Abstract
We studied the status of chromosomes 1 and 19 in 363 astrocytic and oligodendroglial tumors. Whereas the predominant pattern of copy number abnormality was a concurrent loss of the entire 1p and 19q regions (total 1p/19q loss) among oligodendroglial tumors and partial deletions of 1p and/or 19q in astrocytic tumors, a subset of apparently astrocytic tumors also had total 1p/19q loss. The presence of total 1p/19q loss was associated with longer survival of patients with all types of adult gliomas independent of age and diagnosis (P = .041). The most commonly deleted region on 19q in astrocytic tumors spans 885 kb in 19q13.33–q13.41, which is telomeric to the previously proposed region. Novel regions of homozygous deletion, including a part of DPYD (1p21.3) or the KLK cluster (19q13.33), were observed in anaplastic oligodendrogliomas. Amplifications encompassing AKT2 (19q13.2) or CCNE1 (19q12) were identified in some glioblastomas. Deletion mapping of the centromeric regions of 1p and 19q in the tumors that had total 1p/19q loss, indicating that the breakpoints lie centromeric to NOTCH2 within the pericentromeric regions of 1p and 19q. Thus, we show that the copy number abnormalities of 1p and 19q in human gliomas are complex and have distinct patterns that are prognostically predictive independent of age and pathological diagnosis. An accurate identification of total 1p/19q loss and discriminating this from other 1p/19q changes is, however, critical when the 1p/19q copy number status is used to stratify patients in clinical trials.
Keywords: array-CGH, astrocytoma, centromere, deletion, microarray, oligodendroglioma, translocation
The majority of oligodendrogliomas WHO grade II (O) and two-thirds of anaplastic oligodendroglioma WHO grade III (AO), as well as subsets of oligoastrocytomas WHO grade II (OA) and anaplastic oligoastrocytomas WHO grade III (AOA) have concurrent hemizygous deletions of the entire 1p and 19q regions (total 1p/19q loss). This genotype is now considered to be a result of an unbalanced translocation t(1;19)(q10;p10)1,2 and is generally regarded as a hallmark of oligodendroglial tumors (this term encompasses O, AO, OA, and AOA in the following text).3 The exact breakpoint(s) of the t(1;19) translocation are however unknown. Losses of 1p and 19q were correlated with longer survival in AO and AOA.4,5 In contrast to oligodendroglial tumors, astrocytic tumors rarely have total 1p/19q loss. Instead, some of them may have smaller deletions or gains around 1p36 or 19q13.6–8 The impact of 1p/19q status in the survival of astrocytic tumors is not clear.
We have previously reported that astrocytic tumors with total 1p loss have significantly longer overall survival when compared with those with other patterns of 1p alterations or with normal copy number.6 Our data suggested that the astrocytic tumors with total 1p loss may be biologically distinct from those with other types of 1p status. In this study, the copy number status of chromosome 1 and 19, particularly the centromeric regions of 1p and 19q, was extensively studied using array-comparative genomic hybridization (CGH) using a combination of platforms in a series of oligodendroglial and astrocytic tumors. We found that the patterns of chromosomes 1 and 19 alterations are complex. Total 1p/19q loss was significantly associated with longer overall survival of the patients independent of age and diagnosis. A detailed deletion mapping of the 1p/19q centromeric regions suggest that the breakpoint(s) of t(1;19) translocation may be in the pericentromeric regions of 1p and 19q, not disrupting any known protein coding genes.
Materials and Methods
Tumor Materials and Nucleic Acid Extraction
Collection, handling, and DNA/RNA extraction of tumor tissues and the patients' blood samples have been described previously.9 In general, the operations were gross total; no cases with only biopsy were included. The study was approved by the Ethical Committee of the Karolinska Hospital (No. 91:16), Sahlgrenska University Hospital (S339:01) and Cambridge Research Ethics Committee, Cambridge, UK (NRES Cambridgeshire 2 REC reference: 03/115). The following 363 tumors were included in the study: 22 astrocytoma malignancy grade II (prefixed as A), 61 anaplastic astrocytoma malignancy grade III (AA), 183 glioblastoma malignancy grade IV (GB), 34 O, 20 AO, 20 OA, and 23 AOA.
Microsatellite Analysis
Microsatellite analysis (MSA) was performed as described.10 The 32 microsatellite markers used are listed in Supplementary Material, Table S1.
Array-CGH
The 1-Mb array and the 2 versions of the chromosome 1 tile-path array (versions 1 and 2, which contain chromosome 1 tile-path clones and 5-Mb clones only) have been described.6,11 Over the course of this study, 2 versions of the chromosome 19 tile-path array were constructed using the method previously described.6 The first version contains 347 overlapping bacterial artificial chromosome (BAC) clones that cover 68.6% of the euchromatic region of chromosome 19. The second version contains an additional 121 overlapping fosmids that cover 19q13. In addition, an array combining chromosomes 1 and 19 tile path BAC/P1-derived artificial chromosome clones as well as overlapping fosmid clones covering 19q13 (121 fosmids, same as above), pericentromeric regions of 1p (111 fosmids) and 19q (80 fosmids) were also constructed in order to further analyze the centromeric regions of 1p and 19q. A summary of the tile path–array experiments (the number of clones included and cases studied) is in Supplementary Material, Table S2.
A custom oligonucleotide array was designed and manufactured using the eArray system (Agilent Technologies, Santa Clara, California, https://earray.chem.agilent.com/earray/). All available high-density probes for the pericentromeric regions of chromosomes 1 and 19 (chr1: 120 000 126–144 003 112 and chr19: 22 502 407–33 799 497) available at the time of manufacturing were included in duplicate. In addition, selected regions from chromosomes 2, 3, and 5 were included for normalization.
Labeling, hybridization, and data acquisition for all BAC arrays and custom oligonucleotide arrays were performed essentially as described.6 For analysis, the linear ratios of the background-subtracted signal intensities were normalized against the median ratio of either the 1-Mb clones (1-Mb array), 5-Mb clone (tile-path array), or probes from selected regions of chromosomes 2, 3, and 5 (custom oligonucleotide array), averaged between the duplicates, and log2-transformed. The clone/probe positions and the normalized log2 ratios for all tile path and custom oligonucleotide arrays are shown in Supplementary Material, Table S3.
SNP Array
Human CNV370 Genotyping BeadChips (Illumina, San Diego, California) were used to genotype-selected cases according to the manufacturer's recommendations. Briefly, 750 ng of genomic DNA was amplified, fragmented, and hybridized to the array. Products were fluorescently labeled and scanned with the Illumina Beadstation scanner. Raw data were then uploaded in the Beadstudio v3.2 genotyping software (Illumina) for further analysis. First, genotype calls were generated and copy number values and loss of heterozygosity (LOH) were calculated. Then, using the Genome Viewer tool, the log2 R ratio (a measure of the signal intensity at a given locus) and B allele frequency (an estimate of the ratio between the signals of the 2 alleles for each SNP) were examined to locate chromosomal regions with allelic imbalance.
Multiplex PCR
Multiplex PCR was optimized and performed as previously described.6 All primers are listed in Supplementary Material, Table S1.
