Summary
Arteriovenous malformation of the head and neck is a rare vascular anomaly but when present is persistent and progressive in nature and can represent a lethal benign disease. An unusual case is presented of an arteriovenous malformation of the base of tongue in a 32-year-old primigravida at 23.2 weeks of gestation with a history of haemoptysis. The patient was admitted to hospital with 10.7 g/dl of haemoglobin and 32.1% of haematocrit but due to recurrent massive haemoptysis, in the next few days, dropped to 6.7 g/dl of haemoglobin and 20.2% of haematocrit which required immediate blood transfusions. To maintain the upper airways patent the patient underwent tracheostomy; during angiography, showing an arteriovenous malformation with its feeding arteries (lingual artery, internal maxillary artery, and maxillary artery) embolization was made without a significant blood flow reduction. After surgical ligation of the external carotid artery, on the right side, the patient was readmitted for further angiographic evaluation, which confirmed complete occlusion of the carotid artery but, at the same time, revealed the integrity of the arteriovenous malformation perfusion on account of a new feeding artery (left lingual artery). A new superselective catheterization of the lingual artery was performed but due to the effect of progesterone, which causes smooth muscle relaxation and leads to arteriovenous malformation dilatation and rupture, the primigravida again presented haemoptysis. In agreement with the gynaecologists, the patient was given betamethasone to induce foetal lung maturation, and induction of labour was planned at 26 weeks, and a healthy baby was delivered naturally. Over the following days, the patient had no further haemoptysis and so far clinical examination showed no evidence of the original mass (slight haemorrhagic suffusion of the right anterior amygdale region).
Keywords: Tongue, Vascular malformation, Arteriovenous malformations, Pregnancy
Riassunto
Le malformazioni arterovenose del distretto testa-collo sono delle forme cliniche patologiche rare, lentamente progressive che a seconda della sede e della dimensione possono risultare letali. Gli Autori presentano un raro caso di malformazione arterovenosa della base della lingua in una donna di 32 anni primipara alla 23,2 settimana di gestazione con una storia clinica di ripetuti episodi di emottisi nelle settimane antecedenti al ricovero. Pervenuta in regime di urgenza con valori di 10,7 g/dl di emoglobina e 32,1% di ematocrito, a seguito di nuovi episodi di emottisi giungeva a valori di emoglobina di 6,7 g/dl e di ematocrito del 20,2% e veniva pertanto sottoposta a trasfusione. Si eseguiva inoltre una tracheostomia per mantenere la pervietà delle vie aeree ed una angiografia, che mostrava una malformazione arterovenosa alimentata dalle arterie del distretto della carotide esterna di destra. A seguito di un tentativo non riuscito di embolizzazione della malformazione si decideva per una legatura chirurgica della carotide esterna di destra. La gestante veniva successivamente sottoposta a una nuova seduta di angiografia che confermava l’occlusione completa della carotide esterna destra ma evidenziava nel contempo l’integrità della malformazione arterovenosa la cui perfusione veniva assicurata dalla arteria linguale controlaterale ed induceva ad un nuovo tentativo di embolizzazione della linguale sinistra. Per l’incremento dei valori di progesterone che favoriscono la dilatazione e la rottura delle malformazioni arterovenose ed a seguito di nuovi episodi di emottisi, consultati i ginecologi alla paziente veniva somministrato betametasone a dosi tali da favorire la maturazione fetale e indurre il parto alla 26 settimana. Nei giorni seguenti il parto la paziente non ha più presentato episodi di emottisi e la malformazione arterovenosa si è lentamente ridotta fino a scomparire, residuando attualmente soltanto una lieve soffusione emorragica della regione tonsillare anteriore destra.
