Abstract
One of the fundamental challenges facing the development of new chemical entities within the pharmaceutical industry is the extrapolation of key in vivo parameters from in vitro cell culture assays and animal studies. Development of microscale devices and screening assays incorporating primary human cells can potentially provide better, faster and more efficient prediction of in vivo toxicity and clinical drug performance. With this goal in mind, large strides have been made in the area of microfluidics to provide in vitro surrogates that are designed to mimic the physiological architecture and dynamics. More recent advancements have been made in the development of in vitro analogues to physiologically-based pharmacokinetic (PBPK) models – a mathematical model that represents the body as interconnected compartments specific for a particular organ. In this review we highlight recent advancements in human hepatocyte microscale culture, and describe the next generation of integrated devices, whose potential allows for the high throughput assessment of drug metabolism, distribution and pharmacokinetics.
Keywords: Microfluidics, human hepatocyte, bioreactor, coculture
1. INTRODUCTION
Recent advancements in chemistry, such as parallel and combinatorial synthesis, have resulted in a multi-fold increase in the number of compounds that are available for evaluation in new drug discovery. Furthermore, other improvements using a variety of structural chemistry tools, and molecular biology tools, provide the pharmaceutical industry with an unprecedented level of structure-based designs to further guide the synthesis of new chemical entities (NCEs) as potential drug leads. Along with the advancement of chemistry and biology, new automated screening tools have became commercially available which can carry out complex, programmable and adaptable robotic operations to test hundred of thousands of compounds in a speedy and precise manner. As a result, these new factors have worked together to increase our ability to create NCEs that exhibit targeted pharmacological activity. Hence, the task of screening compounds for their pharmacokinetic properties, such as permeability, distribution, and metabolic stability, has become a major challenge in drug discovery. This change, in turn, has compelled the invention and implementation of high-throughput screening methods that predict in vivo pharmacokinetic behavior of NCEs in humans.
One of the most challenging tasks in drug discovery is predicting a NCE’s pharmacokinetic behavior in humans using data derived from in vitro model systems [1, 2]. For an orally administered drug, the primary goal during discovery lead optimization, for a compound with a systemic target, is to improve oral bioavailability and systemic half-life. Therefore, during lead optimization it is essential to identify NCEs with predicted sufficient oral absorption using a variety of in vitro and in vivo assays. It is well recognized that in order for a NCE to achieve reasonable oral absorption, it will need to have adequate aqueous solubility, as well as intestinal permeability [3–5]. Drug absorption through the GI tract following oral administration is a rather complex and dynamic process. Passive diffusion can occur through the cell membranes of enterocytes (transcellular) or the tight junctions between the enterocytes (paracellular) [6–9]. Influx and efflux through various drug transporters also play roles in dictating drug absorption. Since many processes are occurring simultaneously, it is often impossible for a single in vitro model to simulate the entire in vivo process. However, two in vitro screening models, have been developed over the last decade and are currently used by most major pharmaceutical companies in their drug discovery efforts. The first is the Caco-2 human intestinal cell line that is widely used to measure permeability of drug candidates, while the second is a non-cell based assay termed Parallel Artificial Membrane Permeability Assay (PAMPA) used to predict passive, membrane permeability of drug candidates.
Since the liver is one of the most important organs responsible for systemic drug metabolism, hepatic clearance has been another primary focus for lead optimization. Many approaches have been developed to predict hepatic clearance using in vitro methodologies. For example, human liver microsomes (CYP450 containing membrane vesicles) have been used extensively for obtaining metabolic clearance and half-life data for the prediction of human clearance [10, 11]. It was suggested that the presence of plasma in the microsomal mixture might lead to better prediction of in vivo clearance [12, 13], in that it accounts for the effect of protein binding which is present in vivo. Additionally, clearance rates can be corrected by taking into account the free fraction of the test article in plasma [14]. While these corrections may provide a more accurate clearance prediction, they still do not taken into account other factors such as hepatocyte transporter-mediated uptake, which may also play a rate-limiting role [15]. Isolated primary human hepatocytes offer the next step in drug metabolism studies [16–27]. In order to push the envelop of high throughput in vitro screening systems, however, a focus is now being direct to integrate these various systems with microfluidics systems into microscale devices. In this review, we provide an overview of current technological progressions that are aimed at the development of microscale devices.
2. DESIGN OF MICROFLUIDIC SYSTEMS FOR AN INTEGRATED IN VITRO MODEL
Development of a microscale devices and screening assays may provide more efficient prediction of in vivo clearance and half-life in humans. Such microscale devices have the potential to accurately produce physiologically realistic parameters and would more closely model the desired in vivo system being tested. Currently, the gold standard for the pharmaceutical industry is the use of isolated human hepatocytes, either in suspension, or in a static culture configuration, where the cells are attached to a collagen-coated plate. Isolated human hepatocytes, which carry most of the livers detoxification work load, are generally recognized as one of the most relevant first line screens in pre-clinical candidate drug assays. However, serious limitations in predicting human liver responses to drugs still exist [28–37]. Limitations include the rapid loss of liver-specific function during in-vitro culture as well as the scarcity of healthy cells. Significant differences between human and animal metabolism necessitate the use of human cells. However, healthy human livers are used for orthotopic liver transplants and only marginal livers, which are rejected for transplantation, are used as a source for hepatocyte isolation.
The limitations of assays utilizing isolated hepatocytes, as well as the aforementioned goal of integrating multiple assays into a single high throughput device, have driven the development of microscale in-vitro assays with the goal of better modeling in-vivo physiological liver metabolism. In their final embodiment, these microscale devices must integrate a stable, and highly functional hepatocyte cell culture, with a fluidics device that recapitulates the in vivo environment, with respect to cell to volumes ratios, shear stress, and a host of other process and physiologically relevant parameters. As a case in point we focus on the microfluidic device offered by the Hurel Corporation, as it has been described in publication in terms of drug metabolism centric studies. However other devices also exist, and have been oriented toward use in toxicity studies, such as the devices by Park et al., Sudo et al., Toh et al. and Carraro et al. [38–52]. We will also focus on the scientific/technological viability and importance of introducing flow and operating under pharmacokinetically modeled conditions, to engineer cell based assays with higher predictive capabilities, as well as offer up new solutions to other areas studied under the guise of drug metabolism and pharmacokintetics (DMPK).
2.1. Physical Structure – Microscale Perfused Cultures
To better model in vivo conditions several groups are now designing physical replicas of physiologically based in silico pharmacokinetics (PBPK) models [53–60]. These physical models, and resulting cell based assays, are designed to match relevant in vivo parameters. In the realm of DMPK, pharmacokinetic parameters of interest include interactions between cell liquid residence time, liquid to cell ratios, relative size of organs (or tissue compartments), metabolism by cells, shear stress, and the like. By providing a pharmacokinetic-based culture system that mimics the natural state of cells, the predictive value and in-vivo relevance of screening and toxicity assays is enhanced. As a case example, the Hurel microscale culture device [41] comprises a fluidic network of channels segregated into discrete but interconnected compartments. The specific compartment geometry is designed to provide cellular interactions, liquid flow, and liquid residence parameters that correlate with those found for the corresponding cells, tissues, or organs in vivo. In its initial configuration [61–63] the device implemented microfluidics chips with two cell compartments, or compartments, seeded with HepG2 human hepatoma cells and L2 lung cells or 3T3 fibroblasts.
