Abstract
We have shown previously that rotavirus (RV) can infect murine intestinal B220+ cells in vivo (M. Fenaux, M. A. Cuadras, N. Feng, M. Jaimes, and H. B. Greenberg, J. Virol. 80:5219-5232, 2006) and human blood B cells in vitro (M. C. Mesa, L. S. Rodriguez, M. A. Franco, and J. Angel, Virology 366:174-184, 2007). However, the effect of RV on B cells, especially those present in the human intestine, the primary site of RV infection, is unknown. Here, we compared the effects of the in vitro RV infection of human circulating (CBC) and intestinal B cells (IBC). RV infected four times more IBC than CBC, and in both types of B cells the viral replication was highly restricted to the memory subset. RV induced cell death in 30 and 3% of infected CBC and IBC, respectively. Moreover, RV induced activation and differentiation into antibody-secreting cells (ASC) of CBC but not IBC when the B cells were present with other mononuclear cells. However, RV did not induce these effects in purified CBC or IBC, suggesting the participation of other cells in activating and differentiating CBC. RV infection was associated with enhanced interleukin-6 (IL-6) production by CBC independent of viral replication. The infection of the anti-B-cell receptor, lipopolysaccharide, or CpG-stimulated CBC reduced the secretion of IL-6 and IL-8 and decreased the number of ASC. These inhibitory effects were associated with an increase in viral replication and cell death and were observed in polyclonally stimulated CBC but not in IBC. Thus, RV differentially interacts with primary human B cells depending on their tissue of origin and differentiation stage, and it affects their capacity to modulate the local and systemic immune responses.
Rotaviruses (RV) are the main cause of severe gastroenteritis in infants and are responsible for the death of approximately 600,000 young children annually around the world (54 and http://www.who.int/immunization monitoring/burden/rotavirus estimates/en/). At present, two live attenuated vaccines are licensed for use in humans, and both vaccines are highly protective against severe disease in developed countries (60, 69). However, these oral vaccines induce lower levels of protection in children from developing countries, suggesting that the improvement of these vaccine or the development of new RV vaccines is warranted (32). Although the RV immune response has been extensively studied in both animals and humans, the immune factors that correlate with protection for natural infection or vaccination in people remain unclear, and this is an important obstacle for the development of the next generation of RV vaccines (2, 25).
B cells play a critical role in the RV immune response, and both intestinal and systemic immunoglobulins (Ig) are associated with protection (25). For example, in the murine model, B-cell- but not T-cell-deficient mice are unable to establish long-lasting protective immunity against RV reinfection (26). The interaction of RV with B cells has been shown to be peculiar in many ways: the structural viral protein VP6 binds to an important fraction of human naive B cells via surface Ig (55, 58), and VP6 memory B cells (mBC) are enriched in the CD27− IgG+ (58) and CD27+ IgM+ subsets (66). VP6-specific naive B cells and, to a lesser extent, the mBC predominantly use the VH1-46 gene segment (66). Moreover, a massive T-cell-independent B cell activation and humoral response can be detected in vivo after oral RV infection in mice (10). However, the activation of these cells is probably viral strain dependent, since simian rhesus RV (RRV), but not bovine WC3 RV, has been shown to polyclonally stimulate a total antibody-secreting cell (ASC) response in intestinal organ fragment cultures (44). Although the principal immune function of B cells has been associated with the production of Ig, recent reports show that these cells also can play an important role in modulating the immune response independently of Ig through the production of cytokines like tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-10 and their related potential function as antigen-presenting cells (3, 18, 31, 67).
We and others have shown that RVs undergo systemic, extraintestinal replication in both immunodeficient and wild-type mice (16, 24). In addition, a significant antigenemia and viremia is seen in most acutely infected children (8, 9). In particular, we have shown the in vivo replication of homologous and heterologous RV strains in B220+ cells (a marker expressed by B cells or plasmacytoid dendritic cells [pDC]) obtained from murine mesenteric lymph node (24), and recently we also demonstrated that approximately 10% of primary human circulating B cells (CBC) are targets of RV infection in vitro (49). These results suggest that RV has the ability to replicate in primary cells other than intestinal epithelial cells, and that B cells likely represent an important component of this group of RV-permissive nonepithelial cells. However, the detailed analysis of the RV infection of B cells and the consequences this event may have on the B cells themselves, as well as on the immune response in general, has not been undertaken. In this work, we examined if RV has a differential capacity to infect human B cell subsets or B cells derived from blood and intestine and the effect of RV infection on the function of these B cells, which could have a potential impact on the human antiviral immune response.
MATERIALS AND METHODS
Isolation of human B cells from blood and intestine.
Deidentified buffy coats from healthy donors were provided by the Stanford University blood bank. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient with Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden). Cells were washed twice with RPMI medium supplemented with 10% fetal bovine serum (FBS), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). All reagents were from Gibco-BRL, Gaithersburg, MD (complete medium). For most of the experiments, CBC were purified by positive selection using anti-CD19-labeled magnetic microbeads (Miltenyi Biotec, Auburn, CA). For the experiments in which phenotypic changes to CBC were studied, negative selection by rosette formation was used (StemCell Biotech, Vancouver, Canada). The median purity of CD19+ cells after positive or negative selection was 94 (range, 90 to 97%) and 89% (range, 84 to 94%), respectively.
Deidentified sections of human jejunum were obtained from patients undergoing bariatric surgery as treatment for obesity as approved by the Institutional Review Board of Stanford University. Intestinal mononuclear cells (IMC) were isolated by enzyme digestion, filtration, and density gradient centrifugation as previously described by others (63, 64). After resection, the jejunal sections (approximately 150 g) were maintained in a sterile container on ice and phosphate-buffered saline (PBS) (Cellgro, Herndon, VA). All samples were processed within 2 h of surgery. The sections were washed five times with sterile Ca2+- and Mg2+-free PBS to exclude most of the circulating erythrocytes and leukocytes and to reduce sample contamination. Fatty tissue and peritoneal membranes were resected. Samples were cut into 0.3- and 0.5-cm2 sections. To remove epithelial cells, samples were washed twice at 37°C for 10 min with slow agitation. The first wash was with RPMI supplemented with 2% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml fungizone (Gibco), and 1 mM EDTA (Gibco) plus 200 μg/ml of dithiothreitol (DTT) (Sigma-Aldrich, St. Louis, MO), and the second wash was with the same medium but without DTT. After the second wash, the sample was recovered in RPMI plus 10% FBS and kept on ice for 5 min. A volume of 10 ml of sample then was mixed with 15 ml of RPMI supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml fungizone, 3.2 mg/ml collagenase type IA (Sigma-Aldrich), and 0.2 mg/ml DNase (DNAsa, Roche, NJ) (digestion medium). The sample was incubated with the digestion medium for 15 min at 37°C with gentle mixing every 5 min. After digestion, cells were collected on ice using a metallic filter, and the enzymatic process was stopped with the addition of 20 ml of RPMI plus 10% FBS. The intestinal samples underwent six to nine digestion rounds (according to the sample size). Finally, the cells obtained were washed with RPMI plus 10% FBS and filtrated through a 100-μm membrane (BD Bioscience, San Jose, CA). To isolate IMC, a Percoll (GE Healthcare, Uppsala, Sweden) density gradient was performed. Cells obtained from the digestion process were washed and centrifuged, and the pellet was resuspended in 20% Percoll. Subsequently, a 40 to 80% Percoll gradient was deposited from the bottom of the tube. This Percoll gradient was centrifuged at room temperature for 30 min at 850 × g without braking. The top layer of epithelial cells was removed, and IMC were carefully collected and washed twice with RPMI plus 10% FBS and then filtered through a 40-μm membrane (BD Bioscience). Intestinal B cells (IBC) were purified by positive selection with anti-CD19 microbeads (Miltenyi Biotec) by following the manufacturer's protocol. Similarly to CBC, when the RV-induced phenotypic effects on B cells were evaluated, IBC were isolated by negative selection (StemCell, Vancouver, Canada). To remove contaminant epithelial cells present after density gradient separation, an anti-CD104-phycoerythrin (PE) β4 integrin chain expressed exclusively by epithelial and endothelial cells (28), followed by anti-PE microbeads, were included in the negative selection procedure. The median purity of intestinal CD19+ cells was 89 (range, 79 to 93%) and 81% (range, 73 to 90%) after positive and negative selection, respectively.
Production and inactivation of RV.
The vaccine strain RRV (G3 P[3]) and human virus Wa (G1 P1A[8]) were used to infect circulating and intestinal human B cells. Viruses were produced in MA104 cells as previously described (26, 52), with minor modifications. Briefly, a semiconfluent monolayer of MA104 cells was washed three times with serum-free medium and infected at a multiplicity of infection (MOI) of 10 during 1 h at 37°C. The viral inoculum for RRV was removed, but for Wa it was maintained, and serum-free medium with 2 μg/ml trypsin was added. To produce mock-infected cells, the same process was performed without virus. The titer of the virus was determined with MA104 cells as previously described (52).
The chemical inactivation of RRV was done using UV-psoralen (Sigma-Aldrich) as previously described (33). In short, psoralen was added at a concentration of 40 μg/ml. The virus then was placed in ice at a distance of 10 cm and irradiated with high-intensity long-wavelength UV light for 40 min. After this process, less than 10 FFU/ml of RRV was detected. The antigenicity of psoralen-inactivated RRV (iRRV) was tested by measuring the binding capacity of monoclonal antibodies (MAb) against VP6 (1E11 MAb) and trimeric VP7 (159 MAb).
Detection of viral infection and replication in B cells.
