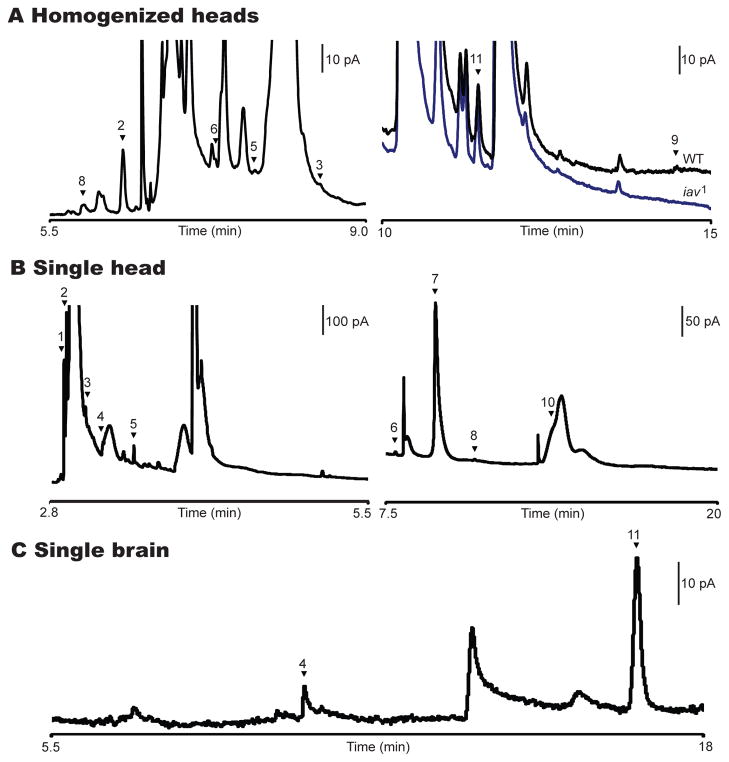
The type of buffer used affects the resolution of the separation of the biogenic amines. By using MEKC and a 25 mM borate/SDS buffer instead of TES/SDS, additional monoamines and metabolites were separated and identified. Borate (at basic pH) forms a complex with analytes possessing vicinal hydroxyl groups, imparting negative charge to the complex, which makes it possible to separate a standard of 14 neurochemicals [24]. Seven of these were later found in homogenized samples from Drosophila heads. Dopamine, tyramine, L-DOPA, and a neurotransmitter analog to the mammalian neurotransmitter norepinephrine, octopamine [13], were detected in addition to previously identified molecules. N-acetylated metabolites N-acetyl dopamine (naDA), N-acetyl octopamine (naOA), and N-acetyl serotonin (na5-HT) were identified; however, serotonin was not observed in these samples. The excellent separation ability of the borate/SDS buffer with MEKC-EC was demonstrated by comparing electropherograms from wild-type Drosophila to a mutant form, inactive (iav1), which expresses lower levels of octopamine and tyramine. As expected, the amounts of naOA, tyramine, and octopamine were reduced in the mutant form vs. the wild type, with tyramine being present at levels below the limit of detection in the mutant (Fig. 2A).
Figure 2.
MEKC-EC separations of homogenates from Drosophila. (A) Enlarged portion of an electropherogram (left) includes peaks naOA (2), naDA (3), na5-HT (5), OA (6), DA (8). Electropherogram (right) compares wild-type (WT - black trace) and mutant (iav1 - blue trace) Drosophila head homogenates emphasizing the internal standard CAT (11) and TA (9). Separation was run with borate buffer. There is no detectable level of TA in the mutant form. (B) Electropherogram of a single head with TES running buffer highlighting L-DOPA (1), naOA (2), naDA (3), naTA (4), na5-HT (5), OA (6), DHBA (7), DA (8), 5-HT (10). TA (9) is not visible on this scale. (C) Electropherogram of hand-dissected brain where naTA (4) and CAT (11) are visible. The working electrode was held at +750 mV vs. an Ag/AgCl reference electrode for all separations. (Reprinted from [21,24], with permission from the American Chemical Society).
The small sample volumes that can be analyzed using CE allow the study of not only a population of flies but also the variability within a population of flies arising from individual fly-to-fly differences. This is accomplished by analyzing one fly head at a time [21]. A new homogenization chamber was fabricated to accommodate the reduced sample volumes. After homogenizing a single fly head in 250 nL of perchloric acid, three individual fly heads were analyzed and compared. This procedure resulted in reproducible identification of nine neurochemicals (Fig. 2B), including N-acetyl tyramine (naTA).
Despite the resolving power of MEKC, unidentified electroactive species coelute with some neurochemicals. To reduce this problem, dissected Drosophila brains have been separated using borate/SDS buffer (Fig. 2C). By removing fly components thought to contain electroactive molecules (e.g., the cuticle, antennae and eyes), the electropherogram becomes easier to interpret. This is observed when comparing Fig. 2A, B with Fig. 2C, where the number of large, overloading, unidentified peaks is significantly reduced in the single-brain electropherogram. In addition, the dopamine from the cuticles is not measured, allowing only the amount of dopamine in the central nervous system (CNS) of the fly to be determined.