Fluorescent In Situ Hybridization
Formaldehyde-fixed paraffin-embedded tumor tissues were sectioned (4 µm), deparaffinized, and pretreated with 10 mM citric acid at 80°C for 2 hours followed by 0.1% pepsin at 37°C. A total of 6 clones, that is, RP4-656M7 (1p12, telomeric to Notch homolog 2 [NOTCH2]), RP11-453B6 (1p11.2, most centromeric BAC), G248P85854G8 (1p11.2), RP11-533N14 (1q21.1), RP11-26D24 (19p12), and RP11-296C17 (19q12) were used for the fluorescent in situ hybridization (FISH) experiments. Clone DNAs were amplified using the Templiphi Amplification Kit (GE Healthcare, Little Chalfont, UK) and labeled with either spectrumgreen- or spectrumorange-coupled dUTP using a Nick Translation Kit (Abbott Molecular, Maidenhead, UK) as per the manufacturer's instruction. The probes were then hybridized to either normal human metaphase chromosomes or pretreated tumor sections for 3 days at 37°C. They were then washed and counter stained with DAPI. Signals were counted in 100 nuclei that had at least 1 signal for each probe.
Statistical Analysis
Statistical analyses were performed using Statistical Package for Social Sciences version 16.0. The log-rank test was used for univariate analysis and Cox regression for multivariate analysis of the potential association between 1p/19q status and the patients' overall survival.
Results
Patterns of Chromosome 1/19 Alterations in Gliomas
A total of 363 gliomas (see Materials and Methods) were analyzed using a 1-Mb array. In this study, we focus on copy number alterations on chromosomes 1 and 19 alone. A detail of the 1-Mb array data for the whole genome will be published elsewhere (Chan, manuscript in preparation).
The patterns of 1p and 19q copy number status were determined and categorized as follows: total 1p loss or total 19q loss was judged when all 1p or 19q clones showed clear evidence of deletion, and the pericentromeric clones of 1q or 19p showed no signs of deletion (eg, monosomy 1 or 19 was not included in this category). Deletion of 1p36 was judged when a terminal or interstitial deletion was limited to any part of 1p36 (telomeric to and including RP1-159A19). Partial deletion of 19q13 was called in a similar manner when any part of 19q13 (telomeric to and including RP11-79M11), but not all of 19q was deleted. All other abnormalities including monosomy or trisomy were classified as other abnormalities.
A summary of the findings for all 363 tumors is provided in Supplementary Material, Table S4. In summary, when the status of 1p/19q in the individual tumors were compared, concurrent total 1p loss and total 19q loss (total 1p/19q loss) were observed in 54 tumors (3/22 A, 3/61 AA, 3/183 GB, 26/34 O, 12/20 AO, 1/20 OA, 6/23 AOA). Two tumors (1 AA and 1 AOA) had total 1p loss and deletion of a portion of but not the entire 19q region (partial 19q loss). Two tumors (2 GB) had total 1p loss and normal 19q. Six tumors (2 AA, 2 GB, and 2 AOA) had normal 1p and total 19q loss. No secondary GB had either total 1p loss, total 19q loss, or 1p36 deletion. Total 1p loss and total 19q loss were strongly associated (P < .001, χ2 test). There were 11 tumors (3 AA, 4 GB, and 4 AOA) that had either total 1p or 19q loss, but not both.
Thus, the predominant patterns of 1p and 19q copy number changes among astrocytic tumors involved partial deletions of one or both chromosomes, most often involving 1p36 or 19q13, while total 1p loss and/or total 19q loss were uncommon. In contrast, the great majority of 1p/19q copy number changes in oligodendroglial tumors were the concurrent total 1p/19q loss, with partial deletions of 1p36 or 19q13 being rare. They were also examined by MSA using up to 32 markers on chromosome 1 when the patients' constitutional DNA was available. Array-CGH and MSA findings correlated well, with array-CGH showing loss or gain where MSA indicated allelic imbalance. There was no evidence of copy number neutral LOH on chromosome 1.
Patients' Survival and 1p/19q Status
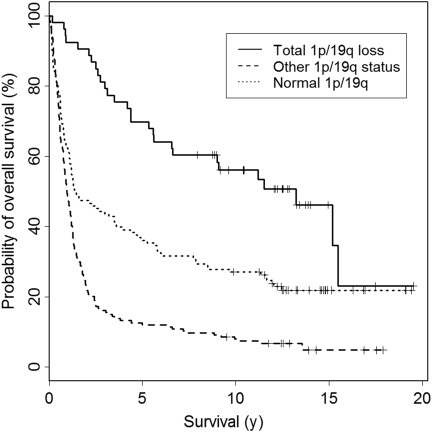
We then tested the patients' overall survival in days calculated from the date of operation for potential association with the 1p/19q status. Only adult patients (16 years of age or older, in total 337 patients) were included in this analysis. The median follow-up of the patients was 4803 days (13.2 years) ranging between 2903 and 7306 days (8.0 and 20.0 years). The 1p/19q status was grouped into the following 3 categories: normal 1p and normal 19q, combined total 1p/19q loss, and any other 1p or 19q status (eg, partial deletion of either or both chromosomal arms) as defined above. In the univariate analysis of adult patients of all diagnoses, patients with total 1p/19q loss showed significantly longer overall survival compared with those with normal 1p/19q, whereas patients with other 1p/19q status had significantly shorter survival than those with normal 1p/19q status (Table 1 and Fig. 1, P < .001). Multivariate analysis including 1p/19q status, age, and histopathological diagnosis for all cases showed that 1p/19q status was an independent prognostic factor (P = .041, Table 1). When tumors of each histological subtype were studied separately, the 1p/19q status was an independent prognostic factor for GBs, but not for the other subtypes. When survival was compared among the patients with tumors that had total 1p/19q loss, WHO grade III and IV tumors tend to have shorter survival when compared with WHO grade II tumors, although the difference was not significant.
Table 1.
Overall survival and the 1p/19q status
| Normal 1p/19q |
Total 1p/19q loss |
Other 1p/19q status |
Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Overall survival | 95% CI | No | Overall survival | 95% CI | No | Overall survival | 95% CI | |||
| A | 7 | 1311 | 1296–1326 | 3 | 4208 | 4021–4395 | 6 | 2627 | 689–4565 | ns | ns |
| AA | 27 | 1730 | 1157–2303 | 3 | nr | nr | 27 | 704 | 580–828 | ns | ns |
| GB | 50 | 218 | 175–261 | 3 | 1091 | 0–2374 | 128 | 280 | 223–337 | 0.002 | 0.004 |
| O | 6 | 4400 | 2328–6472 | 25 | 4832 | 4211–5453 | 0 | — | — | ns | ns |
| AO | 2 | 829 | nr | 12 | 2051 | nr | 2 | 964 | nr | ns | ns |
| OA | 13 | nr | nr | 1 | nr | nr | 2 | nr | nr | ns | ns |
| AOA | 8 | 766 | 0–1889 | 6 | 1596 | 223–2969 | 6 | 707 | 0–1500 | ns | ns |
| Total | 113 | 432 | 314–550 | 53 | 4407 | 2961–5853 | 171 | 346 | 285–407 | <0.001 | 0.041 |
Only patients 16 years of age or older at the time of operation were subjected to survival analysis. Total 1p/19q loss, concurrent total 1p and 19q loss. Other, any other copy number abnormality on 1p and/or 19q. Multivariate analysis, P value for the 1p/19q status in the multivariate model including age and diagnosis. Overall survival in days. A, diffuse astrocytoma; AA, anaplastic astrocytoma; GB, glioblastoma; O, oligodendroglioma grade II; AO, anaplastic oligodendroglioma; OA, oligoastrocytoma; AOA, anaplastic oligoastrocytoma;ns, not significant (P > .100). nr, “not reached” due to more patients alive than deceased.
Fig. 1.
A Kaplan–Meier curve for overall survival of all adult (16 years of age or older) glioma patients divided into 3 subgroups according to the chromosome 1 and 19 status (see Results for definition). The survival differences between the 3 subgroups were statistically significant in univariate (P < .001) and multivariate (P = .041) analyses with total 1p/19q loss being associated with the longest survival.
