Abstract
Localized to the vestibule of the nasal cavity, neurons of the Grueneberg ganglion (GG) respond to cool ambient temperatures. The molecular mechanisms underlying this thermal response are still elusive. Recently, it has been suggested that cool temperatures may activate a cyclic guanosine monophosphate (cGMP) pathway in the GG, which would be reminiscent of thermosensory neurons in Caenorhabditis elegans. In search for other elements of such a cascade, we have found that the cyclic nucleotide-gated ion channel CNGA3 was strongly expressed in the GG and that expression of CNGA3 was confined to those cells that are responsive to coolness. Further experiments revealed that the response of GG neurons to cool temperatures was significantly reduced in CNGA3-deficient mice compared to wild-type conspecifics. The observation that a cGMP-activated non-selective cation channel significantly contributes to the coolness-evoked response in GG neurons strongly suggests that a cGMP cascade is part of the transduction process.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0296-8) contains supplementary material, which is available to authorized users.
Keywords: Olfaction, Thermosensory, Transmembrane guanylyl cyclase, cGMP, c-Fos
Introduction
The perception of odorous molecules is mediated by olfactory sensory neurons (OSNs) located in distinct nasal compartments, including the main olfactory epithelium (MOE), the vomeronasal organ and the septal organ [1–3]. OSNs are generally characterized by the expression of the olfactory marker protein (OMP) and distinct olfactory receptor types as well as axonal projections to defined glomeruli in the olfactory bulb (OB). Recently, it has been discovered that neuronal cells in the Grueneberg ganglion (GG) of the anterior nasal region also express OMP and project their axons to the OB [4–8]. Based on these findings, along with the expression of olfactory receptors [9, 10], GG neurons are considered as chemosensory cells. Recent findings, however, indicate that GG neurons, in addition to a possible chemosensory function [11], respond to cool ambient temperatures [12]. The molecular mechanisms underlying the coolness-induced responses of GG neurons are unknown. In mammals, the registration of changes in the ambient temperature by afferent fibers from the dorsal root ganglion or the trigeminal ganglion is supposed to be mediated by transient receptor potential (TRP) channels, which serve as thermal sensors [13–16]. Among the various TRP subtypes, TRPM8 is considered as an essential element for sensing coolness [17–21]. However, recent studies have shown that TRPM8 is apparently absent from the GG [22]. The nematode Caenorhabditis elegans (C. elegans) detects changes in the environmental temperature via the so-called AFD neurons; in these cells, the thermal transduction process is mediated by a cyclic guanosine monophosphate (cGMP) pathway that comprises transmembrane guanylyl cyclases and cyclic nucleotide-gated (CNG) ion channels [23–25]. Recent approaches to decipher the mechanisms underlying the coolness-induced responses of the GG have led to the observation that a distinct transmembrane guanylyl cyclase, the subtype GC-G, was expressed in the majority of the GG neurons [22, 26]. Importantly, only those GG cells responded to coolness that were endowed with GC-G [12, 22]. These findings point to a possible function of a cGMP cascade in coolness-evoked signaling in the GG. For cGMP-mediated pathways leading to an electrical response of the cell, CNG channels are supposed to play a crucial role [27]. Therefore, in this study, it was investigated whether GG neurons express defined CNG channel subtypes and whether these proteins may be required for coolness-induced responses.
Materials and methods
Mice
This study was performed on mice of wild-type strain C57/BL6J purchased from Charles River (Sulzfeld, Germany). The generation of the CNGA3-deficient mouse strain (CNGA3−/−) has been described previously [28]. All experiments comply with the Principles of Animal Care, publication no. 85-23, revised 1985, of the National Institutes of Health and with the current laws of Germany.
RNA isolation and cDNA synthesis
Isolation of RNA from the GG and subsequent cDNA synthesis were carried out as described elsewhere [9]. For preparation of cDNA from the retina or the MOE, these tissues were dissected, and total RNA was isolated with the NucleoSpin RNA II kit (Macherey–Nagel, Dueren, Germany). Mouse genomic DNA was isolated using the peqGold tissue DNA Mini Kit (Peqlab Biotechnologie, Erlangen, Germany).
Design of oligonucleotide primers
For amplification of sequences encoding distinct CNG subtypes, the following primers were used: CNGA1: 5′-ctacagcctttattggtctac and 5′-tagagactccgtgagctcact; CNGA2: 5′-cgtctgttacactttgcccgtatg and 5′-ggtgtttatcccatctgataggt; CNGA3: 5′-cggaagtacatttacagtctc and 5′-acctcctgttcatctggctct; CNGA4: 5′-gcatagccaagctgatgatct and 5′-gtaagctatcttcagtgcgct; CNGB1: 5′-tcaggaccacggcctacctgct and 5′-gtccaatcatcacagagaaagca; CNGB3: 5′-gctcctgcttgtcaccattgcgt and 5′-ctggtcttgcagggattgcctga. All oligonucleotide primer sequences are given according to the code of the International Union of Biochemistry (IUB code). Oligonucleotide primers were ordered from http://www.biomers.net (Ulm, Germany).
PCR
Polymerase chain reaction (PCR) amplification was performed as described previously [9]. PCR products were cloned into pGem-T plasmids (Promega, Madison, WI) and were subjected to sequence analysis using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Exposure to cool ambient temperatures
Female mice were kept together with their pups in a cage under a 12-h light/dark cycle (light on at 7:00 a.m.). For exposure to a given ambient temperature (15, 22 or 30°C), neonatal pups were transferred (without their mother) to a cage placed in an incubator (CERTOMAT BS-1, B. Braun Biotech International, Melsungen, Germany) adjusted to the desired temperature. The pups were killed directly after exposure by decapitation.