A key roadblock in the development of microscale devices, involving hepatocytes, is the rapid loss of hepatocyte liver-specific function during in-vitro culture and although some conclusions can be drawn with the application of appropriate pharmacokinetic principles, there are still substantial limitations. One concern is that current screening assays utilize cells under conditions that do not replicate their function in their natural setting. Therefore, to create a more “liver like” environment one would need to design an optimized microfluidic system with a potentially long-term, stable culture of primary human hepatocytes. Along these lines there have been previous reports of microreactor systems that couple microfluidics with hepatocyte cultures, by various groups [39, 41, 42, 45, 51, 64].
2.2. Analysis of Fluid dynamics in the Microscale Perfused Culture
When developing an optimized microfluidic system as an in vitro microfluidic surrogate for DMPK assessment of NCEs, special attention needs to given to the fluid dynamics that exist within the in vitro surrogate. The dominant parameters one must address when designing a device are: 1) chemical gradients within the device; 2) flow rates used to assess a variety of DMPK aspects of the new chemical entity; 3) shear stress within a device; 4) efficient mixing given the laminar flow characteristics that are present in a majority of fluidic devices.
Analogous to the liver in vivo, in a microfluidic hepatic based in vitro device, concentration gradients can exist for nutrients, oxygen, metabolic wastes, as well as the NCEs being studied. In vivo, a gradient of oxygen, nutrients and hormones is thought to give rise to zones within the liver, leading to changes in phenotype across the lobule. These phenotypic changes lead to a heterogeneous distribution of drug metabolizing enzymes and transporters in the liver. In vitro, oxygen is rapidly depleted due to its relatively low solubility in cell culture medium and the high metabolic activity of the cells, especially at very high cell to volume ratios. While some work has been done to attempt and recapitulate the in vivo zonation phenomena in vitro, a larger focus has been on ensuring that all cells within a fluidics device receive enough oxygen [65]. When considering these aspects up front, one can ensure that cells remain viable, and reactions that are important for DMPK analysis are not mass transport limited. In this regard it is important to design flow rates, length scales, and cell to volume ratios based on known or predicted cellular requirements. Experimental values of cellular utilization, of oxygen for example, are available in literature and can be used in conjunction with computational fluid dynamics to design proper geometries [66–71]. When designing in this manner, one must be vigilant of the potential boundary conditions that exits, those being either hepatocytes in suspension (with a high surface to volume ratio) thereby having a high consumption rate, or a static plated configuration of hepatocytes (limited by mass transport and a static boundary layer) [72–75]. In order to overcome diffusion limitations in that later case, microfluidics can be applied to add a convective driving force used for the transport of oxygen and nutrients, as well as parent compounds that are to be screened with the in vitro surrogate. It should be noted though, that while there are many benefits due to the addition of flow there lurks the potential hurdle of added shear stress. It has been shown that while a small amount of shear stress is beneficial to cultures of hepatocytes (below 5 dynes/sq cm), while larger amounts are shown to be detrimental (above 5 dynes/sq cm) [65, 76, 77]. To ensure a system that promotes viability and cellular function, a balance must exist between the flow rate applied to the system, as well as the geometry of the system, without disturbing the beneficial transport effects gained through the application of flow. As a final point of consideration, mixing within the device should also be optimized. By their nature, microfluidics devices often exist at very low Reynolds numbers, laminar flow, and thus mixing and transport often occur through diffusion. A wide range of studies have been conducted to induce mixing within microfluidics devices, and include, but are not limited too, the inclusion of baffles, using pulsitile flow, applying electrokinetic driven flow, hydrodynamic focusing, and a whole slew of other techniques [78–85]. This again presents an opportunity, up front, for the use of computational fluid dynamics to ensure the proper design of the device, prior to introducing hepatocytes into the system. By designing a device that provides the proper mixing, and mass transport, while minimizing the amount of shear stress applied to the hepatocytes, one can then potentially provide a device with better in vivo predictive capabilities [71].
2.3. Optimized Human Hepatocyte Culture
Another facet of the microscale device is the generation of a stable long-term culture of human hepatocytes. Optimization of culture conditions to maximize in-vitro adult primary hepatocyte function has been well characterized. Studies have shown that duration of in-vitro function depends upon culture conditions such as the type of substrate used, spatial orientation of the cultured cells, addition of growth factors, and the combinatorial effects of these parameters [69, 86–89]. Many studies have examined the functional response of hepatocytes to the physical and chemical properties of culture substrates. The sandwich model, which incorporates human hepatocytes cultured between two thick layers of collagen, provides an in-vivo like environment that increases certain hepatocyte functions and extends their retention time [90–94]. In one example, primary hepatocytes were cultured in a number of collagen and Matrigel configurations including monolayer, collagen sandwich, Matrigel sandwich or composite (collagen/Matrigel) sandwich. Collagen sandwich and Matrigel cultures yielded superior and comparable albumin secretion for at least 2 weeks. The data also showed that hepatocyte polarity could be manipulated by the overall ECM composition independent of the actual morphologies of the cells in different substrates. A recent study has shown that hepatocytes cultured in a collagen sandwich and the presence of CYP450 inducers produced cells where expression ratios of phase I and Phase II genes closely resembled the in vivo liver [95].
Other methods to maintain function of isolated hepatocytes include the co-culture of primary hepatocytes with nonparenchymal cells. These co-cultures mimic the cell-cell interactions that are important in all facets of embryonic and adult physiology. It has been shown that when primary rat hepatocytes are cultured with fibroblasts, there is a marked increase in hepatocyte function as compared to hepatocytes cultured alone [39, 46, 47, 69, 86, 87, 96]. Recent studies indicate that a model which incorporates both human hepatocytes as well as Caco-2 cells to project oral bioavailability is more favorable than the use of hepatocytes in suspension culture. In general, systems yielding the most promising results are based upon the aggregation of hepatocytes into spheroids, which markedly increases hepatocyte function. In one system, hepatocyte aggregation was induced by plating hepatocytes on low-density fibronectin [26]. Hepatocytes on this ECM initially attach and remain rounded, and over a few days reassemble into spheroids. Such self-aggregated spheroids show an increase in cytochrome P450 1A1 (CYP1a1) activity as compared to hepatocytes that remain in a monolayer configuration. In another study, hepatocyte aggregates and hepatocyte monolayers were cultured in collagen gels [97–99]. Here again, hepatocyte aggregation occurred with increased function as aggregates expressed an average two-fold increase in urea and albumin production compared to monolayer-plated cells. The increase in function seen in this system was attributed to an increase in cell-cell contact also seen in co-cultures, as well as maintenance of spheroid morphology. Thus, to date there are three prevailing hepatocyte culture systems: co-culture, three dimensional networks and aggregate culture. Through these approaches, with a focus towards maximizing homotypic hepatocyte interactions [69, 87, 88], one may potentially derive a long term stable culture, with potential incorporation into a microscale device.