B cell infection was carried out as previously described (49, 52). In general, 0.5 × 106 to 1 × 106 B cells were washed three times with serum-free complete medium and then infected with RRV or Wa at an MOI of 5. Cells were incubated for 45 min at 37°C and washed twice to remove the excess virus, and then they were cultured in complete medium to determine infection by flow cytometry and in serum-free medium to detect viral replication by enzyme-linked immunosorbent assay (ELISA) and MA104 titration at different times postinfection. The RV infection was detected following the expression of intracellular nonstructural protein NSP2 using a MAb against NSP2 previously characterized in our laboratory (MAb 191) (5, 62) and commercially labeled with allophycocyanin (APC) (Chromoprobe Company, http://chromoprobe.com). Briefly, 10 h postinfection (hpi) B cells were washed with PBS-0.5% bovine serum albumin (BSA), 0.02% azide (staining buffer), resuspended, and stained with anti-CD19 PE-Cy7 (SJ25C1 clone), anti-CD20 APC-H7 (L-27 clone), anti-IgD fluorescein isothiocyanate (FITC) (IA6-2 clone), and anti-CD27 PE (MT271 clone). For IBC, anti-CD38 peridinin chlorophyll protein (PerCP)-Cy5.5 (HIT2 clone) also was included to analyze the RV effect on ASC. All antibodies were purchased from BD Bioscience unless otherwise noted. B cells were incubated during 30 min and then fixed and permeabilized using Cytofix/Cytoperm (BD Bioscience) by following the manufacturer's protocol. To block the Fcγ receptors, an FcR blocking reagent from Miltenyi Biotec was added for 5 min at room temperature. The cells then were stained with 0.5 μg/test anti-NSP2 APC and incubated for 30 min at 4°C. Finally, the cells were washed and resuspended in 300 μl of Perm/Wash (BD Bioscience). iRRV was used as an infection control, and an isotype control for anti-NSP2 was used as the staining control for infected B cells. In some experiments, simultaneous intracellular staining with 0.8 μg/test of anti-VP6 PE (MAb 1E11) (Chromoprobe Company) was used. Approximately 20,000 cells were acquired using a FACS Aria II or LSR II cytometer and DIVA software (BD, San Jose, CA).
To determine if B cell infection was productive, the number of viral particles and the relative quantity of VP6 in the supernatant of cell cultures collected 2 and 24 h postinfection was measured by the titration of MA104 cells and ELISA, respectively, as described previously (52).
Sorting of purified infected B cell subsets.
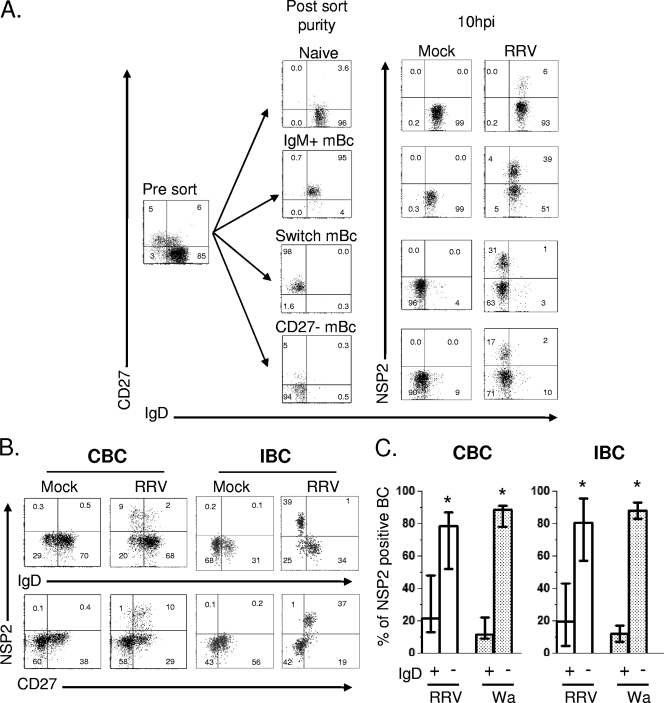
At least 30 million bead-purified CBC were mock or RRV infected as described above. Immediately after infection, cells were stained with anti-CD19, anti-CD27, and anti-IgD and sorted by fluorescence-activated cell sorting (FACS) using a FACS ARIA II (BD). At least 50,000 cells of each of the four subsets of B cells were isolated: CD27− IgD+ (naïve), CD27+ IgD− (switch memory), CD27+ IgD+ (IgM memory) (45), and CD27− IgD− (recently described as a CD27− mBC [23, 71]). Typically, the least-frequent subset was CD27− IgD− (71). Close to 2,000 cells of each purified subset were acquired in the FACS to verify the degree of purity (see the post-sort purity column in Fig. 2A). Approximately 50,000 cells of each subset were cultured for 10 h and then stained with anti-CD19, anti-CD27, anti-IgD, and anti-NSP2 antibodies. A total of 5,000 to 10,000 cells were acquired to identify the percentage of RV NSP2+ cells in mock- or RV-infected cells (see the 10-hpi column in Fig. 2A).
FIG. 2.
RV replication is highly restricted to mBC. (A) Flow cytometry assay to detect the frequency of RV-infected cells in sort-purified subsets of B cells. Microbead-purified CBC were mock infected or were infected with RRV (MOI, 5) for 45 min, stained, sorted by FACS, and cultured for 10 h. The expression of CD19, CD27, IgD, and intracellular NSP2 was evaluated after culture. One representative experiment of three performed is shown. (B) CBC and IBC were purified with beads and then were mock infected or were infected with RRV (MOI, 5). After 10 h, cells were stained with a viability marker, MAb against CD19, CD27, and IgD, and then permeabilized to detect NSP2. For IBC, MAb anti-CD20 and anti-CD38 were included to analyze ASC (data not shown). Dot plots are gated on lymphocytes by size and granularity, CD19+ CBC, or CD19+ CD20+/− IBC. (C) Distribution of RRV- and Wa-infected cells in the B-cell subsets. Results from more than eight experiments for CBC and IBC are presented. Bars represented the medians and ranges. An asterisk indicates statistically significant differences between the frequencies of cells expressing NSP2 in IgD+/− B-cell subsets (P < 0.003, Wilcoxon test).
Cellular viability analysis.
The viability of RV-infected B cells was evaluated using trypan blue exclusion and flow cytometry. Ten hours after infection, cells were collected and a small fraction of them was used for trypan blue staining. The remaining cells were washed twice, resuspended in sterile PBS, and stained with Aqua-Fluorescent reactive dye (Invitrogen, Carlsbad, CA) by following the manufacturer's protocol. Cells then were stained with anti-CD19, anti-CD20, anti-CD27, anti-IgD, and intracellular anti-NSP2, as described above. In some experiments viability changes were detected using a commercial kit (APOPTEST-FITC; Dako Cytomation) that includes staining for annexin V and propidium iodide (PI). B cells treated with a high dose (80 μM) of etoposide (Calbiochem, San Diego, CA) were used as a fast (10 h) B cell death inductor (positive control).
Study of activation markers in B cells.
Negatively purified CBC and IBC cells were mock infected or were infected with iRRV or RRV. Cells stimulated with 50 μg/ml lipopolysaccharide (LPS) (Sigma), 3 μg/ml CpG 2006 (Invivogen), or 10 μg/ml anti-B-cell receptor (anti-BCR) [F(ab′)2 goat anti-human IgA, IgG, and IgM from Jackson Immunoresearch] were used as positive controls. Twelve hours after infection, cells were washed and incubated with anti-CD19 PE-Cy7 (SJ25C1), CD69 APC (FN50 clone), CD40 PE (5C3 clone), CD86 FITC (FUN-1 clone), and HLA-DR APC-Cy7 (L243 clone) for 30 min at 4°C. Finally, cells were washed and fixed with 1% paraformaldehyde (Electron Microscopy Sciences, Washington, PA). Paired PBMC or IMC, from which the B cells were obtained and which were similarly treated, were stained at the same time. For these experiments, mock-infected CBC or IBC were used as controls.
Two-color ELISPOT assay.
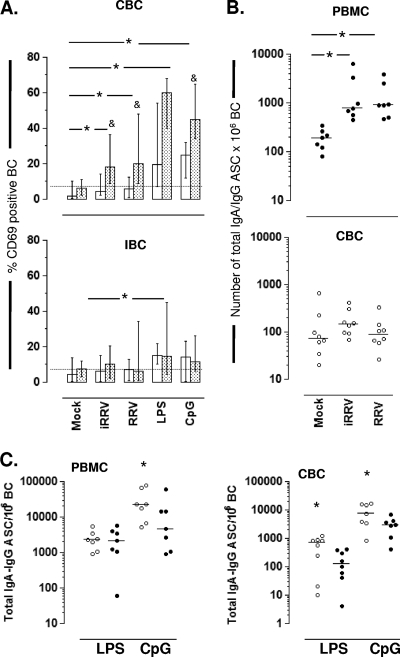
Total ASC were measured by enzyme-linked immunospot (ELISPOT) assay as described previously (61). Briefly, 96-well plates (Immobilon P membrane; MAIPN4510; Millipore, Billerica, MA) were coated with affinity-purified goat anti-human IgA-IgG-IgM (H+L) (KPL, Gaithersburg, MD) at a concentration of 4 μg/ml in PBS. Plates were coated with PBS as a negative control. Plates were incubated overnight at 4°C and blocked for 2 h at 37°C with complete medium prior to use. PBMC, CBC, and polyclonally stimulated CBC were mock infected or were infected with iRRV, RRV, or 10 μg/ml polymyxin B-treated RRV and cultured for 5 days. The virus was maintained during the culture period. Cells then were suspended in complete medium containing 6.3 μg/ml of peroxidase-conjugated goat anti-human IgA antibody (Sigma, St. Louis, MO) and 0.5 μg/ml of phosphatase-conjugated goat anti-human IgG (H+L) antibody (KPL) and distributed into ELISPOT assay plates, prepared as described above, and incubated for 4 h at 37°C in a CO2 incubator. Plates were washed with PBS and developed with an AEC substrate kit for peroxidase (Vector, Burlingame, CA) and subsequently developed with a blue alkaline phosphatase substrate kit (Vector). Human IgA ASC were visualized as red spots and IgG ASC as blue spots in the same wells. The quantity of ASC per well was determined by counting the spots under a dissecting microscope, and the quantity reported is the average count. Background ASC detected in the wells coated with PBS were subtracted from the quantity of total ASC. To report the number of ASC/106 B cells in whole PBMC (see Fig. 4B and C), the frequency of CD19+ cells was determined by flow cytometry.
