Abstract
2-Amino-8-oxo-tetrahydro-4H-chromene-3-carbonitriles were synthesized for the first time from a tandem Michael addition - cyclization reaction between cyclohexane-1,2-dione and benzylidenemalononitriles. An enantioselective synthesis of these compounds was achieved in moderate ee values (up to 63% ee) by using a cinchona alkaloid-derived thiouea catalyst.
Keywords: chromene, tandem reaction, enantioselective, cinchona alkaloid, organocatalysis, 1, 2-cyclohexanedione, benzylidenemalononitrile
Polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives are very important heterocyclic compounds, which frequently exhibit a variety of biological activities.1,2 Among these compounds, 2-amino-4H-chromene-3-carbonitrile derivatives have been reported to possess anticancer, anticoagulant, and fungicidal activities.2 These compounds also find applications as pigments and as potential biodegradable agrochemicals.3 Due to their usefulness, the synthesis of these compounds has attracted a lot of interest.4 Most recently, asymmetric syntheses of some of these 2-amino-4H-pyran-3-carbonitrile derivatives have also been reported.5 Furthermore, the synthesis of partially saturated 2-amino-4H-chromene-3-carbonitrile derivatives has also been reported.6 For example, the cyclization reaction between 1,3-dicarbonyl compounds and benzylidenemalononitriles in the presence of a suitable base gives 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles,6 which have also been demonstrated to have biological activitivities.6a Nonetheless, the synthesis of their 8-oxo analogues has not been reported, although such compounds reportedly are able to alter the lifespan of eukaryotic organisms.7
On the other hand, 1,2-diones are highly reactive compounds and have found many applications in organic synthesis.8 Nevertheless, they have been seldom used in organocatalyzed reactions.9 These few examples are collected below: Göbel and coworkers utilize the dione as an ene-activator in an organocatalyzed Diels-Alder reaction.9a Nair and coworkers reported a lactonization reaction between 1,2-diones and α,β-unsaturated aldehydes catalyzed by an N-heterocyclic carbene.9b In 2006, we reported direct cross aldol reaction of 1,2-diones and ketones catalyzed by proline derivatives.9c Most recently, Rueping and coworkers reported a tandem5,10 Michael-aldol reaction of cyclohexane-1,2-dione and α,β-unsaturated aldehydes.9d
We are interested in developing novel enantioselective methods for the synthesis of 2-amino-4H-pyran-3-carbonitrile derivatives. In this regard, we developed the first enantioselective synthesis of 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles through a tandem Michael addition-cyclization reaction between 2-pyrazolin-5-ones and benzylidenemalononitriles.5b The fact that cyclohexane-1,2-dione may be enolized and used in a Michael addition reaction under organocatalysis9d prompted us to use of this compound as a potential Michael donor in a tandem Michael addition-cyclization reaction with benzylidenemalononitriles. Herein we wish to report the first general and enantioselective synthesis of 2-amino-8-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles on the basis of a tandem Michael addition-cyclization reaction between benzylidenemalononitriles and cyclohexane-1,2-dione.
Cyclohexane-1,2-dione (1) and benzylidenemalononitrile (2a) were adopted as the starting materials for the proposed Michael addition reaction. On the basis of Rueping's results, the reaction of was studied with several readily available organic and inorganic bases as the catalysts. The results are summarized in Table 1.
Table 1.
