Abstract
Neurofibromin, the product of the Nf1 gene, is a guanosine triphosphatase activating protein (GAP) for p21ras (Ras) that accelerates conversion of active Ras-GTP to inactive Ras-GDP. Sensory neurons with reduced levels of neurofibromin likely have augmented Ras-GTP activity. We reported previously that sensory neurons isolated from a mouse model with a heterozygous mutation of the Nf1 gene (Nf1+/−) exhibited greater excitability compared with wild-type mice. To determine the mechanism giving rise to the augmented excitability, differences in specific membrane currents were examined. Consistent with the enhanced excitability of Nf1+/− neurons, peak current densities of both tetrodotoxin-resistant sodium current (TTX-R INa) and TTX-sensitive (TTX-S) INa were significantly larger in Nf1+/− than in wild-type neurons. Although the voltages for half-maximal activation (V0.5) were not different, there was a significant depolarizing shift in the V0.5 for steady-state inactivation of both TTX-R and TTX-S INa in Nf1+/− neurons. In addition, levels of persistent INa were significantly larger in Nf1+/− neurons. Neither delayed rectifier nor A-type potassium currents were altered in Nf1+/− neurons. These results demonstrate that enhanced production of action potentials in Nf1+/− neurons results, in part, from larger current densities and a depolarized voltage dependence of steady-state inactivation for INa that potentially leads to a greater availability of sodium channels at voltages near the firing threshold for the action potential.
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal dominant disease with an incidence of 1 in 3,000 people and is characterized by numerous abnormalities including neurofibromas (benign complex tumors composed of axonal processes, Schwann cells, and mast cells) as well as malignant peripheral nerve sheath tumors (MPNSTs), astrocytomas, and myeloid leukemias (Friedman 1999). Some people with NF1 also experience much more intense painful sensations to stimuli, such as minor injuries, than those without this genetic disorder (Creange et al. 1999; Wolkenstein et al. 2001). Although the mechanisms by which NF1 causes enhanced painful sensation have not been elucidated, it is likely that these abnormal painful states involve the sensitization of small diameter nociceptive sensory neurons.
NF1 results from a heterozygous mutation of the gene that encodes the protein, neurofibromin (NF1+/−). Neurofibromin is expressed abundantly in the nervous system (Daston et al. 1992). The high incidence of learning deficits and exaggerated painful responses (Creange et al. 1999; North et al. 1997; Ozonoff 1999; Trovo-Marqui et al. 2005) indicate that the single active NF1 allele does not generate sufficient functional neurofibromin to fulfill its normal biological role in the nervous system. Neurofibromin facilitates switching the active form of Ras (Ras-GTP) to its inactive form (Ras-GDP) by serving as a GTPase activating protein (GAP). Mutation of NF1 frequently results in enhanced basal and cytokine-stimulated Ras activity in many cell types, including sensory neurons. For example, investigators have shown that the level of Ras-GTP was elevated in MPNSTs and neurofibromas from humans with NF1 (Guha et al. 1996), in mast cells from mice with a heterozygous mutation of Nf1 (Nf1+/−) (Ingram et al. 2001), and in Schwann cells from embryonic mice with a homozygous mutation of Nf1 (Sherman et al. 2000). In addition, sensory neurons from embryonic Nf1−/− mice exhibit increased Ras activity (Klesse and Parada 1998; Vogel et al. 2000). Therefore it is reasonable to speculate that a higher level of Ras-GTP in adult sensory neurons with a heterozygous mutation of the NF1 gene could promote changes in neuronal function.
Previous evidence suggests that the Ras transduction cascade can modulate the activity of ion channels that could contribute to the generation of an action potential (AP). Fitzgerald and Dolphin (1997) demonstrated that microinjection of an activated K-Ras isoform enhanced the voltage-gated calcium current in neurons of the dorsal root ganglia (DRG) from neonatal rats. In addition, we reported previously that small diameter, capsaicin-sensitive sensory neurons isolated from Nf1+/− mice exhibited augmented excitability (Wang et al. 2005). To determine the mechanisms giving rise to the augmented neuronal firing in Nf1+/− neurons, the differences in specific membrane currents were examined in this study. It is possible that the heterozygous deletion of the NF1 gene somehow confers a change in channel activity that leads to increased excitability and may underlie the onset of enhanced painful sensation in people with NF1.
METHODS
Animals
Mice heterozygous for the Nf1 mutation on a background of C57BL/6J were originally developed by Dr. Tyler Jacks (Jacks et al. 1994). All animals were housed, bred, and had free access to food and water in the Indiana University Laboratory Animal Research Center and used in accordance with National Institute of Health Guide for Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80–23, revised 1996).
Isolation and maintenance of sensory neurons
Isolation of sensory neurons from young adult mice (1–2 mo of age) used the procedure developed by Lindsay (1988) with slight modification. The wild-type and Nf1+/− mice used in these studies were littermates. Briefly, male mice were killed by placing them in a CO2 chamber. The isolated spinal column was dissected, the spinal cord was removed, and the dorsal root ganglia (DRGs) were collected in a culture dish filled with sterilized Pucks solution composed of (in mM) 171 NaCl, 6.7 KCl, 1.6 Na2PO4, 0.5 KH2PO4, 6 d-glucose, and 0.01% phenol red, pH 7.3. The ganglia were transferred to a conical tube with Pucks solution containing papain (10 ng/ml). After 10–15 min incubation at 37°C, the ganglia were transferred to another conical tube with F-12 medium containing 1 mg/ml collagenase 1A and 2.5 mg/ml dispase. After 10- to 15-min incubation at 37°C, the tube was centrifuged for 30 s at low speed (∼2,000 × g) after which the enzyme-containing supernatant was removed. The pellet was resuspended in F-12 medium and mechanically dissociated with fire-polished pipettes until all obvious chunks of tissue were gone. Isolated cells were plated onto plastic cover slips that were previously coated with poly-d-lysine and laminin. The cells were maintained in F-12 medium that was supplemented with 10% horse serum, 2 mM glutamine, 100 μg/ml normocin, 50 μg/ml penicillin and streptomycin, 50 μM 5-fluoro-2′-deoxyuridine, and 150 μM uridine at 37°C and 3% CO2. The cells were used within 4–12 h for electrophysiological recordings. All procedures were approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
Electrophysiology
Recordings were made using the whole cell patch-clamp technique as previously described (Hamill et al. 1981; Wang et al. 2005). Briefly, a cover slip with the sensory neurons was placed in a recording chamber where the neurons were bathed in normal Ringers of the following composition (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH. Whole cell currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The whole cell recording configuration was established in normal Ringer. Both capacitance and series resistance compensation (typically 80%) were used. The membrane capacitance was read directly from the patch-clamp amplifier after performing the compensation for capacitance and series resistance. The data were acquired and analyzed using pCLAMP 9.0 (Molecular Devices). Results were not corrected for the liquid junction potential. Only neurons that maintained resting membrane potentials more hyperpolarized than −45 mV were used in this study. At the end of each recording, the neuron was superfused with normal Ringer containing 100 nM capsaicin as sensitivity to this agent is believed to be an indicator of nociceptive sensory neurons (Holzer 1991). Neurons were judged to be capsaicin sensitive if they depolarized in response to 100 nM capsaicin. However, the correlation between capsaicin sensitivity and whether a neuron is a nociceptor is not absolute. Some nociceptive neurons are insensitive to capsaicin, whereas some capsaicin-sensitive neurons are not nociceptors (see Petruska et al. 2000). Therefore this agent was used to define a population of small diameter sensory neurons that could serve a nociceptive function. The results reported here were obtained from capsaicin-sensitive neurons only. All experiments were performed at room temperature (∼23°C).