2.4. Producing an Integrated Scalable Device
With an analysis of the key components needed to create a microscale device, we next turn our attention to the integration of these components into a system which incorporates continuous flow of culture media over isolated hepatocytes cultures. In the past, development of a small-scale bioreactor incorporating rat hepatocytes for drug metabolism studies was first reported by Bader et al [100]. The studies reported that primary rat hepatocytes cultured in a small–scale flat membrane bioreactor in a sandwich configuration maintained drug biotransformation capacity of Uripidil for at least 14 days. A similar study utilizing porcine hepatocytes showed that cells cultured in a flat membrane bioreactor maintained their phase I and phase II activities and responded to inducing drugs over a 3 week period. Studies done with hepatocellular carcinoma cell lines have shown similar results indicating that this model system is useful in studying drug metabolism [62]. Although these initial studies more closely resembled in-vivo resemble physiological conditions, they are still deficient in that they do not mimic physiological conditions accurately enough for predictive studies. Therefore, the resulting assay data is not based on the pattern of drug or toxin exposure that would be found in an animal.
Returning back to our case example of the Hurel Device, we can see that the most promising results lie in an integrated system with optimized microfluic considerations, and a robust coculture system [39, 41]. It was demonstrated with this system that for a wide range of compounds, that the integrated system provides much higher predictive capability then hepatocytes under static conditions, or under flow, without the optimized coculture configuration [39, 41]. In addition, production rates of metabolites, a key capability for metabolism identification groups, is much higher in this system.
3. CURRENT AND FUTURE APPLICATIONS OF MICROSCALE SYSTEMS
3.1. Evaluation of Hepatic Clearance
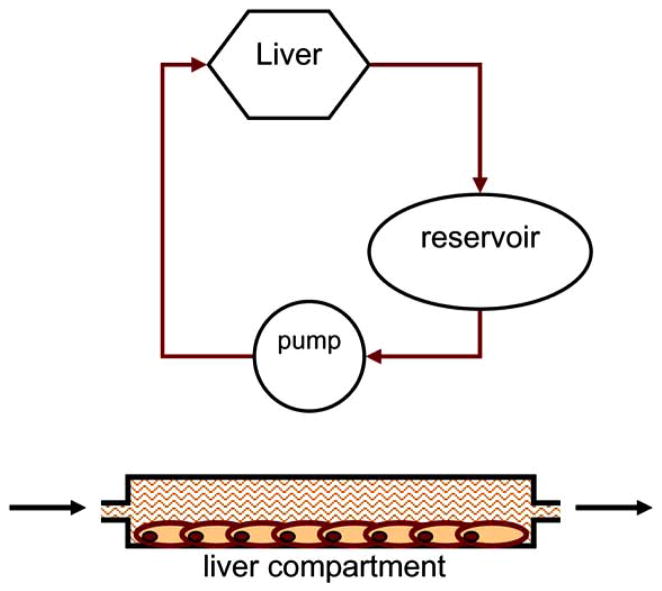
The primary and current use, as aforementioned, of the Hurel microscale system is in the area of drug metabolism, more specifically in the application of predicting in vivo clearance values from in vitro clearance data. Fig. (1) illustrates the relationship between the clearances of drugs by the liver compartment of the microfluidic device and the drug concentrations in the reservoir. The liver compartment in the system functions as the sole eliminating compartment and is connected to a non-eliminating compartment, the reservoir. The liver compartment and the reservoir are connected via the medium flow with a flow rate of Qh in a recirculation loop. Unlike traditional static culture systems, where the predictive hepatic clearance may be obtained by using the experimental intrinsic clearance with the well-stirred model to incorporate the hepatic flow parameter, the microfluidic device itself has a flow component. Therefore, the device itself represents a well-stirred model. The clearance data obtained using the flow device can be scaled up directly to predict the hepatic clearance. Extraction ratio concept is used to scale-up the clearance data obtained using the flow device to the estimated human hepatic clearance as shown below:
where CLh (μL/min/chip) is the clearance of the microfluidic system, C0 and Ct are concentrations (μM) of compound in the reservoir at time 0 and t respectively, AUC0-t is the area under the concentration-time curve from 0 to t, and V is the volume of incubation. The extraction ratio of microfluidic system, Eh, is
Fig. 1.
Top: Diagram of the metabolism microfluidic device comprises of a liver compartment and a reservoir. The liver chamber is the sole elimination compartment of the system. Bottom: the side view of the liver chamber, where the hepatocytes are cultured. The fluid (culture medium or buffer) from and to the reservoir can flow through the liver compartment.
The predicted human hepatic clearance from the flow device can be obtained by up-scaling the extraction ratio:
The predicted data are comparable to the conventional intrinsic clearance calculation [101] with the model compounds tested, indicating that human hepatocytes cultured under flow condition are at least as metabolically active as the ones cultured under a traditional static surrogate system. In addition, the device provides the apparent benefit of avoiding mathematical modeling to predict in vivo parameter value(s), yield higher predictive capability, as aforementioned.