Regions of Chromosome 1 Copy Number Abnormalities
We then studied the regions of copy number abnormalities on chromosomes 1 and 19 using BAC chromosomal tile-path arrays. The number of cases examined by each array-CGH platform is summarized in Supplementary Material, Table S2.
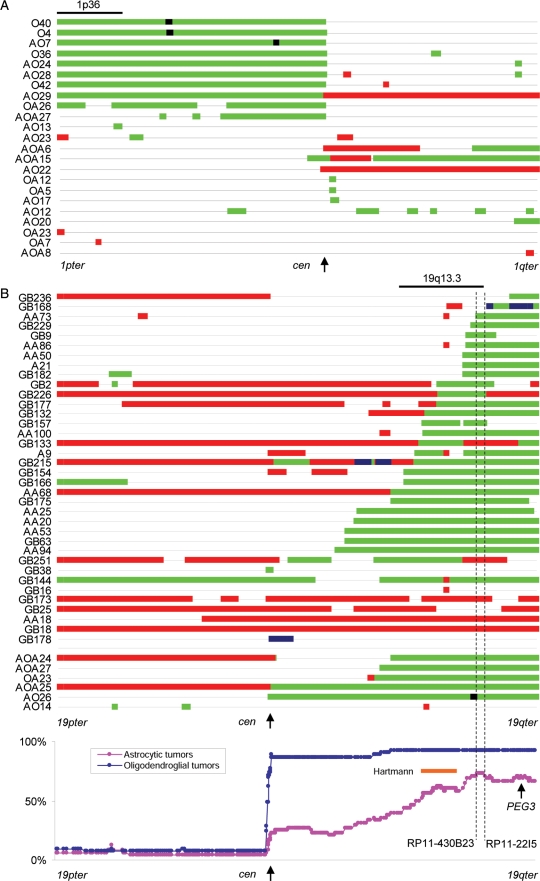
In total, 222 tumors were examined by chromosome 1 tile path array. These include 108 astrocytic tumors we have previously reported, in which we found that small hemizygous or homozygous deletions (HDs) limited to 1p36.22–p36.23 were predominant chromosome 1 abnormalities in these tumors.6 Analysis of 96 oligodendroglial tumors showed that in total 23 tumors had chromosome 1 copy number alterations other than total 1p deletion. Of these, 6 tumors had one or more interstitial deletions on 1p (OA26, AO13, AO23, AO12, AOA15, AOA27; Fig. 2A). These interstitial 1p deletions did not overlap with the commonly deleted region at 1p36.22–1p36.23 reported in astrocytic tumors6 except in the case of OA26, which had 3 large individual regions of deletion on 1p (Fig. 2A). Copy number gains on chromosome 1 were found in 10 tumors. No common recurrent breakpoints for copy number changes were observed. Thus, the patterns of 1p deletion in oligodendroglial tumors contrasts with the data we previously reported for the astrocytic tumors.6
Fig. 2.
(A) A diagram of copy number abnormalities on chromosome 1. Tumors with total 1p deletion as a sole chromosome 1 copy number change are not shown. Copy number status is schematically represented along chromosome 1 (1pter on the left to 1qter on the right) by color-coded bars as follows: black, HD; green, hemizygous deletion; red, copy number gain. (B) A diagram of copy number abnormalities on chromosome 19. Monosomy 19, trisomy 19, or tumors with total 19q deletion as the sole chromosome 19 copy number change are not shown. Copy number status is schematically represented along chromosome 19 (19pter on the left to 19qter on the right) by color-coded bars as follows: black, HD; green, hemizygous deletion; red, copy number gain; blue, amplification. The bottom panel shows the incidence of deletion at each clone plotted along chromosome 19 in astrocytic and oligodendroglial tumors. The minimal deleted region suggested by Hartmann et al.7 is indicated by an orange line. The most frequently deleted region in this study is defined as being between RP11-430B23 and RP11-22I5 (indicated by dotted lines).
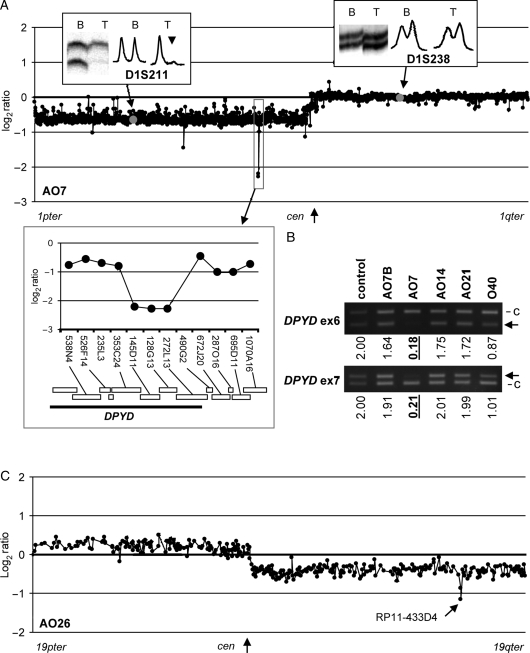
One anaplastic oligodendroglioma, AO7, showed HD at 3 consecutive clones (RP11-145D11, RP11-128G13, and RP11-212L13) on 1p21.3 on a background of total 1p/19q loss (Figs 2A and 3A). These 3 clones contained a part (at least exon 1–12) of the Dihydropyrimidine Dehydrogenase (DPYD) gene (Fig. 3A inset). Multiplex PCR at exons 6 and 7 of DPYD showed copy number values of 0.18 (exon 6) and 0.21 (exon 7), which are well below the theoretical value of a single copy (=1), confirming the HD (Fig. 3B).
Fig. 3.
(A) A chromosome 1 BAC tile path–array plot of AO7. Results of MSA at 2 markers (D1S211, allelic imbalance indicated by an arrow head; D1S238, allelic balance) are shown in insets. The clones harboring each marker are indicated by gray dots in the plot. The region of HD is indicated by a square and enlarged in inset showing locations of clones and genes (modified after Ensembl Genome Browser, http://www.ensembl.org/ Homo_sapiens). (B) Multiplex PCR at DPYD exons 6 and 7. The band representing the target locus is indicated by an arrow in each panel. c, control locus (WI-3306, see Materials and Methods). Calculated copy numbers (normal = 2) are indicated below each lane. HDs (copy number < 0.6) are indicated by underlines. Control, DNA from normal blood; AO7B, blood DNA from AO7. AO14 and AO21 showed no evidence of deletion at this region in the chromosome 1 tile-path array-CGH, whereas O40 had deletion of 1p. The results consistent with the calculated copy number at these 2 exons. (C) A chromosome 19 BAC tile path–array plot of AO26. The homozygously deleted clone (RP11-433D4) is indicated by an arrow.
Two oligodendrogliomas, O4 and O40, had HDs at 3 clones in O4 (RP11-220M1, RP11-116M11, and RP11-218J17) and 4 clones in O40 (RP1-86A18, RP11-295K8, RP11-433N7, and RP11-220M1) in addition to the total 1p/19q loss. These 2 HDs were overlapping at single clone, RP11-220M1, which harbors the Fas (TNFRSF6) associated factor 1 (FAF1) gene and the cyclin-dependent kinase inhibitor 2C (CDKN2C/p18INK4C) gene. Multiplex PCR at exons 1 and 7 of FAF1 and exon 1 of CDKN2C confirmed HD (Supplementary Material, Fig. S1).
The patient's constitutional DNA from AO7, O4, and O40 showed normal copy number of chromosome 1 (data not shown), indicating that these HDs are somatic.