2.2. High-performance liquid chromatography
Just as CE, HPLC has been used to quantify the amount of biogenic amines, their metabolites and their precursors in the Drosophila CNS. These studies have aimed to determine the function of molecules and their localization within the fly head. HPLC is an improved form of column chromatography where solvent is pushed though the column under high pressures (up to 40 MPa). The high pressure allows for faster separation times and smaller column particles, yielding improved resolution. Typically, a C-18 column with an acidic mobile phase and electrochemical detector has been used to separate and detect these compounds [25–29].
Early reports using HPLC demonstrated the separation and the quantification of dopamine, L-DOPA, and α-methyldopa in 1–4-week-old brains and retinas of wild-type and mutant ebony flies, which have a darker pigment and impaired vision [29]. Although the levels of all three analytes were variable over time, the authors reported that these analytes were more abundant in the retina than the brain and more abundant in the mutant ebony fly heads than the wild-type fly heads.
Hardie and Hirsh expanded the number of neurotransmitters analyzed, quantifying dopamine, octopamine, tyramine, and serotonin in the brains and whole heads of Drosophila white-eyed (white) mutants [25]. They noted that nearly 75% of the total dopamine within the white mutant head is located outside the brain. In contrast, the percentages of octopamine, tyramine and serotonin present outside the brain are in the range only 1–37% when compared to the amount in the brain.
HPLC’s quantitative nature was also utilized to examine the role of tyramine in cocaine-sensitization studies of the inactive and the TβHM18 Drosophila mutants [8]. The inactive mutant, named after the flies’ low activity levels, was found to have approximately 60% less tyramine than wild-type flies, despite similar measurable levels of dopamine. While these mutants displayed expected behavioral responses to cocaine upon their first exposure, with repeated cocaine exposure, minimal behavioral sensitization to cocaine was observed. The TβHM18 line has a null mutation in the gene that codes for tyramine β-hydroxylase, the enzyme used to convert tyramine into octopamine. These flies were found to have almost an order of magnitude greater amount of tyramine and near-normal cocaine sensitization when compared to the wild-type fly, ruling out octopamine as the biogenic amine contributing to this cocaine sensitization. These two comparisons showed that tyramine plays a critical role in cocaine sensitization and later helped to confirm the identity of two tyrosine decarboxylase genes [28].
The Meinertzhagen laboratory has further investigated the location and the quantification of biogenic amines within the brain of genetic fly mutants with different eye pigments. They developed a method in the fly to quantify histamine [27], a transmitter known to be located in the eyes of the fly, and compared it, along with the amount of dopamine and serotonin, in white, brown and scarlet mutants, flies with three different eye-pigment mutations [26]. Since scarlet and are the two pigments that control fly-eye color, knocking out one pigment results in a fly with the other eye color, and a knock-out of both pigments results in no eye pigment, white mutants. They measured a significant decrease (in some cases over 50%) in the neurotransmitters of all three Drosophila mutants when compared to wild-type. Similar trends were observed in comparisons of wild-type vs. white mutants of houseflies, blowflies and two species of the flesh fly, signifying that many effects attributed to a mutant gene isolated in a white fly might be from the loss of pigment itself and not the mutated gene. They also noted that, in separations of wild-type fly-head homogenates, 71% of the total dopamine in the head was found in the brain, in contrast to Hardie and Hirsh’s results for white mutant flies.
2.3. Mass spectrometry to study proteins and peptides
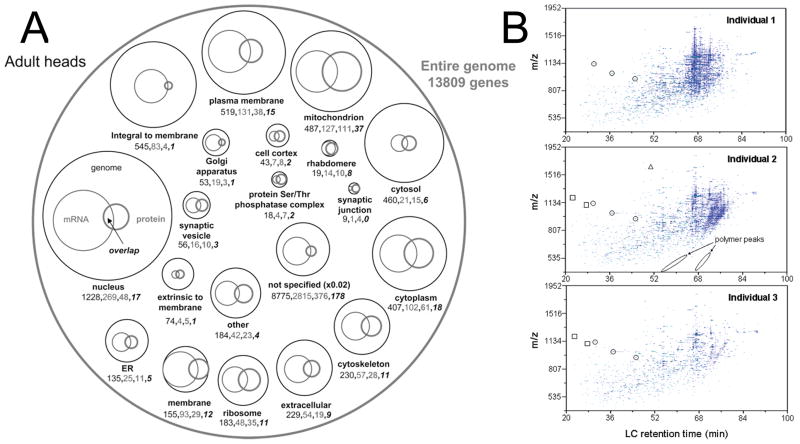
MS studies of the Drosophila proteome have used a variety of methods including matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS [30,31] and ion-trap MS [32]. In addition, a separation step is often added to the analysis including reversed-phase LC [32,33], ion-mobility spectrometry [34–36], or strong-cation-exchange chromatography [33,34]. Fig. 3A summarizes the number of genes, transcripts and proteins that have been observed within the adult Drosophila.
Figure 3.