3.2. Evaluation of Cell Permeability and Bioavailability
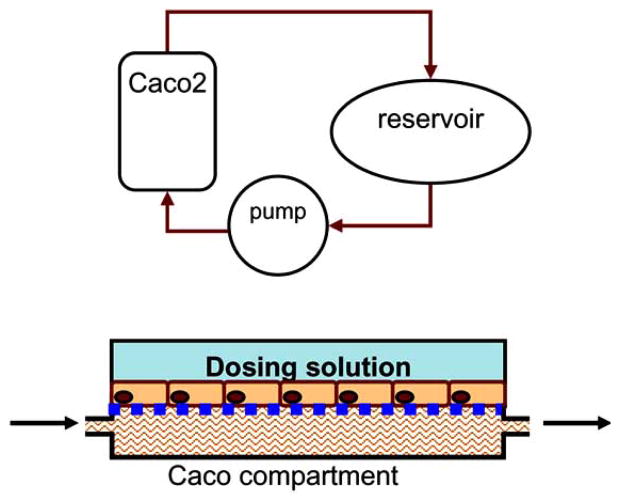
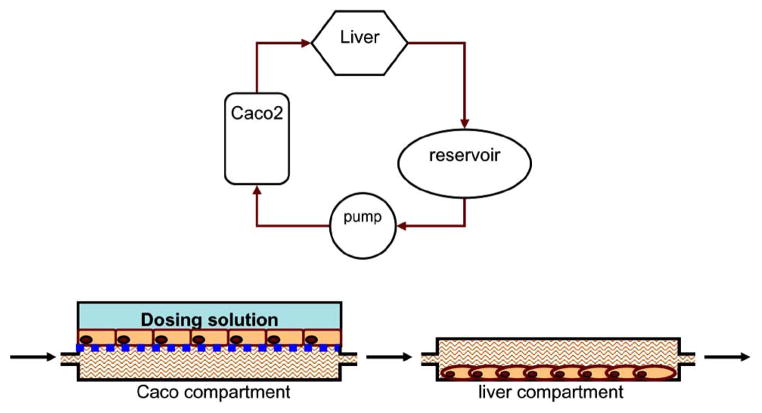
Oral administration of drug remains the most popular route of administration and with the aforementioned increase in NCEs it becomes necessary to develop high throughput approaches for evaluating permeability. These methods have to be effective, low in cost and robust considering the immense number of potential compounds being identified [102]. Biological evaluation of NCE, looking at parameters such as lipophilicity and absorption potential [103] as well as the utilization of systems like immobilized artificial membrane chromatography and parallel artificial membrane permeability assay, has been used in predicating the likely degree of absorption in small intestine. Furthermore, these approaches have led to robotic multi well plate systems which provide high throughput analysis. However, these methods fail to account for many of the physiological aspects of the in vivo absorption and therefore fail to predict absorption accurately [102]. Several animal tissue models have been implemented, but are very difficult to scale down for high throughput evaluation and are very costly requiring large amounts of compound. The most widely used in vitro model for NCE cell permeability determination is cell monolayer cultures with endothelial cells over a diffusion surface. Various different cell types have been employed in these platforms with the most popular cell type being the caco-2 cell. These platforms have been effective, but their scalability has been hindered due to surface area limitation created with smaller well plates. The Hurel micro-device, as illustrated in (Fig. 2), can overcome this limitation allowing for the miniaturization of these cellular models via the precise control over fluid flows possible with micro-fabricated systems. This will facilitate the development of a miniaturized compartment containing the in vitro cellular permeability assay. Computational modeling previously described will be implemented to design a compartment with appropriate volume disruption and flow. Flow through an absorption compartment will result in a more physiologically relevant model considering that there is no static situation in the small intestine and equilibrium values can never be reached. Samples from above and below the endothelial cell diffusion layer will be sampled. The above sample will be used to determine cell permeability and the bottom layer will indicate the bioavailability of the drug. Furthermore, these cellular systems can be established in series with hepatocyte systems to have a robust device that can simultaneously measure bioavailability and drug metabolism (Fig. 3).
Fig. 2.
Top: Diagram of an absorption microfluidic device comprises of an absorption compartment and a reservoir. The absorption chamber has apical and basolateral sides separated by a permeable membrane. Bottom: the side view of the absorption chamber, where Caco-2 cells are cultured on a permeable membrane. The fluid (culture medium or buffer) from and to the reservoir can flow through the absorption compartment. The dosing solution is in contact with the Caco-2 cells from the top.
Fig. 3.
Top: Diagram of a bioavailability microfluidic device comprises of an absorption compartment, a metabolism compartment and a reservoir. Bottom: the side view of the absorption and the metabolism compartment. The fluid (culture medium or buffer) from the reservoir flows through the absorption compartment first, then the metabolism compartment. The drug solution first permeates through the Caco-2 monolayer, and then passes through the liver compartment to encounter the first-pass metabolism.
3.3. Estimation of Volume of Distribution
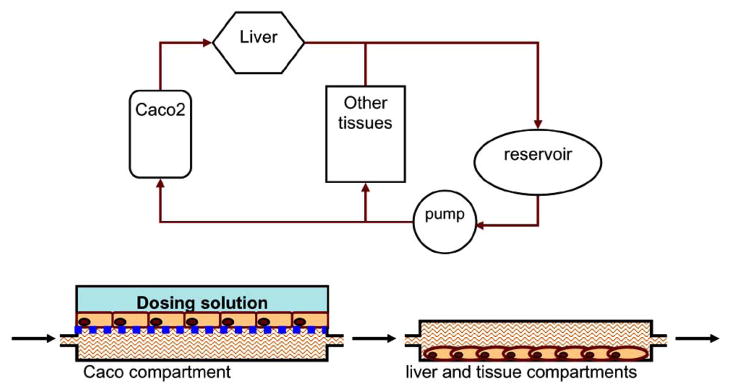
Tissue binding greatly affects drug bioavailability overtime, in many cases determining that drugs half-life, which has a major effect on dosing regiments. At present, the methods used to predict volume of distribution (Vd) include: the extrapolation of animal data [104, 105], physiologically based pharmacokinetic (PBPK) modeling and in silico approaches that employ quantitative structure-pharmacokinetic relationships [106]. Animal models are time consuming and costly, as well as the data extrapolated may not always translate into accurate human Vd. In silico approaches while providing high throughput screening power, are theoretical models that do not account for all the variables that are associated with Vd in vivo. Until this point in vitro models have not been established for assessing Vd and with the amount of throughput demanded by today’s pharmaceutical markets current methods will be costly. A reason for this being, tissue engineering has only recently provided means of culturing adult human cells effectively. Furthermore, the advent Micro-fabricated devices provide the control necessary to develop in vitro platforms for assessing drug Vd. The Hurel device will have a multi- tissue compartment designed to represent plasma distribution into different tissues. The amount of cells will be determined based on their percentage exposure to plasma in vivo. This compartment can be implemented in the chip to measure the volume of distribution a given drug has. The precise control over volume, flow and cell ratios can be established in a compartment to mimic the volume distributions in the body. In this manner a compartment will be created which will represent the tissues in vivo contributing to Vd.
The proposed device (Fig. 4) will be a three compartment device with two flow systems. The first flow system representing the absorption pathway in vivo will have a diffusion layer into the second flow system. The amount of drug in the second flow system will represent the bioavailability of the drug, which will be circulating through two compartments representing the liver and all other tissues in the body. With this design several different DMPK parameters can be assessed simultaneously and to a greater degree of accuracy. The size of the micro-device allows for the design of high throughput systems able to evaluate DMPK characteristics for several NCEs. This micro-device will be the first multi-compartmental micro-device for evaluation of DMPK properties. Overall, the proposed next generation chip is a robust highly integrated micro device designed to facilitate high throughput screening of several different DMPK drug characteristics.
Fig. 4.
Top: Diagram of a pharmacokinetic microfluidic device comprises of an absorption compartment, a metabolism compartment, a biodistribution compartment and a reservoir. The fluid (culture medium or buffer) from the reservoir splits in certain proportion and flows through the absorption compartment or the biodistribution compartment. The fraction flows through the absorption compartment then enter the metabolism compartment simulating the first-pass metabolism. The fraction flows through the biodistribution compartment provides tissue binding effect. Both fractions merge before entering the reservoir. Bottom: the side view of the absorption and the metabolism compartments.
