Abstract
Background
Feedback about performance may optimize motor relearning after stroke.
Objectives
Develop an international collaboration to rapidly test the potential efficacy of daily verbal feedback about walking speed during inpatient rehabilitation after stroke, using a protocol that requires no research funds.
Methods
This phase 2, single-blinded, multicenter trial randomized inpatients to either feedback about self-selected fast walking speed (daily reinforcement of speed, DRS) immediately after a single, daily 10-m walk or to no reinforcement of speed (NRS) after the walk, performed within the context of routine physical therapy. The primary outcome was velocity for a 15.2-m (50-foot) timed walk at discharge. Secondary outcomes were walking distance in 3 minutes, length of stay (LOS), and level of independence (Functional Ambulation Classification, FAC).
Results
Within 18 months, 179 participants were randomized. The groups were balanced for age, gender, time from onset of stroke to entry, initial velocity, and level of walking-related disability. The walking speed at discharge for DRS (0.91 m/s) was greater (P = .01) than that for NRS (0.72 m/s). No difference was found for LOS. LOS for both DRS and NRS was significantly shorter, however, for those who had mean walking speeds >0.4 m/s at entry. The DRS group did not have a higher proportion of FAC independent walkers (P = .1) and did not walk longer distances (P = .09).
Conclusions
An Internet-based collaboration of 18 centers found that feedback about performance once a day produced gains in walking speed large enough to permit unlimited, slow community ambulation at discharge from inpatient rehabilitation.
Keywords: stroke rehabilitation, feedback, walking speed, randomized clinical trial, Functional Ambulation Classification
Introduction
Most recent clinical trials of walking interventions for neurorehabilitation use walking speed as a primary outcome measure and include walking distance over 2 to 6 minutes and level of independence in gait as secondary outcomes.1–5 Speed is a continuous measurement with high reliability and responsiveness.6 Speed also affects energy use and reflects overall locomotor-related ability.7,8 For example, 3 months after stroke, a velocity of 0.8 m/s during a short distance walk correlated with slow unrestricted community ambulation, limited community mobility if more than 0.4 m/s, and unrestricted home mobility if it was from 0.26 to 0.4 m/s.9 An increase in gait velocity that produces a transition to a higher level of ambulation results in better function and quality of life, based on the Stroke Impact Scale, especially for household ambulators.10 Thus, aiming to maximize walking speed during inpatient and outpatient rehabilitation ought to be a therapeutic goal. Few trials, however, have attempted and shown the benefit of practice at higher training speeds, and these used treadmill training.11,12
Many reviews discuss the need for intensive, task-related practice after stroke that is goal oriented and supported by schedules of feedback about performance.13–15 The value of structured verbal feedback to improve walking speed during inpatient rehabilitation after stroke, however, has not been tested. To examine the potential for summary feedback about performance as a therapeutic adjunct, we organized the Stroke Inpatient Rehabilitation With Reinforcement of Walking Speed (SIRROWS) multicenter, randomized clinical trial (MRCT).
A second objective was to determine the feasibility of uniting clinicians in an international network to conduct the RCT, so as to improve the rate of recruitment and obtain more generalizable results. Simplicity of the research design was imperative because we had no research funding to offer centers and wanted to include willing sites even if they did not have extensive clinical trial experience. These potential limitations suggested that we should conduct the trial within the usual activities of each inpatient center. SIRROWS then, tested the hypothesis that feedback about walking speed once a day, compared with no feedback, would lead to faster walking at discharge from inpatient stroke rehabilitation and, in turn, possibly shorten the length of the inpatient rehabilitation stay.
Methods
An open invitation to join the clinical trials network was e-mailed to all members of the World Federation for NeuroRehabilitation and the American Society of Neuro-Rehabilitation and advertised at national meetings and in a journal in 2006. The coordinating center at the University of California Los Angeles (UCLA) received expressions of interest from 47 sites that had inpatient rehabilitation services: 14 within the United States and 33 from 22 other countries. Eight American sites obtained institutional review board (IRB) approval and entered participants. Several others said they could not participate because of high IRB fees charged by their institutions. Also, 14 sites outside the United States obtained IRB approval, and 10 entered participants. Several others were not eligible because they did not have a human subjects protection system. The UCLA study center engaged the site investigators by Email to manage instructions and questions as well as through the study Web site and a quarterly electronic newsletter.