Regions of Chromosome 19 Copy Number Abnormalities
Among the 98 tumors (5 A, 14 AA, 28 GB, 23 O, 15 AO, 2 OA, and 11 AOA) studied by chromosome 19 tile-path array, 49 showed total 19q loss with or without 19p changes (3 A, 4 AA, 2 GB, 23 O, 11 AO, 1 OA, and 5 AOA), 35 partial 19q deletions (2 A, 9 AA, 18 GB, 2 AO, 1 OA, and 3 AO), 3 monosomy 19 (1 GB, 1 AO, and 1 AOA), and 6 other changes (1 AA and 5 GB). The great majority of the partial 19q deletions were overlapping (Fig. 2B). When the incidence of deletion at each clone was compared, an 885 kb region defined by RP11-430B23 and RP11-22I5 (56.78–57.67 Mb, 19q13.33–19q13.41) was most frequently deleted among all astrocytic tumors. GB9 had a single small interstitial deletion encompassing this region. This region was also affected by partial 19q deletions in 5 oligodendroglial tumors.
An anaplastic oligodendroglioma, AO26, showed somatic HD at 2 clones (RP11-10I11 and RP11-433D4) in addition to the total 19q loss (Fig. 2B). The result was reproducible in 2 independent hybridizations. This region does not contain any reported CNVs (http://projects.tcag.ca/variation) and hybridization of the matched blood DNA showed normal copy number status on chromosome 19 (data not shown). This region falls centromeric to the most frequently deleted region defined in this study (see above) and telomeric to that of others7 but does not overlap with either of them. A part of the kallikrein-related peptidase (KLK6-14) gene clusters as well as the sialic acid binding Ig-like lectin 7 (SIGLEC7) gene, SIGLEC9 and the ATP-binding domain 3 (ATPBD3) gene were mapped in this region.
Amplifications on chromosome 19 were found in 3 GBs (Supplementary Material, Fig. S2). GB168 showed multiple small amplicons in the terminal region of 19q. The amplicon with the highest amplitude (RP11-195O11 and RP11-135F15) encompassed a part of the ubiquitin carboxyl-terminal hydrolase 29 (USP29) gene. GB215 had a low-level amplicon that spans 3.85 Mb (RP11-81C10 and RP11-17M22) containing a number of genes including the v-akt murine thymoma viral oncogene homolog 2 (AKT2) gene. GB178 displayed an amplicon encompassing 2.28 Mb at 19q12 (RP11-197B9 to RP11-419O10). The cyclin E1 (CCNE1) gene is mapped to this region, among other protein coding genes.
Breakpoint Mapping in the Pericentromeric Regions of 1p/19q
To map potential breakpoints of the t(1;19) translocation, near-centromeric regions of 1p and 19q were examined using various microarray platforms including a regional fosmid tile-path array, custom oligonucleotide array (Agilent Technologies), and the CNV370 array (Illumina). Twenty-four tumors (10 O, 4 AO, 1 OA, 2 AOA, 3 A, 2AA, and 2 GB) with total 1p/19q loss were studied by a 1p/19q BAC/fosmid array. The region telomeric to the NOTCH2 gene (1p12) showed deletion in all cases, while the data from the region including the centromeric half of NOTCH2 showed a tendency for copy number retention. However, the exact borders of transition were difficult to determine (Supplementary Material, Figs S3 and S4). On 19q, there appeared to be various breakpoints between G248P8271B4 (the most centromeric clone) and G248P8287A5, the region corresponding to chr19:32423876–33037701. For example, some tumors showed copy number loss of the entire 1p or 19q pericentromeric regions (eg, AA34, Supplementary Material, Fig. S3), whereas others showed the patterns suggestive of copy number retention of the pericentromeric regions to varying extents (eg, AO28, Supplementary Material, Fig. S4).
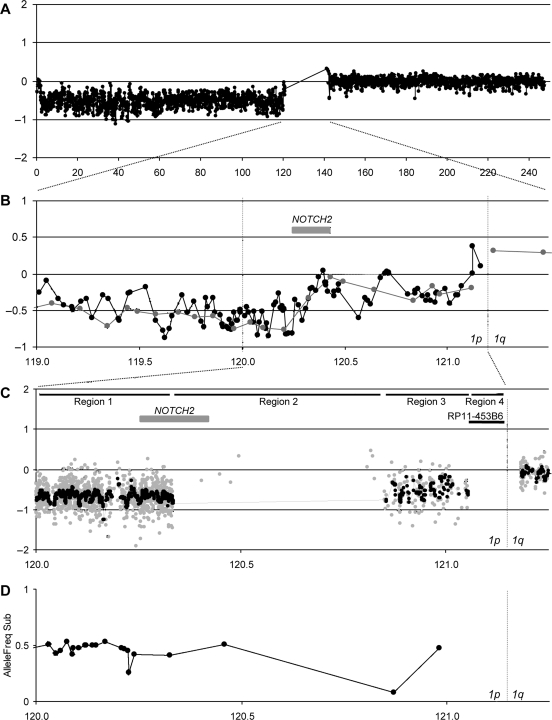
To further elucidate the copy number status in the near-centromeric region, 14 tumors with total 1p/19q loss were examined using a custom 1p/19q pericentromeric oligonucleotide microarray (see Materials and Methods). The region chr1:118994276–120333081 was covered by 1079 probes (average interval 309 bp, defined as Region 1 in Fig. 4C). The chr1:120333082–120851337 region on 1p12 is highly homologous to the chr1:143829712–144000384 region on 1q21 (Region 2 in Fig. 4C, see Discussion) and only 8 sparsely distributed specific probes were available. The chr1:120851338–121052453 region was covered by 154 probes (average interval 1314 bp, Region 3 in Fig. 4C). No unique sequence probes were available in the most centromeric region (chr1:121052454–121186957, Region 4 in Fig. 4C) due to the high prevalence of repetitive sequences. On 19q, the chr19:32929768–33799497 region was covered by 2145 probes (average interval 406 bp, Region 5 in Fig. 5C). The most centromeric region (chr19:32423623–32929767, Region 6 in Fig. 5C) almost entirely consists of repeat sequences and only a single probe was available. The availability of probes for these regions varied (see Materials and Methods). In all 14 tumors examined, the 1p/19q pericentromeric regions covered by a high density of probes showed clear sign of hemizygous deletions, including the 19q pericentromeric region, which had appeared to be retained in the fosmid array analyses (Figs 4C and 5C).
Fig. 4.
Copy number status of the 1p near-centromeric region in AO28. (A) Chromosome 1 BAC array of AO28 showing total 1p loss (see Results for definition). (B) A combined BAC/fosmid array of the 1p near-centromeric region. The corresponding region of (A) is indicated. Gray dots/line, BAC array; black dots/line, fosmid array. The definitions of the Regions (1–4) are described in the text. The position of NOTCH2 is indicated. (C) A custom oligonucleotide array plot of the 1p near-centromeric region. The corresponding region of (B) is indicated. Gray dots, normalized raw log2 ratio of each probe; black dots/line, the moving median of the normalized raw log2 ratio in a 5-kb window. The positions of NOTCH2 and RP11-453B6 (the most centromeric BACs clone used for FISH, see Fig. 6 and Supplementary Material, Fig. S6) are indicated. (D) The B allele frequency plot of the SNP array for the same region as (C). Allelic imbalance (LOH) appears as inconsistent B allele frequencies (BAFs) between the tumor and constitutional DNA. For the presentation, the absolute values of the difference of the BAFs between the tumor and the matched constitutional DNA are plotted only for the informative loci. The values around 0.5 indicate LOH, whereas those close to 0 indicate retention of heterozygosity. All informative loci except one show LOH in this region.
Fig. 5.