4. SUMMARY
Traditional methods of predicting human response to drugs utilize surrogates-typically either static, homogeneous in-vitro cell culture assays or in vivo animal studies. Static in vitro cell culture assays are of limited value because they do not accurately mimic the complex in vivo environment and thus cannot accurately predict human risk. Similarly, while in vivo animal testing can account for these complex intercellular and inter-tissue effects not observable from in-vitro cell-based assays, in-vivo animal studies are extremely expensive, labor-intensive, time consuming, and often the results are of doubtful relevance when correlating human risk. The development of microscale screening assays and devices that can provide better, faster and more efficient prediction of in-vivo toxicity and clinical drug performance is of great interest in a number of fields. A significant body of knowledge currently exists on development of microscale models to better mimic in vivo metabolism. Such a microscale device offers a unique opportunity to study drug metabolism with in vivo relevant parameters which would more closely model the desired in vivo, physiologically realistic, system being tested. In designing a microscale system, special attention must be paid not only to the device itself, but also the cell culture that is introduced within the system. Upon successful integration, such devices have the potential to yield higher predictive capabilities then other non-microscale device alternatives. In addition these microscale devices provide a platform to which other assays can be coupled to potentially provide an “all-in-one” device for assessment of DMPK parameters of a new chemical entity [39, 41, 71].
References
- 1.Chaturvedi PR, Decker CJ, Odinecs A. Prediction of pharmacokinetic properties using experimental approaches during early drug discovery. Curr Opin Chem Biol. 2001;5(4):452–463. doi: 10.1016/s1367-5931(00)00228-3. [DOI] [PubMed] [Google Scholar]
- 2.Korfmacher WA. Lead optimization strategies as part of a drug metabolism environment. Curr Opin Drug Discov Dev. 2003;6(4):481–485. [PubMed] [Google Scholar]
- 3.Rathore R, Jain JP, Srivastava A, Jachak SM, Kumar N. Simultaneous determination of hydrazinocurcumin and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharm Biomed Anal. 2008;46(2):374–380. doi: 10.1016/j.jpba.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell GW, Ritchie DM, Masucci JA, Hageman W, Yan Z. The new pre-preclinical paradigm: compound optimization in early and late phase drug discovery. Curr Top Med Chem. 2001;1(5):353–366. doi: 10.2174/1568026013394949. [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Khan SU, Muhammad NA. Evaluation and selection of bio-relevant dissolution media for a poorly water-soluble new chemical entity. Pharm Dev Technol. 2001;6(4):531–540. doi: 10.1081/pdt-120000291. [DOI] [PubMed] [Google Scholar]
- 6.Motlekar NA, Fasano A, Wachtel MS, Youan BB. Zonula occludens toxin synthetic peptide derivative AT1002 enhances in vitro and in vivo intestinal absorption of low molecular weight heparin. J Drug Target. 2006;14(5):321–329. doi: 10.1080/10611860600613316. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Salamat-Miller N, Johnston TP. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 2005;294(1–2):201–216. doi: 10.1016/j.ijpharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Thanou M, Verhoef JC, Marbach P, Junginger HE. Intestinal absorption of octreotide: N-trimethyl chitosan chloride (TMC) ameliorates the permeability and absorption properties of the somatostatin analogue in vitro and in vivo. J Pharm Sci. 2000;89(7):951–957. doi: 10.1002/1520-6017(200007)89:7<951::AID-JPS13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Lehr CM. Bioadhesion technologies for the delivery of peptide and protein drugs to the gastrointestinal tract. Crit Rev Ther Drug Carrier Syst. 1994;11(2–3):119–160. [PubMed] [Google Scholar]
- 10.Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46–58. [PubMed] [Google Scholar]
- 11.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27(11):1350–1359. [PubMed] [Google Scholar]
- 12.Shibata Y, Takahashi H, Ishii Y. A convenient in vitro screening method for predicting in vivo drug metabolic clearance using isolated hepatocytes suspended in serum. Drug Metab Dispos. 2000;28(12):1518–1523. [PubMed] [Google Scholar]
- 13.Blanchard N, Richert L, Notter B, Delobel F, David P, Coassolo P, Lave T. Impact of serum on clearance predictions obtained from suspensions and primary cultures of rat hepatocytes. Eur J Pharm Sci. 2004;23(2):189–199. doi: 10.1016/j.ejps.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Berezhkovskiy LM, Khojasteh SC, Halladay JS, Hop CE. On the prediction of hepatic clearance using the diluted plasma in metabolic stability assay. J Pharm Sci. 2009;98(6):1922–1927. doi: 10.1002/jps.21582. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Li P, Gallegos R, Uttamsingh V, Xia CQ, Miwa GT, Balani SK, Gan LS. Comparison of intrinsic clearance in liver microsomes and hepatocytes from rats and humans: evaluation of free fraction and uptake in hepatocytes. Drug Metab Dispos. 2006;34(9):1600–1605. doi: 10.1124/dmd.106.010793. [DOI] [PubMed] [Google Scholar]
- 16.Beckers S, Noor F, Muller-Vieira U, Mayer M, Strigun A, Heinzle E. High throughput, non-invasive and dynamic toxicity screening on adherent cells using respiratory measurements. Toxicol In Vitro. 2009 doi: 10.1016/j.tiv.2009.04.018. [Epub ahead of Print] [DOI] [PubMed] [Google Scholar]
- 17.Ainscow EK, Pilling JE, Brown NM, Orme AT, Sullivan M, Hargreaves AC, Cooke EL, Sullivan E, Carlsson S, Andersson TB. Investigations into the liver effects of ximelagatran using high content screening of primary human hepatocyte cultures. Expert Opin Drug Saf. 2008;7(4):351–365. doi: 10.1517/14740338.7.4.351. [DOI] [PubMed] [Google Scholar]
- 18.Fulop AK. Genetics and genomics of hepatic acute phase reactants: a mini-review. Inflamm Allergy Drug Targets. 2007;6(2):109–115. doi: 10.2174/187152807780832247. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson L, Middleton B, Holmgren J, Eirefelt S, Frojd M, Blomgren A, Gustavsson L. An optimized automated assay for determination of metabolic stability using hepatocytes: assay validation, variance component analysis, and in vivo relevance. Assay Drug Dev Technol. 2007;5(3):403–415. doi: 10.1089/adt.2007.059. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Zhuo L. Epigallocatechin gallate and genistein attenuate glial fibrillary acidic protein elevation induced by fibrogenic cytokines in hepatic stellate cells. Int J Mol Med. 2006;18(6):1141–1151. [PubMed] [Google Scholar]