Tissue preparation
For in situ hybridization, heads of mice were dissected in 1× PBS (0.85% NaCl, 1.4 mM KH2PO4, 8 mM Na2HPO4, pH 7.4), embedded in Leica OCT Cryocompound “tissue freezing medium” (Leica Microsystems, Bensheim, Germany) and quickly frozen on dry ice. Sections (12–14 μm) were cut on a CM3050S cryostat (Leica Microsystems, Bensheim, Germany) and adhered to Polysine slides (Menzel, Braunschweig, Germany). For immunohistochemistry, heads of mice were prepared as described above, fixed in 4% paraformaldehyde in 1× PBS for 30 min at 4°C followed by cryoprotection in 25% sucrose (in 1× PBS) at 4°C overnight. Sections (12–25 μm) were cut on a CM3050S cryostat (Leica Microsystems) and adhered to Superfrost Plus microscope slides (Menzel).
In situ hybridization
Digoxigenin- and biotin-labeled antisense riboprobes were generated from partial cDNA clones in pGem-T plasmids encoding mouse CNGA2 (National Center for Biotechnology Information Genbank accession number: NM_007724.2; nucleotide 1,091–2,311), CNGA3 (BC049145.1; nucleotide 1,184–2,153), CNGA4 (NM_001033317.3; nucleotide 627–1,705), GC-G (NM_001081076.1; nucleotide 2,250–3,281), V2r83 (a V2r83-encoding fragment of 2,500 base pairs was amplified from GG cDNA using the primers 5′-atggccagcagacagataag and 5′-actgctcacagaagctgagg), TAAR6 (BC148624.1; nucleotide 209–879), TAAR7D (BC153148.1; nucleotide 233–900), c-Fos (NM_010234; nucleotide 160–1,841) or OMP (NM_011010.2; nucleotide 96–2,062) using the T7/SP6 RNA transcription system (Roche Diagnostics, Mannheim, Germany) as recommended by the manufacturer. After fixation in 4% paraformaldehyde/0.1 M NaHCO3/pH 9.5 for 45 min at 4°C, slices were washed in 1× PBS for 1 min at room temperature, then incubated in 0.2 M HCl for 10 min, in 1% Triton X-100/1× PBS for 2 min and again washed twice in 1× PBS for 30 s. Finally, sections were incubated in 50% formamide/5× SSC (0.75 M NaCl, 0.075 M sodium citrate, pH 7.0) for 10 min. Then tissue was hybridized in hybridization buffer [50% formamide, 25% H2O, 25% Microarray Hybridization Solution Version 2.0 (GE Healthcare, Freiburg, Germany)] containing the probe and incubated in a humid box (50% formamide) at 65°C overnight.
After slides were washed twice in 0.1× SSC for 30 min at 65°C, they were treated with 1% blocking reagent (Roche Diagnostics, Mannheim, Germany) in TBS (100 mM TRIS, 150 mM NaCl, pH 7.5) with 0.3% Triton X-100 for 30 min at room temperature and incubated with an anti-digoxigenin alkaline phosphatase-conjugated antibody (Roche Diagnostics) diluted 1:750 in TBS/0.3% Triton X-100/1% blocking reagent at 37°C for 30 min. After washing twice in TBS for 15 min, slides were rinsed in DAP buffer (100 mM TRIS, pH 9.5, 100 mM NaCl, 50 mM MgCl2). Hybridization signals were visualized using NBT (nitroblue tetrazolium) and BCIP (5-brom-4-chlor-3-indolyl phosphate) as substrates. Sections were mounted in Vectamount mounting medium (Vector Laboratories, Burlingame, CA).
For double-label fluorescent in situ hybridization experiments, fixation, hybridization, washing and blocking were carried out as described above. However, a different hybridization buffer (50% formamide, 2× SSC, 10% dextran sulphate, 0.2 mg/ml yeast t-RNA, 0.2 mg/ml sonicated herring sperm DNA) was used, and sections were simultaneously hybridized with digoxigenin- and biotin-labeled probes. To visualize the probes, sections were incubated for 60 min in TBS/0.3% Triton X-100/1% blocking reagent supplemented with anti-digoxigenin alkaline phosphatase-conjugated antibody (1:500, Roche) and streptavidin-horseradish peroxidase (1:100; TSA Fluorescein System, Perkin Elmer Life Sciences, Boston, MA) at 37°C followed by incubation with HNPP/Fast Red TR solution (HNPP Fluorescent Detection Set, Roche Diagnostics) for 30 min at room temperature. After washing the sections three times for 5 min each with TBS/0.05% Tween 20, biotin-labeled probes were subsequently visualized using the TSA Fluorescein System (Perkin Elmer Life Sciences). After washing the sections again three times for 5 min each with TBS/0.05% Tween 20, they were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/ml in TBS) for 3 min at room temperature and briefly rinsed with H2O. Finally, sections were mounted in 66% glycerol/1× PBS.
Immunohistochemistry
Immunohistochemistry was performed as described previously [9]. To monitor localization of CNGA3, a specific polyclonal antibody (AbmCG3; [28]) generated in rabbit was used at a dilution of 1:500. Secondary detection was carried out by using appropriate secondary antibodies coupled to Alexa dyes (Invitrogen, Carlsbad, CA). Counterstaining was performed for 3 min with propidium iodide (1 μg/ml in 1× PBS). Finally, sections were rinsed with H2O and subsequently mounted in 66% glycerol/1× PBS.
Microscopy and photography
Sections were photographed using a Zeiss Axiophot (Carl Zeiss MicroImaging, Göttingen, Germany). Fluorescence was examined with a SensiCam CCD camera (PCO, Kelheim, Germany) and the Zeiss Axiovision imaging system (Zeiss) with appropriate filter sets.