FIG. 4.
RRV-induced expression of CD69 and ASC differentiation in CBC or IBC. (A) Purified B cells (open bars) derived from either blood (upper) or intestine (lower) and whole mononuclear cells from either blood or the intestine (stippled bars) were mock infected or were infected with iRRV, RRV (MOI, 5), LPS (50 μg/ml), or CpG 2006 (3 μg/ml). After overnight stimulation, the expression of CD69 was analyzed by flow cytometry. The medians and ranges from seven experiments are shown. *, significant differences between mock infection and positive control, iRRV-stimulated, and RRV-stimulated PBMC (P ≤ 0.01, Mann-Whitney test); &, statistically significant differences between purified B cells and B cells as a component of whole mononuclear cells (P < 0.04, Wilcoxon test). (B) PBMC (filled circles; upper) and purified CBC (open circles, lower) were mock infected or were infected with iRRV and RRV (MOI, 5) for 5 days. The numbers of total IgA and IgG ASC were analyzed by two-color ELISPOT assay. Individual paired experiments are shown. Horizontal lines represent the median. An asterisk indicates statistically significant differences between mock-, iRRV-, and RRV-stimulated cells (P ≤ 0.046, Wilcoxon test). (C) PBMC and CBC were treated with LPS (50 μg/ml) or CpG (3 μg/ml) in the presence of mock (open circles) or RRV (filled circles) infection. After 5 days, the frequency of total IgA and IgG ASC was evaluated by ELISPOT assay. An asterisk indicates statistical differences between mock- and RRV-infected CBC (P ≤ 0.016, Wilcoxon test).
Polyclonal stimulation of B cells.
Purified CBC and IBC were washed with serum-free medium twice. A total of 1 × 106 cell/ml were stimulated with 10 μg/ml anti-BCR [F(ab′)2 goat anti-human IgA, IgG, and IgM from Jackson Immunoresearch], 50 μg/ml LPS (Sigma-Aldrich), or 3 μg/ml CpG 2006 oligodeoxinucleotide (Invivogen). After 30 min of incubation at 37°C, CO2 5%, B cells were washed and infected as described above. All polyclonal stimuli were maintained during the culture period. After 10 hpi, B cells were washed and stained to detect intracellular NSP2. In some experiments the stimulation was applied after and not prior to infection.
Measurement of cytokines in B-cell culture supernatants.
To quantify cytokines present in the supernatants of infected CBC and IBC cultures, 72 hpi the supernatants were frozen and stored at −70°C. IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α were detected using a BD cytometric bead array (CBA) human inflammation kit (BD Bioscience) by following the manufacturer's instructions.
Detection of RV-specific cytokine producing circulating and intestinal T cells.
The frequency of RV-specific CD4+ and CD8+ circulating and intestinal T cells was determined as previously described (39, 52). Briefly, 2 × 106 PBMC or IMC were mock or RRV (MOI, 7) infected or treated with the superantigen staphylococcal enterotoxin B (SEB; Sigma-Aldrich) as a positive control (1.25 μg/ml). Anti-CD28 (0.5 μg/ml) and anti-CD49d (0.5 μg/ml) MAbs (Pharmingen, San Diego, CA) were added to each sample as costimulators for 10 h, the last 5 h in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich). The cells were washed and stained with the following antibodies: anti-CD3 Alexafluor 700 (UCHT-1 clone), anti-CD8 Pacific Blue (RPA-T8 clone), anti-CD4 PerCP-Cy5.5 (SK3 clone), anti-CD69 APC (FN50 clone), anti-IFN-γ PE-Cy7 (4S.B3 clone), anti-IL-10 PE (JES3-19F1 clone), anti-TNF-α FITC (Mab11 clone), and anti-IL-2 FITC (5344.111 clone). To exclude dead cells, Aqua-Fluorescent reactive dye (Invitrogen) was added to PBMC and IMC at the beginning of the staining as described above.
To evaluate the potential role of B cells as antigen-presenting cells in this short in vitro memory T-cell assay, a fraction of PBMC or IMC was depleted of B cells using anti-CD19 microbeads (Miltenyi) and stimulated as describe above for unfractionated cells. The efficiency of B cell depletion in PBMC or IMC was ≥98%. After depletion, the cytokine staining was done as described above.
Statistical analysis.
Flow cytometry analysis was performed using FlowJo software (Treestar Inc., Ashland, OR). Statistical analysis was performed with SPSS (Chicago, IL) software, version 17.0, using nonparametric tests. Differences between independent groups were evaluated with Mann-Whitney tests. Differences between paired results were compared with the Wilcoxon test. Significance was established at P < 0.05. Data are shown as medians and ranges unless otherwise noted. For the statistical analysis of studies that quantified cytokines by CBA (see Fig. 5), values below the limit of the sensitivity of the assay were assigned a value of half the sensitivity limit of the assay for each cytokine.
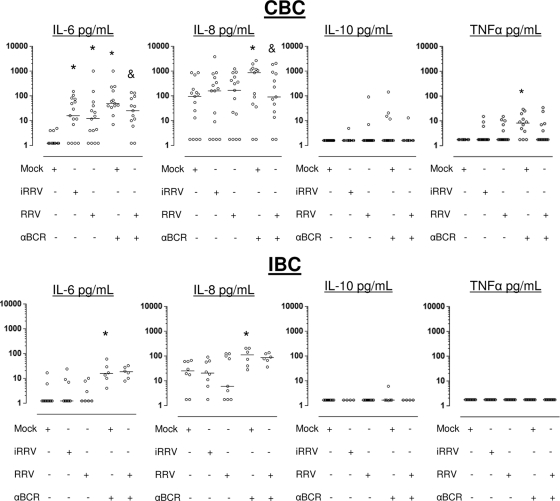
FIG. 5.
RV induces IL-6 secretion in human CBC but not in IBC. Microbead-purified CBC (upper) and IBC (low) were subjected to mock, iRRV, RRV, anti-BCR plus mock, or anti-BCR plus RRV infection. After 72 h of culture, the supernatants were collected and the secretion of IL-6, IL-8, IL-10, and TNF-α was analyzed by CBA. Bars represent the medians. *, statistically significant differences between mock- and iRRV- or RRV-infected cells (P ≤ 0.036, Wilcoxon test); &, significant decrease in the cytokine production between mock- and RRV-infected anti-BCR-treated cells (P ≤ 0.03, Wilcoxon test).
RESULTS
RV preferentially infects human IBC.
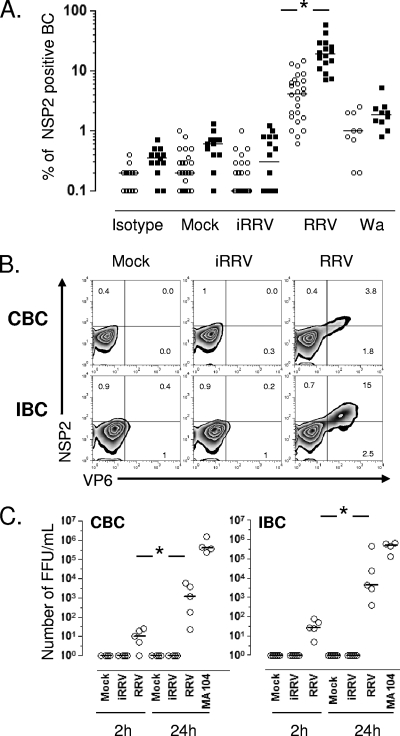
To determine if RV differentially infects human CBC and IBC, purified B cells from adult blood donors or independently from patients undergoing bariatric surgery were mock infected or infected with iRRV, RRV, or Wa at an MOI of 5. The intracellular expression of NSP2 was evaluated by flow cytometry at different times postinfection. Comparable frequencies of RV-infected CBC were seen after anti-NSP2 (MAb 191) or anti-NSP4 (MAb B4-2) staining (n = 2; data not shown), and subsequently only NSP2 staining was employed. No NSP2+ cells were detected 2 hpi in two and one experiments with CBC and IBC, respectively (data not shown). In contrast, significant frequencies of NSP2+ cells were detected in RRV- and Wa-infected but not mock- or iRRV-infected cells 10 hpi (Fig. 1A). IBC were 4-fold more likely to support viral replication than CBC (P < 0.0001, Mann-Whitney test). Significant Wa infection occurred at lower levels than those for RRV in both CBC and IBC. Although no statistical difference was found between intestinal and circulating NSP2+ cells infected with Wa (P = 0.09, Mann-Whitney test), a tendency for higher levels of infection in IBC was seen (Fig. 1A). To confirm these results, CBC and IBC obtained from the same volunteer were infected with RRV (Fig. 1B). After 10 h, the intracellular coexpression of nonstructural protein NSP2 and the viral structural protein VP6 was examined. In agreement with our results, most NSP2+ cells also expressed VP6, and approximately 3-fold-higher levels of double-positive infected cells were present in IBC than in CBC (Fig. 1B).
FIG. 1.