Introduction
“Vascular malformation” is a generalized term used to describe a group of lesions, present at birth, formed by an anomaly of angiovascular or lymphovascular structures that occur in approximately 1% of births, many of which not presenting for treatment 1. There has long since been confusion concerning which lesions should be included in, or excluded from, the category of vascular malformation, as well as regarding a suitable classification scheme within the category. It was not until 1982, when Glovacki and Mulliken described a classification based upon structure, as well as behaviour, that a practical clinical approach to these tumours became possible 2. Based on this classification, vascular malformations can be further subdivided into capillary, venous, lymphatic, arterial or a combination of these channel types 3. Arteriovenous malformation (AVM) is a structural vascular abnormality in which the arterial vasculature connects with the venous vasculature 4. In addition, trauma, ischaemic event secondary to thrombosis, ectasia, hormonal changes and puberty can induce proliferation of the AVM. The AVM usually involves a single vessel when caused by trauma but in the congenital form, it involves multiple vessels. Vascular malformations are commonly present at birth and grow commensurably with the patient, usually not showing clinical significance until later in childhood 5 6. Pregnancy appears to increase the risk of bleeding from AVM. Maternal mortality, associated with untreated AVM, is reported to be 33%. Pregnancy can have marked adverse effects on vascular malformations which can result in serious complications 7. An unusual case of an AVM of the base of tongue is described in a 32-year-old primigravida at 23.2 weeks of gestation with a history of recurrent massive haemoptysis that caused anaemia with Haemoglobin (Hb) values of 6.7 g/dl and haematocrit (HCT) of 20.2% of life-threatening severity both for the mother and foetus.
Case report
A 32-year-old primigravida at 23.2 weeks of gestation was admitted to the Department of Otorhinolaryngological Surgery of the ARNAS Hospital (Palermo, Italy) on September 10, 2007 someone account of episodes of haemoptysis over the last few weeks. Her family history was normal; her medical history consisted of an uncomplicated appendicectomy.
Clinical examination by means of nasal endoscopy with a 0° endoscope, superficial and deep neck palpation were normal; examination of the oral cavity showed swelling of the right side of the tongue and base of tongue with mucosal telangiectasias and elevation of the lateral floor (Fig. 1). No pulsations were detectable.
Fig. 1.

Pharynx/hypopharynx endoscopic examination.
The main laboratory values on admission were: white blood cells (WBC) 9.600/L; red blood cells (RBC) 3.400.000/L; Hb 10.7 g/dl; HCT 32.1%; fibrinogen 307 mg/dl (range 150-400); prothrombin 0.93; International Normalized Ratio (INR) 0.9 (range 0.81-1.17).
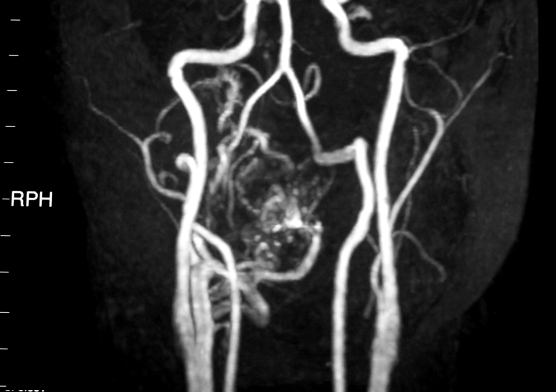
A few days later, the patient presented some recurrent massive haemoptysis which dropped to 7.6 g/dl of Hb and 23.6% of HCT; blood transfusions were immediately required. To maintain the upper airways patent, tracheostomy was performed; to study this mass, computerized tomography (CT) was necessary which showed the vascular content of the mass. Based on the recommendations of the radiologist, the patient was submitted to elective angiography which revealed an AVM with its feeding arteries (lingual artery, internal maxillary artery, and maxillary artery) (Fig. 2). During angiography, embolization with Gelfoam particles of lingual and internal maxillary artery was performed but complete reduction of blood flow was not achieved.
Fig. 2.
Angio CT showing original AVM.
After new massive haemoptysis, consisting of 600 ml of blood in the following 24 hours, surgical ligation of the external carotid artery of the right side was carried out and four days later the patient was readmitted for further angiographic evaluation, which confirmed complete occlusion of the carotid artery but, at the same time, showed the integrity of the AVM perfusion due to a new feeding artery (left lingual artery) (Fig. 3). Further superselective catheterization of the lingual artery on the left side was performed but, due to the effect of progesterone which causes smooth muscle relaxation and leads to AVM dilatation and rupture, the patient again presented haemoptysis, Hb was 6.7 g/dl with 20.2% of HCT and two units of blood were again given. Foetal growth, assessed by serial ultrasonography and sequential biophysical profile scores showed that the foetus was small for date; as pregnancy progressed, the patient’s heart condition began to deteriorate and therefore since AVMs usually show spontaneous postpartum regression, and since in the opinion of the gynaecologists, after labour, the progesterone levels would decrease, the patient was given betamethasone to improve foetal lung maturation, and induction of labour was programmed for 26 weeks. Over the next few days, the patient had no further haemoptysis; after six months, angiographic examination were again performed which showed regression of the mass. So far, further clinical examinations have not shown evidence of the original mass (slight haemorrhagic suffusion of the right anterior amygdale region) (Fig. 4).