Copy number status of the 19q near-centromeric region in AO28. (A) Chromosome 19 BAC array of AO28 showing total 19q loss (see Results for definition). (B) A combined BAC/fosmid array of the 19q near-centromeric region. The corresponding region of (A) is indicated. Gray dots/line, BAC array; black dots/line, fosmid array. The definitions of the Regions (5–6) are described in the text. (C) A custom oligonucleotide array plot of the 19q near-centromeric region. The corresponding region of (B) is indicated. Gray dots, normalized raw log2 ratio of each probe; black dots/line, the moving median of the normalized raw log2 ratio in a 5-kb window. (D) The absolute values of the difference of the B allele frequency between the tumor and the matched constitutional DNA are plotted for the informative loci (see the legend for Fig. 4D). The same region as (C) is shown. All informative loci in the 19q pericentromeric region show LOH, whereas those in the 19p pericentromeric region retained heterozygosity.
In 6 tumors, LOH analysis was performed using an Illumina CNV370 SNP array. On 1p, only a limited number of probes were informative in the Regions 2–4 (centromeric to 120333082), where coverage by Agilent probes was insufficient (see earlier and Fig. 4C and D). The great majority of those loci showed LOH in tumors (with single isolated exceptions, see Fig. 4D). On 19q, a number of probes in the Region 6, where Agilent probes were not available, were informative. All of them showed LOH (Fig. 5D).
Interphase FISH was performed on 2 tumors (AO28 and AA34) that had total 1p/19q loss. The specimen used for FISH contained more than 90% of tumor cells. All 6 probes (see Materials and Methods) showed unique signals at the corresponding chromosomal locations when hybridized to normal metaphase chromosomes (Supplementary Material, Fig. S5). RP4-656M7 (located ∼200 kb telomeric to NOTCH2) showed a modal peak at 1 when compared with RP11-533N14 (1q21.1), which showed a modal peak at 2 in both tumors (left column in Fig. 6 and Supplementary Material, Fig. S5). This indicated that the region corresponding to RP4-656M7 was deleted, which is consistent with the array-CGH result. On the other hand, RP11-453B6, the most centromerically mapped BAC on 1p (Fig. 4C), showed a modal peak at 2 in these 2 tumors, suggesting that this region is retained (middle column in Fig. 6 and Supplementary Material, Fig. S5). G248P85854G8 (1p11.2), a fosmid clone that overlaps with RP11-453B6, also appeared to be retained (data not shown). RP11-296C17 (19q12), located most centromerically on 19q, indicated copy number loss, which is consistent with the oligonucleotide array results (right column in Fig. 6 and Supplementary Material, Fig. S5).
Fig. 6.
FISH results for AO28. Histograms of signals counted from 100 nuclei for each probe set (shown on the top) are shown.
Discussion
The Distinct Patterns of 1p/19q Alterations and Survival
Total 1p/19q loss has almost been considered as hallmark of oligodendrogliomas3,12 and is known to be associated with longer overall and progression-free survival in AOs and AOAs when treated by radiotherapy, with or without chemotherapy.4,5,13–15 The impact of the 1p/19q status in other types of glioma is less well documented. Only a few studies attempted to investigate the association of the 1p/19q status with patients' survival, and the results have not been consistent.8,14,16
The objectives of this study were to investigate the patterns of copy number changes on chromosomes 1 and 19 in diffuse astrocytic and oligodendroglial tumors, to define the targeted regions of these copy number changes, and to evaluate their prognostic value. Our findings show that astrocytic and oligodendroglial tumors generally have distinct patterns of 1p/19q copy number changes, with concurrent total 1p/19q loss being predominantly found among oligodendroglial tumors, whereas other types of chromosome 1 and 19 copy number alterations are rare in oligodendroglial tumors but are more common in astrocytic tumors. Our data show that total 1p/19q loss, but not other patterns of 1p/19q alterations, predicts longer overall survival in all adult astrocytic and oligodendroglial tumors combined, independent of diagnosis and patients' age. This was observed in each histopathological subtype, although the difference did not reach significance except among GBs (Table 1). These findings are in line with the notion that the tumors with a total 1p/19q loss have a distinct biology when compared with those without. However, we also found 9 tumors diagnosed as astrocytic with total 1p/19q loss. These tumors were reviewed histologically. They had been diagnosed as A (3 cases), AA (3 cases), and GB (3 cases). One of the AAs and 2 of the GBs were noted at review to have a small oligodendroglial component. This was not consistent with the tumors being classified as anaplastic oligoastrocytomas, which requires a conspicuous content of tumor cells resembling the tumor cells in an oligodendroglioma.3 However, this raises the issue of how representative of the whole tumor the material examined histologically was. It is conceivable that these 3 cases, while not fulfilling the WHO criteria for oligoastrocytomas really represent such tumors. The same is true for the other 6 cases. Thus, as only a small fraction of any glioma is histologically characterized, and considering the fact that this study showed a significant survival advantage for glioblastoma patients with total 1p and 19q loss, parallel determination of the status of 1p and 19q may provide more accurate prognostic data.
There is a discussion as to whether total 1p/19q loss is a prognostic marker in oligodendroglial tumor patients who do not receive adjuvant treatment.17 It has to be noted that the adjuvant radio- and/or chemotherapy given to the patients in this study was not uniform,18 in particular among the tumors of different pathological diagnosis. This made it difficult to incorporate adjuvant therapy in the survival analysis of this study.
Of note, all 3 As and 1 AA had total 1p/19q loss as the sole copy number change in the 1-Mb analysis, whereas 5 other tumors showed various genomic changes in addition to the total 1p/19q loss. These include 9p loss (3 GB), monosomy 10 (1 GB), and trisomy 7 (1 GB), and CDKN2A HD (1 GB), which are the type of abnormalities frequently observed in GBs without total 1p/19q loss (data not shown). It appears as if there is little difference in genomic profile between GBs with and without total 1p/19q loss, although the number of cases is small.
Methods and Loci for 1p/19q Testing
Our data has implications for the stratification of patients in clinical trials. The most commonly used methods to detect deletions of 1p and 19q are either FISH at a single locus on each chromosomal arm4,5 or LOH analysis using a few microsatellite markers.19 Although these methods are often technically more feasible in the routine clinical laboratories than array-CGH,20 they may not always distinguish partial and total deletions of 1p/19q. When the copy number status of 1p/19q in our tumor series was judged only at a single locus corresponding to the commonly used probes available from a commercial source (1p36.32 and 19q13.32, “virtual FISH”), 8 tumors (2 AA, 4 GB, 1 OA, and 1 AOA) that showed concurrent partial deletions of the 1p36/19q13 loci would have been misjudged as having a total 1p/19q loss (Supplementary Material, Fig. S6A). When the patients' survival of these 8 partial 1p36/19q13 deletion cases and the genuine total 1p/19q loss cases were compared, the patients with genuine total 1p/19q loss survived significantly longer than those with concurrent partial 1p/19q loss (univariate test P < .001, multivariate test with age and diagnosis P = .014, see also Supplementary Material, Fig. S6B), while the partial deletion cases showed a similar survival to cases with other 1p/19q status. Thus, these FISH probes would have wrongly identified 8 patients as potentially having a good prognoses. In order to accurately judge the total 1p/19q loss using FISH, probes for the loci suggested as follows may be used as either additional or alternative probes to those at 1p36.32/19q13.32.