- 21.Smith NF, Hayes A, James K, Nutley BP, McDonald E, Henley A, Dymock B, Drysdale MJ, Raynaud FI, Workman P. Preclinical pharmacokinetics and metabolism of a novel diaryl pyrazole resorcinol series of heat shock protein 90 inhibitors. Mol Cancer Ther. 2006;5(6):1628–1637. doi: 10.1158/1535-7163.MCT-06-0041. [DOI] [PubMed] [Google Scholar]
- 22.Wustner D. Steady state analysis and experimental validation of a model for hepatic high-density lipoprotein transport. Traffic. 2006;7(6):699–715. doi: 10.1111/j.1398-9219.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 23.Jouin D, Blanchard N, Alexandre E, Delobel F, David-Pierson P, Lave T, Jaeck D, Richert L, Coassolo P. Cryopreserved human hepatocytes in suspension are a convenient high throughput tool for the prediction of metabolic clearance. Eur J Pharm Biopharm. 2006;63(3):347–355. doi: 10.1016/j.ejpb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Gego A, Silvie O, Franetich JF, Farhati K, Hannoun L, Luty AJ, Sauerwein RW, Boucheix C, Rubinstein E, Mazier D. New approach for high-throughput screening of drug activity on Plasmodium liver stages. Antimicrob Agents Chemother. 2006;50(4):1586–1589. doi: 10.1128/AAC.50.4.1586-1589.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy A, Heimbach T, Freiwald S, Smith D, Winters R, Michael S, Surendran N, Cai H. Validation of a semi-automated human hepatocyte assay for the determination and prediction of intrinsic clearance in discovery. J Pharm Biomed Anal. 2005;37(2):319–326. doi: 10.1016/j.jpba.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Lechon MJ, Donato T, Ponsoda X, Castell JV. Human hepatic cell cultures: in vitro and in vivo drug metabolism. Altern Lab Anim. 2003;31(3):257–265. doi: 10.1177/026119290303100307. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, Coleman T, Duttaroy A, Cheng S, Hirsch J, Zhang L, Lazard Y, Fischer C, Barber MC, Ma ZD, Zhang YQ, Reavey P, Zhong L, Teng B, Sanyal I, Ruben SM, Blondel O, Birse CE. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol. 2003;21(3):294–301. doi: 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- 28.Obach RS, Kalgutkar AS, Soglia JR, Zhao SX. Can in vitro metabolism-dependent covalent binding data in liver microsomes distinguish hepatotoxic from nonhepatotoxic drugs? An analysis of 18 drugs with consideration of intrinsic clearance and daily dose. Chem Res Toxicol. 2008;21(9):1814–1822. doi: 10.1021/tx800161s. [DOI] [PubMed] [Google Scholar]
- 29.Dickins M. Induction of cytochromes P450. Curr Top Med Chem. 2004;4(16):1745–1766. doi: 10.2174/1568026043387115. [DOI] [PubMed] [Google Scholar]
- 30.Lau YY, Chen YH, Liu TT, Li C, Cui X, White RE, Cheng KC. Evaluation of a novel in vitro Caco-2 hepatocyte hybrid system for predicting in vivo oral bioavailability. Drug Metab Dispos. 2004;32(9):937–942. [PubMed] [Google Scholar]
- 31.Nagasawa M, Ide T, Suzuki M, Tsunoda M, Akasaka Y, Okazaki T, Mochizuki T, Murakami K. Pharmacological characterization of a human-specific peroxisome proliferater-activated receptor alpha (PPARalpha) agonist in dogs. Biochem Pharmacol. 2004;67(11):2057–2069. doi: 10.1016/j.bcp.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Vignati LA, Bogni A, Grossi P, Monshouwer M. A human and mouse pregnane X receptor reporter gene assay in combination with cytotoxicity measurements as a tool to evaluate species-specific CYP3A induction. Toxicology. 2004;199(1):23–33. doi: 10.1016/j.tox.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Garcia M, Rager J, Wang Q, Strab R, Hidalgo IJ, Owen A, Li J. Cryopreserved human hepatocytes as alternative in vitro model for cytochrome p450 induction studies. In Vitro Cell Dev Biol Anim. 2003;39(7):283–287. doi: 10.1290/1543-706X(2003)039<0283:CHHAAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13(4):343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 35.Hawksworth GM. Advantages and disadvantages of using human cells for pharmacological and toxicological studies. Hum Exp Toxicol. 1994;13(8):568–573. doi: 10.1177/096032719401300811. [DOI] [PubMed] [Google Scholar]
- 36.Butterworth BE, Smith-Oliver T, Earle L, Loury DJ, White RD, Doolittle DJ, Working PK, Cattley RC, Jirtle R, Michalopoulos G. Use of primary cultures of human hepatocytes in toxicology studies. Cancer Res. 1989;49(5):1075–1084. [PubMed] [Google Scholar]
- 37.Kaplan WD, Come SE, Takvorian RW, Laffin SM, Gelman RS, Weiss GR, Garnick MB. Pulmonary uptake of technetium 99m macroaggregated albumin: a predictor of gastrointestinal toxicity during hepatic artery perfusion. J Clin Oncol. 1984;2(11):1266–1269. doi: 10.1200/JCO.1984.2.11.1266. [DOI] [PubMed] [Google Scholar]
- 38.Carraro A, Hsu WM, Kulig KM, Cheung WS, Miller ML, Weinberg EJ, Swart EF, Kaazempur-Mofrad M, Borenstein JT, Vacanti JP, Neville C. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed Microdevices. 2008;10(6):795–805. doi: 10.1007/s10544-008-9194-3. [DOI] [PubMed] [Google Scholar]
- 39.Novik E, Maguire TJ, Chao P, Cheng KC, Yarmush ML. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol. 2009;79(7):1036–1044. doi: 10.1016/j.bcp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toh YC, Lim TC, Tai D, Xiao G, van Noort D, Yu H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip. 2009;9(14):2026–2035. doi: 10.1039/b900912d. [DOI] [PubMed] [Google Scholar]
- 41.Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol. 2009;78(6):625–632. doi: 10.1016/j.bcp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, Kamm RD. Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J. 2009;23(7):2155–2164. doi: 10.1096/fj.08-122820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satoh W, Takahashi S, Sassa F, Fukuda J, Suzuki H. On-chip culturing of hepatocytes and monitoring their ammonia metabolism. Lab Chip. 2009;9(1):35–37. doi: 10.1039/b810961c. [DOI] [PubMed] [Google Scholar]
- 44.Korin N, Bransky A, Khoury M, Dinnar U, Levenberg S. Design of well and groove microchannel bioreactors for cell culture. Biotechnol Bioeng. 2009;102(4):1222–1230. doi: 10.1002/bit.22153. [DOI] [PubMed] [Google Scholar]
- 45.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26(1):120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 46.Park J, Li Y, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Radial flow hepatocyte bioreactor using stacked microfabricated grooved substrates. Biotechnol Bioeng. 2008;99(2):455–467. doi: 10.1002/bit.21572. [DOI] [PubMed] [Google Scholar]