RRV and Wa infection of CBC and IBC. Microbead-purified CBC (empty circles) and IBC (filled square) from different patients were mock treated or treated with psoralen-inactivated RRV (iRRV), RRV, or Wa RV (all at an MOI of 5). After 10 h, the cells were stained for surface markers and then permeabilized to detect intracellular viral proteins. (A) Summary of the expression of NSP2 in B cells. Lines represent the median. An asterisk indicates statistically significant differences between the percentage of CBC and IBC infected by RRV (P < 0.0001, Mann-Whitney test). (B) Coexpression of nonstructural (NSP2) and structural (VP6) proteins in paired CBC and IBC purified from the same patient. (C) RRV productively infects human CBC and IBC. Bead-purified CBC and IBC were mock treated or were treated with iRRV or RRV (MOI, 5) for 45 min. After infection, the cells were washed three times. For the first wash, a 1/1,000 dilution of neutralizing MAb (159; anti-VP7) was used. Supernatants at 2 and 24 h were collected, and viral replication was determined by the titration of infectious virus in MA104 cells. A supernatant of RV-infected MA104 cells was used as a positive control. The results of individual experiments are shown, and horizontal lines represent the median. An asterisk indicates statistically significant differences (P < 0.05, Wilcoxon test).
We next examined if RV undergoes a complete viral replication cycle in infected B cells. The relative quantities of infectious virus (Fig. 1C) and VP6 (data not shown) present in infected CBC and IBC supernatants were evaluated by titration on MA104 cells and ELISA at 2 and 24 hpi, respectively. A significant increase of infectious virus and soluble VP6 was present in supernatants of CBC and IBC treated with live RRV at 24 hpi (Fig. 1C and data not shown). Of note, the infection of human primary CBC and IBC with RRV is substantially less productive than the comparable infection of MA104 cells (Fig. 1C). These results indicate that RV preferentially infects human IBC, and that this infection is productive.
RV predominantly infects mBC subsets.
The distribution of B-cell subsets varies according to their localization in the host and in the intestine; unlike the case for blood, there are more antigen-experienced than naïve B cells (21, 22). To determine the susceptibility of B-cell subsets to RV infection, CBC were isolated and mock infected or infected with RRV, and immediately after infection cells were stained with anti-CD19, anti-CD27, and anti-IgD and sorted by FACS. Four subsets of B cells were isolated: CD27− IgD+ (naïve), CD27+ IgD− (switch memory), CD27+ IgD+ (IgM memory) (45), and CD27− IgD− (recently described as a CD27-mBC [23, 71]). All populations were more than 94% pure after sorting (Fig. 2A). Since sorting was performed after infection, virus exposure was comparable for all subsets. At 10 hpi, cells were washed and stained again with anti-CD19, anti-CD27, and anti-IgD MAbs and permeabilized to detect intracellular NSP2. As shown in Fig. 2A, naïve B cells were relatively resistant to RV infection, and 3- to 6-fold higher frequencies of infected cells were observed in the switch, IgM, and CD27-mBC subset populations (n = 3) (Fig. 2A).
To confirm and extend these observations, purified CBC and IBC were infected, and 10 hpi the cells were stained with anti-CD19, anti-CD20, anti-CD27, anti-IgD, anti-CD38, and anti-NSP2 MAbs. As shown in Fig. 2B, RV replication was highly restricted to IgD− CD27+ mBC. Of note, from 20 to 90% of intestinal ASC (CD19+ CD20low CD38high CD27+) supported RRV replication (data not shown). After RRV infection, 52 to 86% and 54 to 92% (median of approximately 80%) of infected cells were present in the memory (IgD−) compartment of CBC and IBC, respectively (Fig. 2B and C). Human Wa RV also preferentially replicated in mBC subsets of CBC and IBC (Fig. 2C). Thus, circulating and intestinal mBC subsets are the principle targets of both heterologous and homologous RV infection, and in the intestine, substantial infection also takes place in CD38high ASC (data not shown).
RV infection diminishes viability of CBC but not of IBC.
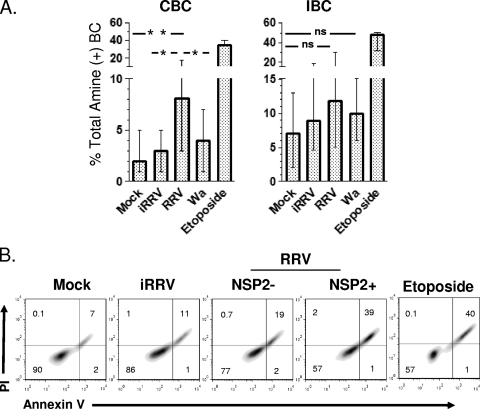
RV infection frequently is cytolytic in cell culture and could cause the death of infected B cells. To determine if RV induces viability alterations in infected CBC and IBC, mock-, iRRV-, RRV-, or Wa-infected cells were stained with several viability markers 10 hpi. The staining of CBC with trypan blue showed a significantly higher frequency of dead cells in RRV-infected than in mock-infected cells, with medians of 11.5 (range, 4.2 to 29%) and 4.3% (range, 0 to 14%), respectively (P = 0.004, Man-Whitney test; n = 13) (data not shown). Trypan blue analysis was not performed on IBC, because the contamination of the preparations with dead epithelial cells confounded the analysis. Moreover, the percentage of fluorescent amine-positive (dead cells) B cells was significantly higher (P = 0.001, Man-Whitney test; n = 10) in RRV-infected CBC (median, 8%; range, 3 to 20%) than mock (median, 2%; range, 1 to 5%)-, iRRV (median 3%; range, 1 to 5%)-, or Wa (median, 4%; range, 1 to 7%)-infected B cells (Fig. 3A). However, no differences were found in the frequency of dead IBC between mock-, iRRV-, RRV-, and Wa-infected cells (Fig. 3A). Etoposide (which, at the high doses used here, can provoke necrosis [53]) induced comparable frequencies of dead cells in both CBC and IBC (Fig. 3A).
FIG. 3.
RV-induced death in CBC and IBC. Bead-purified CBC and IBC were mock infected or were infected with iRRV, RRV, or Wa RV (MOI, 5) or with 80 μM etoposide as a positive control. The viability of B cells was evaluated by flow cytometry after 10 h. (A) Frequency of amine+ CBC and IBC. Medians and ranges from at least 12 experiments for CBC and IBC are shown. For Wa RV, six experiments are included. The double asterisk indicates statistically significant differences between results for mock- and RRV-infected B cells (P = 0.005, Wilcoxon test). An asterisk indicates statistically significant differences between iRRV- and RRV-infected B cells or between RRV- and Wa-infected cells (P = 0.01, Wilcoxon test). ns, nonsignificant differences. (B) CBC were mock infected or were infected with iRRV, RRV (MOI, 5), or 80 μM etoposide. After 10 h, the expression of PI, annexin V, and NSP2 was evaluated by flow cytometry in B cells. For RRV-infected cells, gates on NSP2+ and NSP2− cells are shown. One representative experiment of three performed is presented.
To further study the mechanism of death generated by RRV in CBC, infected CBC were stained with annexin V (an apoptosis marker) and propidium iodide (PI) (a necrosis marker). As seen in Fig. 3B, levels of staining comparable to those described above for the fluorescent amine were detected with these markers. The analysis of infected cells (Fig. 3B) showed a predominant frequency of annexin V and PI double-positive cells, suggesting that necrosis, more likely than apoptosis (15, 19), is the primary mechanism for RV-induced B-cell death. These results suggest that RRV induces viability alterations in a significant fraction of CBC, while IBC, which are more readily infected, are relatively resistant to RV-induced death early (10 h) after infection. No differences were seen between mock- and Wa-infected B cells, probably because of the lower frequency of cells supporting viral infection (Fig. 3A).
RV induces B-cell activation and differentiation to ASC by an indirect mechanism.
T-independent massive B cell activation has been reported after RV infection in vivo and in vitro in the mouse model (6, 10). To determine if RV induces the activation of human CBC and IBC in vitro, the expression levels of CD69, CD40, CD86, and HLA-DR were examined by flow cytometry after the mock, iRRV, or RRV infection of negatively purified CBC, IBC, or the respective PBMC and IMC preparations from which B cells were purified. Cell stimulation with LPS, CpG 2006, or, in some cases, anti-BCR were used as positive controls for B-cell activation. LPS and CpG 2006 significantly increased the percentage of CD69+ B cells present in purified CBC and PBMC (Fig. 4A, top). However, after RV infection, B-cell activation was not observed in purified circulating or intestinal B cells (Fig. 4A, top and bottom, open bars). Strikingly, the frequency of activated B cells was significantly higher after the RV infection of PBMC than that for purified CBC derived from the PBMC (Fig. 4A, top, stippled bars). RV replication was not a critical requirement of activation, since iRRV increased CD69 expression in PBMC as well as live RRV (Fig. 4A, top). In contrast, RV and CpG 2006 did not activate IBC and IMC, and only a low but significant activation (Fig. 5A, bottom) was detected after the LPS treatment of purified IBC and whole IMC (P < 0.03, Mann-Whitney test). The anti-BCR treatment of purified CBC and IBC, used as positive controls, resulted in the enhanced expression of CD69 (median for CBC, 26%; range, 15 to 77%; median for IBC, 13%; range 3 to 20%; n = 4; data not shown). A not significant but consistent increase in CD86 expression was noted in CBC infected with RV, but no changes were observed when HLA-DR or CD40 was analyzed (data not shown). Taken together, these findings indicate that RV induced the activation of B cells in the circulation when they were present as a component of PBMC and not when they were purified. On the other hand, IBC were not activated by RV treatment (except for low-level activation with LPS) even when exposed as a component of IMC.
Since RV induced B-cell activation in PBMC, we wished to determine if RV also could induce BC differentiation into ASC. For this purpose, PBMC and CBC were mock infected or were infected with iRRV or RRV, and the frequency of total IgA and IgG ASC was measured by two-color ELISPOT assay after 5 days of culture. In agreement with CD69 expression analysis (Fig. 4A), the RRV infection of PBMC significantly increased the frequency of IgA/IgG ASC in the culture (Fig. 4B, top). This effect was not dependent on RV replication and was not explained by the LPS contamination of the RRV preparation, since iRRV- and polymyxin B-treated RRV supernatant induced similar frequencies of IgA/IgG ASC in the culture (Fig. 4B, top, and data not shown, respectively). In contrast to the effect of the virus on PBMC, the RV infection of purified CBC did not result in notable ASC differentiation (Fig. 4B, bottom), suggesting that factor(s) from another cell(s) present in the PBMC population likely was necessary for RV-mediated circulating B-cell activation and differentiation to ASC.