Mass-spectrometric measurements of the Drosophila proteome. (A) Venn diagram of the known adult Drosophila genome (thin black circles and numbers), mRNA transcripts (thin grey circles and numbers), proteome (thick grey circles and bold numbers), and the overlap between mRNA transcripts and proteome (bold black Italic numbers). Circle size corresponds to the number of known genes, transcripts, and proteins listed below the circle. (B) LC-IMS-MS analysis of three digested individual flies. Many of these features are common within all three individuals but some examples of the differences have been labeled. Circled features designate peptides found in all three individuals, boxes only two individuals, and triangles only one individual. (Reprinted from [35,36], with permission from the American Chemical Society).
Initial proteomic methods have been used to understand the basic biology of Drosophila. Taraszka et al. [35] were able to map the proteomes of three individual Drosophila heads, identifying 197 proteins and finding at least 101 proteins in all three samples. While the other 96 proteins might not be expressed in all three fly samples, it could also be that the flies were using different proteins for different functions at the time of sacrifice. Fig. 3B shows examples of these differences.
More globally, differences have been observed in the Drosophila proteome lifespan. The fly proteome has been investigated over 60 days, at 7-day increments [34]. Approximately 1700 different proteins were identified and their changes in regulation compared between three different age groups: young (1–21-day-old flies), middle (22–42-day-old flies), and old (43–60-day-old flies). In these comparisons, a significant difference in protein regulation was observed for the young vs. middle groups. When the proteins experiencing an order of magnitude change or more in abundance were considered, 30 proteins were down-regulated while 12 proteins were up-regulated in the middle-aged group. These proteins were found to be associated with metabolism, development, reproduction or defense response.
Proteomic methods have yielded insight into Parkinson’s disease. Flies expressing mutated A30P [33], mutated A53T [37] or normal human [32] α-synuclein genes all display symptoms of Parkinson’s disease and have been investigated. These symptoms include decreased locomotor ability, formation of Lewy body-like inclusions in the brain, and degeneration of dopaminergic neurons with age. The symptoms are most severe for the flies with the A30P mutation, followed by the A53T mutated flies, and lastly the normal human α-synuclein mutated flies. All three of these mutated flies have had their proteomes compared to wild-type flies. Of note, the levels of 49 proteins in the A30P flies and 24 proteins in the A53T flies were significantly altered. Most of these proteins are associated with the actin cytoskeleton, mitochondria and membrane. In the human α-synuclein mutant flies, only 12 protein changes were observed, mostly related to metabolism and cellular signaling. These protein changes correlate with the severity of the Parkinson’s symptoms seen in the mutated flies and might lead to general insight about the protein alterations associated with this disease.
Complementary to the genomic and proteomic work, Drosophila neuropeptides have also been investigated using MALDI-TOF-MS. Predel et al. characterized the adult-fly peptidome using this technique and could identify 32 different neuropeptides in the Drosophila CNS [31]. Not only did this reveal the occurrence of these neuropeptides, but it also depicted their morphological distribution.
Recently, Kravitz and co-workers improved upon this method by combining both MALDI-TOF-MS and electrospray ionization (ESI) quadrupole time-of-flight (QTOF)-MS. Using the Drosophila galactosidase-4-upstream activating sequences (GAL4-UAS) gene-targeting system, subsets of cells were genetically labeled to aid in sample preparation (explanation of the GAL4-UAS system is beyond the scope of this review, but see [38]). They could successfully identify 42 neuropeptides encoded by 18 different genes in adult Drosophila brain extract [39].
The larval Drosophila peptidome has also been investigated using both one- and two-dimensional (1D and 2D) capillary LC (cLC) followed by QTOF-MS. Baggerman et al. could identify 38 peptides using the 2D technique vs. 28 using the 1D technique [40,41]. Their results demonstrated the increased efficiency of 2D LC-QTOF-MS over its 1D counterpart.
Yew, Cody, and Kravitz [42] applied atmospheric pressure ionization (API) to studying cuticular pheromones by using direct analysis in real-time MS. Since this technique can provide near-instantaneous analysis of samples, pheromones from live, awake Drosophila could be chemically investigated over a long period of time. Flies were immobilized by a vacuum applied through a pipette tip and probed with a metal pin attached to a micromanipulator while still allowing the fly the ability to interact behaviorally with other flies. Pheromone levels were found to be increased in females vs. males, in females after courtship, and as one moves closer to the genitals of the male fly. While this work beautifully shows the spatial and the temporal resolution that atmospheric pressure MS can provide, it lacks the ability to measure analytes from inside the fly.
3. Analytical techniques to measure physiology of intact flies
Recently, analytical tools have been developed to record chemical measurements in adult Drosophila and Drosophila larvae in real time. The ability to acquire direct physiological information will bridge the gap between observed fly behavior and the chemical signaling pathways that underlie those behaviors. Work has also been done to develop technologies for manipulating individual Drosophila embryos to study development. These tools will enable us to answer questions about the functions in an individual organism that whole-tissue homogenization and pooled sampling methods cannot address.