Our array-CGH data indicated that the region between RP5-944F13 and RP11-25F16 on 1p (69.88–77.88 Mb, 1p31.1) and the region between RP11-298M15 and CTD-2527I21 on 19q (38.09–40.34 Mb, 19q13.12–q13.2) are seldom deleted among the 20 tumors in the series with a partial deletion of 1p and/or 19q (3 AA, 12 GB, 1 AO, 1 OA, 3 AOA, Supplementary Material, Fig. S6A). No partial 1p/19q deletion cases had deletions of these 2 regions at the same time. Therefore, FISH probes in these 2 regions should detect only genuine total 1p/19q loss. We therefore recommend the use of FISH probes from these 2 regions as alternative loci for the 1p/19q testing in a routine clinical laboratory. Although this needs to be validated in a larger study, this has a potential to serve as a reliable and practical alternative to the commonly used FISH probes or array-CGH in the routine laboratories.
Target Genes on Chromosome 1
In a previous study of 108 astrocytic tumors, we identified a minimal commonly deleted region on 1p36 as well as overlapping HD within this region in 9 GBs.6 In the current study, we examined 96 oligodendroglial tumors with a chromosome 1 BAC tile path array. Only 1 OA showed a partial deletion involving 1p36.23–p36.33, in addition to 2 other large interstitial deletions on 1p (1p36.11–p31.3 and 1p31.1–p12, Fig. 2A). No HD at 1p36 was found in any of the oligodendrogliomas or oligoastrocytomas. It appears that interstitial deletions involving 1p36 is an almost specific genetic change in astrocytic tumors.
On the other hand, we found somatic HD in more centromeric regions of 1p in 3 oligodendrogliomas (2 O and 1 AO). All 3 are associated with total 1p/19q loss (Fig. 2A). One of the HDs includes a part of the DPYD gene, which encodes dihydropyrimidine dehydrogenase (DPD). DPD is best known as an enzyme involved in the catabolism of 5-fluorouracil (5-FU). 5-FU treatment for the cancer patients heterozygous for the mutated allele may cause serious and often fatal toxicity due to partial deficiency of DPD. Individuals homozygous or compound heterozygous for mutations may manifest various symptoms such as convulsive disorders, motor, and/or mental retardation.21 In a small phase II study of 5-FU treatment in gliomas, 1 oligodendroglioma showed a complete radiological response, while no other types of glioma responded.22 Although the association between the sensitivity to 5-FU and the status of DPYD needs to be established, 5-FU may be effective in a subset of oligodendrogliomas with DPD deficit. The role of DPYD inactivation in tumorigenesis is less clear. A common fragile site FRA1E has been mapped to the 370 kb region within DPYD spanning intron 8–18, which overlaps with the HD in AO7.23 Whether the deletion of DPYD is involved in gliomagenesis or if it is a bystander effect due to the structural vulnerability of the region remains to be seen.
Another 2 HDs on 1p in our series involved CDKN2C/p18INK4C and FAF1. Mutations/HDs of CDKN2C/p18INK4C have been reported in AOs and GBs.24–27 p18INK4C belongs to the G1 cyclin-dependent kinase inhibitor family and has been show to suppress growth when reconstituted in the CDKN2C null glioma cells.26 A cooperative role of CDKN2C with CDKN2A in tumor suppression through a p16INK4A-E2F1-p18INK4C feedback circuit has been demonstrated, consolidating CDKN2C's role in gliomagenesis.26 FAF1 is a member of the Fas death-inducing signaling complex and enhances Fas-mediated apoptosis28 (references therein). It is plausible that loss of FAF1 may lead to the development of tumor by diminishing apoptosis. Altomare et al. found frequent deletions of FAF1 and down-regulation of the protein product in asbestos-induced malignant mesothelioma in Arf (+/ − ) mice. They suggested that loss of FAF1, which is a regulator of the NF-κB pathway, may contribute to tumorigenesis via aberrant NF-κB signaling.28 However, it is possible that CDKN2c, which is mapped in close proximity to FAF1, may also have been codeleted with FAF1 in their model. The significance of FAF1 loss in tumorigenesis remains to be clarified.
Target Genes/Regions on Chromosome 19
The target genes/regions of 19q deletion in gliomas have been elusive29 (references therein). An extensive LOH mapping of 19q using polymorphic markers in oligodendroglial and astrocytic tumors resulted in several commonly deleted regions and candidate tumor suppressor genes, including epithelial membrane protein 3 (EMP3),30 glucocorticoid receptor DNA binding factor 1 (GRLF1, also known as p190RhoGAP),31 zinc finger protein 296 (ZNF296, also known as ZNF342),32 and paternally expressed 3 (PEG3).33 No somatic mutations have been found in these genes.
The most commonly deleted region in astrocytic tumors defined in our study spans 885 kb (56.78–57.67 Mb), which lies telomeric to but not overlapping with the regions suggested by others (50.7–54.4 Mb, Fig. 2B).29 This region contains at least 34 genes, many of which belong to the members of the Krüppel-type zinc finger transcription factor proteins. Chromosome 19 harbors 266 of ∼800 total human genes of this family located within 11 clusters.34 The commonly deleted region defined in our study overlaps with 2 of the clusters.
The reason for inconsistencies in the proposed target regions of 19q deletions is unclear. The partial deletions of 19q in many of our astrocytic tumors span large regions, including the regions suggested by others (Fig. 2B). In a study of 141 gliomas using an SNP array, Beroukhim et al. identified a large region of loss at 19q13.41, which centered at 55.2 Mb in 31% of cases. Their analysis of the data sets from 2 other SNP studies of gliomas35,36 also found a similar region of frequent loss at 19q13.41 (36%) centered at 55.3 Mb. This region falls between our region and that proposed by Hartmann et al.7 The large number of genes/transcripts contained in these regions poses a challenge for anyone who wishes to identify the target gene(s).
Chromosome 19 has a high density of repeat elements, most notably the Alu repeat, which is known for its association with chromosomal rearrangements.34,37 Chromosome 19 is also known for its high prevalence of duplication structures of 2 types, one with tandemly clustered gene families and the other with large intrachromosomal segmental duplications.34 It is possible that such sequence characteristics of chromosome 19 may predispose to chromosomal rearrangements, resulting in complex patterns of deletions.
One anaplastic oligodendroglioma (AO26) had an HD in the region containing a part of the tissue kallikrein gene clusters (KLK6-14) as well as SIGLEC7, SIGLEC9, and ATPBD3 (Fig. 3C). The tissue KLK gene family consists of 15 genes that share considerable sequence similarities, all of which are clustered at 19q13.33 (56.01–56.28 Mb), all encoding tissue-specific serine proteases38 (references therein). KLK3, also known as PSA, is a well-established biomarker for prostate cancer. KLK6-9 and 14 are preferentially expressed in central nervous system. Down-regulation of several members of the KLK family has been reported in a subset of breast cancers. SIGLECs are transmembrane proteins that are predominantly expressed on the cell surface of immune cells and considered to regulate their functions.39 Their potential role in carcinogenesis is unclear. ATPBD3 (ATP-binding domain protein 3) forms a complex with urm1 and is involved in tRNA modification.40 The significance of these genes in oligodendrogliomas is unknown.
Two amplicons on 19q found in this study are noteworthy. GB215 showed low-level amplification of AKT2, a gene encoding a serine/threonine kinase which is involved in the phosphatidylinositol 3-kinase (PI3K) pathway. AKT2 is an oncogene known to be amplified in a variety of cancers including ovarian cancers.41 As GB215 has no mutation or HD of PTEN, AKT2 may serve as an alternative mechanism to disrupt the PI3K pathway. GB178 had an amplicon including CCNE1. Cyclin E1 is a G1 cyclin that is involved in the G1/S progression of the cell cycle (RB1 pathway). GB178 has no mutations/HD/amplification at CDKN2A, RB1, or CDK4, while it harbors a TP53 mutation.9 It appears that the amplification of CCNE1 may be an alternative way of disrupting the RB1 pathway, although it occurs infrequently.