Subjects
Patients admitted to an inpatient facility because they qualified for stroke rehabilitation were eligible if they were at least 35 years of age with unilateral hemiparesis (strength on the British Medical Council Scale ≤ 4/5 at the ankle, knee, and hip). Participants had to be able to follow simple instructions for reinforcement about walking speed and take at least 5 steps with no more than maximal assistance of 1 person. Exclusion criteria included prior stroke with residual impairment and pain on stepping, dyspnea or angina on modest exertion, or other premorbid limitations on walking. Entry could be delayed after admission if a person satisfied all other selection criteria but was not yet able to take 5 steps or was experiencing a transient medical problem. In this case, a daily examination was performed, and the patient was randomized immediately after meeting criteria. Eligibility was determined by the treating physician-investigator during routine inpatient admission assessment. All participants had to sign an informed consent patterned after the one approved by the IRB at UCLA and tailored to each institution. The trial was registered at www.ClincialTrials.gov (NCT0042480).
Randomization
After obtaining informed consent, the site investigator entered the patient’s descriptor information into an online patient entry form. On submission, a computer-generated system provided immediate notification of the randomization result. Because of a software problem with the automatic system approximately half way through the trial, manual randomization was adopted, and investigators were notified within 24 hours by e-mail of the result. Assignment was carried out by a random permuted block design with a block size of 4. Stratification by site was a prerandomization factor.
Interventions
All participants received the site’s conventional inpatient rehabilitation. They also performed a daily 10-m walk (or shorter distance walk until 10 m was feasible) as part of a physical therapy session. The experimental group received feedback about walking speed (daily reinforcement of speed, DRS) after each day’s 10-m walk. The DRS participants were timed after being told to walk as quickly as they felt was safe, and then, they were given specific feedback and encouragement concerning speed. For example, “Very good! You walked that in (number of) seconds.” Then, (a) “This is better by (number of) seconds” or (b) “This shows you are holding your own” or (c) “I believe that you will soon be able to walk a bit faster.” The control group was not timed during its daily 10-m walk and received no information about walking speed (no reinforcement of speed, NRS).
Measures
Baseline descriptors were time from onset of stroke, stroke type and general location (determined by radiological or clinical information), age, race, and gender. Stroke severity was assessed at baseline by the National Institutes of Health Stroke Scale (NIHSS), disability by the modified Rankin Scale (mRS), and whether human assistance was needed to walk based on the Functional Ambulation Classification (FAC).16
The primary outcome measure, as well as an interim measurement, was self-selected walking speed (m/s) at discharge, performed with instructions to walk as fast as the participant deemed to be safe over a flat, 15.2-m (50-foot) walkway. The distance was greater than the daily timed walk. Participants were given an extra 2 m (6 feet) at each end of the walkway to allow a steady pace to be achieved without acceleration at the start and deceleration at the end. The stopwatch started when the lead foot began to cross the start line and ended when the lead foot crossed the finish line. For patients who were unable to walk 15.2 m but could take 5 steps, the test was performed over a shorter, feasible distance, until a 15.2-m walk could be accomplished. The treating physical therapist could assist the participant as necessary but did not provide any feedback or encouragement. The participant’s usual assistive and orthotic devices were permitted for all evaluations. Walking speed was assessed at study entry and at 2-week intervals during inpatient rehabilitation for up to a maximum of 8 weeks and at inpatient discharge. We encouraged follow-up evaluations at 3 and 6 months after discharge. The FAC was also recorded with each walking test of velocity. The average of 2 trials of the 15.2-m walk was recorded at discharge and follow-up assessments. To minimize fatigue, only a single trial was performed at baseline and during inpatient rehabilitation.