We next examined if RV could modulate the differentiation of B cells to ASC induced by various polyclonal stimulations. CBC and PBMC were treated with the Toll-like receptor (TLR) agonists LPS and CpG 2006 as well as anti-BCR, and they were simultaneously infected with RRV. All stimuli were maintained during the time course of the experiment, as described in Materials and Methods. High frequencies of ASC were observed following LPS treatment and even more so after the CpG 2006 treatment of mock-infected PBMC or CBC (Fig. 4C, open circles). A significant decrease in the frequency of ASC generation was observed with concomitant RV infection in CpG-stimulated PBMC and CBC and LPS-treated CBC (Fig. 4C, filled circles). Taken together, these findings show that RV can inhibit the differentiation of polyclonally stimulated CBC to ASC.
RV induces IL-6 secretion in human CBC but not in IBC.
Recent studies have shown that cytokines released by B cells can play important roles in modulating the immune response (36, 47, 48, 73). We analyzed, by CBA, the pattern of cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α) produced by CBC and IBC 72 h after RV infection. In general, higher levels of IL-6 and IL-8 were found in supernatants of CBC than in those of IBC (Fig. 5). RRV and iRRV significantly enhanced the secretion of IL-6 from CBC but not from IBC (P < 0.01 and P > 0.2, respectively, Wilcoxon test) (Fig. 5, top and bottom). Comparable IL-6 secretion was obtained after the infection of CBC with cesium-purified RRV, excluding the possibility that this cytokine was induced by cellular components present in supernatants (n = 3) (data not shown). IL-8 was not induced by RRV or iRRV in these experiments. IL-10 and TNF-α were induced irregularly in CBC but not in IBC (Fig. 5).
We next examined if RV could modulate the secretion of cytokines induced by polyclonally stimulated B cells. After polyclonal stimulation with anti-BCR (or LPS and CpG 2006; data not shown) of mock-infected cells, a significant increase in the secretion of IL-6 and IL-8 in CBC and IBC, and TNF-α only in CBC, was observed (Fig. 5). Interestingly, a significant decrease in the IL-6 and IL-8 secretion was observed in anti-BCR-treated CBC infected with RV compared to that of noninfected anti-BCR-treated control cells (P = 0.02 and P = 0.03, respectively, Wilcoxon test; n = 13), and this suppressive response was not present in anti-BCR-treated infected IBC (Fig. 5). A low level of production of TNF-α (median, 9 pg/ml; range, 1.8 to 30; n = 12; Fig. 5) and IL-10 (median, 20 pg/ml; range, 5 to 183; n = 10; data not shown) was provoked by the anti-BCR and CpG 2006 stimulation of CBC, respectively. Of note, a detectable but not significant decrease in TNF-α in anti-BCR-stimulated CBC also was obtained after RV infection (Fig. 5, top). In contrast, IL-12p70 and IL-1β were not detected in the cultures of CBC or IBC with any of the stimulations (data not shown). In summary, CBC and IBC secrete cytokines differentially after RV infection, with CBC being more responsive, and RV decreases the secretion of IL-6 and IL-8 from anti-BCR-stimulated CBC but not IBC (Fig. 5).
Polyclonal stimulation induces a significant increase in RRV infection and cell death in CBC but not in IBC.
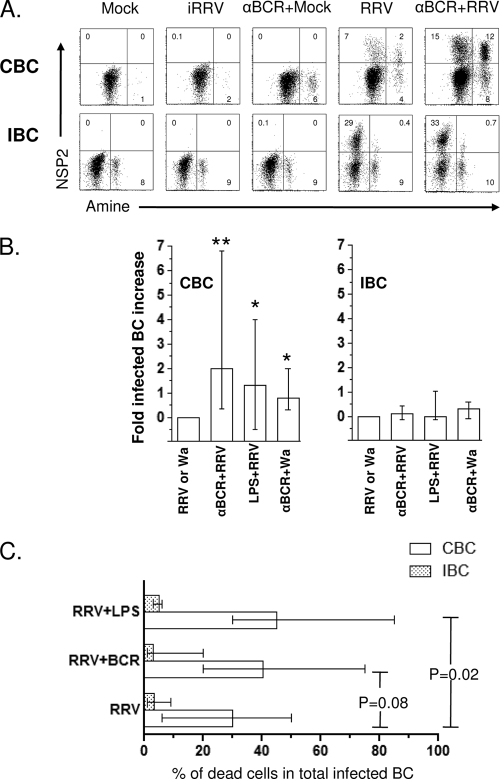
Since RV can modulate the frequency of ASC and the secretion of IL-6 and IL-8 in polyclonally stimulated B cells (Fig. 4 and 5, respectively), we examined the effect of B-cell activation on viral replication and cell viability. CBC and IBC were left untreated or were treated with anti-BCR, LPS, or CpG 2006 and then mock infected or infected with iRRV, RRV, or Wa. Anti-BCR, LPS, or CpG 2006 treatment was maintained during the incubation. No NSP2+ B cells were detected in polyclonally stimulated cells that were mock infected or infected with iRRV. As seen in Fig. 6A, the polyclonal stimulation with anti-BCR increased 3-fold the percentage of CBC that support RRV replication. LPS treatment had a significant stimulatory effect on the RRV infection as well (Fig. 6B). In contrast, no increase in the frequency of NSP2+ cells was detected in IBC treated with these same stimuli (Fig. 6A and B). A comparable increase in the Wa-infected cell number was observed in polyclonally stimulated CBC (n = 5) (Fig. 6B). Interestingly, when viral infection and cell death were analyzed together, a significant positive relation (n = 6, P = 0.02, Mann-Whitney test) between the increase in RV-infected cell number and CBC death was found in LPS-treated cells (Fig. 6C). Although no significant relationship between cell death and RV replication was seen in anti-BCR-stimulated CBC, a similar tendency was noted (Fig. 6A and C). In contrast to CBC, RV-infected anti-BCR and LPS-stimulated IBC did not have an increase in viral replication and RV-induced cell death (Fig. 6).
FIG. 6.
Polyclonal stimulation induces increases in RRV infection and cell death in CBC but not in IBC. (A) Purified CBC and IBC were treated with 10 μg/ml of anti-BCR antibodies before RRV infection (MOI, 5). Viability and CD19 and NSP2 expression were assessed by flow cytometry at 10 hpi. A representative of 14 experiments is shown. (B) Enhancement in viral replication after polyclonal stimulation is presented as the fold increase. The bars show the medians and ranges from 8 to 12 experiments for CBC and 6 to 12 experiments for IBC. For Wa RV, five experiments are presented. * and **, statistically significant differences between RRV and anti-BCR plus RRV (P = 0.0009) and between RRV and LPS plus RRV or Wa and anti-BCR plus Wa (P = 0.03, Mann-Whitney test), respectively. (C) Purified CBC and IBC were infected with RRV or RRV plus polyclonal stimulation and then cultured for 10 h. Cells were stained to evaluate the coexpression of amine viability marker and NSP2. Bars represent the median frequency of double-positive (NSP2+ and amine+) CBC (empty bars) and IBC (stippled bars). P values (Mann-Whitney test) are shown (n = 6 to 12 experiments).
B cells do not play a role in the stimulation of RV-specific memory circulating and intestinal CD4+ and CD8+ T cells.
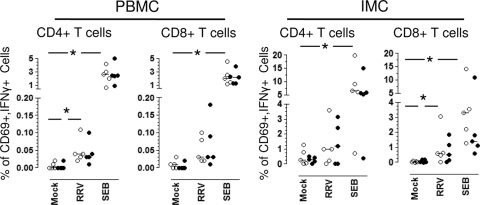
To characterize the role of B cells as antigen-presenting cells, an in vitro T-cell stimulation assay was carried out using PBMCs and IMCs that were not depleted or that were depleted of B cells with microbeads. The frequencies of RV-specific T cells producing IFN-γ, IL-2, TNF-α, or IL-10 were evaluated by intracellular cytokine detection. In agreement with our previous results (39), a low but significant level of difference was found in the frequency of RV-specific IFN-γ-producing CD4+ T cells in PBMCs stimulated with RRV (median, 0.04%; range, 0.02 to 0.11%) compared to that for mock-infected cells (median, 0.01%; range, 0.00 to 0.02%; P = 0.04, Wilcoxon test; n = 5) (Fig. 7). Interestingly, a substantial difference also was found in intestinal CD8+ T cells either mock (median, 0.04%; range, 0.0 to 0.08%; P = 0.04, Wilcoxon test) or RRV stimulated (median, 0.58%; range, 0.04 to 3.08%) (Fig. 7). After the depletion of B cells from PBMCs or IMCs, no differences were found in the frequency of IFN-γ-producing RV-specific CD4+ or CD8+ cells compared to that of nondepleted whole PBMCs or IMCs, suggesting that B cells do not participate significantly as antigen-presenting cells in this short in vitro memory assay (Fig. 7). However, an important decrease in the frequency of SEB-stimulated intestinal CD8+ T cells producing IFN-γ was observed after B-cell depletion, which could be due to the reduction of HLA I-expressing cells (HLA molecules are necessary for adequate superantigen stimulation). No important differences were found in the RV-specific T cells producing IL-10, TNF-α, or IL-2 observed in PBMC or IMC either left nondepleted or depleted of B cells (data not shown).
FIG. 7.