3.1. Individual-adult-fly measurements
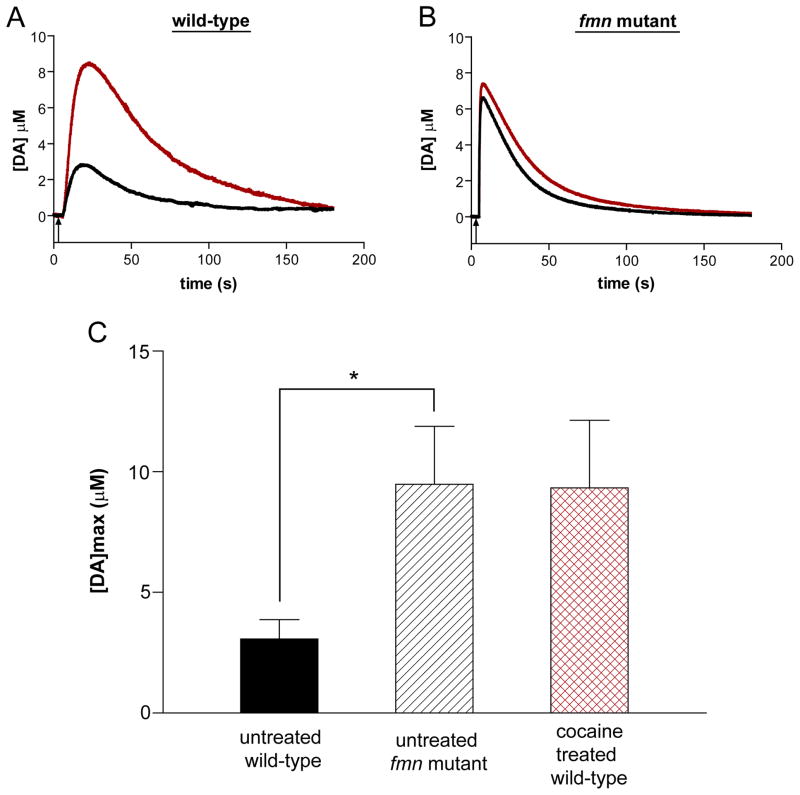
EC has been used for in vivo measurements of electroactive biogenic amines in model systems (e.g., rats, mice, and primates), but, until very recently, these measurements were not feasible in an organism as small as Drosophila [43–45]. Our laboratory has developed a method to monitor dopamine in the fly CNS using background-subtracted fast-scan cyclic voltammetry (FSCV) with carbon-fiber microelectrodes [11]. This method has been successfully used to investigate uptake of exogenously applied dopamine by the Drosophila dopamine transporter (DAT). Fig. 4(A and B) compares the concentration of dopamine measured before and after treatment with cocaine, known to block dopamine uptake by the DAT, in a wild-type fly vs. a fumin (fmn) mutant fly that lacks a functional DAT. While the peak dopamine concentration, [DA]max increased 3-fold after cocaine treatment for the wild-type fly, uptake remained unchanged for the fmn mutant. When the average [DA]max of multiple flies was considered (Fig. 4C), the [DA]max of untreated wild-type flies was significantly lower than that of fmn mutant flies. Interestingly, the [DA]max for cocaine-treated wild-type flies was not significantly different from that of the untreated fmn mutant flies, which supports existing evidence that cocaine effectively blocks the Drosophila dopamine transporter. These measurements validate the use of this in vivo method in flies as a model system to study drug-addiction mechanisms.
Figure 4.
Investigating dopamine-transporter function. (A) Representative concentration trace of exogenously applied 1.0 mM dopamine in wild-type Drosophila before (black line) and after (red line) cocaine application. An increase in dopamine concentration in the adult wild-type fly was observed following a 5 min exposure to 1.0 mM cocaine. Black arrow corresponds to a 1.0 s dopamine application beginning at 5.0 s. (B) Representative concentration trace of exogenously applied 1.0 mM dopamine in the fmn mutant before (black line) and after (red line) cocaine application. No significant change was observed in the adult fmn mutant fly. (C) Comparison of baseline [DA]max for the untreated wild-type and fmn mutant (mean ± SEM; Student’s t-test, p = 0.02 (*), n = 9) and the treated wild-type fly after application of 1.0 mM cocaine. The difference in [DA]max between untreated fmn mutants and wild-type flies treated with cocaine is not significantly different (mean ± SEM; Student’s t-test, p = 1.0, n = 6–9).
In addition to EC techniques, calcium imaging has been employed for making intact, whole-fly measurements using genetically-encoded fluorescent proteins. Fluorescent proteins that measure calcium changes (an accepted indicator of electrical activity) can be genetically expressed in specific neurons to target a tissue of interest in the Drosophila brain using the GAL4-UAS system. This methodology has been explored using several calcium-sensitive fluorescent proteins, including cameleon 2.1, camgaroo 2 and G-CaMP. Fiala and co-workers labeled the mushroom body calyx and antennal lobe in the Drosophila CNS with cameleon 2.1 and measured odor-evoked calcium signals in vivo from both regions [46]. Moreover, this technique can be altered to target any brain region of interest for which a GAL4 “driver line” exists [47]. Based upon previously published work on dissected mushroom bodies by Davis and co-workers, the GAL4-UAS system was used to label the mushroom bodies with camgaroo 2, and the intensity changes of the fluorescent Ca2+ reporter in response to acetylcholine application were recorded in an intact fly [48,49]. Axel and colleagues employed two-photon calcium microscopy to image the antennae lobes of flies that expressed G-CaMP in their projection neurons [50]. Using this technique, they were able to link odor-induced calcium changes to specific regions of the antennal lobe. Each odor elicited a distinct pattern that appears to be conserved between different organisms of the same fly line. These calcium-imaging techniques could be used for quantitative investigation of olfactory learning and memory in the Drosophila mushroom bodies and antennae lobes.