Secondary outcomes were distance walked in 3 minutes at discharge and length of stay (LOS) for inpatient rehabilitation. The distance of the walkway for the 3-minute walk varied across sites according to available space, but most used a 30.5 m (100-foot) straight walkway and 180° turns. The distance traveled in meters was recorded as well as the number of rests. The test was carried out after 4 weeks of inpatient rehabilitation (if applicable), at discharge, and if feasible at 3 and 6 months postdischarge. The primary and secondary walking outcomes were measured by a blinded therapist who did not work on the inpatient unit and who performed the tests on all participants at her site. Because improvement of the level of independence for mobility is a major goal of inpatient rehabilitation, the walking speed at discharge was anticipated to affect the LOS.
Computer-Based Data Entry
A manual of operations was provided to each user. The study Web site familiarized the principal investigator and the blinded observer with procedures and data entry forms. The online database was created using a simple Wiki-based engine. The database was password protected. Each PI and blinded observer had different login codes to enter data. Each site had access to only their data. A patient status page showed investigators and assessors which outcomes to collect at each time point; it included direct links to the relevant data entry form, which made the database simple to navigate. Alternately, forms could be selected from a drop-down menu.
Statistical Design
SIRROWS, which we considered to be a phase 2 RCT, had a 2-group, randomized, repeated measures design with treatment blinding. Given the minimal risk to participants, we did not engage an external data monitoring and safety committee. The primary end point was walking speed at discharge. The reinforcement received by the experimental DRS group and the greater awareness about walking speed over time on the part of the therapist were anticipated to improve walking speed by 25% or by at least 0.2 m/s over the control NRS group. This gain would be at the upper level of differences found in prior trials that aimed to increase walking speed at various intervals after stroke (see Discussion). For an anticipated effect size of 0.4, a 2-tailed α of .01, and power of 0.9, we anticipated needing a sample size of 150 participants in each group, as long as dropouts were no more than 15%. We planned a futility analysis when about 50 participants from each arm of the trial had discharge data.
An analysis of covariance was planned for walking speed, LOS, and walking distance carried out by the general linear model and by Huber’s robust analysis of covariance if outliers were present. Additional interaction terms to be introduced included time from onset of stroke to entry and the initial NIHSS. In addition, the admission mRS and FAC were divided into a binary result and assessed by Fisher’s exact test. The mRS was divided into ≤2 (slight to no disability) versus ≥3 (moderate to severe disability). The FAC was divided into ≥4 (independent on level surfaces or better) versus <4 (0 = unable to walk; 1 and 2 = needs physical assistance; 3 = walks with supervision). Baseline descriptor data were compared by t tests.
Results
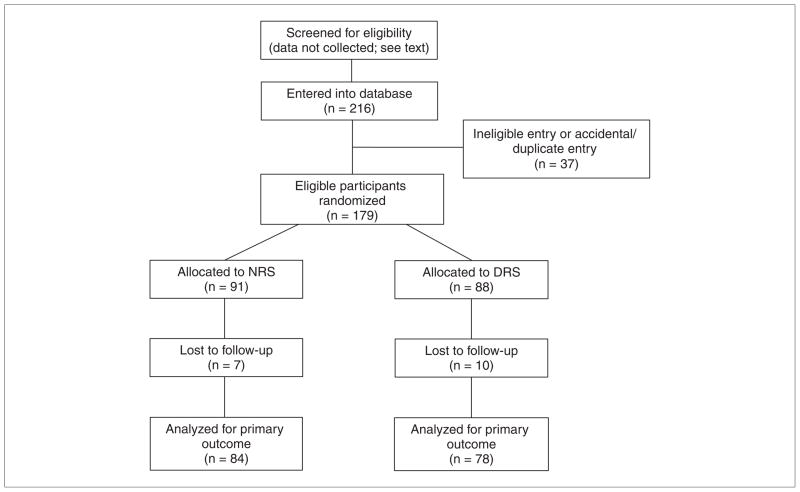
Eighteen sites enrolled 179 eligible patients between July 2007 and February 2009 (Figure 1). About 10% of participants in each group dropped out prior to the primary outcome assessment. Sites gradually came online. For example, by November 2007, 25 sites had IRB approval, 13 had randomized participants, and 62 participants were enrolled. By April 2008, 17 sites had randomized 103 participants. We did not ask investigators to ascertain the number of participants who were screened at admission for inpatient rehabilitation, did not meet entry criteria, or refused to participate because this information would have required a part-time coordinator at each site for complete ascertainment. We anticipated that about 15% of all admissions would be eligible. No adverse events or safety concerns related to the daily walking task were reported on the database form. The planned interim analysis for walking speed was carried out by the study statistician without unblinding the RCT and determined that no more than 75 participants would be needed in each arm to show a difference, if one existed. We looked for significant differences in the results of walking speed before the interim analysis, which corresponded to the time we had to switch from immediate computer randomization to e-mailing the result, and after the analysis, suggesting that this change in the randomization procedure did not lead to any systematic bias.