Results
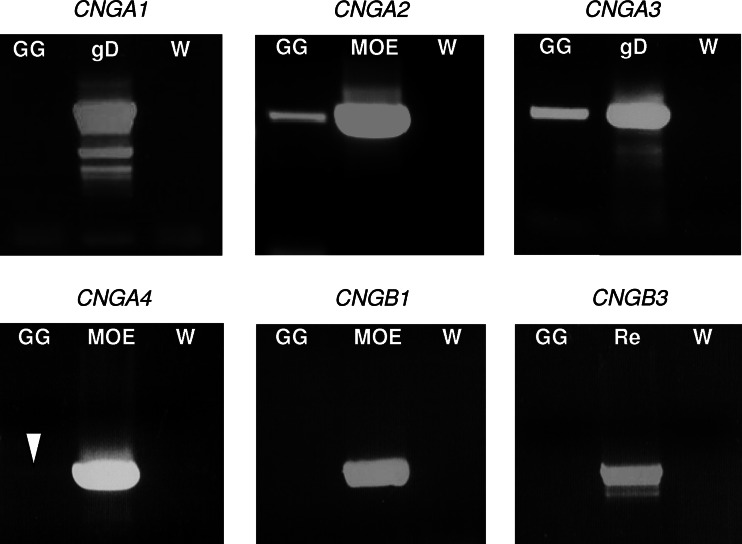
As an initial step to assess whether GG neurons express CNG channels, PCR experiments were performed. Out of the six known mammalian CNG subtypes (CNGA1 through CNGA4, CNGB1 and CNGB3), only the principal subunits CNGA1, CNGA2 and CNGA3 form functional channels on their own [29]. In PCR approaches with primer pairs specific for coding sequences of these three subunits, amplicons of the expected size were amplified from mouse genomic DNA (CNGA1 and CNGA3) or MOE cDNA (CNGA2) in control experiments (Fig. 1). Using cDNA from the GG of neonatal mice as template, no amplicon was obtained for CNGA1, but appropriate PCR products were amplified for CNGA2 and CNGA3 (Fig. 1). For CNGA2, however, amplification was rather weak. Regarding the modulatory CNG subunits (CNGA4, CNGB1, CNGB3), PCR approaches with specific primers gave no amplification for CNGB1 and CNGB3 from GG cDNA; a faint amplification was observed for CNGA4 (Fig. 1). In control PCR experiments, fragments of the expected size were amplified for CNGB1 and CNGB3 from cDNA of the MOE or the retina, respectively (Fig. 1).
Fig. 1.
Identification of CNG channel subunits expressed in the GG by PCR experiments. In PCR approaches with specific primer pairs matching to the coding sequence of CNG channel subtypes CNGA1, CNGA2, CNGA3, CNGA4, CNGB1 and CNGB3, no amplicons of the expected molecular size were obtained from GG cDNA of several-day-old postnatal animals for CNGA1, CNGB1 and CNGB3. In control experiments, using the same primers, such PCR products were easily amplified from genomic DNA (gD) or cDNA of the MOE or retina (Re), respectively (PCR reactions without template are indicated by “W”). With primers for CNGA2, CNGA3 or CNGA4 (arrowhead), PCR products of the predicted size were obtained from GG cDNA
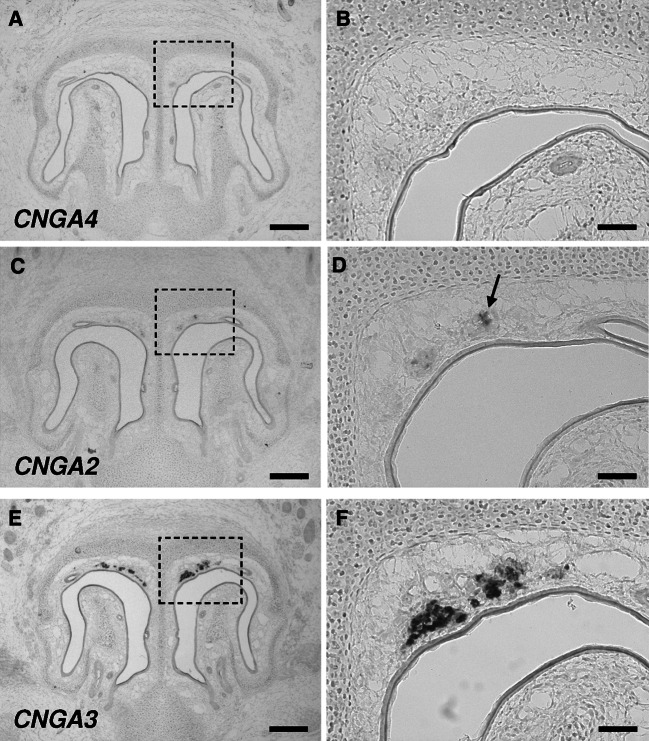
To investigate potential expression of CNGA2, CNGA3 and CNGA4 in the GG in more detail, in situ hybridization experiments were conducted with antisense riboprobes specific for these CNG subunits. Using probes for CNGA2 or CNGA4, no cells (CNGA4; Fig. 2a, b) or only a few of them (CNGA2; Fig. 2c, d) were labeled; in the latter case, labeling was weak. The same probes intensely stained cells in the MOE (supplemental Fig. 1), which are known to express CNGA2 and CNGA4 [30–32]. Thus, these results of in situ hybridization experiments revealed that CNGA2 and CNGA4 are not significantly expressed in the GG. In contrast, hybridizing sections through the GG with an antisense probe for CNGA3 led to numerous signals (Fig. 2e, f), indicating that many GG cells express CNGA3. The specificity of these signals was confirmed by control experiments in which the corresponding sense probe did not label cells in the GG (supplemental Fig. 2).
Fig. 2.