Fig. 3.
Angio CT after ligation of external carotid artery confirmed integrity of AVM.
Fig. 4.
Oral cavity examination six months after labour.
Discussion
AVMs are composed of a central nidus with anomalous arteriovenous shunts and a network of surrounding collateral vessels 4. The short circuit or shunting between the high pressure arterial and low pressure venous system accounts for much of the clinical presentation, anatomical changes, and progression of the lesions 5.
AVMs are usually present at birth but commonly manifest in childhood or adolescence. As AVM has a gradual onset and progression, it is rarely associated with an enlarged heart and high output cardiac failure 8. There are series of cases described by different Authors but one of the largest series was reported by Kohout et al. 5 who reported on 81 AVMs located in the head and neck area. The majority of these were localized over the cheek (31%) and the ear (16%). Others were localized on the nose (10%), the forehead (10%), the upper lip (7%), the mandible (5%), the neck (5%), the scalp (4%) and the maxilla (4%) 9–13.
AVMs can also occur following trauma; Darlow et al. reported an AVM in the maxillary sinus following a blunt trauma to the paranasal region 14.
AVMs occur with the same frequency in both sexes 2, the size may increase rapidly secondary to infection, trauma, ligation, attempted excision or via hormonal influences such as during pregnancy and puberty, as the case described here.
Few data are currently available regarding AVMs in pregnancy. According to Robinson and Sabiston, women with AVMs in pregnancy that were most likely to bleed tended to be younger (20-25 years) and were usually primiparous, with bleeding being most common between 15 and 20 weeks of gestation (as the patient described herein) but which could occur at any stage including during labour and in the puerperium 15. Heart failure may occur in documented cases of AVM in pregnancy, the exact mechanism of which is often unclear. In normal pregnancy, cardiac output has been shown to be increased by 30-40% by the 24th week, which is mediated by an increase both in stroke volume and heart rate 16–18. It has been estimated that the coexistence of pregnancy and AVM may result in a 150% increase in cardiac output above normal levels. A number of cases of high output cardiac failure have been reported in pregnancy related to AVM and, therefore, beginning a pregnancy with pre-existing AVMs can be dangerous 19 20. Our patient showed no evidence of heart failure at any stage.
Many AVMs show spontaneous postpartum regression, as documented in this case and by Elliot et al., speculation remains concerning the reasons for this 7. Oestrogens are associated with arterial spider telangiectasias and, therefore, it is postulated that the changes in hormonal balance in pregnancy with resulting venodilation may be responsible. Progesterones have been related to increased venous distensibility during pregnancy and during the menstrual cycle and, clearly, this may be a reasonable hypothesis for the relationship of AVM and pregnancy 18 20 21.
The natural history of AVMs is documented by a clinical staging system introduced by Schobinger: Stage I (quiescence), Stage II (expansion), Stage III (destructive), Stage IV (decompensation) 22.
Plain radiography and computed tomography scans have a limited role as diagnostic tools in high-flow vascular malformations; the diagnosis is usually made with Doppler ultrasonography. Magnetic resonance imaging (MRI) which has become the investigation of choice since it depicts the extent and lack of invasion of these lesions, providing multiplanar images and differentiating between high- and low-flow lesions 23. Angiography is useful in poorly defined cases and for embolization before surgery as in the present case before arterial ligation 24 25. It demonstrates the flow characteristics, feeding vessels, and dangerous anastomoses. Characteristic angiography findings are marked hypertrophy and tortuosity in the feeding vessels. The appearance of the nidus (centre) of the lesion varies from large tortuous vessels to innumerable small vessels appearing as an intense blush. Collateral vessels usually have a ‘cork screw’ appearance. When contrast is used, parenchymal staining is generally absent 26.