- 47.Nahmias Y, Berthiaume F, Yarmush ML. Integration of technologies for hepatic tissue engineering. Adv Biochem Eng Biotechnol. 2007;103:309–329. doi: 10.1007/10_029. [DOI] [PubMed] [Google Scholar]
- 48.King KR, Wang S, Irimia D, Jayaraman A, Toner M, Yarmush ML. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7(1):77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kane BJ, Zinner MJ, Yarmush ML, Toner M. Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem. 2006;78(13):4291–4298. doi: 10.1021/ac051856v. [DOI] [PubMed] [Google Scholar]
- 50.Khademhosseini A, Yeh J, Eng G, Karp J, Kaji H, Borenstein J, Farokhzad OC, Langer R. Cell docking inside microwells within reversibly sealed microfluidic channels for fabricating multiphenotype cell arrays. Lab Chip. 2005;5(12):1380–1386. doi: 10.1039/b508096g. [DOI] [PubMed] [Google Scholar]
- 51.Sivaraman A, Leach JK, Townsend S, Iida T, Hogan BJ, Stolz DB, Fry R, Samson LD, Tannenbaum SR, Griffith LG. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab. 2005;6(6):569–591. doi: 10.2174/138920005774832632. [DOI] [PubMed] [Google Scholar]
- 52.Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, Griffith LG. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78(3):257–269. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 53.Shono Y, Jantratid E, Janssen N, Kesisoglou F, Mao Y, Vertzoni M, Reppas C, Dressman JB. Prediction of food effects on the absorption of celecoxib based on biorelevant dissolution testing coupled with physiologically based pharmacokinetic modeling. Eur J Pharm Biopharm. 2009;73(1):107–114. doi: 10.1016/j.ejpb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 54.von Kleist M, Huisinga W. Pharmacokinetic-pharmacodynamic relationship of NRTIs and its connection to viral escape: an example based on zidovudine. Eur J Pharm Sci. 2009;36(4–5):532–543. doi: 10.1016/j.ejps.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Hunt CA, Ropella GE, Yan L, Hung DY, Roberts MS. Physiologically based synthetic models of hepatic disposition. J Pharmacokinet Pharmacodyn. 2006;33(6):737–772. doi: 10.1007/s10928-006-9031-3. [DOI] [PubMed] [Google Scholar]
- 56.Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–1034. doi: 10.2165/00003088-200645100-00005. [DOI] [PubMed] [Google Scholar]
- 57.Cai H, Stoner C, Reddy A, Freiwald S, Smith D, Winters R, Stankovic C, Surendran N. Evaluation of an integrated in vitro-in silico PBPK (physiologically based pharmacokinetic) model to provide estimates of human bioavailability. Int J Pharm. 2006;308(1–2):133–139. doi: 10.1016/j.ijpharm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 58.van der Merwe D, Brooks JD, Gehring R, Baynes RE, Monteiro-Riviere NA, Riviere JE. A physiologically based pharmacokinetic model of organophosphate dermal absorption. Toxicol Sci. 2006;89(1):188–204. doi: 10.1093/toxsci/kfj014. [DOI] [PubMed] [Google Scholar]
- 59.Theil FP, Guentert TW, Haddad S, Poulin P. Utility of physiologically based pharmacokinetic models to drug development and rational drug discovery candidate selection. Toxicol Lett. 2003;138(1–2):29–49. doi: 10.1016/s0378-4274(02)00374-0. [DOI] [PubMed] [Google Scholar]
- 60.Dobrev ID, Andersen ME, Yang RS. In silico toxicology: simulating interaction thresholds for human exposure to mixtures of trichloroethylene, tetrachloroethylene, and 1,1,1-trichloroethane. Environ Health Perspect. 2002;110(10):1031–1039. doi: 10.1289/ehp.021101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog. 2004;20(1):338–345. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- 62.Viravaidya K, Sin A, Shuler ML. Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnol Prog. 2004;20(1):316–323. doi: 10.1021/bp0341996. [DOI] [PubMed] [Google Scholar]
- 63.Park TH, Shuler ML. Integration of cell culture and microfabrication technology. Biotechnol Prog. 2003;19(2):243–253. doi: 10.1021/bp020143k. [DOI] [PubMed] [Google Scholar]
- 64.Yates C, Shepard CR, Papworth G, Dash A, Beer Stolz D, Tannenbaum S, Griffith L, Wells A. Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Adv Cancer Res. 2007;97:225–246. doi: 10.1016/S0065-230X(06)97010-9. [DOI] [PubMed] [Google Scholar]
- 65.Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng. 2001;73(5):379–389. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]
- 66.Kellner K, Liebsch G, Klimant I, Wolfbeis OS, Blunk T, Schulz MB, Gopferich A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80(1):73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 67.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA. 2009;106(37):15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ledezma GA, Folch A, Bhatia SN, Balis UJ, Yarmush ML, Toner M. Numerical model of fluid flow and oxygen transport in a radial-flow microchannel containing hepatocytes. J Biomech Eng. 1999;121(1):58–64. doi: 10.1115/1.2798043. [DOI] [PubMed] [Google Scholar]
- 69.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Probing heterotypic cell interactions: hepatocyte function in microfabricated co-cultures. J Biomater Sci Polym Ed. 1998;9(11):1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- 70.Foy BD, Rotem A, Toner M, Tompkins RG, Yarmush ML. A device to measure the oxygen uptake rate of attached cells: importance in bioartificial organ design. Cell Transplant. 1994;3(6):515–527. doi: 10.1177/096368979400300609. [DOI] [PubMed] [Google Scholar]
- 71.Maguire T, Novik E, Chao P, Cheng KC, Yarmush ML. Computational Fluid Dynamic Analysis of a Microfluidic DMPK Device. (In Process) 2009 [Google Scholar]
- 72.Chen X, Murawski A, Patel K, Crespi CL, Balimane PV. A novel design of artificial membrane for improving the PAMPA model. Pharm Res. 2008;25(7):1511–1520. doi: 10.1007/s11095-007-9517-8. [DOI] [PubMed] [Google Scholar]
- 73.Li C, Nair L, Liu T, Li F, Pichardo J, Agrawal S, Chase R, Tong X, Uss AS, Bogen S, Njoroge FG, Morrison RA, Cheng KC. Correlation between PAMPA permeability and cellular activities of hepatitis C virus protease inhibitors. Biochem Pharmacol. 2008;75(5):1186–1197. doi: 10.1016/j.bcp.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 74.Avdeef A, Kansy M, Bendels S, Tsinman K. Absorption-excipient-pH classification gradient maps: sparingly soluble drugs and the pH partition hypothesis. Eur J Pharm Sci. 2008;33(1):29–41. doi: 10.1016/j.ejps.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Avdeef A, Bendels S, Di L, Faller B, Kansy M, Sugano K, Yamauchi Y. PAMPA--critical factors for better predictions of absorption. J Pharm Sci. 2007;96(11):2893–2909. doi: 10.1002/jps.21068. [DOI] [PubMed] [Google Scholar]