Expression of CNGA3 in numerous GG cells. a–f In situ hybridization experiments with antisense probes specific for CNGA4 (a, b), CNGA2 (c, d) or CNGA3 (e, f) on coronal sections through the GG of pups. b, d and f Show higher magnifications of the boxed areas in a, c and e. These experiments revealed that CNGA4 is absent from the GG, whereas CNGA2 is weakly expressed by a subset of cells in the GG (arrow in d). CNGA3, however, is strongly expressed in numerous GG cells. Scale bars a, c, e = 200 μm; b, d, f = 50 μm
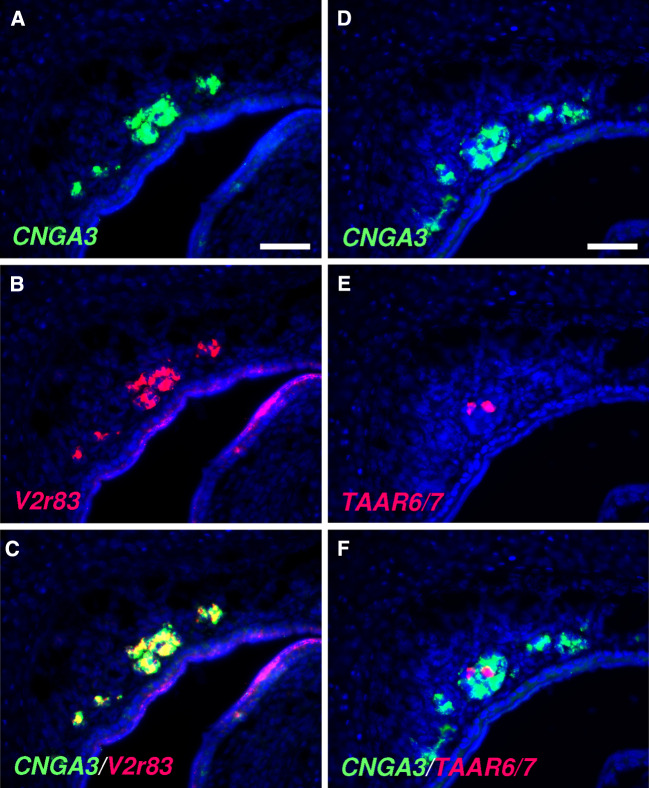
To analyze whether CNGA3 is expressed by OMP-positive neurons in the GG, double-labeling experiments were conducted. By two-color in situ hybridization, it was found that CNGA3 is expressed in a large subset of OMP-positive GG neurons (Fig. 3). OMP-positive GG cells can be subdivided into two distinct subpopulations: one is characterized by the expression of receptor V2r83 and is activated by coolness, whereas the other expresses members of the trace amine-associated receptor (TAAR) family and apparently does not respond to cool ambient temperatures [10, 12]. In this regard, double-staining in situ hybridization experiments revealed that CNGA3 is expressed by V2r83-positive GG neurons (Fig. 4a–c). This finding is in line with the observation that the number of V2r83-expressing GG neurons in newborn mouse pups (approximately 653 cells per individual [10]) is almost identical to the number of CNGA3-positive cells in the GG (about 666 cells per individual; as determined by in situ hybridization experiments with a CNGA3-specific antisense probe in which the number of labeled cells in three animals was counted). By contrast, GG cells expressing the two most abundant TAAR subtypes (TAAR6 and TAAR7; [10]) lack co-expression of CNGA3 (Fig. 4d–f). It has been shown recently that V2r83-positive GG cells also express the transmembrane guanylyl cyclase G (GC-G) and the cGMP-dependent phosphodiesterase 2A (PDE2A; [22]). These findings suggest that CNGA3 is co-expressed with these two cGMP-associated signaling elements. In fact, double-staining in situ hybridization experiments demonstrated that CNGA3 is expressed in a substantial number of GC-G-positive GG neurons (Fig. 5). Thus, it can be concluded that CNGA3 is expressed in coolness-sensitive GG cells, which are endowed with a cGMP-mediated signaling cascade.
Fig. 3.

CNGA3 is expressed in a large subpopulation of OMP-positive GG neurons. a–c Two-color in situ hybridization on a coronal section through the GG from an early postnatal stage using antisense RNA probes for CNGA3 (a, red) and OMP (b, green). The overlay (c) demonstrates expression of CNGA3 in the overwhelming majority of OMP-positive GG neurons. The section was counterstained with DAPI (blue). Scale bar = 50 μm
Fig. 4.
Expression of CNGA3 in a receptor-specific subset of GG neurons. a–c Double-labeling in situ hybridization on a coronal section through the GG of an early postnatal mouse with antisense riboprobes for CNGA3 (a, green) and V2r83 (b, red). The merged image (c) demonstrates expression of CNGA3 by V2r83-positive GG neurons. d–f In situ hybridization with probes for CNGA3 (d, green), TAAR6 and TAAR7 (e, red) revealed absence of CNGA3 from TAAR-positive GG neurons (f). Sections were counterstained with DAPI (blue). Scale bars = 50 μm
Fig. 5.
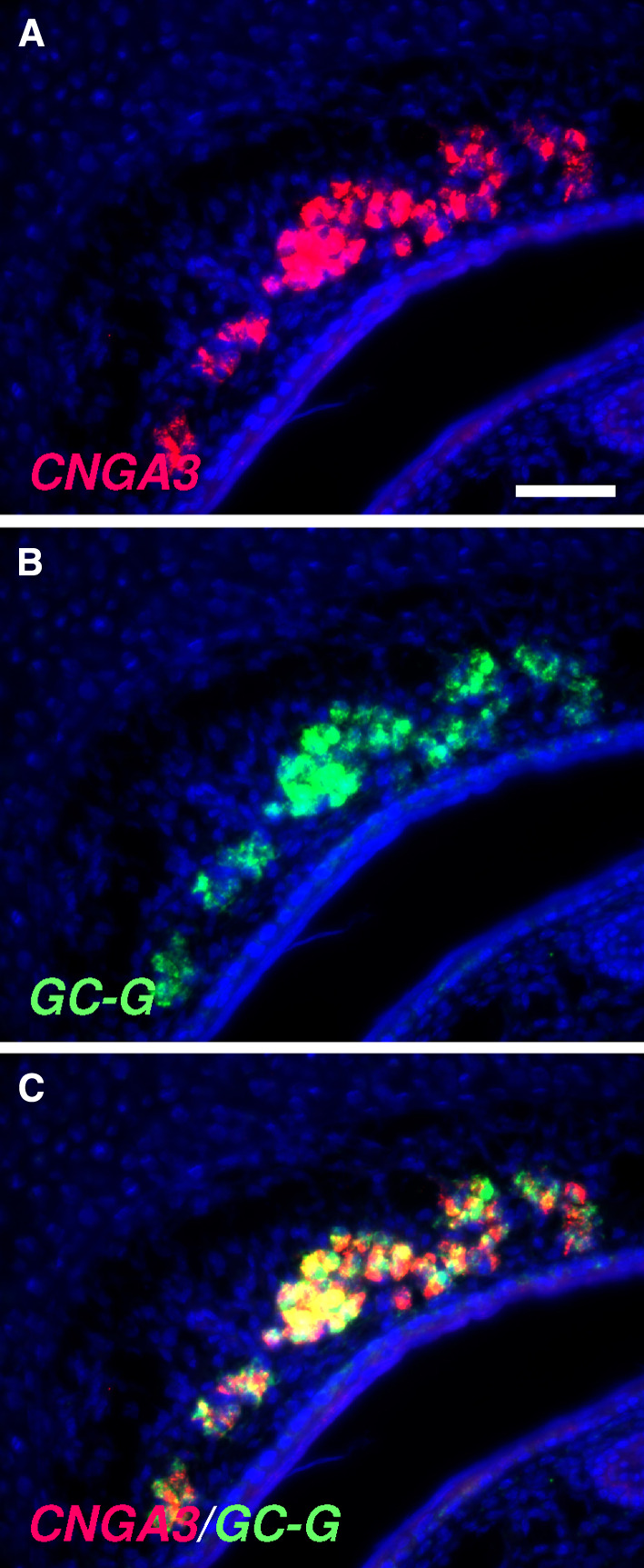
Co-expression of CNGA3 and GC-G in the GG of pups. a–c Double fluorescent in situ hybridization with antisense probes for CNGA3 (a, red) and GC-G (b, green) reveals co-expression of these two signaling elements in GG neurons (merged image in c). The section was counterstained with DAPI (blue). Scale bar = 50 μm
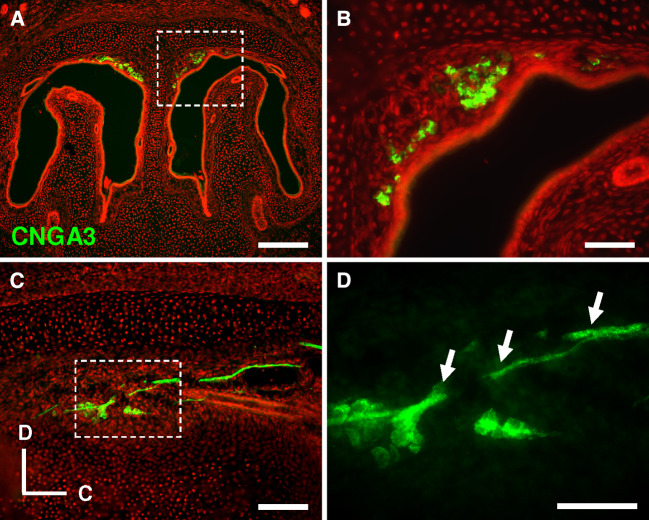
