Abstract
The indole–diketopiperazine bridge is an important structural feature of many bispyrrolidinoindoline and epipolythiodiketopiperazine fungal metabolites. Organocatalytic conjugate addition of diketopiperazines to indoles was achieved in good to excellent yields through electrophilic indolenine intermediates generated under mild conditions. Screening of catalysts and solvents at different temperatures was performed in order to achieve high product yields.
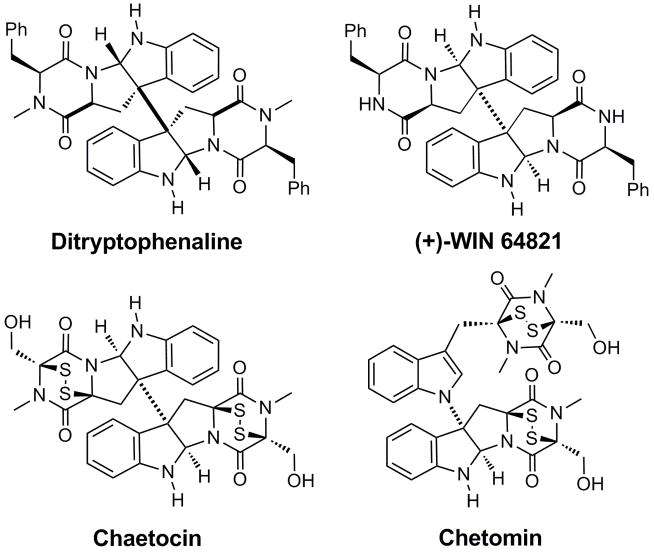
The indole – diketopiperazine bridge is an important structural feature of many complex alkaloids of fungal origin that display a spectrum of interesting biological activity. Such alkaloids contain cyclotryptophan motifs of considerable structural diversity often fused to N-alkylated or sulfenylated diketopiperazine rings. Ditryptophenaline1 (Figure 1), extracted from several strains of Aspergillus flavus, incorporates 3a,3a′-bispyrrolidinoindoline core and shows cytotoxicity in several cell lines. Alkaloid (+)-WIN 64821,2–6 also isolated from Aspergillus sp., is a potent competitive substance P antagonist against the human neurokinin 14–6 and cholecystokinin type B receptors.7 Other indole alkaloids derived from two tryptophan units and with two additional α-amino acids in diketopiperazine rings include (+)-asperazine8 and (+)-gliocladin C.9 Sporidesmins, toxic secondary metabolites produced by filamentous fungi of Chaetomium or Pithomyces sp. also contain cyclotryptophan motifs along with the disulfide or polysulfide bridges spanning diketopiperazine rings. Chaetocin and chetomin are two examples of dimeric epidithiodiketopiperazine (ETP) fungal metabolites,10,11 that incorporate an indole system fused to the ETP core (Figure 1). The potent biological activity of these natural products is attributed to the redox properties of the disulfide bridges.11,12 Apart from cytotoxic and immunomodulatory activities, chetomin also shows promising antiangiogenic action: it suppresses the neovascularization in solid tumors by disrupting the transcription of the vascular endothelial growth factor (VEGF) gene.11,13
Figure 1.
Natural products with cyclotryptophan motifs incorporating indole – diketopiperazine linkages
Despite their striking architecture and interesting biological activity, many dimeric ETPs have remained elusive synthetic targets, with total synthesis of 11,11′-dideoxyverticillin A, a potent tyrosine kinase inhibitor, being the only reported example to date.14 This highlights the challenges involved in the construction of such stereochemically complex, densely functionalized structures bearing highly sensitive to bases and reducing agents functional groups. Direct organocatalytic methods for the formation of the indole – diketopiperazine linkage could facilitate development of succinct routes for the efficient assembly of the key structural blocks of dimeric ETPs and bispyrrolidinoindoline alkaloids.
The lower reactivity of β-amido esters and diketopiperazines in α-alkylation reactions, as compared to aldehydes and ketones, remains the main challenge in the development of direct and efficient procedures for their alkylation. Although many examples of organocatalytic alkylations of aldehydes and ketones can be found in the recent literature,15 none so far have been reported for substituted β-amido esters and diketopiperazines. Organocatalytic transformations of the piperazine-2,5-dione ring system are known to be more challenging as compared to aldehydes, ketones and esters due to its lower reactivity toward electrophiles and greater steric bulk.16 In this communication, we report the first direct, organocatalytic coupling of substituted diketopiperazines and indoles.