Breakpoint Mapping of Putative t(1;19) Translocation
We attempted to locate the putative breakpoints of the t(1;19) translocation by identifying those associated with total 1p/19q loss using the combination of the BAC/fosmid array, oligonucleotide array, SNP array, and FISH. NOTCH2 is mapped on 1p12, close to the centromere. NOTCH signals are involved in nervous system development.42 NOTCH2 amplification has been found in some medulloblastoma/PNETs, and introduction of a truncated form of NOTCH2 enhanced the proliferation of a medulloblastoma cell line.43 These findings make NOTCH2 an attractive candidate for the target of the t(1;19) translocation.16 Our BAC/fosmid array analysis showed a pattern suggesting copy number retention in the region both at and centromeric to the NOTCH2 gene. However, the oligonucleotide arrays indicated copy number loss in the region centromeric to NOTCH2. Similar findings using custom BAC and oligonucleotide arrays have recently been reported.16,44 This discrepancy is most likely due to the cross-hybridization of the BAC/fosmid probes to the NOTCH2NL region on 1q, which contains a 165 kb inverse duplication of the NOTCH2 region.45 Such cross-hybridization could give rise to a false copy number retention pattern by the BAC/fosmid array. Only unique sequence probes were included in our oligonucleotide arrays, and as a consequence there were no probes covering these homologous regions (Fig. 4C). The probes centromeric to the NOTCH2-homologous region (154 probes) detected deletions, indicating that the breakpoints on 1p were centromeric to those probes and did not involve NOTCH2. In line with this, Boulay et al.46 studied exon 1 of NOTCH2 in oligodendrogliomas and found loss of one copy. Our FISH analysis using the most centromerically mapped BAC probe on 1p (a region further centromeric to the most unique centromeric oligonucleotide probe available, Fig. 4C) suggested that this region may be retained. However, because of the high prevalence of alpha-satellite repeats in this region, this result needs to be interpreted with caution. Boulay et al.46 has reported the presence of intragenic HDs within NOTCH2 in oligodendrogliomas, although the presence of a truncated transcript has never been demonstrated. Mutations of NOTCH2 have been reported in several cancers, including lymphomas and a glioma cell line.47,48 Benetkiewicz et al.44 examined sequences of all exons of NOTCH2 in 4 oligodendrogliomas and found no mutations.
The fosmid tile-path array data on 19q indicated normal copy number in various parts of the 19q centromeric region (chr19: 32423876–33037701, see Results) in several oligodendrogliomas (Fig. 5B). Benetkiewicz et al.44 used a high-density oligonucleotide array and reported similar findings. However, our SNP array analysis showed LOH in this region (Fig. 5D). SNP array analysis is less likely to be influenced by the presence of repetitive sequences than array-CGH where the results are entirely dependent on the specificity of hybridization. Our FISH experiments in selected cases confirmed the SNP array data (Fig. 6). The discrepancy may be due to the fact that more than 99% of the sequence in this region of 19q consists of repetitive elements, mainly of alpha-satellite repeats (www.repeatmasker.org). These repetitive sequences could cause cross-hybridization that gives rise to an incorrect apparent copy number retention pattern as has been observed for the 1p centromeric region. No known protein-coding genes are mapped in this region. We suggest, from our study and others, that the breakpoints of t(1;19)(q10;p10) are likely to be located within the centromeric–pericentromeric regions of 1p/19q, not disrupting any protein-coding genes known so far, including NOTCH2. The role of NOTCH2 in the development of oligodendrogliomas as a target of other genetic alterations however requires further investigation.
The identification of the t(1;19)(q10;p10) translocation caused a paradigm shift from the conventional tumor suppressor gene theory to the idea of an oncogenic fusion gene as a molecular target of this abnormality. However, the findings we and others have observed so far make the presence of such a fusion gene unlikely. The presence of families that carry constitutional balanced t(1;19) translocations provides an intriguing addition to the story (Vazquez-Cardenas et al.49 and references therein). The members of these families do not appear to have any phenotypical abnormalities associated with the translocation. The breakpoints of those constitutional balanced t(1;19) translocations are within the centromere of chromosomes 1 and 19. These data suggest that the disruption of chromosomes 1 and 19 centromeres alone may not necessarily be pathogenic. Taken together, the possibility still remains that there may be a gene(s) on 1p and/or 19q targeted by the loss of 1p/19q as a result of unbalanced t(1;19) translocation, which is involved in the pathogenesis of these tumors. Mukasa et al.50 compared expression profiles between oligodendroglial tumors with and without 1p/19q LOH and found that nearly 60% of the genes whose expressions were significantly decreased in tumors with 1p LOH were located on chromosomes 1 or 19. The reduced expression possibly due to either haploinsufficiency50 or loss of the unmethylated allele in imprinted genes (as exemplified in DIRAS3 on 1p3151) in one or more of the genes on 1p/19q may be involved in the pathogenesis of tumors with this abnormality.
Our study indicated that the presence of total 1p/19q loss is associated with longer survival in adult gliomas independent of pathological diagnosis. Thus, the biological significance of t(1;19) is unquestionable. Currently, however, all options are still open regarding its molecular targets. The search for the target(s) of t(1;19)—or 1p/19q loss—continues.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online.
Funding
This work was supported by grants from Cancer Research UK, Samantha Dickson Brain Tumour Trust, Jacqueline Seroussi Memorial Foundation for Cancer Research, and Cambridge Fund for the Prevention of Disease (CAMPOD).
Acknowledgments
We would like to thank the Mapping Core, Map Finishing, and Microarray Facility groups of the Wellcome Trust Sanger Institute, Hinxton, UK, for initial clone supply and verification, Rachel Cooper for technical assistance in the version 1 chromosome 1 array production, the Cambridge Genomic Services in the Department of Pathology, University of Cambridge, for printing of the arrays.
Conflict of interest statement. None declared.