To visualize the subcellular localization of the CNGA3 protein in GG neurons, immunohistochemical approaches with a CNGA3-specific antibody were conducted. In these experiments, it was found that the somata of numerous GG cells were intensely stained (Fig. 6a, b). In CNGA3-deficient (CNGA3−/−) mice, no labeled cells were detectable in the GG (supplemental Fig. 3b), confirming the specificity of the staining in Fig. 6a, b. On sagittal sections (Fig. 6c, d), it became evident that the CNGA3 protein is localized to the somata and the axonal processes of GG neurons.
Fig. 6.
The CNGA3 protein is localized to the soma and the axon of GG neurons. a Immunohistochemical staining with a CNGA3-specific antibody on a coronal section through the GG of a CNGA3+/+ pup. b High magnification image of the boxed area in a. The soma of GG neurons is clearly stained. c–d Using the anti-CNGA3 antibody for immunostaining on sagittal sections [caudal (C) is to the right and dorsal (D) to the top] through the GG of wild-type animals, in addition to the soma, the CNGA3 protein was also found in axonal processes of GG neurons (arrows in d). The boxed area in c is shown at a higher magnification in d. Sections were counterstained with propidium iodide (red, a–c). Scale bars a = 200 μm; b, d = 50 μm; c = 100 μm
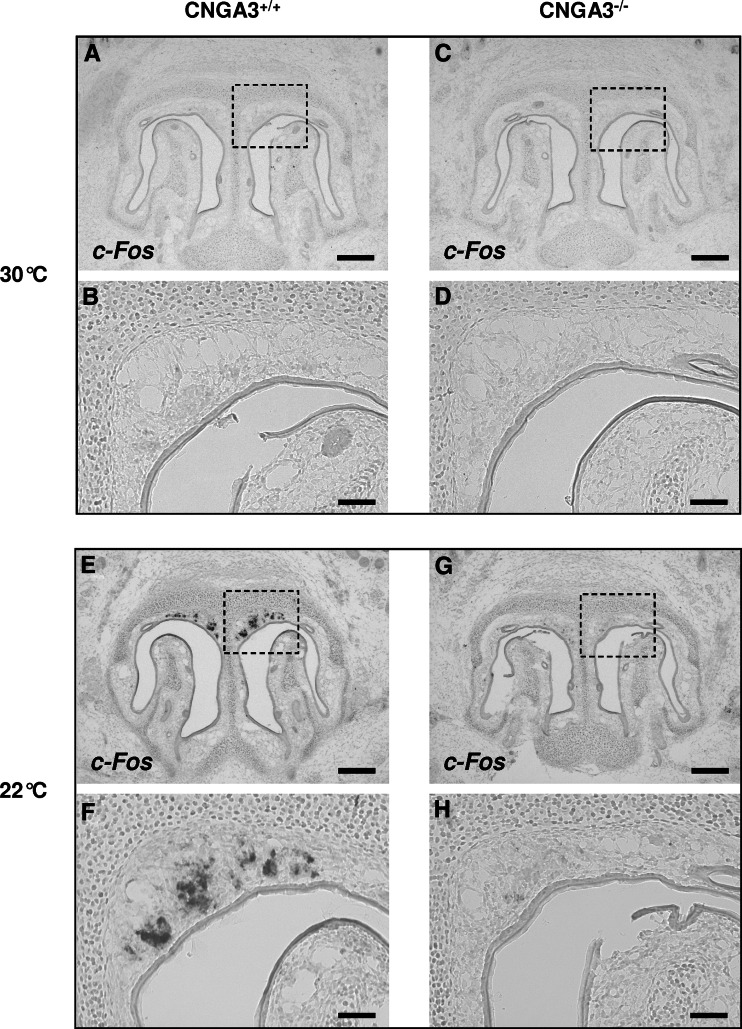
Monitoring the expression of the activity-dependent gene c-Fos, it was recently found that GG neurons respond to cool ambient temperatures (22°C or below) [12]. To approach the question whether the CNGA3 channel may be involved in mediating coolness-induced responses of GG neurons, we set out to compare the GG of wild-type and CNGA3-deficient mice concerning their responsiveness to cool ambient temperatures. In control experiments, it was verified that the GG of CNGA3−/− mice expresses signaling elements characteristic of coolness-responding GG cells (OMP, V2r83 and GC-G) to a similar extent as the GG of wild-type (CNGA3+/+) animals (supplemental Fig. 4). Next, neonatal pups were exposed to a warm ambient temperature (30°C) for 2 h, and the GG was subsequently analyzed for c-Fos expression by in situ hybridization. As documented in Fig. 7a–d, no hybridization signals were observed in either wild-type or CNGA3-deficient mice. When the same experiment was conducted at a cool ambient temperature (22°C), significant differences were noticed between CNGA3+/+ and CNGA3−/− animals: in the GG of CNGA3+/+ individuals, a larger number of intensely stained c-Fos-positive cells (about 569 cells per individual; as determined by counting the number of labeled cells in three animals) were detectable, indicating activation of these cells by coolness (Fig. 7e, f). By contrast, in the GG of CNGA3−/− mice, labeling was comparatively faint or even absent (Fig. 7g, h); i.e., compared to wild-type animals, only a few cells were c-Fos-positive (if there were stained cells at all in CNGA3−/− mice). Moreover, in many cases, even in these cells, labeling was weak. As for 22°C, also at 15°C, clear differences regarding the c-Fos expression in the GG were observed between wild-type and CNGA3−/− mice (supplemental Fig. 5). When heterozygous (CNGA3+/−) pups were exposed to cool temperatures (22°C), c-Fos signals were similar to those in wild-type conspecifics (supplemental Fig. 6). Thus, in summary, these findings suggest that the cyclic nucleotide-gated ion channel CNGA3 significantly contributes to the generation of coolness-induced responses in GG neurons.
Fig. 7.