3.2. Individual-larva measurements
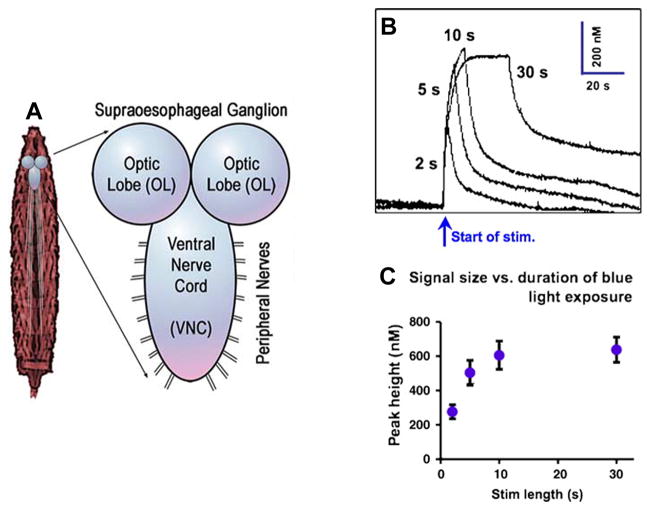
There has been recent progress in the development of techniques for measuring neurotransmitters from individual Drosophila larvae by combining EC and optical stimulation methods (Fig. 5A). Channelrhodopsin-2 is a light-activated cation-selective ion channel that, when placed under the control of a GAL4-UAS system and crossed with flies of a “driver line” specific to serotonin (Tph-GAL4), will produce larvae that release serotonin upon exposure to blue light [51].
Figure 5.
In vivo measurements in Drosophila larvae following optical stimulation. (A) Diagram of neuromuscular anatomy of a third-instar larva. (B) Representative traces of evoked peak serotonin concentration varying with duration of blue-light stimulus (2, 5, 10, and 30 s). (C) Pooled data (mean ± SEM, n = 6) shows an increase in peak height with increasing duration of blue-light exposure. Peak height appears to plateau after 10 s; peak height at 30 s is not significantly different from that at 10 s (Student’s t-test, 2 tailed, p = 0.78). (Reprinted from [52,54], with permission from the Society for Neuroscience and Elsevier).
Recently, Venton and colleagues utilized these transgenic larvae to measure serotonin release from neurons located in an isolated larva ventral nerve cord (VNC) using FSCV with a microelectrode [52]. The extracellular serotonin concentration in the VNC was found to consistently vary in the range 280–640 nM with the duration of exposure to blue light [Fig. 5(B and C]. Inhibition of the serotonin transporter with cocaine and fluoxetine confirmed that the removal of serotonin from the extracellular space was due to transport, and demonstrated the potential use of this model system for studying basic serotonin-signaling mechanisms.
The Drosophila larva model system also has potential use in other areas. A novel sampling technique has been developed to obtain nL volumes of hemolymph from individual Drosophila larvae for chemical analysis. Hemolymph contains amino acids (e.g., glutamate and glutamine) that are thought to play a role in neurodegeneration. This technique extracts 50–300 nL of hemolymph from a single Drosophila larva then, following derivatization with fluorescamine, its amino-acid content is quantified using CE with laser-induced fluorescence detection [53]. In a demonstration of this technique, Shippy and co-workers compared genderblind (gb) larvae, mutants developed previously by collaborators [54] that contain approximately half the normal extracellular glutamate concentration, to wild-type larvae. Overall, the gb mutants were found to have 38% lower glutamate levels than the wild-type larvae with 13 amino acids in total being successfully separated and quantified from each larva hemolymph (n = 10–17). These initial findings support the continued development of this technique in quantifying amino-acid levels from individual Drosophila hemolymph to better understand their role in human disease.
3.3. Controlling individual-fly-embryo development using microfluidics
There is increasing interest in using Drosophila embryos to study mechanisms of development and gene function. One powerful method of silencing a gene of interest is called RNA interference (RNAi). Cells are exposed to specifically-designed double-stranded RNA (dsRNA) that, once inside the cell, is cleaved into smaller dsRNA pieces (siRNAs) by endogenous enzymes. The siRNA then binds to an RNA-induced silencing complex (RISC), where it becomes unwound. The unwound siRNA guides the RISC to the corresponding messenger RNA (mRNA), whereby the RISC destroys the mRNA, thus eliminating the coding of that particular gene and the subsequent function of the gene [55,56].