Figure 1.
CONSORT diagram of flow of participants through the randomized controlled trial
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; DRS, daily reinforcement of walking speed; NRS, no reinforcement of walking speed.
Descriptive Analysis
Ischemic stroke accounted for 81% of admissions, and 51% of strokes affected the left hemisphere. Baseline characteristics are shown in Table 1. No significant differences were found between the 2 groups in terms of mean age, gender (59% males), time poststroke, stroke severity (NIHSS), initial walking speed, and proportion of people with mRS ≤ 2 and FAC ≥ 4. On average, the initial self-selected fast walking speed was 0.45 m/s; 68% of all participants walked at less than 0.5 m/s at study entry. The proportion of patients who walked slower than 0.5 m/s was not significantly different between the 2 groups. Overall, 76% were non-Hispanic Caucasians, 7% African or African American, 14% Asian, and 3% Hispanic.
Table 1.
Baseline Summary of SIRROWS Participants
| DRS, Mean (SD) | NRS, Mean (SD) | P Value | |
|---|---|---|---|
| Number of participants | 88 | 91 | .91 |
| Age (years) | 62.9 (12.6) | 65.1 (11.9) | .85 |
| Stroke onset to entry (days) | 27.3 (78) | 30.2 (53.5) | .78 |
| Initial walking speed (m/s) | 0.45 (0.37) | 0.46 (0.34) | .98 |
| NIHSS | 6.4 (3.5) | 6.6 (3.1) | .74 |
| mRS (≥2) | 99% | 97% | .62 |
| FAC (≥4) | 4.9% | 4.8% | 1.00 |
Abbreviations: SIRROWS, Stroke Inpatient Rehabilitation With Reinforcement of Walking Speed; SD, standard deviation; DRS, daily reinforcement of walking speed; NRS, no reinforcement of walking speed; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; FAC, Functional Ambulation Classification.
Primary Analysis
Table 2 shows the outcomes for the experimental DRS and the control NRS groups at discharge. Box plots strongly suggested that the walking speed data were skewed, so the data were transformed as log(speed at discharge + 0.5), which reduced the skewness to a negligible amount. For statistical modeling, we used this transformation and the covariates of treatment group, age, site, and time from stroke onset to study entry. The results were unchanged when carried out by the general linear model and by Huber’s robust analysis of covariance. Additional interaction terms—admission NIHSS, mRS, and FAC—did not alter the results. With the transformed model, a standard residual analysis did not show any large deviations. We did not make adjustments for missing data (about 10% of participants in each group) for walking velocity because SIRROWS was a phase 2 trial and the missing data appeared to have occurred randomly, unrelated to the interventions.
Table 2.
Outcome Data at Discharge From Inpatient Rehabilitation
| DRS, Mean (SD), 95% CI | NRS, Mean (SD), 95% CI | P Value | |
|---|---|---|---|
| Walking speed (m/s) | 0.91 (0.57), 0.21, 2.03 | 0.72 (0.44), 0.14, 1.58 | .01 |
| Length of rehabilitation stay (days) | 42.8 (34.7) | 40.4 (28.7) | .62 |
| Walking distance (m) | 131.9 (75.4) | 112.2 (61.0) | .09 |
| FAC ≥ 4 | 36% | 24% | .12 |
Abbreviations: SD, standard deviation; DRS, daily reinforcement of walking speed; NRS, no reinforcement of walking speed; CI, confidence interval; FAC, Functional Ambulation Classification.