To isolate the potassium current (IK), neurons were superfused with a Ringer solution wherein NaCl was substituted with equimolar N-methyl-glucamine (NMG) chloride and was composed of (in mM) 140 NMG chloride, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with KOH. Recording pipettes were pulled from borosilicate glass tubing and typically had resistances of 2–5 MΩ when filled with the following solution (in mM): 140 KCl, 5 MgCl2, 4 ATP, 0.3 GTP, 2.5 CaCl2, 5 EGTA (calculated free Ca2+ concentration of ∼100 nM, MaxChelator), and 10 HEPES, adjusted pH at 7.3 with KOH. The membrane was held at −60 mV; this value was chosen so that current measurements could be ascertained at a voltage that reflected the normal resting potential in these sensory neurons. The data were acquired at 500 Hz. Leak subtraction was not used for the measurement of IK so that any effects on the holding current could be determined. Activation of IK was determined by voltage steps 300 ms in duration, which were applied at 5-s intervals in +10-mV increments from −80 to +60 mV. Steady-state inactivation of IK was measured by applying a 15-s conditioning prepulse (−100 to +20 mV in +20-mV increments) after which the voltage was stepped to +60 mV for 200 ms; a 20-s interval separated each acquisition. The fast inactivating IK known as IA (Connor and Stevens 1971; Neher 1971) was isolated by subtraction of the currents obtained for a conditioning prepulse to −40 mV from those obtained for a prepulse to −100 mV. Initially, a 4-s prepulse to −100 mV was applied followed by voltage steps of 500 ms that ranged from −80 to +40 mV in +20-mV increments at 15-s intervals. This was followed by an identical voltage protocol using a prepulse to −40 mV. IA was obtained by digital subtraction of these current traces as shown in Fig. 3. Inactivation of IA was determined by using a series of 4-s prepulses that ranged from −100 to −40 mV (+10-mV increments) that were immediately followed by a 200-ms step to −40 mV. The peak values of IK were determined isochronally. At the end of each recording, the neuron was exposed to 100 nM capsaicin.
Fig. 3.
The rapidly inactivating IA current in wild-type and Nf1+/− neurons is not different. Representative current traces for a wild-type neuron exhibiting IA are shown in A, top. The voltage protocol used to elicit the currents are shown below the traces; neurons were held at −100 mV, 500 ms steps were from −80 to +40 mV in 20 mV increments. B: representative current traces for the same neuron as in A except that a 4 s prepulse to −40 mV preceded the voltage steps. C: the A-type of IK obtained by the subtraction of the traces in B from those in A. The G/Gmax-voltage relations for activation and inactivation of IA are summarized in D. The results for activation were obtained from 14 wild-type and 11 Nf1+/− neurons; inactivation from 8 wild-type and 6 Nf1+/− neurons. The data points have been fitted by the Boltzmann relation and are shown as continuous lines.
To isolate the sodium current (INa), a Ringer solution composed of (in mM) 110 NaCl, 30 TEACl, 0.1 CaCl2, 5 MgCl2, and 10 HEPES, pH adjusted to 7.4 with HCl and TEAOH was used. The osmolality was adjusted to 300–310 mosM/l using glucose. The pipettes were filled with a Cs fluoride-based solution composed of (in mM) 110 CsF, 25 CsCl, 10 NaCl, 5 MgCl2, 4 ATP, 0.3 GTP, 1 CaCl2, 10 EGTA, 10 glucose, and 10 HEPES at pH 7.3 adjusted with CsOH. The data were acquired at 10 kHz and filtered at 5 kHz. Leakage currents were subtracted by using the P/4 protocol. Pipettes used for the recording of INa had an average resistance of 2.02 ± 0.04 MΩ (n = 20, range: 1.7–2.2 MΩ). The mean series resistance before compensation was 5.4 ± 0.4 MΩ (n = 20). For the peak values of TTX-R INa, the uncompensated series resistance gave a voltage error of approximately −2.2 ± 0.3 mV (n = 20). Activation of INa was determined by using a holding voltage at −100 mV with voltage steps of 30 ms applied at 10-s intervals in +5-mV increments from −80 to +40 mV; a 5-s interval separated each prepulse sweep. After obtaining the control recording of total INa, the superfusate was changed to Ringer containing 500 nM TTX and superfused for the appropriate times. TTX-S INa was obtained by digital subtraction of the current traces recorded before and after TTX treatment; these values were used to determine the current-voltage relation for activation. Steady-state inactivation was determined by applying a 200-ms conditioning prepulse that ranged from −100 to +10 mV in +10-mV increments after which the voltage was stepped to 0 mV for 30 ms; a 5-s interval separated each prepulse sweep. However, to determine the steady-state inactivation of TTX-S INa, the prepulse inactivation protocol described by Cummins and Waxman (1997) was used to rapidly separate TTX-S and TTX-R components. Briefly, the TTX-R INa obtained by using a prepulse (typically −35 to −40 mV) that selectively induced inactivation of TTX-S currents was digitally subtracted from the total INa obtained without the prepulse to yield the TTX-S INa.