While using cells for high-throughput screens is useful, embryos are ideal model systems for studying development and gene function because they possess physiological content with greater biological complexity; however, until recently, performing RNAi on embryos was a tedious process that required a skilled technician to inject each embryo by hand individually. Solgaard and colleagues have developed a microfluidic device coupled with a computer-controlled injection system to inject Drosophila embryos with dsRNA for high-throughput RNAi screens [57]. This microelectromechanical systems (MEMS)-based device has been automated to detect embryos on a glass slide, followed by rapid injection of 60 pL RNAi aliquots into each embryo with 98% reliability. Although preliminary prototypes require initial manual injector alignment to the device, it has great potential for future development into a fully automated process and has already been adapted for various embryo applications where controlled microinjections of small molecules, such as drugs or proteins, are necessary [58,59].
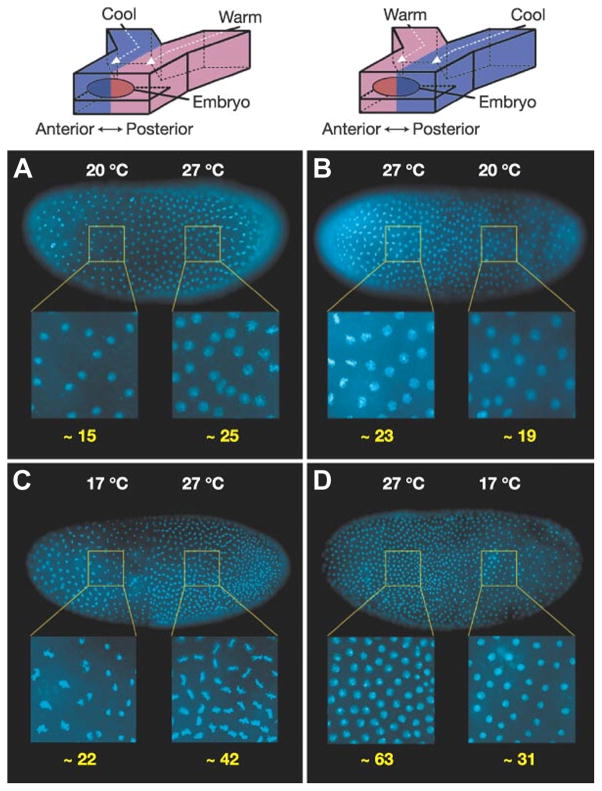
Microfluidic technologies have also been utilized to develop devices capable of spatial and temporal control of developing Drosophila embryos. Ismagilov and co-workers [60,61] have used a ‘Y’ junction device to investigate a compensatory regulation mechanism displayed by developing embryos towards external perturbations in temperature (Fig. 6, top). When the anterior and posterior sides of an embryo were exposed to an extreme temperature gradient using two laminar streams held at different temperatures, the warmer half of the embryo had a higher number of nuclei, and was therefore developing more rapidly than the cooler half [Fig. 6A and B)]. When the temperature difference between anterior and posterior sides was increased from 7°C to 10°C, the difference in the rate of development between the two sides also increased [Fig. 6(C and D)]. Also, the Even-skipped gene (a gene that codes for segmentation during early embryonic development) was expressed sooner in the warmer region of the embryo, causing the usual seven-stripe segmentation pattern to develop in the wrong order. Interestingly, despite the different developmental rates forced upon the two regions of the embryo, when allowed to come to room temperature, the embryos displayed the completed stripe pattern correctly and developed into normal larvae, suggesting Drosophila have a compensation mechanism to counteract extreme environmental conditions during embryo development. This device has since been adapted to allow for easier attachment of the embryos [62]. Continued modifications that enhance the ability to apply external gradients to an immobilized embryo will pave the way for future studies on the mechanisms of biochemical networks during development.
Figure 6.
Microfluidic devices in the analysis of Drosophila embryos. The rate of development in each half of the embryo exposed to a T-step is affected by temperature, as demonstrated by the difference in nuclear density (number of nuclei in enlarged areas shown underneath in yellow numbering). (A, B) Embryos exposed to a T-step of 20°C/27°C for 140 min. (A) Anterior half 20°C, posterior half 27°C. (B) Anterior half 27°C, posterior half 20°C. (C, D) Identical set-up to A and B with embryos exposed to a greater T-step of 17°C/27°C for 150 min. In all images, higher nuclear density was observed in the warmer half of the embryo. (Reprinted from [60], with permission from Nature Publishing Group).
4. Conclusions and future trends of Drosophila research
Several analytical-chemistry techniques, including CE, HPLC, MS, EC and microfluidics, have recently been developed to address Drosophila melanogaster. These advances have allowed researchers to begin quantifying neurochemicals and to obtain spatiotemporal information in this mass-limited model organism.
Far from the fly being an experimental organism used by biologists only, adult flies, fly larvae and embryos of flies are all currently being studied in many scientific fields. The need for the future is to build better connections between biologists and analytical chemists to develop technology in the direction of problem-driven research. Further miniaturization is a critical component of the analytical aspect of this work, as the physical methods are still relatively large compared to the extremely small sample.