Both groups significantly increased their gait speed at discharge relative to baseline. However, the change in gait speed was greater for the DRS group in the primary analysis (P = .01). The mean change scores from baseline (DRS = 0.45, standard deviation [SD] = 0.35; NRS = 0.27, SD = 0.29) also significantly favored the DRS group (P = .01) at discharge. The difference between groups was retained at 3 months postdischarge (P = .03), although the number of participants with data was reduced by 50% (DRS = 35; NRS = 48). By 6 months, only 35% of participants had velocity data.
Secondary Analyses
Unadjusted group means are given in Table 2 for walking distance and LOS. Walking distance in 3 minutes showed somewhat larger gains for the DRS group, but P = .09. The proportion of DRS compared with NRS participants who were independent ambulators was also at this near threshold for significance. Feedback had no measurable effect on LOS. LOS, however, was greater (P < .0001) for the non-US sites, at 55.7 days (SD = 32.2), versus US sites, at 19.4 days (SD = 11.0), which may have limited the utility of this parameter. Of note, analysis revealed that LOS was highly associated with the initial walking speed of participants in both groups; patients who walked <0.5 m/s at baseline had a longer LOS (P = .02). We examined for a baseline walking speed that might predict the LOS. Based on a tree structure classification analysis (CART), the most robust difference in each group occurred at 0.42 m/s, which would suggest, as anticipated, that greater locomotor disability may be the primary factor for a longer LOS.
Discussion
SIRROWS is the first international MRCT to test an intervention to improve walking speed during inpatient rehabilitation for stroke. The results of the trial are novel. A single daily bout of feedback about performance for that day’s walking speed over a short distance led to significantly faster speeds at discharge compared with well-matched participants who did not receive feedback. Indeed, the velocity attained by the experimental group gave the average participant the ability to walk at a pace that is associated with the capacity for unlimited slow community mobility.1
The increase in walking speed from baseline in SIR-ROWS was considerably better for both groups than prior RCTs of walking interventions, although prior studies usually started after inpatient rehabilitation had been completed.1,17 The mean change scores from baseline to discharge were 0.25 m/s for the NRS and 0.46 m/s for the DRS group. The DRS group doubled its speed between entry and discharge. The difference in favor of DRS at discharge was 0.19 m/s, which exceeds the minimal clinically important difference of >0.16 m/s, at least for comfortable walking speed in patients who had a similar initial speed within the early time frame of the SIRROWS trial.18
Other studies help put the DRS mean change and final walking velocity into general perspective. A Cochrane analysis of RCTs of overground training for patients whose time since stroke onset was longer than in SIRROWS revealed increases in walking speed resulting from the experimental treatment of up to 0.1 m/s.17 These gains are considerably smaller than those achieved by the DRS over the NRS arm of SIRROWS. A few studies included onset of gait training within the SIRROWS time frame of inpatient rehabilitation. One of the SIRROWS sites, for example, previously reported mean increases in walking speed in 226 nonambulators, on admission for stroke rehabilitation, of 0.19 m/s and a gain of 0.28 m/s in 147 patients who initially walked at 0.41 m/s.19 A recent 3-arm, phase 2 RCT examined different intensities of physical therapy for inpatients at a mean of 35 days poststroke.20 Half of the patients were not yet able to walk to obtain a baseline walking speed, and the other half had a mean velocity of 0.2 m/s. Mean velocities improved to 0.30 m/s in the arm that received the usual amount of therapy and to 0.55 m/s in the group with 15 h of additional therapy for 6 weeks. Results improved up to 0.1 m/s across the groups after an additional 6 weeks of usual or more intensive treatment. Thus, most participants did not achieve community-level walking speeds. Another RCT probably included a more homogeneous and possibly more impaired group of patients than SIRROWS during acute stroke rehabilitation.21 Walking speed after 6 weeks increased from that at entry, when patients could not walk, to 0.40 m/s for a group randomized to receive extra lower-extremity training, and to 0.20 m/s for the control groups. Mean speeds increased to 0.58 and 0.46 m/s at 12 weeks, respectively, with further increases to 0.65 and 0.55 m/s at 20 weeks, which was at the end of the interventions. Thus, the mean walking speeds achieved in this sample were similar to that of the NRS group but lower than that of the DRS group over a similar time frame.