Tandem Michael addition-cyclization reaction between 1,2-cyclohexanedione (1) and benzylidenemalononitriles (2)a
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | 2/3 | solvent | catalyst | time (h) |
yield (%)b |
| 1 | Ph | a | toluene | DABCO | 27 | 75 |
| 2 | Ph | a | toluene | DBU | 30 | 43 |
| 3 | Ph | a | toluene | Et3N | 38 | 67 |
| 4 | Ph | a | toluene | K2CO3 | 48 | 29 |
| 5 | Ph | a | toluene | NaHCO3 | 72 | 0 |
| 6 | Ph | a | CH2Cl2 | DABCO | 24 | 71 |
| 7 | Ph | a | THF | DABCO | 28 | 74 |
| 8 | Ph | a | EtOAc | DABCO | 27 | 64 |
| 9 | Ph | a | acetone | DABCO | 29 | 51 |
| 10 | Ph | a | EtOH | DABCO | 30 | 61 |
| 11 | 4-ClC6H4 | b | toluene | DABCO | 24 | 73 |
| 12 | 4-BrC6H4 | c | toluene | DABCO | 30 | 59 |
| 13 | 4-CNC6H4 | d | toluene | DABCO | 23 | 45 |
| 14 | 4-NO2C6H4 | e | toluene | DABCO | 22 | 64 |
| 15 | 3-BrC6H4 | f | toluene | DABCO | 20 | 65 |
| 16 | 4-CH3C6H4 | g | toluene | DABCO | 25 | 67 |
| 17 | thiophen-2-yl | h | toluene | DABCO | 26 | 79 |
| 18 | CH3(CH2)5 | i | toluene | DABCO | 32 | 39 |
All reactions were conducted with the indicated arylidenemalononitrile (2, 0.30 mmol), 1,2-cyclohexanedione (1, 0.32 mmol), and the catalyst (10 mmol %, 0.03 mmol) in the specified solvent (1.5 mL) at room temperature.
Yield of isolated product after column chromatography.
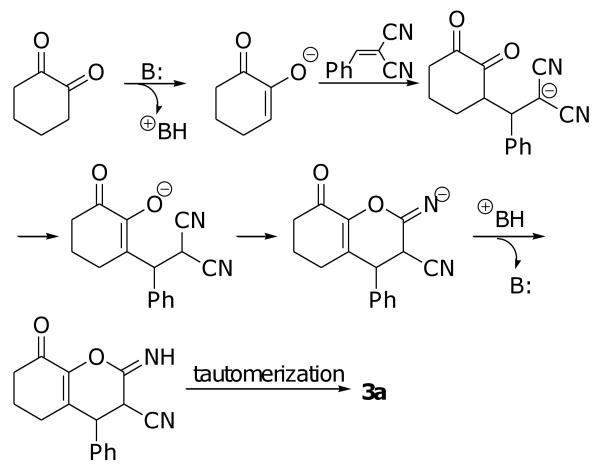
As shown in Table 1, when the reaction was carried out with 10 mol % DABCO as the base catalyst in toluene at room temperature for 27 h (entry 1), a product, which was identified to be 3a, was isolated in 75% yield. Unlike Rueping's results,9d no formation of bicyclic products was observed.11 Formation of 3a may be rationalized by the tandem enolization-Michael addition-enolization-cyclization reaction-tautomerization sequence as shown in Scheme 1. DBU, Et3N, and K2CO3 also catalyze this reaction (entries 2-4), albeit in lower efficiency. However, weaker base NaHCO3 cannot catalyze the reaction, as no product could be obtained (entry 5). Further screening of the reaction solvents identified that toluene (entry 1), CH2Cl2 (entry 6), and THF (entry 7) are good solvents for this reaction, while EtOAc, acetone, and EtOH (entries 8-10) are worse ones. Under the optimized conditions, several substituted benzylidenemalononitriles were applied as the substrate. As shown by the results in Table 1, acceptable to good yields (45-73%) were obtained with benzylidenemalononitriles with an electron-withdrawing group substituent at the para position (entries 11-14). Benzylidenemalononitriles with a meta substituent (entry 15) or an electron-donating group (entry 16) also led to good results. Heterocyclic thiophen-2-ylmethylidenemalononitrile gave the highest yield of 79% of the desired product (entry 17). Alkylidenemalononitrile 2i also reacted to produce the expected product 3i, although the yield is lower and the reaction is sluggish (entry 18). However, other 1,2-diones, such as butane-2,3-dione and 3-methylcyclopentane-1,2-dione, do not yield the expected product (data not shown), with the former gives no product at all and the latter some unidentified products.
Scheme 1.