The genetics of larvae and adult flies needs to be considered, as expression can vary and specific methods in larvae cannot always be carried out in the adult. Both models are extremely important and need to be integrated.
The continued development of tools capable of measuring neurotransmitters, neuropeptides, and amino acids in the size-limited regions of the fly will further our ability to utilize this genetically-advantageous model system in our attempts to improve understanding of human physiological functions.
Acknowledgments
The authors thank the National Institutes of Health (grant 5R01GM078385–02) and the Swedish National Science Foundation (VR) for support. AGE is supported by a Marie Curie Chair from the European Union’s Sixth Framework Programme.
Abbreviations
- DAT
Dopamine transporter
- GAL4-UAS
Galactosidase-4-upstream activating sequences
- RNAi
RNA interference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waddell S, Quinn WG. Trends Genet. 2001;17:719. doi: 10.1016/s0168-9525(01)02526-4. [DOI] [PubMed] [Google Scholar]
- 2.Kim YC, Lee HG, Han KA. J Neurosci. 2007;27:7640. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. J Neurosci. 2003;23:10495. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber B, Stocker RF. Chem Sens. 2007;32:65. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- 5.Rubin GM, et al. Science. Vol. 287. Washington, DC: 2000. p. 2204. [Google Scholar]
- 6.Li H, Chaney S, Forte M, Hirsh J. Curr Biol. 2000;10:211. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 7.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. J Neurosci. 2005;25:7377. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClung C, Hirsh J. Curr Biol. 1999;9:853. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- 9.Bergquist J, Sciubisz A, Kaczor A, Silberring J. J Neurosci Methods. 2002;113:1. doi: 10.1016/s0165-0270(01)00502-7. [DOI] [PubMed] [Google Scholar]
- 10.Bainton RJ, Tsai LTY, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Curr Biol. 2000;10:187. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 11.Makos MA, Kim YC, Han KA, Heien ML, Ewing AG. Anal Chem. 2009;81:1848. doi: 10.1021/ac802297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. J Neurobiol. 2003;54:618. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 13.Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Curr Biol. 2008;18:159. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 14.Adams MD, et al. Science. Vol. 287. Washington, DC: 2000. p. 2185. [Google Scholar]
- 15.Sokolowski MB. Nat Rev Genet. 2001;2:879. doi: 10.1038/35098592. [DOI] [PubMed] [Google Scholar]
- 16.Lee HG, Kim YC, Dunning JS, Han KA. PLoS ONE. 2008;3:e1391. doi: 10.1371/journal.pone.0001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholz H, Ramond J, Singh CM, Heberlein U. Neuron. 2000;28:261. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 18.Panda S, Hogenesch JB, Kay SA. Nature (London) 2002;417:329. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 19.Celotto AM, Palladino MJ. Mol Interv. 2005;5:292. doi: 10.1124/mi.5.5.9. [DOI] [PubMed] [Google Scholar]
- 20.Marsh JL, Thompson LM. BioEssays. 2004;26:485. doi: 10.1002/bies.20029. [DOI] [PubMed] [Google Scholar]
- 21.Powell PR, Paxon TL, Han KA, Ewing AG. Anal Chem. 2005;77:6902. doi: 10.1021/ac050963m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar JS, Pabbati CP, Lorenz RM, He M, Fiorini GS, Chiu DT. Anal Chem. 2006;78:6948. doi: 10.1021/ac0613131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ream PJ, Suljak SW, Ewing AG, Han KA. Anal Chem. 2003;75:3972. doi: 10.1021/ac034219i. [DOI] [PubMed] [Google Scholar]
- 24.Paxon TL, Powell PR, Lee HG, Han KA, Ewing AG. Anal Chem. 2005;77:5349. doi: 10.1021/ac050474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardie SL, Hirsh J. J Neurosci Methods. 2006;153:243. doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Borycz J, Borycz JA, Kubow A, Lloyd V, Meinertzhagen IA. J Exp Biol. 2008;211:3454. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- 27.Borycz J, Vohra M, Tokarczyk G, Meinertzhagen IA. J Neurosci Methods. 2000;101:141. doi: 10.1016/s0165-0270(00)00259-4. [DOI] [PubMed] [Google Scholar]
- 28.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. J Biol Chem. 2005;280:14948. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 29.Ramadan H, Alawi AA, Alawi MA. Cell Biol Int. 1993;17:765. doi: 10.1006/cbir.1993.1138. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin MA, Medzihradszky KF, Lock CM, Fisher B, Settineri TA, Burlingame AL. Anal Chem. 2001;73:1707. doi: 10.1021/ac0011080. [DOI] [PubMed] [Google Scholar]