Circulating and intestinal B cells are not critical antigen-presenting cells for RV-specific T cells. Whole (open circles) or B-cell-depleted (filled circles) PBMC or IMC were mock stimulated or stimulated with RRV or SEB, as described in Materials and Methods. After 10 h of culture (brefeldin A was added for the last 5 h), the cells were stained with a viability marker, MAb against CD3, CD4, CD8, CD69, and IFN-γ. The frequency of RV-specific IFN-γ-producing T cells was analyzed by flow cytometry. Dead cells were excluded from the analysis by viability staining. Individual experiments are shown. Lines represent the medians. An asterisk indicates significant differences between mock- and RV- or SEB-stimulated cells (P ≤ 0.04, Wilcoxon test).
DISCUSSION
We studied the interaction of RV with human CBC and IBC and have several conclusions. (i) RV preferentially infects IBC instead of CBC, and in B cells from both locations replication is highly restricted to antigen-experienced memory B-cell subsets. (ii) The RV infection of CBC was associated with the necrotic cell death of a significant fraction of CBC, but comparably infected IBC were relatively resistant to cell death for the short term. (iii) The RV infection of PBMC, but not purified B cells and not IMC or purified IBC, induced the expression of B-cell activation markers and the differentiation of B cells to ASC. (iv) RV-infected CBC, but not IBC, produced IL-6. (v) RV infection decreased the number of ASC generated after the LPS and CpG stimulation of CBC. (vi) RV infection of polyclonally BCR-stimulated CBC, but not IBC, decreased the production of IL-6 and IL-8. (vii) The frequency of CBC, but not IBC, supporting RV replication and cell mortality increased when cells were activated via BCR or TLR-4. (viii) Finally, B cells do not function as important antigen-presenting cells for RV in a short memory T-cell stimulation assay with PBMC and IMC, and probably there are more RV-specific memory T cells in the intestine than in the circulation of nonacutely infected adults.
As we reported previously (49), RRV infected a relatively small fraction of total blood B cells (Fig. 1). Interestingly, 4-fold-greater frequencies of RRV infection were observed when IBC, rather than CBC, were examined (Fig. 1A and B). Frequencies of Wa-infected CBC and IBC were consistently lower than those of RRV (Fig. 1A), but a similar tendency for the increased infection of IBC was seen. This can be explained by the fact that mBC and ASC, which are the most permissive targets of RV infection (Fig. 2), are present in intestinal samples at higher levels than in the circulation (11, 22). In our samples, less than 0.5% (range, 0.08 to 1.2%; n = 5) of the CBC expressed ASC markers, while 20% (range, 4.4 to 42%; n = 16; data not shown) of IBC expressed those markers. However, we cannot rule out the additional possibility that, compared to circulating mBC, intestinal mBC have a higher susceptibility to RV infection. Another possible explanation for the increased susceptibility of IBC is that the receptor used by RV to enter B cells also is important for intestinal homing and hence is present at greater frequency in IBC. However, the integrin α4β7, one of the best-characterized intestinal homing receptors (4, 12, 35), is not a good candidate, because it is expressed by both naive B cells (59) and mBC. In addition, neither blocking MAb anti-α4β7 nor recombinant MadCAM-1 was able to inhibit the RRV infection of IBC (n = 3) (data not shown).
The RV infection of primary human B cells was productive (Fig. 1C), but not as productive as that in the highly permissive MA104 cell line. In contrast to the flow cytometry results, which show the greater infection of IBC, the relative amount of VP6 or the number of infectious particles detected in the supernatants of RRV-infected CBC and IBC 24 hpi were not significantly different (Fig. 1C and data not shown). This apparent discrepancy likely is explained by differences in the sensitivity of the techniques used (52). However, since there are higher frequencies of total ASC in intestinal samples, it also seems possible that RV-specific ASC were present in the intestinal B-cell samples, which, during the 10-h incubation period, could secrete neutralizing Ab that reduced the number of infectious particles present in these samples, thereby artificially lowering the observed viral yields.
RV infected higher frequencies of antigen-experienced B cells compared to naive B cells from both CBC and IBC (Fig. 2). In the circulation, however, because of the substantially higher percentage of naïve B cells present (Fig. 2), the total number of potentially infectible B cells in the naïve subset would be appreciable, although still less than that in the combined memory compartments. This selective viral replication in the memory compartment may influence how RV modulates the immune response, since it recently has been reported that the profile of cytokines released by the different subsets of B cells varies substantially. For example, human mBC but not naïve cells produced TNF-α and IL-12 after stimulation with CD40L and anti-BCR (17, 27). Moreover, the supernatant of mBC but not naïve B cell cultures induced IFN-γ production by T cells (17, 27). Of note, TNF-α and IFN-γ have been reported to be in the plasma of children with febrile acute RV infection (42). Interestingly, despite the fact that RV antigen and infectious virus are present in the plasma of a high percentage of recently infected children or mice, it has been impossible to detect RV-infected blood B cells in these samples (7, 49). Our findings of the preferential RV infection of antigen-experienced B cells provide one possible explanation to this observation, since young children have low frequencies of mBC, which are more susceptible to RV infection and, if infected, would quickly die, preventing their detection in circulation (Fig. 3).
Contrary to Epstein-Barr virus (EBV), which persistently infects B cells and in which they inhibit apoptotic pathways (1), RV induces a fast lytic cycle in many cell types (43). Here, we demonstrated that RV affected the viability of CBC but not that of IBC (Fig. 3A), even though 4-fold-higher percentages of IBC were infected. RV probably induces the cell death of B cells by necrosis and not by apoptosis, because the same percentage of RV-infected B cells expressed the three death markers used (annexin V, PI [Fig. 3B], and Aqua-Fluorescence; data not shown); however, these results need to be confirmed in future studies. Of note, prior studies of RV effects on intestinal epithelial cell viability have indicated that the primary mechanism of cell death in this case is by apoptosis (14). The fact that RV-infected IBC are more resistant to death could explain, at least in part, why large numbers of activated B cells accumulate in intestinal lymphoid organs during RV infection in mice (10).
In agreement with previous reports of RV-infected splenocytes from mice and PBMC from children with acute RV-induced gastroenteritis (6, 70), RV induced the CD69 expression of a significant fraction of CBC when cells were infected as a component of whole PMBC, but there was low or no activation and ASC differentiation observed when purified CBC or IBC were infected (Fig. 4). iRRV had an effect similar to that of live RRV (Fig. 4B). This result may have implications for the design of future inactivated RV vaccines. Taken together, these results suggest that RV induces the activation and differentiation of CBC by an indirect mechanism that probably is not dependent on T cells, because similar levels of B-cell activation were observed in a T-cell-deficient mouse model (10) but could be dependent on innate immune cells in the PBMC compartment, such as pDC. Viruses can modulate B- and T-cell responses through their interaction with pDC (68). A mechanism for B-cell activation and Ig secretion mediated by cytokines produced by pDC, especially IFN-α and IL-6, has been described for influenza virus in vitro (41). Moreover, sustained signals through the type I IFN-α/β receptor are necessary for massive B cell activation after West Nile virus infection in vivo in mice (56). Given that we showed that in human PBMC infected with RRV pDC produce type I IFN (49), it seems reasonable to hypothesize that cytokines secreted by pDC mediate RV B-cell activation and differentiation to ASC (Fig. 4).
Although RV and iRRV promoted the development of ASC from PBMC (Fig. 4B), the RV infection of LPS- or CpG-stimulated CBC significantly decrease the number of total ASC obtained after culture (Fig. 4C). The final outcome of the systemic humoral response in an RV-infected individual probably will be determined by a balance between these opposite effects. In addition, the polyclonal activation of B cells has been seen in other viral infections, such as hepatitis C virus (HCV), HIV, and papillomavirus (37, 57, 72), and it has been suggested that it is a mechanism used by the virus to evade the immune response by the activation of B cell clones of irrelevant specificities (51).
Significant RV-induced B cell activation has been seen in murine IMC derived from Peyer's patches or mesenteric lymph node (6). Furthermore, increases in the number of total Igs and ASC from Peyer's patches and mesenteric lymph node have been reported after the oral live RRV but not WC3 (bovine RV) inoculation of suckling mice, suggesting that this effect is viral strain dependent (44). In contrast, we did not detect the enhanced expression of activation markers after the RRV infection of B cells obtained from intestine (Fig. 4A), even in the presence of saturating doses of strong polyclonal stimuli (Fig. 4A). The reason for this discrepancy is unknown. However, differences in isolation methods (mechanic versus enzymatic), the host involved (human versus mouse), and the characteristics of the tissue used may be implicated. Of note, differences in the activation of B cells from human and murine origin have been described. In fact, it is well know that naive human B cells do not respond to stimulation with CpG or LPS, while murine cells are highly activated with these toll agonists (40) because of the differential expression of TLR in human and murine B cells (34). A disparity in the expression of other receptors also could explain why, contrary to its activation of CBC, RV does not efficiently activate human IBC. For example, inhibitory receptors present on a subset of mBC from tonsils (50) make these cells respond less efficiently to polyclonal stimulation than that for circulating mBC.
After RV infection, purified CBC secreted only small amounts of IL-6, while IBC did not secrete any of the cytokines tested (Fig. 5). Although other RV-infected cells, like monocyte-derived DC (52), have been reported to produce IL-6, CBC may be partially responsible of the enhanced serum levels of IL-6 observed in children with acute RV gastroenteritis (42). Since the increased level of IL-6 secretion in vitro was independent of viral replication (Fig. 5), the high levels of antigenemia observed during acute RV infection could be triggering the B-cell production of this cytokine. After polyclonal stimulation with anti-BCR and RV infection, a significant decrease of IL-6 and IL-8 secretion by CBC was noted (Fig. 5) compared to that of cytokine secretion in BCR-stimulated mock-infected CBC. Both the low level of production of cytokines generated by RV alone and the inhibition of BCR-induced cytokine production could be explained by an RV-dependent blockage of the nuclear accumulation of NF-κB in infected cells (a critical transcription factor in B cells for the expression of some proinflammatory cytokines genes, including IL-6 and IL-8 [3]), as has been shown recently for RV (29, 38).