Proposed mechanism for the formation of product 3
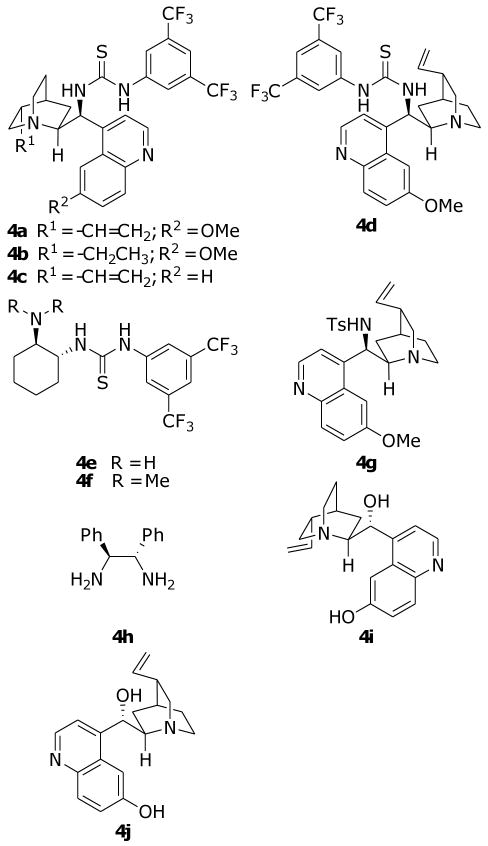
Since one stereogenic center is created at the 4-position of product 3 during this reaction, it was our intention to develop an asymmetric version of this reaction by using chiral Lewis base organocatalysts. Thus, with 1 and 2a as the model substrates, several readily available chiral Lewis base organocatalysts (Figure 1) were screened. The results of this screening are collected in Table 2. As detailed in Table 2, out of the ten catalyst screened, only the quinine-derived thiourea catalysts 4a, 4b, and 4c are giving slightly higher ee values (about 35% ee) when they were applied in toluene at room temperature (entries 1-3), while the rest all generate ee values no greater than 30% (entries 4-10). It should be pointed out that catalysts 4d, 4e, 4f, 4g, and 4j (entries 4-7, and 10) produce the other enantiomer as the major product as compared to the rest catalysts. Because catalyst 4a yields the best yields and the highest ee values among the three best catalysts, further optimizations were focused on catalyst 4a. Screening different organic solvents revealed that most of these solvents (entries 11-16) produce worst ee values of the product than toluene does (entry 1). Only in ethanol a higher ee value of 40% was achieved (entry 17). Nevertheless, the attempt to further increase the ee value through lowering the reaction temperature failed in this solvent, since at 0 °C the same ee value was obtained (entry 18). We then went back to toluene and tried to increase the ee value by employing the temperature effects. To our pleasure, with toluene, the ee value of the product 3a may be increased to 63% at 0 °C (entry 19). However, further dropping of the temperature (to -15 °C) proves to have detrimental effects on both the reactivity and the enantioselectivity of this reaction (entry 20). Once the optimized reaction conditions were found, the other benzylidenemalononitriles were applied to this reaction under these conditions. The results are collected in Table 3.
Figure 1.
Chiral catalyst screened for the enantioselective synthesis of 3a
Table 2.
Catalst screening for the enantioselective synthesis of 3aa
 | |||||
|---|---|---|---|---|---|
| entry | solvent | catalyst | time (h) | yield (%)b | ee (%)c |
| 1 | toluene | 4a | 26 | 56 | 35 |
| 2 | toluene | 4b | 30 | 41 | 35 |
| 3 | toluene | 4c | 30 | 47 | 34 |
| 4 | toluene | 4d | 28 | 37 | 30d |
| 5 | toluene | 4e | 240 | 21 | 1d |
| 6 | toluene | 4f | 40 | 34 | 27d |
| 7 | toluene | 4g | 26 | 70 | 25d |
| 8 | toluene | 4h | 168 | 31 | 3 |
| 9 | toluene | 4i | 28 | 61 | 16 |
| 10 | toluene | 4j | 28 | 72 | 3d |
| 11 | CH2Cl2 | 4a | 30 | 43 | 18 |
| 12 | Et2O | 4a | 28 | 55 | 17 |
| 13 | EtOAc | 4a | 28 | 35 | 25 |
| 14 | THF | 4a | 40 | 33 | 33 |
| 15 | CHCl3 | 4a | 38 | 33 | 6 |
| 16 | CH3CN | 4a | 28 | 31 | 15 |
| 17 | EtOH | 4a | 28 | 53 | 40 |
| 18e | EtOH | 4a | 72 | 58 | 40 |
| 19e | toluene | 4a | 28 | 64 | 63 |
| 20f | toluene | 4a | 28 | 43 | 59 |
Unless otherwise indicated, all reactions were conducted with benzylidenemalononitrile 2a (0.30 mmol), cyclohexane-1,2-dione (0.32 mmol), and the catalyst (10 mmol %, 0.030 mmol) in the specified solvent (1.5 mL) at room temperature.