The size of the effect in SIRROWS was also greater than velocity increases obtained with more complex interventions, such as robotic assists. For example, a Cochrane review of RCTs through 2007 found that mean walking velocity increased only 0.08 m/s for robotic-trained participants.22 A subsequent RCT compared the Lokomat to overground training in slow walkers (0.34 m/s at onset) starting about 3 to 4 months after onset of stroke. Velocity increased in the robotic group by only 0.06 m/s and in the conventional group by 0.18 m/s at the end of 24 sessions of therapy.5
The remarkably fast mean velocities achieved by the DRS group in SIRROWS cannot be compared with high confidence to any of these trials, however, because of differences in the population sampled, the types and intensities of interventions, entry criteria, and time from onset of stroke to the start of rehabilitation. The modest improvements after prior attempts to improve walking speed in comparison to the SIRROWS result, however, suggest that even modest feedback and reinforcement about daily walking speed alone offers a conceptually sound, practical, low-technology, and no-cost intervention to potentially improve walking speed and related functional outcomes. If confirmed by additional studies, similar feedback should be included in the control and experimental arms of trials that deploy more costly and technologically complex walking interventions.23
Defining the type, timing, and frequency of feedback about the results of motor performance after stroke or other neurological diseases is a work in progress. For example, Boysen et al24 examined the effects of repeated encouragement and verbal instructions about physical activity given at discharge from inpatient rehabilitation and at 3-month intervals for 12 months, then every 6 months, compared with a control group that received information about the possible benefits of being physically active but did not receive specific instruction. No significant increase in physical activity was found in these patients with mild impairments. Perhaps, the verbal encouragement was not frequent enough to produce sufficient motivation to change behavior. SIRROWS used daily verbal reinforcement on a more targeted behavior—walking speed—with a more captive research group during inpatient rehabilitation. The frequency and specificity of feedback may be critical for success.
The secondary outcomes of SIRROWS were less responsive to feedback. LOS was longer for most non-US sites. Thus, the potential confounder of wide variances in LOS among sites may have lessened the likelihood of finding a shorter LOS in the DRS group. By controlling for time from onset of stroke to entry and for LOS, however, this discrepancy did not alter the significant results for walking speed. Also, secondary outcomes for walking distance and the proportion of DRS patients who were rated as independent ambulators did not reveal a statistically significant difference. With P values in the range of 0.1 within a phase 2 discovery trial, one should probably not reject an intervention or a measurement from further study, especially when the primary outcome suggests efficacy. Walking speed and distance often, in a laboratory setting, increase in unison after stroke, because patients use similar walking speeds for both tests.25 The FAC also usually improves in parallel with faster speeds, but once patients are independent walkers, speed may decrease.18
The results of SIRROWS need to be confirmed by a more rigorous RCT because we designed this trial as a stage 3 intervention23 within the context of a phase 2 study. We had to plan the design in ways that were less optimal than other recent large trials for walking-related outcomes3,4 because we lacked financial resources to optimally manage SIRROWS and engaged rehabilitation sites that varied in experience and infrastructure. We aimed to make the instructions, data acquisition, and management so easy that the trial could be completed with integrity with only an electronic Internet-based data entry strategy and within the usual daily therapy plans of centers. We could not anticipate being able to increase the complexity of feedback, define the type or amount of daily physical therapy, or collect neuroimaging studies, quality of life scales, and other data that could be confounded by language and cultural differences. We also could not count on all sites to obtain outpatient measurements beyond the day of discharge, given likely differences in convenience of accessibility. Indeed, we encountered a sharp drop in collected data after discharge.