Consistently with the decrease in the IL-6 production and ASC differentiation in polyclonally stimulated RV-infected CBC, the stimulation via BCR or TLR-4 of CBC but not IBC significantly increased the percentage of infected CBC (Fig. 6A and B). This could be due to the rapid redistribution and affinity changes of some RV receptors caused by the stimuli (30, 65). In preliminary experiments to determine if the increase in the percentage of infected cells after CBC stimulation was due to an increase in virus entry or an increase in viral replication, we infected targets with RV before or after anti-BCR treatment. In four of six experiments, an increase was obtained when CBC were infected before but not after BCR stimulation (data not shown). In two experiments the increase was obtained in both conditions. These results suggest that both mechanisms are involved. In contrast, even though IBC were more efficiently infected by RRV, the pretreatment with anti-BCR or anti-LPS did not significantly increase the frequency of infected IBC and did not affect their IL-6 and IL-8 secretion (Fig. 5 and 6). This could be related to the fact that, unlike CBC, most IBC are mBC or activated B cells that are highly susceptible to RV infection but were refractory to further activation and associated increased replication (20, 22). This may be a novel mechanism of local immune resistance and the modulation of RV pathogenesis.
In agreement with our previous results (49), the depletion of CBC did not modify the frequency of RV-specific memory CD4+ or CD8+ T cells secreting IFN-γ in PBMC after short-term stimulation (10 h) (Fig. 7). The modification of the frequencies of RV-specific T cells in B-cell-depleted IMC also was not detected (Fig. 7). This finding seems to be at odds with a previous report that supports the role of transferred murine systemic purified B cells in the presentation of RV antigen (13). An explanation for this discrepancy could be that in the short stimulation assay we used, B cells respond slower than pDC, which quickly produce type I IFN (46) and control the production of IFN-γ from T cells, as we have shown recently (49). Prior studies of humans have noted a relative paucity of RV-specific CD8+ T cells in the circulation of both acutely infected and immune subjects (39). It is of interest that the present studies indicate that many more RV-specific class I-restricted T cells appear to be resident in the intestine of healthy RV immune adults than in the circulation (Fig. 7). These preliminary findings should be confirmed and extended in future studies.
In conclusion, these findings suggest that RV differentially interacts with primary human B cells, depending on their tissue of origin and differentiation stage, and this differential interaction likely plays a role in modulating the immune response. Moreover, we describe a relatively efficient model of the RV infection of primary human intestinal cells with a heterologous virus in which one out five B cells and, especially, 20 to 90% of intestinal ASC (CD19+ CD20low CD38high CD27+) supported RV replication. Most studies of the viral cycle have been performed in cell lines, and future studies using this primary cell model may shed light on basic aspects of RV replication and virus immunopathology.
Acknowledgments
This study was supported in part by a VA Merit Award and NIH grants RO1 AI021362-26 and P30DK56339. Carlos F. Narváez was funded by the Stanford University Medical School and the Programa de Apoyo a Doctorados Nacionales of Colciencias, Colombia.
We thank Luz Stella Rodríguez, Emily M. Deal, Xiaosong He, and Ningguo Feng for their helpful comments.
Footnotes
Published ahead of print on 17 February 2010.
REFERENCES
- 1.Anderton, E., J. Yee, P. Smith, T. Crook, R. E. White, and M. J. Allday. 2008. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt's lymphoma. Oncogene 27:421-433. [DOI] [PubMed] [Google Scholar]
- 2.Angel, J., M. A. Franco, and H. B. Greenberg. 2007. Rotavirus vaccines: recent developments and future considerations. Nat. Rev. Microbiol. 5:529-539. [DOI] [PubMed] [Google Scholar]
- 3.Baccam, M., S. Y. Woo, C. Vinson, and G. A. Bishop. 2003. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-kappa B, AP-1, and C/EBP. J. Immunol. 170:3099-3108. [DOI] [PubMed] [Google Scholar]
- 4.Bargatze, R. F., M. A. Jutila, and E. C. Butcher. 1995. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3:99-108. [DOI] [PubMed] [Google Scholar]
- 5.Bass, D. M., E. R. Mackow, and H. B. Greenberg. 1990. NS35 and not vp7 is the soluble rotavirus protein which binds to target cells. J. Virol. 64:322-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blutt, S. E., S. E. Crawford, K. L. Warfield, D. E. Lewis, M. K. Estes, and M. E. Conner. 2004. The VP7 outer capsid protein of rotavirus induces polyclonal B-cell activation. J. Virol. 78:6974-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blutt, S. E., M. Fenaux, K. L. Warfield, H. B. Greenberg, and M. E. Conner. 2006. Active viremia in rotavirus-infected mice. J. Virol. 80:6702-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 9.Blutt, S. E., D. O. Matson, S. E. Crawford, M. A. Staat, P. Azimi, B. L. Bennett, P. A. Piedra, and M. E. Conner. 2007. Rotavirus antigenemia in children is associated with viremia. PLoS Med. 4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blutt, S. E., K. L. Warfield, D. E. Lewis, and M. E. Conner. 2002. Early response to rotavirus infection involves massive B cell activation. J. Immunol. 168:5716-5721. [DOI] [PubMed] [Google Scholar]
- 11.Brandtzaeg, P., and F. E. Johansen. 2005. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 206:32-63. [DOI] [PubMed] [Google Scholar]
- 12.Butcher, E. C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209-253. [DOI] [PubMed] [Google Scholar]
- 13.Coffin, S. E., S. L. Clark, N. A. Bos, J. O. Brubaker, and P. A. Offit. 1999. Migration of antigen-presenting B cells from peripheral to mucosal lymphoid tissues may induce intestinal antigen-specific IgA following parenteral immunization. J. Immunol. 163:3064-3070. [PubMed] [Google Scholar]
- 14.Chaïbi, C., J. Cotte-Laffitte, C. Sandre, A. Esclatine, A. L. Servin, A. M. Quero, and M. Geniteau-Legendre. 2005. Rotavirus induces apoptosis in fully differentiated human intestinal Caco-2 cells. Virology 332:480-490. [DOI] [PubMed] [Google Scholar]
- 15.Chen, S., A. C. Cheng, M. S. Wang, and X. Peng. 2008. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J. Gastroenterol. 14:2174-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford, S. E., D. G. Patel, E. Cheng, Z. Berkova, J. M. Hyser, M. Ciarlet, M. J. Finegold, M. E. Conner, and M. K. Estes. 2006. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J. Virol. 80:4820-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duddy, M., M. Niino, F. Adatia, S. Hebert, M. Freedman, H. Atkins, H. J. Kim, and A. Bar-Or. 2007. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 178:6092-6099. [DOI] [PubMed] [Google Scholar]
- 18.Duddy, M. E., A. Alter, and A. Bar-Or. 2004. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J. Immunol. 172:3422-3427. [DOI] [PubMed] [Google Scholar]
- 19.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 20.Fagarasan, S., and T. Honjo. 2003. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 3:63-72. [DOI] [PubMed] [Google Scholar]
- 21.Farstad, I. N., T. S. Halstensen, A. I. Lazarovits, J. Norstein, O. Fausa, and P. Brandtzaeg. 1995. Human intestinal B-cell blasts and plasma cells express the mucosal homing receptor integrin alpha 4 beta 7. Scand. J. Immunol. 42:662-672. [DOI] [PubMed] [Google Scholar]
- 22.Farstad, I. N., J. Norstein, and P. Brandtzaeg. 1997. Phenotypes of B and T cells in human intestinal and mesenteric lymph. Gastroenterology 112:163-173. [DOI] [PubMed] [Google Scholar]
- 23.Fecteau, J. F., G. Cote, and S. Neron. 2006. A new memory CD27− IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J. Immunol. 177:3728-3736. [DOI] [PubMed] [Google Scholar]
- 24.Fenaux, M., M. A. Cuadras, N. Feng, M. Jaimes, and H. B. Greenberg. 2006. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J. Virol. 80:5219-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco, M. A., J. Angel, and H. B. Greenberg. 2006. Immunity and correlates of protection for rotavirus vaccines. Vaccine 24:2718-2731. [DOI] [PubMed] [Google Scholar]
- 26.Franco, M. A., and H. B. Greenberg. 1995. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 69:7800-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagro, A., D. Servis, A. M. Cepika, K. M. Toellner, G. Grafton, D. R. Taylor, S. Branica, and J. Gordon. 2006. Type I cytokine profiles of human naive and memory B lymphocytes: a potential for memory cells to impact polarization. Immunology 118:66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauthier, R., C. Harnois, J. F. Drolet, J. C. Reed, A. Vezina, and P. H. Vachon. 2001. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am. J. Physiol. Cell Physiol. 280:C1540-C1554. [DOI] [PubMed] [Google Scholar]
- 29.Graff, J. W., K. Ettayebi, and M. E. Hardy. 2009. Rotavirus NSP1 inhibits NFkappaB activation by inducing proteasome-dependent degradation of beta-TrCP: a novel mechanism of IFN antagonism. PLoS Pathog. 5:e1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham, K. L., F. E. Fleming, P. Halasz, M. J. Hewish, H. S. Nagesha, I. H. Holmes, Y. Takada, and B. S. Coulson. 2005. Rotaviruses interact with alpha4beta7 and alpha4beta1 integrins by binding the same integrin domains as natural ligands. J. Gen. Virol. 86:3397-3408. [DOI] [PubMed] [Google Scholar]
- 31.Gray, D., M. Gray, and T. Barr. 2007. Innate responses of B cells. Eur. J. Immunol. 37:3304-3310. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg, H. B., and M. K. Estes. 2009. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 136:1939-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groene, W. S., and R. D. Shaw. 1992. Psoralen preparation of antigenically intact noninfectious rotavirus particles. J. Virol. Methods 38:93-102. [DOI] [PubMed] [Google Scholar]
- 34.Gururajan, M., J. Jacob, and B. Pulendran. 2007. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One 2:e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamann, A., D. P. Andrew, D. Jablonski-Westrich, B. Holzmann, and E. C. Butcher. 1994. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 152:3282-3293. [PubMed] [Google Scholar]
- 36.Harris, D. P., L. Haynes, P. C. Sayles, D. K. Duso, S. M. Eaton, N. M. Lepak, L. L. Johnson, S. L. Swain, and F. E. Lund. 2000. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1:475-482. [DOI] [PubMed] [Google Scholar]
- 37.He, B., X. Qiao, P. J. Klasse, A. Chiu, A. Chadburn, D. M. Knowles, J. P. Moore, and A. Cerutti. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 176:3931-3941. [DOI] [PubMed] [Google Scholar]
- 38.Holloway, G., T. T. Truong, and B. S. Coulson. 2009. Rotavirus antagonizes cellular antiviral responses by inhibiting the nuclear accumulation of STAT1, STAT2, and NF-kappaB. J. Virol. 83:4942-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaimes, M. C., O. L. Rojas, A. M. Gonzalez, I. Cajiao, A. Charpilienne, P. Pothier, E. Kohli, H. B. Greenberg, M. A. Franco, and J. Angel. 2002. Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J. Virol. 76:4741-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janeway, C. A., Jr., and R. Medzhitov. 1999. Lipoproteins take their toll on the host. Curr. Biol. 9:R879-R882. [DOI] [PubMed] [Google Scholar]
- 41.Jego, G., A. K. Palucka, J. P. Blanck, C. Chalouni, V. Pascual, and J. Banchereau. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225-234. [DOI] [PubMed] [Google Scholar]
- 42.Jiang, B., L. Snipes-Magaldi, P. Dennehy, H. Keyserling, R. C. Holman, J. Bresee, J. Gentsch, and R. I. Glass. 2003. Cytokines as mediators for or effectors against rotavirus disease in children. Clin. Diagn. Lab. Immunol. 10:995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2002. Rotaviruses. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Raven Publishers, Philadephia, PA.