Only a few strategies for the direct construction of the indole – diketopiperazine bridge are known to date. The most commonly used is the method pioneered by the groups of Kametani and Somei (Figure 2).17,18 This remarkable reaction involves coupling of the nucleophilic substrate with gramine in refluxing acetonitrile in the presence of trialkylphosphine. This reaction has received a number of applications, for example, in the synthesis of paraherquamide A19 and B,20,21 by Williams and coworkers as well as in the mechanistic study of the Morin rearrangement,22 although it uses toxic trialkylphosphine and generally gives product in moderate yields (40–70%). An interesting modification of this reaction has been reported by Hart and Magomedov, where treatment of substituted diketopiperazine with Li2CuCl4 followed by the addition of gramine methosulfate gave coupling product in 54% yield. 23
Figure 2.
Alkylation of piperazine-2,5-dione 2a with gramine 1a
Our first goal was to improve the efficiency and increase product yields in the coupling of gramine with substituted diketopiperazines. Hence, we directed our initial efforts to exploration of the feasibility of the reaction between gramine 1a and ethyl ester 2a using inexpensive, readily available quinine as Lewis base (Figure 2). The results were encouraging as higher yields were obtained when quinine was used in place of tributylphosphine. To further explore the scope of this transformation, three substrates 2a–c were tested at temperatures ranging from ambient to the temperature of the reflux of acetonitrile (Table 1). No reaction was taking place at room temperature, and low yields were obtained at 50 °C (Table 1, Entries 1–4), presumably due to low rate of formation of 3-methylene indolenine intermediate from gramine. However, further increase of the temperature to 82 °C dramatically improved the reaction. The products 3a–3c formed in good to excellent yields, although as expected, the reaction showed decrease in yields with the increase of the steric bulk of the ester groups in substrates 2a–2c (Table 1, Entries 5–7). To compensate for such a decrease, longer reaction time and larger excess of 1a were used. The use of chiral quinine opened a possibility for an asymmetric induction during the catalytic cycle. Unfortunately, the observed enantioselectivity was rather low, with ee ranging from 10–25%. The use of gramine-N-oxide 1b24 or 1-Boc-gramine 1c25 (Figure 3) did not improve product yield or enantioselectivity.
Table 1.
Effect of substrate and temperature in the quinine-promoted organocatalytic alkylation of substituted diketopiperazines with gramine
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Eq. of 1a | Time (h) | Temp. (°C) | Yielda (%) |
| 1 | 2a | 2.0 | 24 | rt | NR |
| 2 | 2b | 2.0 | 24 | rt | NR |
| 3 | 2a | 2.0 | 24 | 50 | 10 |
| 4 | 2b | 2.0 | 24 | 50 | <5 |
| 5 | 2a | 2.0 | 24 | 82 | 92 |
| 6 | 2b | 3.5 | 48 | 82 | 81 |
| 7 | 2c | 4.0 | 48 | 82 | 72 |
Yield of purified product after chromatographic separation.
Figure 3.

Gramine-N-oxide 1b and 1-Boc-protected gramine 1c as substrates for organocatalytic α-alkylation of diketopiperazines
In a search for donors that generate indolenine intermediates at ambient temperature and tolerating indole ring and methylene bridge substituents, we turned to arylsulfonyl indoles that have been recently reported to promote proline-catalyzed α-alkylation of aldehydes.15,26,27 Two types of arylsulfonyl indoles were chosen: one with the phenyl group at the indolyl position, which could facilitate determination of the diastereomeric ratio of the products, and the substrate 5c with unsubstituted methylene bridge28 (vide infra). The sulfonyl group at the benzylic position of 3-substituted indoles constitutes a good leaving group, which under basic or acidic conditions allows for a facile generation of an electrophilic indolenine intermediate. To generate such intermediates from the arylsulfonyl indoles, a method that involves treatment with KF on basic alumina29 is often used. Because β-amido esters are usually compatible with such bases, we sought to develop a simple and general protocol for the alkylation of substituted diketopiperazines with arylsulfonyl indoles using cinchona alkaloids as catalysts.