References
- 1.Griffin CA, Burger P, Morsberger L, et al. Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65:988–994. doi: 10.1097/01.jnen.0000235122.98052.8f. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. 4th. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 4.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 5.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 6.Ichimura K, Vogazianou AP, Liu L, et al. 1p36 is a preferential target of chromosome 1 deletions in astrocytic tumours and homozygously deleted in a subset of glioblastomas. Oncogene. 2008;27:2097–2108. doi: 10.1038/sj.onc.1210848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann C, Johnk L, Kitange G, et al. Transcript map of the 3.7-Mb D19S112–D19S246 candidate tumor suppressor region on the long arm of chromosome 19. Cancer Res. 2002;62:4100–4108. [PubMed] [Google Scholar]
- 8.Idbaih A, Marie Y, Pierron G, et al. Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol. 2005;58:483–487. doi: 10.1002/ana.20607. [DOI] [PubMed] [Google Scholar]
- 9.Ichimura K, Bolin MB, Goike HM, Schmidt EE, Moshref A, Collins VP. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res. 2000;60:417–424. [PubMed] [Google Scholar]
- 10.Ichimura K, Schmidt EE, Miyakawa A, Goike HM, Collins VP. Distinct patterns of deletion on 10p and 10q suggest involvement of multiple tumor suppressor genes in the development of astrocytic gliomas of different malignancy grades. Genes Chromosomes Cancer. 1998;22:9–15. doi: 10.1002/(sici)1098-2264(199805)22:1<9::aid-gcc2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.McCabe MG, Ichimura K, Liu L, et al. High-resolution array-based comparative genomic hybridization of medulloblastomas and supratentorial primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 2006;65:549–561. doi: 10.1097/00005072-200606000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrey LE, Louis DN, Paleologos N, et al. Survey of treatment recommendations for anaplastic oligodendroglioma. Neuro-Oncology. 2007;9:314–318. doi: 10.1215/15228517-2007-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 14.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 15.Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol. 2006;24:4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 16.Idbaih A, Marie Y, Lucchesi C, et al. BAC array CGH distinguishes mutually exclusive alterations that define clinicogenetic subtypes of gliomas. Int J Cancer. 2008;122:1778–1786. doi: 10.1002/ijc.23270. [DOI] [PubMed] [Google Scholar]
- 17.Weller M, Berger H, Hartmann C, et al. Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res. 2007;13:6933–6937. doi: 10.1158/1078-0432.CCR-07-0573. [DOI] [PubMed] [Google Scholar]
- 18.Backlund LM, Nilsson BR, Goike HM, et al. Short postoperative survival for glioblastoma patients with a dysfunctional Rb1 pathway in combination with no wild-type PTEN. Clin Cancer Res. 2003;9:4151–4158. [PubMed] [Google Scholar]
- 19.Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res. 2001;7:839–845. [PubMed] [Google Scholar]
- 20.Yip S, Iafrate AJ, Louis DN. Molecular diagnostic testing in malignant gliomas: a practical update on predictive markers. J Neuropathol Exp Neurol. 2008;67:1–15. doi: 10.1097/nen.0b013e31815f65fb. [DOI] [PubMed] [Google Scholar]
- 21.van Kuilenburg AB, Haasjes J, Richel DJ, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705–4712. [PubMed] [Google Scholar]
- 22.Stewart DJ, Dahrouge S, Soltys K. A phase II study of 5-fluorouracil plus folinic acid in malignant gliomas in adults. J Neurooncol. 1995;23:249–252. doi: 10.1007/BF01059957. [DOI] [PubMed] [Google Scholar]
- 23.Hormozian F, Schmitt JG, Sagulenko E, Schwab M, Savelyeva L. FRA1E common fragile site breaks map within a 370kilobase pair region and disrupt the dihydropyrimidine dehydrogenase gene (DPYD) Cancer Lett. 2007;246:82–91. doi: 10.1016/j.canlet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Husemann K, Wolter M, Buschges R, Bostrom J, Sabel M, Reifenberger G. Identification of two distinct deleted regions on the short arm of chromosome 1 and rare mutation of the CDKN2C gene from 1p32 in oligodendroglial tumors. J Neuropathol Exp Neurol. 1999;58:1041–1050. doi: 10.1097/00005072-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Pohl U, Cairncross JG, Louis DN. Homozygous deletions of the CDKN2C/p18INK4C gene on the short arm of chromosome 1 in anaplastic oligodendrogliomas. Brain Pathol. 1999;9:639–643. doi: 10.1111/j.1750-3639.1999.tb00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedemeyer R, Brennan C, Heffernan TP, et al. Feedback circuit among INK4 tumor suppressors constrains human glioblastoma development. Cancer Cell. 2008;13:355–364. doi: 10.1016/j.ccr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLendon R, Network CGAR, McLendon RFA, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altomare DA, Menges CW, Pei J, et al. Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc Natl Acad Sci USA. 2009;106:3420–3425. doi: 10.1073/pnas.0808816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann C, Mueller W, von Deimling A. Pathology and molecular genetics of oligodendroglial tumors. J Mol Med. 2004;82:638–655. doi: 10.1007/s00109-004-0565-9. [DOI] [PubMed] [Google Scholar]
- 30.Kunitz A, Wolter M, van den Boom J, et al. DNA hypermethylation and aberrant expression of the EMP3 gene at 19q13.3 in Human Gliomas. Brain Pathol. 2007;17:363–370. doi: 10.1111/j.1750-3639.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf RM, Draghi N, Liang X, et al. p190RhoGAP can act to inhibit PDGF-induced gliomas in mice: a putative tumor suppressor encoded on human chromosome 19q13.3. Genes Dev. 2003;17:476–487. doi: 10.1101/gad.1040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong C, Bollen AW, Costello JF. The contribution of genetic and epigenetic mechanisms to gene silencing in oligodendrogliomas. Cancer Res. 2003;63:7600–7605. [PubMed] [Google Scholar]
- 33.Trouillard O, Aguirre-Cruz L, Hoang-Xuan K, Marie Y, Delattre JY, Sanson M. Parental 19q loss and PEG3 expression in oligodendrogliomas. Cancer Genet Cytogenet. 2004;151:182–183. doi: 10.1016/j.cancergencyto.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Grimwood J, Gordon LA, Olsen A, et al. The DNA sequence and biology of human chromosome 19. Nature. 2004;428:529–535. doi: 10.1038/nature02399. [DOI] [PubMed] [Google Scholar]
- 35.Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66:11502–11513. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 36.Kotliarov Y, Steed ME, Christopher N, et al. High-resolution global genomic survey of 178 gliomas reveals novel regions of copy number alteration and allelic imbalances. Cancer Res. 2006;66:9428–9436. doi: 10.1158/0008-5472.CAN-06-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolomietz E, Meyn MS, Pandita A, Squire JA. The role of Alu repeat clusters as mediators of recurrent chromosomal aberrations in tumors. Genes Chromosomes Cancer. 2002;35:97–112. doi: 10.1002/gcc.10111. [DOI] [PubMed] [Google Scholar]
- 38.Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 39.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 40.Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci USA. 2008;105:18255–18260. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 42.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 43.Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 44.Benetkiewicz M, Idbaih A, Cousin PY, et al. NOTCH2 is neither rearranged nor mutated in t(1;19) positive oligodendrogliomas. PLoS ONE. 2009;4:e4107. doi: 10.1371/journal.pone.0004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory SG, Barlow KF, McLay KE, et al. The DNA sequence and biological annotation of human chromosome 1. Nature. 2006;441:315–321. doi: 10.1038/nature04727. [DOI] [PubMed] [Google Scholar]
- 46.Boulay JL, Miserez AR, Zweifel C, et al. Loss of NOTCH2 positively predicts survival in subgroups of human glial brain tumors. PLoS ONE. 2007;2:e576. doi: 10.1371/journal.pone.0000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SY, Kumano K, Nakazaki K, et al. Gain-of-function mutations and copy number increases of Notch2 in diffuse large B-cell lymphoma. Cancer Sci. 2009;100:920–926. doi: 10.1111/j.1349-7006.2009.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sivasankaran B, Degen M, Ghaffari A, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res. 2009;69:458–465. doi: 10.1158/0008-5472.CAN-08-2610. [DOI] [PubMed] [Google Scholar]
- 49.Vazquez-Cardenas A, Vasquez-Velasquez AI, Barros-Nunez P, Mantilla-Capacho J, Rocchi M, Rivera H. Familial whole-arm translocations (1;19), (9;13), and (12;21): a review of 101 constitutional exchanges. J Appl Genet. 2007;48:261–268. doi: 10.1007/BF03195221. [DOI] [PubMed] [Google Scholar]
- 50.Mukasa A, Ueki K, Matsumoto S, et al. Distinction in gene expression profiles of oligodendrogliomas with and without allelic loss of 1p. Oncogene. 2002;21:3961–3968. doi: 10.1038/sj.onc.1205495. [DOI] [PubMed] [Google Scholar]
- 51.Riemenschneider MJ, Reifenberger J, Reifenberger G. Frequent biallelic inactivation and transcriptional silencing of the DIRAS3 gene at 1p31 in oligodendroglial tumors with 1p loss. Int J Cancer. 2008;122:2503–2510. doi: 10.1002/ijc.23409. [DOI] [PubMed] [Google Scholar]