Yield of isolated product after column chromatography.
Determined by HPLC analysis on a ChiralCel OD-H column.
The opposite enantiomer was obtained as the major product in these cases.
The reaction was conducted at 0 °C.
The reaction was conducted at -15 °C.
Table 3.
Enantioselective synthesis of 2-amino-8-oxo-tetrahydro-4H-chromene-3-carbonitrilesa
 | |||||
|---|---|---|---|---|---|
| entry | R | 3 | time (h) | yield (%)b | ee (%)c |
| 1 | Ph | 3a | 28 | 64 | 63 |
| 2 | 4-ClC6H4 | 3b | 24 | 60 | 58 |
| 3 | 4-BrC6H4 | 3c | 30 | 55 | 57 |
| 4 | 4-CNC6H4 | 3d | 24 | 51 | 43 |
| 5 | 4-NO2C6H4 | 3e | 26 | 55 | 48 |
| 6 | 3-BrC6H4 | 3f | 24 | 63 | 52d |
| 7 | 4-CH3C6H4 | 3g | 28 | 49 | 50 |
| 8 | thiophen-2-yl | 3h | 30 | 37 | 47e |
| 9 | CH3(CH2)5 | 3i | 96 | 12 | 9d |
All reactions were conducted with the benzylidenemalononitrile (0.30 mmol), cyclohexane-1,2-dione (0.32 mmol), and catalyst 4a (10 mol %, 0.030 mmol) in toluene (1.5 mL) at 0 °C.
Yield of isolated product after column chromatography.
Unless otherwise specified, ee values were determined by HPLC analysis on a ChiralCel OD-H column.
Determined by HPLC analysis on a ChiralPak AD-H column.
Determined by HPLC analysis on a ChiralPak AS column.
As shown in Table 3, benzylidenemalononitriles with various substituents all produce the desired product in mediocre to good ee values (43-63% ee, entries 1-7). The yields (49-64%) obtained are usually low because of the formation of some unidentified products in the reaction. The heterocyclic thiophen-2-ylmethylidenemalononitrile also gives the expected product in 47% ee and 37% yield (entry 8). However, the alkylidenemalononitrile 2i reacts very slowly and leads to poor ee value of the product (entry 9). Again, butane-2,3-dione and 3-methylcyclopentane-1,2-dione failed to yield the desired products (data not shown).
In summary, we have developed the first general synthesis of 2-amino-8-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitriles by employing a tandem Michael addition-cyclization reaction between cyclohexane-1,2-dione with benzylidenemalononitriles with DABCO as the catalyst. An enantioselective version of this reaction was also realized (with ee value up to 63%) by using a quinine-derived thiourea catalyst.
Supplementary Material
Acknowledgments
This research is financially supported by the Welch Foundation (Grant No. AX-1593) and partly by the National Institute of General Medical Sciences (Grant No. 1SC1GM082718-01), for which the authors are most grateful.