We first performed the reaction with substrate 2c and 5a in dichloromethane as the solvent. The presence of a phenyl group at the indolyl position produces diastereomeric products whose d.r. could be readily determined by 1H NMR spectroscopy. This would provide an opportunity to study the effect of different organocatalysts on the diastereoselectivity of the reaction. Gratifyingly, the reaction proceeded in high yields with moderate amounts of cinchona alkaloids (Table 2, Entry 1). After the successful reaction of the arylsulfonyl indole 5a with the diketopiperazine 2a we focused on optimizing the reaction conditions by varying solvents, temperatures and substituents on aryl indoles and diketopiperazine. Substituents at the indole ring (C-2 position) and at the bridging carbon are well tolerated, suggesting its wide scope (Table 2, Entries 10–11). We found that 80 mg of KF/alumina per 0.1 mmol of sulfone was needed to obtain good yields. Next, the effects of catalyst loading, temperature and solvents were studied in the reactions with substrate 2c. The catalyst 4b gave the best yields with good diastereomeric ratios at room temperature. Lowering the reaction temperature to 0 °C resulted in a decrease of product yield but did not improve stereoselectivity of the reaction. Even with the relatively bulky substrate 2c, high yields were obtained after increasing its stoichiometric amount from three to five equivalents (Table 2, Entry 7). Finally, arylsulfonyl indole 5c, with methylene group at the indolyl position in the reactions with substrate 2c gave product in 90% yield (Table 2, Entry 12), illustrating that this transformation could be readily used in the synthesis of natural products with cyclotryptophan motifs.
Table 2.
Scope of α-alkylation of diketopiperazines 2b–2c with arylsulfonyl indoles 5a–5c catalyzed by cinchona alkaloids 4a–4c
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Indole | DKP | Solvent | Catalyst | Temp. (°C) | Time (h) | Yielda (%) | d.r. |
| 1 | 5a | 2c | CH2Cl2 | 4a | rt | 48 | 55 | 5:1 |
| 2 | 5b | 2c | CH2Cl2 | 4a | rt | 72 | 70 | 2.6:1 |
| 3 | 5a | 2c | CH2Cl2 | 4b | rt | 48 | 72 | 4.5:1 |
| 4 | 5a | 2c | Toluene | 4b | 0 | 120 | NR | - |
| 5 | 5a | 2c | Toluene | 4b | rt | 120 | 46 | 3.8:1 |
| 6 | 5b | 2c | CH2Cl2 | 4b | rt | 72 | 75 | 3:1 |
| 7 | 5a | 2c | CH2Cl2 | 4b | rt | 72 | 89b | 4.5:1 |
| 8 | 5a | 2b | CH2Cl2 | 4c | rt | 48 | 71 | 1:1 |
| 9 | 5a | 2b | Toluene | 4c | rt | 48 | 54 | 1:1 |
| 10 | 5a | 2c | CH2Cl2 | 4c | rt | 48 | 70 | 2.7:1 |
| 11 | 5b | 2c | CH2Cl2 | 4c | rt | 72 | 70 | 2.5:1 |
| 12 | 5c | 2c | CH2Cl2 | 4b | rt | 48 | 90 | N/A |
Yield of purified product after chromatographic separation.
5 eq. of 2c was used.
We also performed the reactions using different stoichiometries of diketopiperazine and arylsulfonyl indole in order to determine the best ratio of reactants. Interestingly, it was found that the best yields were obtained when the arylsulfonyl indole, as opposed to carboxylated diketopiperazine, was used as the limiting reagent.
In conclusion, a new strategy for the direct organocatalytic coupling of substituted diketopiperazines with indoles has been developed, through the use of 3-(-1-arylsulfonylalkyl)indoles as convenient precursors to indolenine intermediates and cinchona alkaloids as organocatalysts. This method could provide an efficient means for rapid construction of bispyrrolidinoindoline scaffolds of alkaloids and epidithiodiketopiperazine fungal metabolites. Further exploration of the mechanism of this reaction and development of its enantioselective variant are under investigation.
Supplementary Material
Figure 4.

Cinchona alkaloid Lewis bases used in this study
Acknowledgments
We thank the National Science Foundation (CHE-0748838) and the National Institutes of Health (R21 CA129388) for funding.