Web-based, neurorehabilitation MRCTs with spare protocols can include sites from any place in the world, enabling a large number of investigators to contribute to the development of everyday, evidence-based practices despite living in different health care cultures. This opportunity can increase the number of patients available to trials, enrich the daily practices of participating clinicians, and increase the ability to generalize the results of studies across populations. If collaborative networks like SIRROWS can enter large numbers of participants over relatively short periods, they can also test promising therapies for common and uncommon impairments and disabilities, develop dose–response curves prior to a phase 3 RCT, and use more sophisticated interventions and outcome measurements that are culturally relevant.26,27
Conclusion
Immediate reinforcement about daily walking speed during inpatient stroke rehabilitation may lead to clinically significant gains in walking speed. Further trials by a network of collaborating sites can build on the SIRROWS results by examining greater frequency of feedback during inpatient care and add feedback during the first 3 to 6 months of out-patient rehabilitation to determine the optimal dose of this strategy as well as examine longer-term outcomes.
Acknowledgments
We thank the American Society of Neurorehabilitation and the World Federation for NeuroRehabilitation for promoting this trial.
Funding
Support for the data management was provided by NIH grants to B. Dobkin, R21-HD050838 and R01-HD46740. Partial support to Chonnam National University was provided by Grant No. A084869 of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, and by the Brain Korea 21 Project, Center for Biomedical Human Resources.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
The following is a list of SIRROWS investigators. Germany: St. Mauritius Therapieklinik, Düsseldorf, Birgit Köepping, MD, Klaus-Martin Stephan, Julia Bohland. India: All India Institute of Medical Sciences, New Delhi, Shiv L. Yadav, MD. Italy: IRCCS San Raffaele Hospital, Milan, Mauro Comola, MD, Silvia Mammi, Andrea Tettamanti, Roberto Gatti; Fondazione Instituto San Raffaele-Giglio, Giuseppe Galardi, MD, Francesca Rubino, Cristina Boccagni; IRCCS San Camillo, Venezia, Paolo Tonin, MD, Carla Zucconi, Vincenzo Iaia, Michela Agostini. Japan: Morinomiya Hospital, Osaka, Ichiro Miyai, MD, PhD, Hajime Yagura, Megumi Shima. Korea: Chonnam National University Medical School & Hospital, Sam-Gyu Lee, MD, PhD, Jae-Young Han, In-Sung Choi. Nigeria: Federal Medical Center, Abeokuta, Mayowa Owolabi, MD, Michael Ogunlana. Turkey: Gazi University Faculty of Medicine, Ankara, Gulcin Kaymak Karatas, MD, Ayca Utkan, MD; Ankara University Faculty of Medicine, Ankara, Gunes Yavuzer, MD, PhD, Vildan Binay. USA: Kessler Foundation Research Center, West Orange, NJ, Anna Barrett, MD, Cristin McKenna, MD, PhD, Kirk Endersby, Daniel Kalemba; Mayo Clinic, Rochester, MN, Allen W. Brown, MD, Bart Hanson, Sarah Reynolds, Steve Finnie, Mary Gordon; St Luke’s and Whiteside Institute for Clinical Research, Duluth, MN, Ed Crisostomo, MD, Lora Lockett, Summer Arce; St Luke’s Hospital, Bethlehem, PA, Robert Coni, DO, Jason Kundek, Kristin Lapp, Kristen Kramer; Burke Rehabilitation Hospital, NY, Pasquale Fonzetti, MD, PhD, Sandra Alexandrou, Diana Zondorak, Jennifer Matuszewski; Kernan Hospital, Baltimore, MD, Glenn Kehs, MD, Gertrude Morrison, RN; Rehabilitation Hospital of Rhode Island, North Smithfield, RI, Stephen Mernoff, MD, Jacqueline Brennan, John Warrington, Tracy Jackson, Caryn Sullivan, Jeanne Pascale; Vanderbilt Stallworth Rehabilitation Hospital, Nashville, TN, Shelby Nye, DPT, Adrian A. Jarquin-Valdivia, Sarah Kelley, Amanda Burnett, Kelly Merrell.