Footnotes
Supplementary data: Supplementary data associated with this article can be found, in the online version, at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, Surgenor AE. Bioorg Med Chem. 2006;14:4792–4802. doi: 10.1016/j.bmc.2006.03.021. [DOI] [PubMed] [Google Scholar]; (b) Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) El-Tamany ES, El-Shahed FA, Mohamed BH. J Serb Chem Soc. 1999;64:9–18. [Google Scholar]; (d) Zaki MEA, Soliman HA, Hiekal OA, Rashad AE. Z Naturforsch C. 2006;61:1–5. doi: 10.1515/znc-2006-1-201. [DOI] [PubMed] [Google Scholar]; (e) Ismail ZH, Aly GM, El-Degwi MS, Heiba HI, Ghorab MM. Egypt J Biotech. 2003;13:73–82. [Google Scholar]
- 2.(a) Konishi K, Kuragano T, Nohara A. Nippon Noyaku Gakkaishi. 1990;15:241–244. [Google Scholar]; (b) Liao SY, Qian L, Miao TF, Shen Y, Zheng KC. J Theor Comput Chem. 2009;8:143–155. [Google Scholar]; (c) Kemnitzer W, Jiang S, Wang Y, Kasibhatla S, Crogan-Grundy C, Bubenik M, Labrecque D, Denis R, Lamothe S, Attardo G, Gourdeau H, Tseng B, Drewe J, Cai SX. Bioorg Med Chem Lett. 2008;18:603–607. doi: 10.1016/j.bmcl.2007.11.078. [DOI] [PubMed] [Google Scholar]; (d) Kemnitzer W, Drewe J, Jiang S, Zhang H, Crogan-Grundy C, Labreque D, Bubenick M, Attardo G, Denis R, Lamothe S, Gourdeau H, Tseng B, Kasibhatla S, Cai SX. J Med Chem. 2008;51:417–423. doi: 10.1021/jm7010657. [DOI] [PubMed] [Google Scholar]; (e) Bonsignore L, Loy G, Secci D, Calignano A. Eur J Med Chem. 1993;28:517–520. [Google Scholar]; (f) Andreani LL, Lapi E. Boll Chim Farm. 1960;99:583–586. [PubMed] [Google Scholar]
- 3.(a) Hafez EAA, Elnagdi MH, Elagamey AGA, Ei-Taweel FMAA. Heterocycles. 1987;26:903–907. [Google Scholar]; (c) Witte EC, Neubert P, Roesch A. Ger Offen DE3427985. 1986. [Google Scholar]; Chem Abstr. 1986;104:224915. [Google Scholar]; (b) Morinaka Y, Takahashi K. Jpn Kokai Tokkyo Koho JP52017498. 1977. [Google Scholar]; Chem Abstr. 1977;87:102299. [Google Scholar]
- 4.(a) Bloxham J, Dell CP, Smith CW. Heterocycles. 1994;38:399–408. [Google Scholar]; (b) Elagamey AGA, Sawllim SZ, El-Taweel FMA, Elnagdi MH. Collect Czech Chem Commun. 1988;53:1534–1538. [Google Scholar]; (c) Ballini R, Bosica G, Conforti ML, Maggi R, Mazzacani A, Righi P, Sartori G. Tetrahedron. 2001;57:1395–1398. [Google Scholar]; (d) Jin TS, Zhang JS, Liu LB, Wang AQ, Li TS. Synth Commun. 2006;36:2009–2015. [Google Scholar]; (e) Zhang AQ, Zhang M, Chen HH, Chen J, Chen HY. Synth Commun. 2007;37:231–235. [Google Scholar]
- 5.(a) Wang XS, Yang GS, Zhao G. Tetrahedron: Asymmetry. 2008;19:709–714. [Google Scholar]; (b) Gogi S, Zhao CG. Tetrahedron Lett. 2009;52:2252–2255. doi: 10.1016/j.tetlet.2009.02.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Kumar D, Reddy VB, Sharad S, Dube U, Kapur S. Eur J Med Chem. 2009;44:3805–3809. doi: 10.1016/j.ejmech.2009.04.017. [DOI] [PubMed] [Google Scholar]; (b) Wang XS, Wu JR, Li Q, Tu SJ. J Chem Res. 2009:234–236. [Google Scholar]; (b) Harb AFA, Hesien AHM, Metwally SA, Elnagdi MH. Liebigs Ann Chem. 1989:585–588. [Google Scholar]; (c) Martin N, Pascual C, Seoane C, Soto JL. Heterocycles. 1987;26:2811–2816. [Google Scholar]; (d) Zayed SE, Abou Elmaged EI, Metwally SA, Elnagdi MH. Collect Czech Chem Commun. 1991;56:2175–2182. [Google Scholar]; (e) Elnagdi MH, Adbel-Motaleb RM, Mustafa M, Zayed MF, Kamel EM. J Heterocycl Chem. 1987;24:1677–1681. [Google Scholar]