Attenuation of coolness-induced responses in the GG of CNGA3-deficient mice. a–h In situ hybridization experiments with an antisense probe for c-Fos on coronal sections through the GG of wild-type (left panel) or CNGA3-deficient (right panel) pups exposed to a warm (30°C; a–d) or a cool (22°C; e–h) ambient temperature for 2 h. b, d, f and h Show higher magnifications of the boxed areas in a, c, e and g. At 30°C (a–d), no c-Fos expression was detectable in the GG of both wild-type (a, b) and CNGA3−/− (c, d) individuals. Upon exposure to 22°C, strong c-Fos expression was observed in the GG of wild-type pups (e, f), whereas c-Fos expression was hardly detectable in the GG of CNGA3-deficient individuals (g, h). The data shown in a–h are representative of four independent experiments. For each of these four independent experiments, a “novel” litter was used. From each of these litters, one to three animals were used for each temperature tested. Scale bars a, c, e, g = 200 μm; b, d, f, h = 50 μm
Discussion
Recent experimental evidence indicates that neuronal cells in the GG are responsive to chemical [11] as well as thermal stimuli [12]. The molecular mechanisms underlying this sensory capacity are unknown. Regarding the response to cool temperatures, it is interesting to note that GG neurons lack expression of the coolness-sensitive ion channel TRPM8 [22], which is considered to render nerve fibers of the somatosensory system responsive to coolness [19–21]. Therefore, it was proposed that the responsiveness of GG neurons to coolness may be due to an alternative pathway, possibly mediated by the second messenger cGMP [22]. Such a mechanism would be reminiscent of thermosensory signaling in the nematode C. elegans, which involves transmembrane guanylyl cyclases and CNG channels [23–25, 33]. This concept received support from the finding that a distinct transmembrane guanylyl cyclase (subtype GC-G) and the phosphodiesterase PDE2A are expressed in those GG neurons that respond to coolness [22]. In the present study, it was found that the cGMP-activated CNG subunit CNGA3 is expressed in numerous GG neurons. Apart from a weak expression of CNGA2, no further CNG subunits were found to be expressed in the GG. Vertebrate CNG channels are supposed to function as hetero-oligomers of cyclic nucleotide-activated subunits (CNGA1, CNGA2 or CNGA3) with modulatory subunits (CNGA4, CNGB1 or CNGB3) [29, 34]. Thus, the observation that the majority of GG neurons seems to express CNGA3 only raises the question whether a homomeric CNGA3 channel may operate in GG cells. Interestingly, in heterologous expression systems, it has been observed previously that CNGA3 forms functional channels in the absence of other CNG subunits [35, 36]. The specific expression in only the coolness-sensitive and V2r83-positive GG neurons (Fig. 4) suggests that CNGA3 might play a role in coolness-evoked signaling in these cells. The finding that coolness-induced c-Fos expression was significantly reduced in CNGA3-knockout mice compared to wild-type conspecifics strongly indicates that CNGA3 is a crucial element in the mechanism mediating the responses of GG neurons to cool temperatures. Such a thermosensory pathway involving cGMP-gated ion channels would be indeed reminiscent of neurons in C. elegans, in which thermosensation is mediated by a cGMP cascade encompassing CNG channels [23–25, 33]. Hence, a pathway related to the thermosensory signaling cascade of C. elegans might operate in coolness-sensitive GG cells. Since responses of thermosensory neurons in mammals are generally supposed to be accomplished via temperature-sensitive TRP channels [14–16], the present finding that CNGA3 contributes to coolness-induced signaling may point to an alternative, cGMP-dependent transduction cascade for thermal responses in mammals. Furthermore, because activation of CNG channels is known to cause an influx of cations, consequently leading to a depolarization of the plasma membrane [29], it can be assumed that in GG neurons, coolness evokes receptor potentials conceivably eliciting the generation of action potentials that might be conveyed to the brain via axonal processes of GG cells.