Footnotes
Supplementary Material
Synthetic procedures, characterization and NMR spectra of compounds 3a–c and 6a–c are available. Supplementary data associated with this article is available with its online version at doi:00.0000/j.tetlet.2009.00.0000.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Springer JP, Buchi G, Kobbe B, Demain AL, Clardy J. Tetrahedron Lett. 1977:2403–2406. [Google Scholar]
- 2.Movassaghi M, Schmidt MA, Ashenhurst JA. Angew Chem-Int Edit. 2008;47:1485–1487. doi: 10.1002/anie.200704960. [DOI] [PubMed] [Google Scholar]
- 3.Barrow CJ, Cai P, Snyder JK, Sedlock DM, Sun HH, Cooper R. J Org Chem. 1993;58:6016–6021. [Google Scholar]
- 4.Oleynek JJ, Sedlock DM, Barrow CJ, Appell KC, Casiano F, Haycock D, Ward SJ, Kaplita P, Gillum AM. J Antibiot. 1994;47:399–410. doi: 10.7164/antibiotics.47.399. [DOI] [PubMed] [Google Scholar]
- 5.Popp JL, Musza LL, Barrow CJ, Rudewicz PJ, Houck DR. J Antibiot. 1994;47:411–419. doi: 10.7164/antibiotics.47.411. [DOI] [PubMed] [Google Scholar]
- 6.Sedlock DM, Barrow CJ, Brownell JE, Hong A, Gillum AM, Houck DR. J Antibiot. 1994;47:391–398. doi: 10.7164/antibiotics.47.391. [DOI] [PubMed] [Google Scholar]
- 7.Hiramoto MSM, Miyata H, Saita Y. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 1994;36:557–569. [Google Scholar]
- 8.Govek SP, Overman LE. J Am Chem Soc. 2001;123:9468–9469. doi: 10.1016/j.tet.2007.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overman LE, Shin Y. Org Lett. 2007;9:339–341. doi: 10.1021/ol062801y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McInnes AG, Taylor A, Walter JA. J Am Chem Soc. 1976;98:6741–6741. doi: 10.1021/ja00437a074. [DOI] [PubMed] [Google Scholar]
- 11.Block KM, Wang H, Szabo LZ, Polaske NW, Henchey LK, Dubey R, Kushal S, Laszlo CF, Makhoul J, Song Z, Meuillet EJ, Olenyuk BZ. J Am Chem Soc. 2009 doi: 10.1021/ja807601b. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardiner DM, Waring P, Howlett BJ. Microbiology-UK. 2005;151:1021–1032. doi: 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]
- 13.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang BQ, Wang JM, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Ashenhurst JA, Movassaghi M. Science. 2009;324:238–241. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaikh RR, Mazzanti A, Petrini M, Bartoli G, Melchiorre P. Angew Chem-Int Edit. 2008;47:8707–8710. doi: 10.1002/anie.200803947. [DOI] [PubMed] [Google Scholar]
- 16.Dubey R, Polaske NW, Nichol GS, Olenyuk B. Tetrahedron Lett. 2009;50:4310–4313. doi: 10.1016/j.tetlet.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kametani T, Kanaya N, Ihara M. J Am Chem Soc. 1980;102:3972–3975. [Google Scholar]
- 18.Somei M, Karasawa Y, Kaneko C. Heterocycles. 1981;16:941–949. [Google Scholar]
- 19.Williams RM, Cao JH, Tsujishima H, Cox RJ. J Am Chem Soc. 2003;125:12172–12178. doi: 10.1021/ja036713+. [DOI] [PubMed] [Google Scholar]
- 20.Cushing TD, Sanzcervera JF, Williams RM. J Am Chem Soc. 1993;115:9323–9324. [Google Scholar]
- 21.Cushing TD, SanzCervera JF, Williams RM. J Am Chem Soc. 1996;118:557–579. [Google Scholar]
- 22.Freed JD, Hart DJ, Magomedov NA. J Org Chem. 2001;66:839–852. doi: 10.1021/jo0013406. [DOI] [PubMed] [Google Scholar]
- 23.Hart DJ, Magomedov N. J Org Chem. 1999;64:2990–2991. doi: 10.1021/jo990147c. [DOI] [PubMed] [Google Scholar]
- 24.Henry DW, Leete E. J Am Chem Soc. 1957;79:5254–5256. [Google Scholar]
- 25.Chauder B, Larkin A, Snieckus V. Org Lett. 2002;4:815–817. doi: 10.1021/ol017310m. [DOI] [PubMed] [Google Scholar]
- 26.Ballini R, Palmieri A, Petrini M, Shaikh RR. Adv Synth Catal. 2008;350:129–134. [Google Scholar]
- 27.Palmieri A, Petrini M. J Org Chem. 2007;72:1863–1866. doi: 10.1021/jo062538e. [DOI] [PubMed] [Google Scholar]
- 28.Licari JJ, Hartzel LW, Dougherty G, Benson FR. J Am Chem Soc. 1955;77:5386–5387. [Google Scholar]
- 29.Ballini R, Palmieri A, Petrini M, Torregiani E. Org Lett. 2006;8:4093–4096. doi: 10.1021/ol061604w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.