Data analysis
All values represent the means ± SE. The voltage dependence for activation of IK or INa was determined by fitting the conductance-voltage curve with the Boltzmann relation wherein G/Gmax = 1/[1 + exp(V0.5 − Vm)/k], where G is the conductance [G = I/(Vm − ERev)], Gmax is the maximal conductance obtained from the Boltzmann fit under control conditions, V0.5 is the voltage for half-maximal activation, Vm is the membrane potential, and k is a slope factor. ERev is the reversal potential. For IK, the calculated EK value of −84 mV was used. For INa, ENa was determined for each neuron wherein the current values around the reversal potential were fit with a linear regression line to determine the voltage at which the current was zero. This reversal potential was used in the Boltzmann calculation. The average value for ENa for wild-type TTX-S was 45.5 ± 1.2 mV (n = 5) versus Nf1+/− 50.2 ± 3.4 mV (P = 0.25, Student's t-test) and for TTX-R, ENa for wild-type neurons was 42.9 ± 2.2 mV (n = 8) versus Nf1+/− 47.6 ± 1.9 mV (n = 9; P = 0.12 Student's t-test). The Boltzmann parameters were determined for each individual neuron and were used to calculate the means ± SE. Fits were performed using SigmaPlot 9.0 (Systat Software, San Jose, CA). To fit the inactivation curves, the Boltzmann relation G/Gmax = c + {(1 − c)/[1 + exp(V0.5 − Vm)/k]} was used where c is the fraction of noninactivating current. For IK, c is defined as the peak current obtained at +60 mV for the prepulse to +20 mV, whereas for INa, c is defined by the peak current obtained at 0 mV for the prepulse to +10 mV. The other parameters are as defined in the preceding text. Statistical differences between the control recordings and those obtained under various treatment conditions were determined by using either a Student's t-test or an ANOVA. When a significant difference was obtained with an ANOVA, post hoc analyses were performed using a Tukey test. Values of P < 0.05 were judged to be statistically significant.
Chemicals
Tissue culture supplies were purchased from Invitrogen (Carlsbad, CA). Papain was purchased from Worthington Biochemical (Lakewood, NJ), and dispase was obtained from Roche Diagnostics (Indianapolis, IN). All other chemicals were obtained from Sigma Chemical (St Louis, MO). Capsaicin was dissolved in 1-methyl-2-pyrrolidinone (MPL) to obtain a 1 mM stock solution that was then diluted with normal Ringer to yield final concentration of 100 nM. Previous studies from this laboratory have shown that MPL does not affect either IK or INa (Zhang et al. 2002, 2006a,b).
RESULTS
Delayed-rectifier like potassium currents in wild-type and Nf1+/− sensory neurons are not different
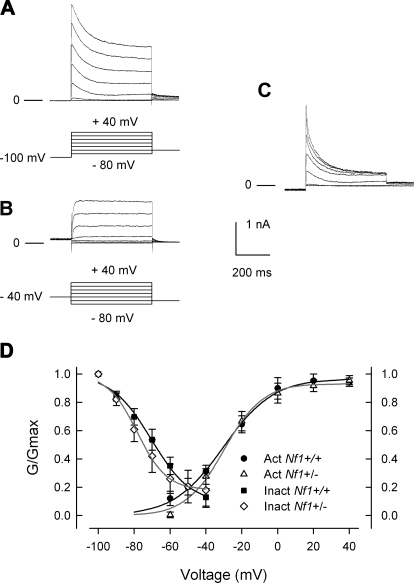
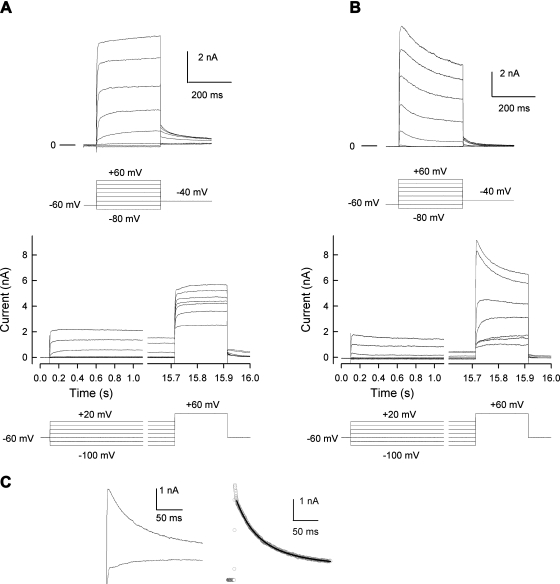
Exposure of small diameter rat sensory neurons to pro-inflammatory agents suppresses IK and leads to enhanced excitability (Evans et al. 1999; Nicol et al. 1997; Zhang et al. 2002). Because neurons isolated from Nf1+/− mice exhibited augmented excitability compared with wild-type mice (Wang et al. 2005), experiments were performed to determine whether IK recorded from Nf1+/− neurons was different from that of wild-type neurons. In these recordings from mouse neurons, two distinct phenotypes of IK were observed. One exhibited rapid activation with little time-dependent inactivation, whereas the other type showed rapid activation with more rapid inactivation kinetics. Both phenotypes of IK were observed in each genotype. Figure 1A, top, shows representative current traces that exhibited rapid activation with little time-dependent inactivation. These currents were obtained from a Nf1+/− neuron wherein the peak IK was 7.21 nA, which occurred at the end of the voltage step. Figure 1A, bottom, demonstrates the results obtained for steady-state inactivation of IK in this neuron. Note that there is little reduction in the current during the prepulses even after 15 s. Figure 1B, top, illustrates representative currents that showed rapid activation with faster inactivation kinetics and were obtained from a different Nf1+/− neuron. The peak IK measured at +60 mV was 10.49 nA and occurred at ∼70 ms. The steady-state inactivation for this neuron is shown in Fig. 1 B, bottom. Note the large amount of current decay during the prepulse. Figure 1C, left, compares the inactivation kinetics for the traces obtained for the step to +60 mV after prepulses to −100 mV from the neurons shown in A and B. The right panel represents the subtraction of the slowly inactivating trace (A) from the more rapidly inactivating trace (B); this yields a rapidly inactivating current that has many of the hallmarks of IA. The decay of the current shown in Figure 1C, right, was fit with a double exponential where the slow τ1 was 97 ms and the fast τ2 was 33 ms (A1 was 1,696, A2 was 1,659, correlation coefficient was 0.989). These time constants are consistent with the values described for those obtained for isolated IA currents (see following text). Thus it is possible that the more rapid inactivation of IK as typified by the neuron shown in B results from the contribution of IA-type currents to the total outward IK.
Fig. 1.
Mouse sensory neurons exhibit 2 distinct phenotypes of IK. A, top: IKs recorded from a Nf1+/− neuron that exhibited rapid activation with little time-dependent inactivation. Bottom: the currents obtained with the steady-state inactivation protocol for this same neuron. The prepulses for the steady-state inactivation are continuous steps but have been broken at the indicated times for presentation purposes. B, top: the IKs recorded from an Nf1+/− neuron that exhibited rapid activation with faster inactivation kinetics. Bottom: the currents obtained with the steady-state inactivation protocol for this neuron. The voltage protocols used are illustrated at the bottom of each panel. The lines labeled 0 represents the zero-current level. C, left: inactivation kinetics for IK obtained for the step to +60 mV after prepulses to −100 mV from the neurons shown in A and B, bottom. Right: the subtraction of the slowly inactivating trace (A) from the more rapidly inactivating trace (B) and illustrates the rapidly inactivating current. The line through the data points represents the double exponential fit to the decay of the rapidly inactivating current wherein the fitting parameters were A1, 1,696; τ1, 97 ms; A2, 1,659; τ2, 33 ms; C, 0.59 nA; correlation coefficient, 0.989.