The functional relevance of coolness-induced responses in GG neurons is so far elusive. Based on the observation that GG neurons project their axons to so-called “necklace” glomeruli in the OB and due to the finding that the number of GG cells peaks in perinatal stages, it has been proposed that the GG could be involved in mother/child interactions [4, 7, 10]. Interestingly, in pups, coolness-evoked responses of the GG were only observed in the absence of the dam, when pups are exposed to cool ambient temperatures [12]. Therefore, it is conceivable that the coolness-induced responses of the GG provide an alerting signal for pups, indicating the absence of their mother. Such a signal could make the pups search or call for their mother. However, in CNGA3-deficient pups, in which coolness-induced responses of the GG are diminished, we have not made any spontaneous observations regarding aberrant pup or maternal behavior (our unpublished observations). In addition, in a previous study [28], it was found that CNGA3−/− mice were normal in their appearance and body weight and that there was no significant difference in mortality between wild-type and knockout animals, indicating that suckling and thriving of pups, which are dependent on close mother/child interactions, are similar in wild-type and CNGA3-deficient mice. Concerning a potential function of thermosensory signaling in the GG, it has been speculated recently that coolness-induced responses in the GG of pups might be associated with the so-called “huddling” behavior, which allows pups to minimize heat loss because of physical contact with littermates [12]. However, in preliminary experiments, it was found that similar to wild-type conspecifics, also CNGA3-deficient pups clearly huddle (our unpublished observations). Thus, further studies are required for a better understanding of the functional implications of coolness-induced responses in the GG.
In C. elegans, CNG channels are expressed in distinct sensory neuron types, including the so-called AWC neurons [23, 24, 33, 37]. Interestingly, these neurons not only respond to temperature but also to chemical stimuli, and both chemo- and thermosensation rely on cGMP signaling via CNG channels [23, 24, 33]. Based on the analogy to AWC neurons, it is tempting to speculate that cGMP-associated signaling proteins in GG neurons might not only be involved in coolness-evoked responses but also contribute to chemosensory signaling. Importantly, in this regard, CNGA3 is crucial for chemoresponsiveness of another cell type in the nose of mice, the so-called GC-D neurons [38–40]. Thus, the signaling elements contributing to chemosensation in GC-D neurons (CNGA3 and the transmembrane guanylyl cyclase GC-D) [39, 40] are closely related or even identical to cGMP-associated proteins (GC-G and CNGA3) in GG neurons ([22, 26], this study). Moreover, both GC-D neurons and GG neurons express the cGMP-dependent phosphodiesterase PDE2A [22, 26, 41]. Accordingly, CNGA3 could also contribute to the recently described chemosensory signaling of GG neurons [11].
By immunohistochemistry, during the course of the present study, the CNGA3 protein was observed to be localized to distinct compartments of GG neurons, including the soma and the axon. This observation is consistent with previous findings that have demonstrated that in GC-D neurons of the MOE, CNGA3-specific immunostaining was not confined to apical sensory compartments but was also found throughout the cell body [38]. Since CNG channels are known transmembrane proteins [27, 29, 34], it can be assumed that at least a substantial portion of the CNGA3 protein in GG neurons is localized to the cell membrane. Thus, there might be different fractions of the CNGA3 protein in the soma of GG neurons: a membranous and a cytoplasmic fraction; thereby, the cytoplasmic one could represent freshly synthesized protein on its way to the cell membrane or the axon, whereas the membranous fraction is presumably involved in the conductance of electrical currents. Concerning the presence of CNGA3 in GG axons, a further potential function of this protein in GG neurons has to be envisioned. In this context, in addition to their principal function as transducers of photoreception, chemo- and thermosensation [24, 27, 29, 33, 34], it has been suggested that CNG channels also influence axonal pathfinding. For example, the convergence pattern of axonal projections onto glomeruli in the murine OB is markedly altered in OSNs of the MOE which are deficient for the CNG subtype CNGA2 [42, 43]. Similarly, in C. elegans, mutants of CNG channels showed disrupted axonal projections of distinct sensory neuron types [23, 44]. Thus, CNGA3 might be also involved in axonal outgrowth and/or guidance of GG neurons. In this context, it was recently reported that Semaphorin proteins, which act as axonal growth cone guidance molecules, affect growth cone repulsion in a CNG-dependent manner via the Semaphorin receptor Neuropilin in Xenopus spinal neurons [45]. Interestingly, GG neurons are not only endowed with CNGA3 (this study), but also co-express the Neuropilin subtype Neuropilin-2 [4]. Therefore, the remarkable presence of CNGA3 protein in axons of GG neurons supports the notion that CNGA3 might be involved in axonal outgrowth and/or pathfinding of GG axons. Most recently, in this regard, it has been reported that an antibody for CNGA3 labels “unusual whip-like” structures in the GG that are closely adjacent to GG neurons [26]. The nature of these stained structures is yet unclear; nevertheless, it can be speculated that they represent proximal segments of axons/axon bundles and/or other processes originating from GG neurons.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
The authors would like to thank Anne Ullrich and Bela Zimmer for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations
- cGMP
Cyclic guanosine monophosphate
- CNG
Cyclic nucleotide-gated
- DAPI
4′,6-Diamidino-2-phenylindole
- GG
Grueneberg ganglion
- MOE
Main olfactory epithelium
- OB
Olfactory bulb
- OMP
Olfactory marker protein
- OSNs
Olfactory sensory neurons
- PCR
Polymerase chain reaction
- TAAR
Trace amine-associated receptor
- TRP
Transient receptor potential
References