- 7.Goldfarb DS. U.S. Patent US 2009163545. 2009
- 8.For selected examples, see: Svennebring A, Nilsson P, Larhed M. J Org Chem. 2007;72:5851–5854. doi: 10.1021/jo0708487.Held I, Xu S, Zipse H. Synthesis. 2007:1185–1196.Wu S, Fluxe A, Janusz JM, Sheffer JB, Browning G, Blass B, Cobum K, Hedges R, Murawsky M, Fang B, Fadayel GM, Hare M, Djandjighian L. Bioorg Med Chem Lett. 2006;16:5859–5863. doi: 10.1016/j.bmcl.2006.08.057.Ding Y, Girardet J, Smith KL, Larson G, Prigaro B, Lai VCH, Zhong W, Wu JZ. Bioorg Med Chem Lett. 2005;15:675–678. doi: 10.1016/j.bmcl.2004.11.028.Curiel D, Cowley A, Beer PD. Chem Commun. 2005:236–238. doi: 10.1039/b412363h.Zhao Z, Wisnoski DD, Wolkenberg SE, Leister WH, Wang Y, Lindsley CW. Tetrahedron Lett. 2004;45:4873–4876. doi: 10.1021/ol049682b.Lindsley CW, Wisnoski DD, Wang Y, Leister WH, Zhao Z. Tetrahedron Lett. 2003;44:4495–4498.Maruoka H, Kashige N, Miake F, Yamaguchi T. Chem Pharm Bull. 2005;53:1359–1361. doi: 10.1248/cpb.53.1359.Trost BM, Schroeder GM. J Am Chem Soc. 2000;122:3785–3786.
- 9.For examples, see, Schuster T, Bauch M, Duerner G, Göbel MW. Org Lett. 2000;2:179–181. doi: 10.1021/ol991276i.Nair V, Vellalath S, Poonoth M, Mohan R, Suresh E. Org Lett. 2006;8:507–509. doi: 10.1021/ol052926n.Samanta S, Zhao CG. Tetrahedron Lett. 2006;47:3383–3386.Rueping M, Kuenkel A, Tato F, Bats JW. Angew Chem Int Ed. 2009;48:3699–3702. doi: 10.1002/anie.200900754.
- 10.For some leading examples of chincona alkaloid-catalyzed tandem reactions, see: Tan B, Chua PJ, Li Y, Zhong G. Org Lett. 2008;10:2437–2440. doi: 10.1021/ol8007183.Biddle MM, Lin M, Scheidt KA. J Am Chem Soc. 2007;129:3830–3831. doi: 10.1021/ja070394v.Zu LS, Wang J, Li H, Xie HX, Jiang W, Wang W. J Am Chem Soc. 2007;129:1036–1037. doi: 10.1021/ja067781+.Wang B, Wu F, Wang Y, Liu X, Deng L. J Am Chem Soc. 2007;129:768–769. doi: 10.1021/ja0670409.Wang Y, Liu XF, Deng L. J Am Chem Soc. 2006;128:3928–3930. doi: 10.1021/ja060312n.Dudding T, Hafez AM, Taggi AE, Wagerle TR, Lectka T. Org Lett. 2002;4:387–390. doi: 10.1021/ol017087t.Tan B, Chua PJ, Zeng X, Lu M, Zhong G. Org Lett. 2008;10:3489–3492. doi: 10.1021/ol801273x.Tan B, Shi Z, Chua PJ, Zhong G. Org Lett. 2008;10:3425–3428. doi: 10.1021/ol801246m.For an excellent review on organocatalyzed tandem reactions, see: Enders D, Grondal C, Hüttl MRM. Angew Chem Int Ed. 2007;46:1570–1581. doi: 10.1002/anie.200603129.
- 11.The reaction does produces some unidentified products, which lowers the yield of the reaction.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.