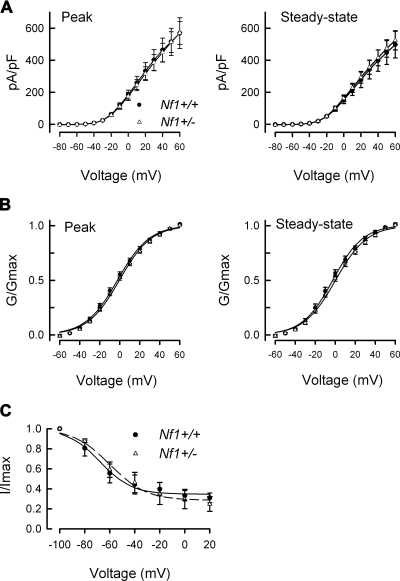
To minimize the variance of currents obtained from these different neurons, currents were normalized for cell surface area and expressed as current density (pA/pF). The current density-voltage relations for 11 wild-type and 13 Nf1+/− neurons are summarized in Fig. 2A. The current density-voltage relations for the peak (left) and steady-state (right) IKs in wild-type and Nf1+/− neurons were nearly identical (the average peak current density in wild-type and Nf1+/− neurons was 569.2 ± 96.3 and 570.3 ± 71.1 pA/pF, respectively, for the step to +60 mV). The current values were transformed to conductance (G), the conductance-voltage relation was fit with the Boltzmann relation, and the conductance for each neuron was then normalized to the maximal value of G (Gmax) obtained from the fit. The G/Gmax-voltage relation is summarized in Fig. 2B and indicates there is little difference in either the peak or steady-state values between the two genotypes. The Boltzmann fitting parameters, V0.5 and k, for the peak and steady-state measurements are shown in Table 1. Similar to IK activation, there were no significant differences in the properties of steady-state inactivation for IK between neurons of the two genotypes (see Fig. 2C). For example, in wild-type cells, IK was inactivated by 70.2 ± 5.4% (n = 7) after the conditioning prepulse to +20 mV, which was not different from the 74.5 ± 7%, (n = 6) in Nf1+/− neurons. The Boltzmann parameters for inactivation are summarized in Table 1. Based on the close overlap between the relations in the wild-type and Nf1+/− neurons for the current-voltage, the G/Gmax-voltage, and steady-state inactivation it seems unlikely that either the total IK or the voltage dependence of activation/inactivation of IK is altered in Nf1+/−neurons and therefore differences in IK do not account for the enhanced excitability previously shown (Wang et al. 2005).
Fig. 2.
The peak and steady-state IKs in wild-type and Nf1+/− sensory neurons are not different. A, left: the current density-voltage relations obtained for the peak IKs from wild-type and Nf1+/− sensory neurons. Right: the current density-voltage relations obtained for the steady-state IKs from wild-type and Nf1+/− sensory neurons. The steady-state values were measured at the end of the voltage step. B: the conductance-voltage relations for the peak (left) and the steady-state (right) measurements. The points in B have been fitted by the Boltzmann relation and are shown as the continuous lines. The values in each panel of A and B represent the means ± SE obtained from 11 wild-type and 13 Nf1+/− neurons. C: the steady-state inactivation of IK in 7 wild-type and 6 Nf1+/− sensory neurons. The steady-state inactivation voltage protocol is shown in Fig. 1, A and B. Currents were normalized to the maximal value of G obtained for the −100 mV prepulse. The data points have been fitted by the Boltzmann relation and are shown as ---.
Table 1.
Boltzmann fitting parameters for IK
| V0.5, mV | k, mV | n | ||||
|---|---|---|---|---|---|---|
| A. Activation | ||||||
| Peak | ||||||
| Wild type | −2.3 ± 1.7 | 15.1 ± 0.6 | 11 | |||
| Nf1+/− | −0.4 ± 1.4 | 15.3 ± 0.6 | 13 | |||
| Steady state | ||||||
| Wild type | −2.6 ± 2.1 | 14.1 ± 0.7 | 11 | |||
| Nf1+/− | 0.3 ± 1.6 | 15.2 ± 0.6 | 13 | |||
| IA type | ||||||
| Wild type | −27.0 ± 5.8 | 15.5 ± 2.7 | 14 | |||
| Nf1+/− | −25.0 ± 4.1 | 13.8 ± 2.6 | 11 | |||
| B. Inactivation | ||||||
| IK | ||||||
| Wild-type | −56.0 ± 7.2 | 12.5 ± 2.7 | 7 | |||
| Nf1+/− | −59.9 ± 6.9 | 13.7 ± 3.2 | 6 | |||
| IA type | ||||||
| Wild-type | −71.1 ± 2.6 | 9.6 ± 1.2 | 8 | |||
| Nf1+/− | −77.3 ± 2.2 | 7.7 ± 1.2 | 6 | |||
All values are means ± SE.
A-type IK does not contribute to the augmented excitability of Nf1+/− neurons
The rapidly inactivating type of IK known as IA controls neuronal excitability by its modulation of the frequency of firing (Connor and Stevens 1971; Neher 1971). Because Nf1+/− neurons fire APs at a higher frequency when stimulated by a depolarizing ramp of current compared with neurons from wild-type mice (Wang et al. 2005), experiments were performed to determine whether IA currents were different in neurons from the two genotypes. Interestingly, not every small diameter, capsaicin-sensitive sensory neurons of either genotype exhibited an obvious IA-type current. As shown in Fig. 3, IA was isolated (described in methods) by subtracting the currents obtained at a holding potential of −100 mV (A) from those obtained at −40 mV (B); the results for a representative wild-type neuron are shown in C. All isolated IA traces, regardless of genotype had a peak current that rapidly decayed over time and then reached a stable plateau (Fig. 3C). When the currents were normalized to cell capacitance, the pA/pF-voltage relations were not different (data not shown). In wild-type neurons, the peak value IA was 238.7 ± 94.7 pA/pF (for the step to +40 mV, n = 14) and was not significantly different from that in Nf1+/− neurons (340.9 ± 114.7 pA/pF, n = 11, P = 0.49 Student's t-test). The G/Gmax-voltage relations are summarized in Fig. 3D and demonstrate the similarities in voltage dependence for both activation and inactivation of IA in these two genotypes. The relations for G/Gmax-voltage were fitted by the Boltzmann relation and are summarized in Table 1 wherein the values for V0.5 and k for the activation of IA in the two genotypes were not different.