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22:649–660. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 2.Dobkin B, Apple D, Barbeau H, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair. 2003;17:153–167. doi: 10.1177/0888439003255508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobkin BH, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan PW, Sullivan KJ, Behrman AL, et al. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7:39. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidler J, Nichols D, Pelliccino M, et al. Multi-center randomized clinical trial evaluating the effectiveness of the Lokomat in sub-acute stroke. Neurorehabil Neural Repair. 2009;23:5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 6.Kollen B, Kwakkel G, Lindeman E. Hemiplegic gait after stroke: is measurement of maximum speed required? Arch Phys Med Rehabil. 2006;87:358–363. doi: 10.1016/j.apmr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neuro-rehabil Neural Repair. 2008;22:672–675. doi: 10.1177/1545968308318837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reisman D, Rudolph K, Farquhar W. Influence of speed on walking economy poststroke. Neurorehabil Neural Repair. 2009;23:529–534. doi: 10.1177/1545968308328732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry J, Garrett M, Gromley J, Mulroy S. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 10.Schmid A, Duncan P, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 11.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients. Stroke. 2002;33:553–558. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 12.Laufer Y, Dickstein R, Chefez Y, Marcovitz E. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation: a randomized study. J Rehabil Res Dev. 2001;38:69–78. [PubMed] [Google Scholar]
- 13.Carr J, Shepherd R. Neurological Rehabilitation: Optimizing Motor Performance. Oxford, UK: Butterworth Heinemann; 1998. [Google Scholar]
- 14.Byl NN, Pitsch EA, Abrams GM. Functional outcomes can vary by dose: learning-based sensorimotor training for patients stable poststroke. Neurorehabil Neural Repair. 2008;22:494–504. doi: 10.1177/1545968308317431. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian S, Massie C, Malcolm M, Levin M. Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence [published online ahead of print October 27, 2009] Neu-rorehabil Neural Repair. doi: 10.1177/1545968309349941. [DOI] [PubMed] [Google Scholar]
- 16.Mehrholz J, Wagner K, Rutte K, Meiner D, Pohl M. Predictive validity and responsiveness of the Functional Ambulation Category in hemiparetic patients after stroke. Arch Phys Med Rehabil. 2007;88:1314–1319. doi: 10.1016/j.apmr.2007.06.764. [DOI] [PubMed] [Google Scholar]
- 17.States R, Pappas E, Salem Y. Overground physical therapy for gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev. 2009;(3):CD006075. doi: 10.1002/14651858.CD006075.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollen B, Kwakkel G, Lindeman E. Time dependency of walking classification in stroke. Phys Ther. 2006;86:618–625. [PubMed] [Google Scholar]
- 19.Rabadi M, Blau A. Admission ambulation velocity predicts length of stay and discharge disposition following stroke in an acute rehabilitation hospital. Neurorehabil Neural Repair. 2005;19:20–26. doi: 10.1177/1545968304272762. [DOI] [PubMed] [Google Scholar]
- 20.Cooke EV, Tallis R, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: phase I randomized controlled trial [published online ahead of print August 24, 2009] Neurore-habil Neural Repair. doi: 10.1177/1545968309343216. [DOI] [PubMed] [Google Scholar]
- 21.Kwakkel G, Wagenaar R, Twisk J, Lankhorst G, Koetsier J. Intensity of leg and arm training after primary middle cerebral artery stroke: a randomised trial. Lancet. 1999;354:191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 22.Mehrholz J, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2007;(4):CD006185. doi: 10.1002/14651858.CD006185.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Dobkin BH. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil Neural Repair. 2009;23:197–206. doi: 10.1177/1545968309331863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boysen G, Krarup L-H, Zeng X, et al. ExStroke pilot trial of the effect of repeated instructions to improve physical activity after ischemic stroke: a multinational randomised controlled clinical trial. BMJ. 2009;339:b2810. doi: 10.1136/bmj.b2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobkin BH. Short-distance walking speed and timed walking distance: redundant measures for clinical trials? Neurology. 2006;66:584–586. doi: 10.1212/01.wnl.0000198502.88147.dd. [DOI] [PubMed] [Google Scholar]
- 26.Cheeran B, Cohen L, Dobkin B, et al. The restorative neurosciences in stroke: driving the translational research pipeline from basic science to the rehabilitation of people after stroke. Cumberland Consensus Group. Neurorehabil Neural Repair. 2009;23:97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobkin BH. Collaborative models for translational neuroscience research. Neurorehabil Neural Repair. 2009;23:633–640. doi: 10.1177/1545968309338290. [DOI] [PMC free article] [PubMed] [Google Scholar]