Treatment is rarely indicated for an asymptomatic AVM. Once diagnosis is made, the patient should be closely followed up at 6-month or yearly intervals. Intervention should often be delayed until there are signs and symptoms of pain, bleeding, ulceration, infection, or concern for endangering vital structures (Schobinger Stage II–III) 4. Ligation or proximal embolization of feeding vessels should be avoided. This will lead to rapid recruitment of flow from nearby arteries and denies access to embolization. In the present patient, in fact, the angiographic evaluation made after ligation of the external carotid artery confirmed the integrity of the AVM perfusion because as a result of a new feeding artery (contralateral lingual artery).
Superselective arterial or retrograde venous embolization may be used as first choice treatment for for AVM non amenable to surgery. Materials used for embolization include coils, PVA foam, gel foam, methyl methacrylate and silicone spheres. Gelfoam particles were favoured in our case because of easy handling and precise targeted delivery associated with minimal risk of migration 27–29 any may hope for long-term success is total resection of the tissue involved with the AVM in the presence of complications. Ignoring residual and unresponsive anomalous channels only invites further collateral formation, shunting, and expansion.
Conclusion
Much has now been learnt about the radiographic appearance, natural history, and response to treatment of vascular malformations. This knowledge now allows clinicians, using a team approach to form appropriate treatment plans but during pregnancy the evolution of the AVM becomes unpredictable; in fact, the effect of progesterone can lead in a short period to AVM dilatation and its rupture which can result in severe complications both for the lives of the woman and the fetus. As demonstrated by the case described here and according to the international literature, the choice of treatment, when possible, should be postponed until labour. Despite all the known treatments only the reduction of hormone concentration, after labour, can stabilize the AVM and allows, in many cases, a normal life undergoing “watch and wait” follow-up every six months.
References
- 1.Watzinger F, Gossweiner S, Wagner A, Richling B, Millesi-Schobel G, Hollmann K. Extensive facial vascular malformations and hemangiomas: a review of the literature and case reports. J Craniomaxillofac Surg 1997;25:335-43. [DOI] [PubMed] [Google Scholar]
- 2.Glovacki MJB, Mulliken JB. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412-22. [DOI] [PubMed] [Google Scholar]
- 3.Kaban LB, Mulliken JB. Vascular anomalies of the maxillofacial region. J Oral Maxillofac Surg 1986;44:203-13. [DOI] [PubMed] [Google Scholar]
- 4.Seccia A, Salgarello M, Farallo E, Falappa PG. Combined radiological and surgical treatment of arteriovenous malformations of the head and neck. Ann Plast Surg 1999;43:359-66. [DOI] [PubMed] [Google Scholar]
- 5.Kohout MP, Hansen M, Pribaz JJ, Mulliken JB. Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg 1998;102:643-54. [DOI] [PubMed] [Google Scholar]
- 6.Boyd JB, Mulliken JB, Kaban LB, Upton J 3rd, Murray JE. Skeletal changes associated with vascular malformations. Plast Reconstr Surg 1984;74:789-97. [DOI] [PubMed] [Google Scholar]
- 7.Elliott JA, Rankin RN, Inwood MJ, Milne JK. An arteriovenous malformation in pregnancy: a case report and review of the literature. Am J Obstet Gynecol 1985;152:85-8. [DOI] [PubMed] [Google Scholar]
- 8.Gomes MMR, Bernatz PE. Arteriovenous fistulas. A review and ten year experience at the Mayo Clinic. Mayo Clin Proc 1970;45:81-102. [PubMed] [Google Scholar]
- 9.Jackson IT, Carreno R, Potparic Z, Hussain K. Hemangiomas, vascular malformations and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg 1993;91:1216-30. [DOI] [PubMed] [Google Scholar]