To determine whether altered kinetics of IA in Nf1+/− neurons could account for the enhanced AP firing, the decay of the current for the voltage step to +40 mV was fitted with an exponential function by using Clampfit 9. In six wild-type neurons, the decay kinetics were well fitted with a double exponential having a fast tau (τ) of 34 ± 13 ms and a slow τ of 246 ± 75 ms. Similarly, in four of five Nf1+/− neurons, the decay of IA was well fitted wherein the fast τ was 52 ± 18 ms and the slow τ was 470 ± 151 ms. These values were not significantly different (P = 0.44 and 0.29 for the fast and slow τs, respectively, Student's t-test). In one of the five Nf1+/− cells, the decay phase was best fitted with a single exponential wherein τ was 160 ms and was within the range observed for the slow τs for the two exponential fits. Taken together, it is unlikely that IA contributes to the augmented neuronal excitability observed previously for Nf1+/− neurons.
TTX-R and TTX-S INa are augmented in Nf1+/− neurons
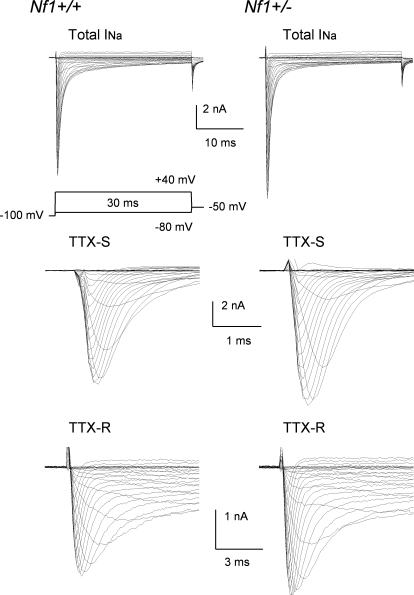
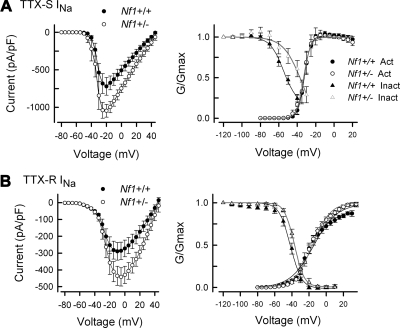
The properties of sodium channels, such as distribution, density, trafficking as well as the threshold of activation and repriming characteristics can all influence the firing patterns of sensory neurons (Blair and Bean 2002, 2003; Herzog et al. 2001; Schild and Kunze 1997; Waxman et al. 1999). To determine whether the enhanced firing of Nf1+/− neurons results from alterations of INa, studies were performed to measure the properties of this current in both wild-type and Nf1+/− sensory neurons. In small diameter sensory neurons, tetrodotoxin (TTX) can be used to separate total INa into those currents that are sensitive (TTX-S) and resistant (TTX-R) to blockage by this toxin (Caffrey et al. 1992; Campbell 1993; Elliott and Elliott 1993; Ogata and Tatebayashi 1992; Roy and Narahashi 1992). Representative traces for total, TTX-S, and TTX-R INa in wild-type neurons and Nf1+/− neurons are shown in Fig. 4. For the wild-type neuron (left), the peak value for the total INa was −10.41 nA and occurred at −15 mV, whereas the peak current for the Nf1+/− neuron (right) was −12.38 nA and occurred at −10 mV. The current density for TTX-S INa in Nf1+/− neurons was significantly larger compared with the values determined from wild-type neurons (Fig. 5A, left). The average peak current density in Nf1+/− neurons was −1,047 ± 85 pA/pF (n = 6) for the step to −20 mV and was significantly larger than the −721 ± 118 pA/pF for the wild-type neurons (n = 5, Student's t-test). Figure 5A, right, represents the G/Gmax-voltage relation for both activation and inactivation. These results show that the voltage dependence for activation of TTX-S INa in Nf1+/− neurons was not different from that in wild-type neurons (see Table 2). However, the V0.5 for steady-state inactivation of TTX-S INa determined in Nf1+/− neurons was shifted to more depolarized values by 12 mV (see Table 2) although the value of k was unchanged. These results suggest that the rightward shift in the inactivation for TTX-S INa may, in part, contribute to the greater current densities in Nf1+/− neurons.
Fig. 4.
Representative traces of INa obtained from a wild-type (left) and Nf1+/− neuron (right). Top: total INa, middle: TTX sensitive (TTX-S) INa, bottom: TTX resistant (TTX-R) INa. TTX-S was obtained by digital subtraction of the traces for TTX-R INa from those of the total INa.
Fig. 5.
The current density of both TTX-R and TTX-S INa in Nf1+/− neurons is significantly larger than those in wild-type neurons. A: the current density for TTX-S INa was significantly larger in the 6 Nf1+/− neurons compared with 5 wild-type neurons. The values were significantly different between −20 and +20 mV (P < 0.05, Student's t-test). Right: the G/Gmax-voltage relation for TTX-S INa in these neurons and shows that there is no difference in the voltage-dependence of activation; however, the steady-state inactivation of the 12 Nf1+/− neurons was shifted to more depolarizing potentials compared with the 10 wild-type neurons. The values for inactivation were significantly different for the prepulse voltages of −50 and −40 mV (P < 0.05, Student's t-test). The continuous lines through the points are the Boltzmann fits for the wild-type (black line) and Nf1+/− (gray line) neurons. B, left: the current density for TTX-R INa obtained from 9 Nf1+/− neurons was significantly larger compared with 8 wild-type neurons. The current values were significantly different between 0 and +25 mV (P < 0.05, Student's t-test). Right: the G/Gmax-voltage relation for TTX-R INa in these neurons and shows that there is no difference in the voltage dependence of activation; however, the steady-state inactivation of the Nf1+/− neurons was shifted to more depolarizing potentials. The continuous lines through the points are the Boltzmann fits for the wild-type (black line) and Nf1+/− (gray line) neurons. The values of G/Gmax for the Nf1+/− neurons were significantly different from the wild-type neurons for prepulse voltages from −60 to −30 mV (P < 0.05, Student's t-test). The fitting parameters are described in Table 2.
Table 2.
Boltzmann fitting parameters for INa
| Activation |
Inactivation |
|||||
|---|---|---|---|---|---|---|
| TTX-S INa | V0.5, mV | k, mV | n | V0.5, mV | k, mV | n |
| Wild type | −31.7 ± 2.2 | 3.0 ± 0.6 | 5 | −56.6 ± 1.2 | 6.3 ± 1.3 | 10 |
| Nf1+/− | −33.2 ± 2.3 | 2.8 ± 0.5 | 6 | −44.5 ± 3.4* | 4.6 ± 1.4 | 12 |
| TTX-R INa | ||||||
| Wild type | −13.6 ± 2.7 | 13.1 ± 1.4 | 8 | −42.3 ± 1.7 | 6.4 ± 1.0 | 12 |
| Nf1+/− | −15.8 ± 1.7 | 10.0 ± 1.0 | 10 | −36.3 ± 1.8* | 4.8 ± 0.4 | 12 |
Values are means ± SE.