- 44.Khoury, C. A., K. A. Brown, J. E. Kim, and P. A. Offit. 1994. Rotavirus-specific intestinal immune response in mice assessed by enzyme-linked immunospot assay and intestinal fragment culture. Clin. Diagn. Lab. Immunol. 1:722-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 47.Lund, F. E. 2008. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 20:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund, F. E., B. A. Garvy, T. D. Randall, and D. P. Harris. 2005. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr. Dir. Autoimmun. 8:25-54. [DOI] [PubMed] [Google Scholar]
- 49.Mesa, M. C., L. S. Rodriguez, M. A. Franco, and J. Angel. 2007. Interaction of rotavirus with human peripheral blood mononuclear cells: plasmacytoid dendritic cells play a role in stimulating memory rotavirus specific T cells in vitro. Virology 366:174-184. [DOI] [PubMed] [Google Scholar]
- 50.Moir, S., J. Ho, A. Malaspina, W. Wang, A. C. DiPoto, M. A. O'Shea, G. Roby, S. Kottilil, J. Arthos, M. A. Proschan, T. W. Chun, and A. S. Fauci. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montes, C. L., E. V. Acosta-Rodriguez, M. C. Merino, D. A. Bermejo, and A. Gruppi. 2007. Polyclonal B cell activation in infections: infectious agents' devilry or defense mechanism of the host? J. Leukoc. Biol. 82:1027-1032. [DOI] [PubMed] [Google Scholar]
- 52.Narvaez, C. F., J. Angel, and M. A. Franco. 2005. Interaction of rotavirus with human myeloid dendritic cells. J. Virol. 79:14526-14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novo, E., F. Marra, E. Zamara, L. Valfre di Bonzo, L. Monitillo, S. Cannito, I. Petrai, A. Mazzocca, A. Bonacchi, R. S. De Franco, S. Colombatto, R. Autelli, M. Pinzani, and M. Parola. 2006. Overexpression of Bcl-2 by activated human hepatic stellate cells: resistance to apoptosis as a mechanism of progressive hepatic fibrogenesis in humans. Gut 55:1174-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parashar, U. D., C. J. Gibson, J. S. Bresse, and R. I. Glass. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12:304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parez, N., A. Garbarg-Chenon, C. Fourgeux, F. Le Deist, A. Servant-Delmas, A. Charpilienne, J. Cohen, and I. Schwartz-Cornil. 2004. The VP6 protein of rotavirus interacts with a large fraction of human naive B cells via surface immunoglobulins. J. Virol. 78:12489-12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purtha, W. E., K. A. Chachu, H. W. T. Virgin, and M. S. Diamond. 2008. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J. Virol. 82:10964-10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Racanelli, V., M. A. Frassanito, P. Leone, M. Galiano, V. De Re, F. Silvestris, and F. Dammacco. 2006. Antibody production and in vitro behavior of CD27− defined B-cell subsets: persistent hepatitis C virus infection changes the rules. J. Virol. 80:3923-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rojas, O. L., C. F. Narvaez, H. B. Greenberg, J. Angel, and M. A. Franco. 2008. Characterization of rotavirus specific B cells and their relation with serological memory. Virology 380:234-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rott, L. S., M. J. Briskin, and E. C. Butcher. 2000. Expression of alpha4beta7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J. Leukoc. Biol. 68:807-814. [PubMed] [Google Scholar]
- 60.Ruiz-Palacios, G. M., I. Perez-Schael, F. R. Velazquez, H. Abate, T. Breuer, S. C. Clemens, B. Cheuvart, F. Espinoza, P. Gillard, B. L. Innis, Y. Cervantes, A. C. Linhares, P. Lopez, M. Macias-Parra, E. Ortega-Barria, V. Richardson, D. M. Rivera-Medina, L. Rivera, B. Salinas, N. Pavia-Ruz, J. Salmeron, R. Ruttimann, J. C. Tinoco, P. Rubio, E. Nunez, M. L. Guerrero, J. P. Yarzabal, S. Damaso, N. Tornieporth, X. Saez-Llorens, R. F. Vergara, T. Vesikari, A. Bouckenooghe, R. Clemens, B. De Vos, and M. O'Ryan. 2006. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J Med. 354:11-22. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki, S., M. C. Jaimes, T. H. Holmes, C. L. Dekker, K. Mahmood, G. W. Kemble, A. M. Arvin, and H. B. Greenberg. 2007. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J. Virol. 81:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaw, R. D., P. T. Vo, P. A. Offit, B. S. Coulson, and H. B. Greenberg. 1986. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology 155:434-451. [DOI] [PubMed] [Google Scholar]
- 63.Smythies, L. E., M. Sellers, R. H. Clements, M. Mosteller-Barnum, G. Meng, W. H. Benjamin, J. M. Orenstein, and P. D. Smith. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 115:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smythies, L. E., L. M. Wahl, and P. D. Smith. 2006. Isolation and purification of human intestinal macrophages, chapter 7, unit 76B. In J. E. Coligan, B. E. Bierer, D. H. Margulies, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology. Wiley Interscience, New York, NY. [DOI] [PubMed]
- 65.Sriramarao, P., R. G. DiScipio, R. R. Cobb, M. Cybulsky, G. Stachnick, D. Castaneda, M. Elices, and D. H. Broide. 2000. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of shear flow. Blood 95:592-601. [PubMed] [Google Scholar]
- 66.Tian, C., G. K. Luskin, K. M. Dischert, J. N. Higginbotham, B. E. Shepherd, and J. E. Crowe, Jr. 2008. Immunodominance of the VH1-46 antibody gene segment in the primary repertoire of human rotavirus-specific B cells is reduced in the memory compartment through somatic mutation of nondominant clones. J. Immunol. 180:3279-3288. [DOI] [PubMed] [Google Scholar]
- 67.Vanden Bush, T. J., C. M. Buchta, J. Claudio, and G. A. Bishop. 2009. Cutting edge: importance of IL-6 and cooperation between innate and adaptive immune receptors in cellular vaccination with B lymphocytes. J. Immunol. 183:4833-4837. [DOI] [PubMed] [Google Scholar]
- 68.Varani, S., M. Cederarv, S. Feld, C. Tammik, G. Frascaroli, M. P. Landini, and C. Soderberg-Naucler. 2007. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J. Immunol. 179:7767-7776. [DOI] [PubMed] [Google Scholar]
- 69.Vesikari, T., D. O. Matson, P. Dennehy, P. Van Damme, M. Santosham, Z. Rodriguez, M. J. Dallas, J. F. Heyse, M. G. Goveia, S. B. Black, H. R. Shinefield, C. D. Christie, S. Ylitalo, R. F. Itzler, M. L. Coia, M. T. Onorato, B. A. Adeyi, G. S. Marshall, L. Gothefors, D. Campens, A. Karvonen, J. P. Watt, K. L. O'Brien, M. J. DiNubile, H. F. Clark, J. W. Boslego, P. A. Offit, and P. M. Heaton. 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl. J Med. 354:23-33. [DOI] [PubMed] [Google Scholar]
- 70.Wang, Y., P. H. Dennehy, H. L. Keyserling, K. Tang, J. R. Gentsch, R. I. Glass, and B. Jiang. 2007. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J. Virol. 81:3904-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirths, S., and A. Lanzavecchia. 2005. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 35:3433-3441. [DOI] [PubMed] [Google Scholar]
- 72.Yang, R., F. M. Murillo, M. J. Delannoy, R. L. Blosser, W. H. t. Yutzy, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2005. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J. Immunol. 174:7912-7919. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, X., E. Deriaud, X. Jiao, D. Braun, C. Leclerc, and R. Lo-Man. 2007. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J. Exp. Med. 204:1107-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]