- 1.Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol. 2007;42:463–480. doi: 10.1080/10409230701693359. [DOI] [PubMed] [Google Scholar]
- 3.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- 4.Fuss SH, Omura M, Mombaerts P. The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur J Neurosci. 2005;22:2649–2664. doi: 10.1111/j.1460-9568.2005.04468.x. [DOI] [PubMed] [Google Scholar]
- 5.Koos DS, Fraser SE. The Grueneberg ganglion projects to the olfactory bulb. Neuroreport. 2005;16:1929–1932. doi: 10.1097/01.wnr.0000186597.72081.10. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer J, Hass N, Schwarzenbacher K, Besser S, Breer H. A novel population of neuronal cells expressing the olfactory marker protein (OMP) in the anterior/dorsal region of the nasal cavity. Histochem Cell Biol. 2006;125:337–349. doi: 10.1007/s00418-005-0077-x. [DOI] [PubMed] [Google Scholar]
- 7.Roppolo D, Ribaud V, Jungo VP, Lüscher C, Rodriguez I. Projection of the Gruneberg ganglion to the mouse olfactory bulb. Eur J Neurosci. 2006;23:2887–2894. doi: 10.1111/j.1460-9568.2006.04818.x. [DOI] [PubMed] [Google Scholar]
- 8.Storan MJ, Key B. Septal organ of Gruneberg is part of the olfactory system. J Comp Neurol. 2006;494:834–844. doi: 10.1002/cne.20858. [DOI] [PubMed] [Google Scholar]
- 9.Fleischer J, Schwarzenbacher K, Besser S, Hass N, Breer H. Olfactory receptors and signalling elements in the Grueneberg ganglion. J Neurochem. 2006;98:543–554. doi: 10.1111/j.1471-4159.2006.03894.x. [DOI] [PubMed] [Google Scholar]
- 10.Fleischer J, Schwarzenbacher K, Breer H. Expression of trace amine-associated receptors in the Grueneberg ganglion. Chem Senses. 2007;32:623–631. doi: 10.1093/chemse/bjm032. [DOI] [PubMed] [Google Scholar]
- 11.Brechbühl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- 12.Mamasuew K, Breer H, Fleischer J. Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci. 2008;28:1775–1785. doi: 10.1111/j.1460-9568.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- 13.Hensel H. Thermoreception and temperature regulation. Monogr Physiol Soc. 1981;38:1–321. [PubMed] [Google Scholar]
- 14.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 15.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Woolf CJ. Pain TRPs. Neuron. 2005;46:9–12. doi: 10.1016/j.neuron.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 17.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 18.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 19.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 20.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Fleischer J, Mamasuew K, Breer H. Expression of cGMP signaling elements in the Grueneberg ganglion. Histochem Cell Biol. 2009;131:75–88. doi: 10.1007/s00418-008-0514-8. [DOI] [PubMed] [Google Scholar]
- 23.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans . Neuron. 1996;17:695–706. doi: 10.1016/S0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans . Neuron. 1996;17:707–718. doi: 10.1016/S0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 25.Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans . Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CY, Fraser SE, Koos DS. Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway. J Comp Neurol. 2009;516:36–48. doi: 10.1002/cne.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 28.Biel M, Seeliger M, Pfeifer A, Kohler K, Gerstner A, Ludwig A, Jaissle G, Fauser S, Zrenner E, Hofmann F. Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3. Proc Natl Acad Sci USA. 1999;96:7553–7557. doi: 10.1073/pnas.96.13.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biel M. Cyclic nucleotide-regulated cation channels. J Biol Chem. 2009;284:9017–9021. doi: 10.1074/jbc.R800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig J, Margalit T, Eismann E, Lancet D, Kaupp UB. Primary structure of cAMP-gated channel from bovine olfactory epithelium. FEBS Lett. 1990;270:24–29. doi: 10.1016/0014-5793(90)81226-E. [DOI] [PubMed] [Google Scholar]
- 32.Liman ER, Buck LB. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994;13:611–621. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 33.Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, Kimura KD, Inada H, Matsumoto K, Mori I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans . Science. 2008;320:803–807. doi: 10.1126/science.1148922. [DOI] [PubMed] [Google Scholar]
- 34.Pifferi S, Boccaccio A, Menini A. Cyclic nucleotide-gated ion channels in sensory transduction. FEBS Lett. 2006;580:2853–2859. doi: 10.1016/j.febslet.2006.03.086. [DOI] [PubMed] [Google Scholar]
- 35.Bönigk W, Altenhofen W, Müller F, Dose A, Illing M, Molday RS, Kaupp UB. Rod and cone photoreceptor cells express distinct genes for cGMP-gated channels. Neuron. 1993;10:865–877. doi: 10.1016/0896-6273(93)90202-3. [DOI] [PubMed] [Google Scholar]
- 36.Weyand I, Godde M, Frings S, Weiner J, Müller F, Altenhofen W, Hatt H, Kaupp UB. Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nature. 1994;368:859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- 37.Cho SW, Choi KY, Park CS. A new putative cyclic nucleotide-gated channel gene, cng-3, is critical for thermotolerance in Caenorhabditis elegans . Biochem Biophys Res Commun. 2004;325:525–531. doi: 10.1016/j.bbrc.2004.10.060. [DOI] [PubMed] [Google Scholar]
- 38.Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci USA. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 40.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juilfs DM, Fülle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng C, Feinstein P, Bozza T, Rodriguez I, Mombaerts P. Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit. Neuron. 2000;26:81–91. doi: 10.1016/S0896-6273(00)81140-X. [DOI] [PubMed] [Google Scholar]
- 43.Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–1069. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Coburn CM, Mori I, Ohshima Y, Bargmann CI. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: a distinct pathway for maintenance of sensory axon structure. Development. 1998;125:249–258. doi: 10.1242/dev.125.2.249. [DOI] [PubMed] [Google Scholar]
- 45.Togashi K, von Schimmelmann MJ, Nishiyama M, Lim CS, Yoshida N, Yun B, Molday RS, Goshima Y, Hong K. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.