P < 0.05 Student's t-test. TTX-S and TTX-R, tetrodotoxin sensitive and resistant, respectively.
In addition, augmentation of TTX-R INa has been proposed to be an important underlying mechanism in neuronal sensitization (England et al. 1996; Gold et al. 1996, 2002; Jeftinija 1994; Tanaka et al. 1998; Waxman et al. 1999; Zhang et al. 2002). Therefore the possible contribution of TTX-R INa to the augmented INa measured from Nf1+/− neurons was examined. TTX-R INa was isolated by using the voltage protocols described in the preceding text in the presence of 500 nM TTX. The current density of TTX-R INa in Nf1+/− neurons was significantly larger than that measured in wild-type neurons. As illustrated in Fig. 5B, left,, the average peak value of TTX-R INa measured for the step to −5 mV in Nf1+/− neurons was −442 ± 55 pA/pF (n = 9), which was significantly larger than that in wild-type neurons (−287 ± 52 pA/pF, n = 8, Student's t-test). The G/Gmax-voltage relations for activation and inactivation in the wild-type and Nf1+/− sensory neurons are shown in Fig. 5B, right. The voltage dependence for activation of TTX-R INa in Nf1+/− neurons is nearly the same as wild-type neurons (see Table 2). However, the V0.5 for TTX-R INa determined for steady-state inactivation in Nf1+/− neurons was shifted to more depolarized values by 6 mV (see Table 2) although the value of k was unchanged.
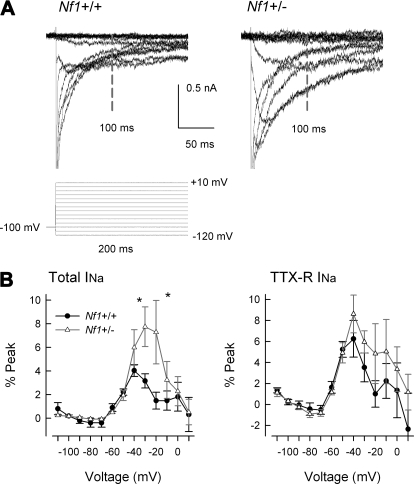
Because the inactivation of TTX-S and TTX-R INa in Nf1+/− neurons was altered whereas the activation was not, this depolarizing shift in the inactivation of INa resulted in a larger window current suggesting that over this voltage range there are more conducting sodium channels. To examine a potential consequence of this idea, the levels of the persistent INa were measured. In recordings to assess the inactivation of INa, measurements of the current 100 ms after the onset of the prepulse indicated that there was greater current in the Nf1+/− neurons compared with the wild-type (see Fig. 6). Representative traces of the persistent INa are shown in Fig. 6A where the vertical bar indicates the point at which the current was determined; the peaks have been truncated to better illustrate the persistent component. The results obtained under control recording conditions (total INa) and after treatment with TTX (TTX-R INa) for the two genotypes are summarized in Fig. 6B. In wild-type neurons, the persistent INa had an average value of −0.42 ± 0.08 nA (n = 10, data not shown) and peaked at −40 mV, whereas in Nf1+/− neurons the persistent current had an average value of −0.68 ± 0.32 nA (n = 12) that peaked at −20 mV. Values for the persistent INa in wild-type neurons are consistent with previous observations (Baker and Bostock 1997; Cepeda et al. 1995; Crill 1996; French et al. 1990; Kiernan et al. 2003; Wu et al. 2005). For the TTX-R persistent INa, there was no difference between the two genotypes. In wild-type neurons, the current had a peak value of −0.48 ± 0.15 nA (n = 12, data not shown) at −40 mV and in Nf1+/− neurons the average value was of −0.51 ± 0.11 nA (n = 12) that peaked at −40 mV. To account for cell-to-cell variance in the levels of total INa, the persistent INa was normalized as the percentage of the maximal transient current for each neuron. When measured as the percent of the peak transient current, the persistent INa was significantly larger (Student's t-test) for the −30- and −20-mV steps compared with wild-type neurons (left); however, there was no difference for TTX-R INa (right). For wild-type neurons at −30 mV, the persistent INa was 3.2 ± 0.6% of the peak (n = 10, range: −0.5–6.0%), whereas for Nf1+/− neurons, the persistent INa was 7.8 ± 1.7% (n = 12, range: 2.6–17.6%). Not all Nf1+/− neurons exhibited an augmented persistent current; 3 of the 12 Nf1+/− neurons had values less than the average value for the wild-type neurons (∼3%). This observation is consistent with previous results demonstrating that not all large or small diameter sensory neurons exhibit persistent INa (Baker and Bostock 1997; Keirnan et al. 2003). Therefore it is possible that the Nf1 mutation somehow modifies the inactivation properties of the channels that give rise to the total INa and may account for the enhanced levels of excitability observed in Nf1+/− neurons (Wang et al. 2005).
Fig. 6.
Nf1+/− sensory neurons exhibit larger persistent INa compared with wild-type neurons. A: representative current traces for the total INa obtained from a wild-type neuron (left) and from an Nf1+/− neuron (right). The currents were evoked by a series of 200 ms voltage pulses that ranged from −120 to +10 mV. The values for the persistent INa were obtained 100 ms after the onset of the prepulse (noted by the vertical bar labeled 100 ms). The peak currents have been truncated for clarity of the persistent INa. B: summary for the voltage dependence of the persistent INa measured as the percent of the maximum transient current for wild-type (n = 10) and Nf1+/− (n = 12) neurons. Left: the persistent current for the total INa; right: current remaining after exposure to TTX. *, significant difference between wild-type and Nf1+/− neurons (P < 0.05).
DISCUSSION
In this report, we show that the augmented excitability exhibited by sensory neurons isolated from Nf1+/− mice likely results from the significantly larger current densities for both TTX-S and TTX-R INa as well as elevated levels of persistent INa. Although the voltage dependence for activation of INa was not different between the genotypes, the voltage dependence for inactivation is shifted to more depolarized voltages in Nf1+/− neurons. Such a shift would increase the existing window current and thereby contribute to the elevated AP firing in Nf1+/− neurons.