- 10.Malan E, Azzolini A. Congenital arteriovenous malformations of the face and scalp. J Cardiovasc Surg 1968;9:109-40. [PubMed] [Google Scholar]
- 11.Chen MT, Horng SY, Yeong EK, Pan QD. Treatment of high-flow vascular malformations in the head and neck with arterial ligation followed by sclerotherapy. Ann Plast Surg 1996;36:147-53. [DOI] [PubMed] [Google Scholar]
- 12.Svendsen PA, Wikholm G, Fogdestam I, Anniko M, Mendel L. Direct puncture of large arteriovenous malformations in the head and neck for embolization and subsequent reconstructive surgery. Scand J Plast Reconstr Surg Hand Surg 1994;28:131-5. [DOI] [PubMed] [Google Scholar]
- 13.Erdmann MW, Jackson JE, Davies DM, Allison DJ. Multidisciplinary approach to the management of head and neck arteriovenous malformations. Ann R Coll Surg Engl 1995;77:53. [PMC free article] [PubMed] [Google Scholar]
- 14.Darlow LD, Murphy JB, Berrios RJ, Park Y, Feldman RS. Arteriovenous malformations of the maxillary sinus: An unusual clinical presentation. Oral Surg Oral Med Oral Pathol 1988;66:21-3. [DOI] [PubMed] [Google Scholar]
- 15.Robinson LA, Sabiston DC Jr. Syndrome of congenital internal mammary-to-pulmonary arteriovenous fistula associated with mitral valve prolapse. Arch Surg 1981;116:1265-73. [DOI] [PubMed] [Google Scholar]
- 16.Dutta DC. Hypertensive Disorders in Pregnancy. In: Dutta DC, editor. Text Book of Obstetrics. 6th Edition. Kolkata: New Central Book Agency; 2004. p. 205. [Google Scholar]
- 17.Dutta DC. Physiological changes during pregnancy. In Dutta DC, editor. Text Book of Obstetrics. 6th Edition. Kolkata: New Central Book Agency; 2004. p. 52. [Google Scholar]
- 18.Mc Clausland AM, Hyman C, Windsor T, Trotter AD. Venous distensibility during pregnancy. Am J Obstet Gynecol 1961;81:472-9. [DOI] [PubMed] [Google Scholar]
- 19.Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 4th Edition. New York: Churchill Livingstone; 2002. [Google Scholar]
- 20.Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anaesthesiol 1965;26:393. [DOI] [PubMed] [Google Scholar]
- 21.Clapp JF, Seaward BL, Sleamaker RH. Maternal physiological adaptations to early human pregnancy. Am J Obstet Gynecol 1988;156:1456. [DOI] [PubMed] [Google Scholar]
- 22.Cumming C. Cummings: Otolaryngology: Head & Neck Surgery. 4th Edition. London: Elsevier; 2005. [Google Scholar]
- 23.Kakimoto N, Tanimoto K, Nishiyama H, Murakami S, Furukawa S, Kreiborg S. CT and MR imaging features of oral and maxillofacial hemangioma and vascular malformation. Eur J Radiol 2005;55:108-12. [DOI] [PubMed] [Google Scholar]
- 24.Lee CY, Yim MB, Benndorf G. Traumatic pseudoaneurysm of the pharyngeal artery: an unusual cause of hematemesis and hematochezia after craniofacial trauma. Surg Neurol 2006;66:444-6. [DOI] [PubMed] [Google Scholar]
- 25.Baqain ZH, Thakkar C, Kalavrezos N. Superselective embolization for control of facial haemorrhage. Int J Care Injured 2004;35:435-8. [DOI] [PubMed] [Google Scholar]
- 26.Nussel F, Wegmuller H, Huber P. Comparison of magnetic resonance angiography, magnetic resonance imaging and conventional angiography in cerebral arteriovenous malformation. Neuroradiology 1991;33:56-61. [DOI] [PubMed] [Google Scholar]
- 27.Remonda L, Schroth G, Caversaccio M, Ladrach K, Lovblad KO, Zbaren P. Endovascular treatment of acute and subacute hemorrhage in the head and neck. Arch Otolaryngol Head Neck Surg 2000;126:1255-62. [DOI] [PubMed] [Google Scholar]
- 28.Rogers SN, Patel M, Beirne JC, Nixon TE. Traumatic aneurysm of the maxillary artery: the role of interventional radiology. A report of two cases. Int J Oral Maxillofac Surg 1995;24:336-9. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez F, Delgado F, Ramos M. Pseudoaneurysm of the superficial temporal artery treated by embolization: report of a case. J Oral Maxillofac Surg 2000;58:819-21. [DOI] [PubMed] [Google Scholar]