- 31.Predel R, Wegener C, Russell WK, Tichy SE, Russell DH, Nachman RJ. J Comput Neurol. 2004;474:379. doi: 10.1002/cne.20145. [DOI] [PubMed] [Google Scholar]
- 32.Xun Z, Kaufman TC, Clemmer DE. J Proteome Res. 2008;7:3911. doi: 10.1021/pr800207h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xun Z, Sowell RA, Kaufman TC, Clemmer DE. J Proteome Res. 2007;6:348. doi: 10.1021/pr060488o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowell RA, Hersberger KE, Kaufman TC, Clemmer DE. J Proteome Res. 2007;6:3637. doi: 10.1021/pr070224h. [DOI] [PubMed] [Google Scholar]
- 35.Taraszka JA, Gao X, Valentine SJ, Sowell RA, Koeniger SL, Miller DF, Kaufman TC, Clemmer DE. J Proteome Res. 2005;4:1238. doi: 10.1021/pr050037o. [DOI] [PubMed] [Google Scholar]
- 36.Taraszka JA, Kurulugama R, Sowell RA, Valentine SJ, Koeniger SL, Arnold RJ, Miller DF, Kaufman TC, Clemmer DE. J Proteome Res. 2005;4:1223. doi: 10.1021/pr050038g. [DOI] [PubMed] [Google Scholar]
- 37.Xun Z, Sowell RA, Kaufman TC, Clemmer DE. Mol Cell Proteomics. 2008;7:1191. doi: 10.1074/mcp.M700467-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy JB. Genesis. 2002;34:1. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 39.Yew JY, Wang Y, Barteneva N, Dikler S, Kutz-Naber KK, Li L, Kravitz EA. J Proteome Res. 2009;8:1271. doi: 10.1021/pr800601x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggerman G, Boonen K, Verleyen P, De Loof A, Schoofs L. J Mass Spectrom. 2005;40:250. doi: 10.1002/jms.744. [DOI] [PubMed] [Google Scholar]
- 41.Baggerman G, Cerstiaens A, De Loof A, Schoofs L. J Biol Chem. 2002;277:40368. doi: 10.1074/jbc.M206257200. [DOI] [PubMed] [Google Scholar]
- 42.Yew JY, Cody RB, Kravitz EA. Proc Natl Acad Sci USA. 2008;105:7135. doi: 10.1073/pnas.0802692105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benoit-Marand M, Jaber M, Gonon F. Eur J Neurosci. 2000;12:2985. doi: 10.1046/j.1460-9568.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 44.Burmeister JJ, Pomerleau F, Huettl P, Gash CR, Wemer CE, Bruno JP, Gerhardt GA. Biosens Bioelectron. 2008;23:1382. doi: 10.1016/j.bios.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Quintero JE, Day BK, Zhang Z, Grondin R, Stephens ML, Huettl P, Pomerleau F, Gash DM, Gerhardt GA. Exp Neurol. 2007;208:238. doi: 10.1016/j.expneurol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud JM, Buchner E, Galizia CG. Curr Biol. 2002;12:1877. doi: 10.1016/s0960-9822(02)01239-3. [DOI] [PubMed] [Google Scholar]
- 47.Fiala A, Spall T. Sci STKE. 2003:l6. doi: 10.1126/stke.2003.174.pl6. [DOI] [PubMed] [Google Scholar]
- 48.Wright NJD. J Neurosci Methods. 2006;155:77. doi: 10.1016/j.jneumeth.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 49.Yu DH, Baird GS, Tsien RY, Davis RL. J Neurosci. 2003;23:64. doi: 10.1523/JNEUROSCI.23-01-00064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Cell. 2003;112:271. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 51.Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Curr Biol. 2006;16:1741. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 52.Borue X, Cooper S, Hirsh J, Condron B, Venton BJ. J Neurosci Methods. 2009;179:300. doi: 10.1016/j.jneumeth.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piyankarage SC, Augustin H, Grosjean Y, Featherstone DE, Shippy SA. Anal Chem. 2008;80:1201. doi: 10.1021/ac701785z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. J Neurosci. 2007;27:111. doi: 10.1523/JNEUROSCI.4770-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Nature (London) 1998;391:806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 56.Hannon GJ. Nature (London) 2002;418:244. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 57.Zappe S, Fish M, Scott MP, Solgaard O. Lab Chip. 2006;6:1012. doi: 10.1039/b600238b. [DOI] [PubMed] [Google Scholar]
- 58.Cornell E, Fisher WW, Nordmeyer R, Yegian D, Dong M, Biggin MD, Celniker SE, Jin J. Rev Sci Instrum. 2008;79:7. doi: 10.1063/1.2827516. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Liu X, Gelinas D, Ciruna B, Sun Y. PLoS One. 2007;2:e862. doi: 10.1371/journal.pone.0000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. Nature (London) 2005;434:1134. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lucchetta EM, Munson MS, Ismagilov RF. Lab Chip. 2006;6:185. doi: 10.1039/b516119c. [DOI] [PubMed] [Google Scholar]
- 62.Dagani GT, Monzo K, Fakhoury JR, Chen CC, Sisson JC, Zhang XJ. Biomed Microdev. 2007;9:681. doi: 10.1007/s10544-007-9077-z. [DOI] [PubMed] [Google Scholar]
- 63.Coulom H, Birman S. J Neurosci. 2004;24:10993. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rein K, Zöckler M, Mader MT, Grübel C, Heisenberg M. Curr Biol. 2002;12:227. doi: 10.1016/s0960-9822(02)00656-5. [DOI] [PubMed] [Google Scholar]