Surprisingly, there were no differences in the current densities for either the rapidly inactivating IA or the delayed rectifier-like IKs. These observations are in contrast to studies examining IKs in neurofibromin-deficient Schwann cells (SCs). Previous work showed that IKs were upregulated in SCs, which may play an important role in the tumorogenesis of neurofibromas. However, the currents modulated as a consequence of these mutations appeared to be different. Both normal isolated human SCs and neurofibromin-deficient human MPNST cell lines exhibited a prominent rapidly inactivating IA the densities of which were the same; however, in tumor cells, IA exhibited slower inactivation kinetics (Fieber 1998). These mutant cells exhibited a TEA- and 4-AP-sensitive noninactivating type IK that was not observed in the normal SCs. In contrast, SCs isolated from Nf1−/− embryonic mice had a greater current density for IA-like currents compared with wild-type mice (Xu et al. 2002). The origins for the differences in the types of IK in the neurofibromin-deficient SCs are unclear; they may arise from differences in species (human vs. mouse), developmental stage of the SCs (adult vs. embryonic) or be related to other alterations intrinsic to the malignant tumor cells. We found no differences in IK for sensory neurons isolated from wild-type and Nf1+/− mice, suggesting that there are significant differences in the regulation of membrane currents by neurofibromin in SCs and sensory neurons.
Our observations that the current density for INa was significantly larger in Nf1+/− sensory neurons raise an important question as to whether the increased current densities result from differences in the levels of expression of sodium channels or in altered modulatory activities of signaling pathways. It is well documented that inflammatory mediators such as prostaglandin E2, 5-HT, and endothelin-1 augment the peak amplitude of TTX-R INa by shifting the voltage dependence for activation to more hyperpolarized voltages (England et al. 1996; Gold et al. 1996; Zhou et al. 2002). Because the voltage dependences for activation of TTX-S or TTX-R INa were not different between the genotypes, our results imply that the larger current densities in Nf1+/− neurons did not result from posttranslational modifications mediated by intracellular signaling pathways. However, tumor necrosis factor α, via activation of p38 MAP kinase, enhanced TTX-R INa without altering the voltage dependences for either activation or inactivation in sensory neurons isolated from the mouse (Jin and Gereau 2006). In addition to the transient TTX-S and TTX-R INa, sensory neurons from Nf1+/− mice exhibited significantly increased persistent INa as measured 100 ms after the onset of prepulse voltage steps. For the persistent INa (total INa conditions), the currents were significantly enhanced by about twofold at −30 and −20 mV. Our previous study (Wang et al. 2005) determined that the firing threshold for Nf1+/− neurons was approximately −32 mV compared with −26 mV for the wild-type neurons (based on APs evoked with a depolarizing current ramp). Thus over this voltage range, an augmented persistent INa that overlapped the firing threshold could have a significant impact on neuronal excitability. Consistent with this notion, previous reports demonstrated that activation of a persistent INa led to increased AP firing (Cepeda et al. 1995; Crill 1996; Do and Bean 2003; French et al. 1990; Stafstrom et al. 1982, 1984; Taddese and Bean 2002; Theiss et al. 2007; Wu et al. 2005). Conditions associated with the Nf1+/− genotype could produce transcriptional modifications that alter levels of expression for critical modulators of channel activity. Consistent with this idea are studies examining the interactions between the conducting α subunit of TTX-S sodium channels and the auxiliary β subunits. Overexpression of β2, β3, or β4 with Nav1.1 or Nav1.2 in heterologous expression systems had little effect on peak current amplitudes; however, expression of these β subunits gave rise to a threefold increase in the persistent INa (Aman et al. 2009; Qu et al. 2001). The β3 subunit was expressed in high levels in small and medium diameter sensory neurons of the DRG (Qu et al. 2001). Thus the elevated levels of persistent INa observed in the Nf1+/− sensory neurons might result from increased expression of key β subunits. Currently very little is understood about potential differences in the efficacies of different signaling cascades in sensory neurons of Nf1+/− mice with reduced activities of neurofibromin. Thus the regulation by intracellular signaling pathways of INa and other membrane currents important in controlling excitability in Nf1+/− mice could be an important area for future investigation.
Previous studies have established important connections between neurofibromin and cyclic AMP signaling. In PC12 cells deficient in PKA, treatment with NGF increased the levels of both mRNA and saxitoxin binding for type II sodium channels; however, recordings showed no detectable INa in these cells, indicating that PKA was critical in the expression of functional channels (Ginty et al. 1992). Although early studies demonstrated that NGF rapidly increased intracellular levels of cyclic AMP (Higuchi et al. 2003; Knipper et al. 1993), establishing a causal link has been elusive. Recent studies in PC12 cells showed that NGF elevated cyclic AMP levels through activation of soluble rather than transmembrane adenylyl cyclase and that this cyclic AMP increased the activity of the small G protein, Rap1 (Stressin et al. 2006). Furthermore, enhancement of IK in neurofibromin-deficient SCs was dependent on activation of the cyclic AMP signaling pathway (Fieber 1998; Xu et al. 2002), suggesting an important interaction between neurofibromin and cyclic AMP. However, the role of neurofibromin in regulating the activity of the cyclic AMP pathway appears to depend on cell type. In SCs, cyclic AMP was mitogenic and cyclic AMP levels were negatively regulated by neurofibromin (Kim et al. 2001), whereas in astrocytes, cyclic AMP was anti-mitogenic and neurofibromin positively regulated cyclic AMP production (Dasgupta et al. 2003). Likewise, in isolated cortical neurons, increased cyclic AMP elevated the levels of phosphorylated Erk and activation of Rap1, whereas in cortical astrocytes, this elevation in cyclic AMP decreased phosphorylated Erk (Dugan et al. 1999). The exact nature of the interaction between neurofibromin and adenylyl cyclase remains unclear.
Taken together, the role of neurofibromin in regulating/modulating the activities of a variety of signaling pathways is poorly understood. It is difficult to mechanistically infer the regulation of intracellular transduction cascades based on results obtained in other cell systems. Thus understanding the role of neurofibromin in regulating the expression levels of ion channels as well as the modulation of their activity will be the focus of future investigations. A better appreciation of the mechanisms underlying the enhanced neuronal excitability in sensory neurons with the Nf1 mutation, similar to the human disorder NF1, and how this sensitization may be modified in inflammation or injury could lead to better therapies for the painful conditions associated with NF1.
GRANTS
This investigation was conducted in a facility constructed with support from National Institutes of Health Grant C06 RR-015481-01; this work was supported by NIH Grant NS-051668 to C. M. Hingtgen.
DISCLOSURES
The authors have no conflicts of interest regarding this report.
ACKNOWLEDGMENTS
We thank Dr. Ted Cummins for discussions about sodium channels.
REFERENCES
- Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J Neurosci 29: 2027–2042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J Neurophysiol 77: 1503–1513, 1997 [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 22: 10277–10290, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci 23: 10338–10350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res 592: 283–297, 1992 [DOI] [PubMed] [Google Scholar]
- Campbell DT. Single-channel current/voltage relationships of two kinds of Na+ channel in vertebrate sensory neurons. Pfluegers 423: 492–496, 1993 [DOI] [PubMed] [Google Scholar]
- Cepeda C, Chandler SH, Shumate LW, Levine MS. Persistent Na+ conductance in medium-sized neostriatal neurons: characterization using infrared videomicroscopy and whole cell patch-clamp recordings. J Neurophysiol 74: 1343–1348, 1995 [DOI] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol 213: 21–30, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creange A, Zeller J, Rostaing-Rigattieri S, Brugieres P, Degos JD, Revuz J, Wolkenstein P. Neurological complications of neurofibromatosis type 1 in adulthood. Brain 122: 473–481, 1999 [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996 [DOI] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 17: 3503–3514, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Dugan LL, Gutmann DH. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci 23: 8949–8954, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron 8: 415–428, 1992 [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- Dugan LL, Kim JS, Zhang Y, Bart RD, Sun Y, Holtzman DM, Gutmann DH. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem 274: 25842–25848, 1999 [DOI] [PubMed] [Google Scholar]
- Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol 463: 39–56, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol 495: 429–440, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurons. J Physiol 516: 163–178, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber LA. Ionic currents in normal and neurofibromatosis type 1-affected human Schwann cells, induction of tumor cell K current in normal Schwann cells by cyclic AMP. J Neurosci Res 54: 495–506, 1998 [DOI] [PubMed] [Google Scholar]
- Fitzgerald EM, Dolphin AC. Regulation of rat neuronal voltage-dependent calcium channels by endogenous p21-ras. Eur J Neurosci 9: 1252–1261, 1997 [DOI] [PubMed] [Google Scholar]
- French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol 95: 1139–1157, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet 89: 1–6, 1999 [PubMed] [Google Scholar]
- Ginty DD, Fanger GR, Wagner JA, Maue RA. The activity of cAMP-dependent protein kinase is required at a posttranslational level for induction of voltage-dependent sodium channels by peptide growth factors in PC12 cells. J Cell Biol 116: 1465–1473, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA 93: 1108–1112, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E2 modulates TTX-R INa in rat colonic sensory neurons. J Neurophysiol 88: 1512–1522, 2002 [DOI] [PubMed] [Google Scholar]
- Guha A, Lau N, Huvar I, Gutmann D, Provias J, Pawson T, Boss G. Ras-GTP levels are elevated in human NF1 peripheral nerve tumors. Oncogene 12: 507–513, 1996 [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol 86: 1351–1364, 2001 [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. EMBO J 22: 1790–1800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991 [PubMed] [Google Scholar]
- Ingram DA, Hiatt K, King AJ, Fisher L, Shivakumar R, Derstine C, Wenning MJ, Diaz B, Travers JB, Hood A, Marshall M, Williams DA, Clapp DW. Hyperactivation of p21(ras) and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med 194: 57–69, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet 7: 353–361, 1994 [DOI] [PubMed] [Google Scholar]
- Jeftinija S. Bradykinin excites tetrodotoxin-resistant primary afferent fibers. Brain Res 665: 69–76, 1994 [DOI] [PubMed] [Google Scholar]
- Jin X, Gereau RW., 4th Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26: 246–255, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Baker MD, Bostock H. Characteristics of late Na(+) current in adult rat small sensory neurons. Neuroscience 119: 653–660, 2003 [DOI] [PubMed] [Google Scholar]
- Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J Neurosci 21: 1110–1116, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesse LJ, Parada LF. p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J Neurosci 18: 10420–10428, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Beck A, Rylett J, Breer H. Neurotrophin induced cAMP and IP3 responses in PC12 cells. Different pathways. FEBS Lett 324: 147–152, 1993 [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 8: 2394–2405, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol 58: 36–53, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol 77: 167–176, 1997 [DOI] [PubMed] [Google Scholar]
- North KN, Riccardi V, Samango-Sprouse C, Ferner R, Moore B, Legius E, Ratner N, Denckla MB. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology 48: 1121–1127, 1997 [DOI] [PubMed] [Google Scholar]
- Ogata N, Tatebayashi H. Comparison of two types of Na+ currents with low-voltage-activated T-type Ca2+ current in newborn rat dorsal root ganglia. Pfluegers 420: 590–594, 1992 [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Cognitive impairment in neurofibromatosis type 1. Am J Med Genet 89: 45–52, 1999 [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol 84: 2365–2379, 2000 [DOI] [PubMed] [Google Scholar]
- Qu Y, Curtis R, Lawson D, Gilbride K, Ge P, DiStefano PS, Silos-Santiago I, Catterall WA, Scheuer T. Differential modulation of sodium channel gating and persistent sodium currents by the beta1, beta2, and beta3 subunits. Mol Cell Neurosci 18: 570–580, 2001 [DOI] [PubMed] [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci 12: 2104–2111, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol 78: 3198–3209, 1997 [DOI] [PubMed] [Google Scholar]
- Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem 275: 30740–30745, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Crill WE. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res 236: 221–226, 1982 [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Flatman JA, Crill WE. Properties of subthreshold response and action potential recorded in layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol 52: 244–263, 1984 [DOI] [PubMed] [Google Scholar]
- Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem 281: 17253–17258, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 33: 587–600, 2002 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport 9: 967–972, 1998 [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurons. J Physiol 580: 507–522, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovo-Marqui AB, Goloni-Bertollo EM, Valerio NI, Pavarino-Bertelli EC, Muniz MP, Teixeira MF, Antonio JR, Tajara EH. High frequencies of plexiform neurofibromas, mental retardation, learning difficulties, and scoliosis in Brazilian patients with neurofibromatosis type 1. Braz J Med Biol Res 38: 1441–1447, 2005 [DOI] [PubMed] [Google Scholar]
- Vogel KS, El-Afandi M, Parada LF. Neurofibromin negatively regulates neurotrophin signaling through p21ras in embryonic sensory neurons. Mol Cell Neurosci 15: 398–407, 2000 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nicol GD, Clapp DW, Hingtgen CM. Sensory neurons from Nf1 haploinsufficient mice exhibit increased excitability. J Neurophysiol 94: 3670–3676, 2005 [DOI] [PubMed] [Google Scholar]
- Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve 22: 1177–1187, 1999 [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplege A. Quality-of-life impairment in neurofibromatosis type 1: a cross-sectional study of 128 cases. Arch Dermatol 137: 1421–1425, 2001 [DOI] [PubMed] [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic v neurons participate in burst generation and control of membrane excitability. J Neurophysiol 93: 2710–2722, 2005 [DOI] [PubMed] [Google Scholar]
- Xu Y, Chiamvimonvat N, Vazquez AE, Akunuru S, Ratner N, Yamoah EN. Gene-targeted deletion of neurofibromin enhances the expression of a transient outward K+ current in Schwann cells: a protein kinase A-mediated mechanism. J Neurosci 22: 9194–9202, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol 96: 1042–1052, 2006a [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J Physiol 544: 385–402, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol 575: 101–113, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Davar G, Strichartz G. Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1. J Neurosci 22: 6325–6330, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]