- 76.Kan P, Miyoshi H, Ohshima N. Perfusion of medium with supplemented growth factors changes metabolic activities and cell morphology of hepatocyte-nonparenchymal cell coculture. Tissue Eng. 2004;10(9–10):1297–1307. doi: 10.1089/ten.2004.10.1297. [DOI] [PubMed] [Google Scholar]
- 77.Kan P, Miyoshi H, Yanagi K, Ohshima N. Effects of shear stress on metabolic function of the co-culture system of hepatocyte/nonparenchymal cells for a bioartificial liver. ASAIO J. 1998;44(5):M441–444. doi: 10.1097/00002480-199809000-00023. [DOI] [PubMed] [Google Scholar]
- 78.Wen CY, Yeh CP, Tsai CH, Fu LM. Rapid magnetic microfluidic mixer utilizing AC electromagnetic field. Electrophoresis. 2009;30(24):4179–4186. doi: 10.1002/elps.200900400. [DOI] [PubMed] [Google Scholar]
- 79.Yu B, Lee RJ, Lee LJ. Microfluidic methods for production of liposomes. Methods Enzymol. 2009;465:129–141. doi: 10.1016/S0076-6879(09)65007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park SG, Lee SK, Moon JH, Yang SM. Holographic fabrication of three-dimensional nanostructures for microfluidic passive mixing. Lab Chip. 2009;9(21):3144–3150. doi: 10.1039/b913817j. [DOI] [PubMed] [Google Scholar]
- 81.Yan D, Yang C, Miao J, Lam Y, Huang X. Enhancement of electrokinetically driven microfluidic T-mixer using frequency modulated electric field and channel geometry effects. Electrophoresis. 2009;30(18):3144–3152. doi: 10.1002/elps.200900162. [DOI] [PubMed] [Google Scholar]
- 82.Ding WK, Shah NP. Effect of homogenization techniques on reducing the size of microcapsules and the survival of probiotic bacteria therein. J Food Sci. 2009;74(6):M231–236. doi: 10.1111/j.1750-3841.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- 83.Koh CG, Zhang X, Liu S, Golan S, Yu B, Yang X, Guan J, Jin Y, Talmon Y, Muthusamy N, Chan KK, Byrd JC, Lee RJ, Marcucci G, Lee LJ. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J Control Release. 2009;141(1):62–69. doi: 10.1016/j.jconrel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam YC, Gan HY, Nguyen NT, Lie H. Micromixer based on viscoelastic flow instability at low Reynolds number. Biomicrofluidics. 2009;3(1):14106. doi: 10.1063/1.3108462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tabeling P. A brief introduction to slippage, droplets and mixing in microfluidic systems. Lab Chip. 2009;9(17):2428–2436. doi: 10.1039/b904937c. [DOI] [PubMed] [Google Scholar]
- 86.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 87.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog. 1998;14(3):378–387. doi: 10.1021/bp980036j. [DOI] [PubMed] [Google Scholar]
- 88.Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J Biomed Mater Res. 1997;34(2):189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 89.Bhatia SN, Toner M, Tompkins RG, Yarmush ML. Selective adhesion of hepatocytes on patterned surfaces. Ann N Y Acad Sci. 1994;745:187–209. doi: 10.1111/j.1749-6632.1994.tb44373.x. [DOI] [PubMed] [Google Scholar]
- 90.Novik EI, Barminko J, Maguire TJ, Sharma N, Wallenstein EJ, Schloss RS, Yarmush ML. Augmentation of EB-directed hepatocyte-specific function via collagen sandwich and SNAP. Biotechnol Prog. 2008;24(5):1132–1141. doi: 10.1002/btpr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng. 2008;14(2):27–236. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 92.Kang YH, Berthiaume F, Yarmush ML. Long-term stable cultures of rat hepatocytes: an in vitro model to study acute and chronic hepatic inflammation. Tissue Eng. 2002;8(4):681–693. doi: 10.1089/107632702760240599. [DOI] [PubMed] [Google Scholar]
- 93.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10(13):1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 94.Moghe PV, Berthiaume F, Ezzell RM, Toner M, Tompkins RG, Yarmush ML. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials. 1996;17(3):373–385. doi: 10.1016/0142-9612(96)85576-1. [DOI] [PubMed] [Google Scholar]
- 95.Kienhuis AS, Wortelboer HM, Maas WJ, van Herwijnen M, Kleinjans JC, van Delft JH, Stierum RH. A sandwich-cultured rat hepatocyte system with increased metabolic competence evaluated by gene expression profiling. Toxicol In Vitro. 2007;21(5):892–901. doi: 10.1016/j.tiv.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 96.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci U S A. 2009;106(37):15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishikawa Y, Tokusashi Y, Kadohama T, Nishimori H, Ogawa K. Hepatocytic cells form bile duct-like structures within a three-dimensional collagen gel matrix. Exp Cell Res. 1996;223(2):357–371. doi: 10.1006/excr.1996.0091. [DOI] [PubMed] [Google Scholar]
- 98.Parsons-Wingerter PA, Saltzman WM. Growth versus function in the three-dimensional culture of single and aggregated hepatocytes within collagen gels. Biotechnol Prog. 1993;9(6):600–607. doi: 10.1021/bp00024a006. [DOI] [PubMed] [Google Scholar]
- 99.Hirai Y, Takebe K, Nakajima M, Takashina M, Iizuka M. Extended expression of liver functions of hepatocytes in collagen-contained cell aggregates (cell packs) Cytotechnology. 1991;6(3):209–217. doi: 10.1007/BF00624759. [DOI] [PubMed] [Google Scholar]
- 100.Bader A, Knop E, Fruhauf N, Crome O, Boker K, Christians U, Oldhafer K, Ringe B, Pichlmayr R, Sewing KF. Reconstruction of liver tissue in vitro: geometry of characteristic flat bed, hollow fiber, and spouted bed bioreactors with reference to the in vivo liver. Artif Organs. 1995;19(9):941–950. doi: 10.1111/j.1525-1594.1995.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 101.Lau YY, Sapidou E, Cui X, White RE, Cheng KC. Development of a Novel in Vitro Model to Predict Hepatic Clearance Using Fresh, Cryopreserved, and Sandwich-Cultured Hepatocytes. Drug Metab Dispos. 2002;30(12):1446–1454. doi: 10.1124/dmd.30.12.1446. [DOI] [PubMed] [Google Scholar]
- 102.Balimane PV, Chong S, Morrison RA. Current methodologies used for evaluation of intestinal permeability and absorption. J Pharmacol Toxicol Methods. 2000;44(1):301–312. doi: 10.1016/s1056-8719(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 103.Houston JB, Upshall DG, Bridges JW. A re-evaluation of the importance of partition coefficients in the gastrointestinal absorption of anutrients. J Pharmacol Exp Ther. 1974;189(1):244–254. [PubMed] [Google Scholar]
- 104.Lin JH, Sugiyama Y, Awazu S, Hanano M. In vitro and in vivo evaluation of the tissue-to-blood partition coefficient for physiological pharmacokinetic models. J Pharmacokinet Biopharm. 1982;10(6):637–647. doi: 10.1007/BF01062545. [DOI] [PubMed] [Google Scholar]
- 105.Sawada Y, Hanano M, Sugiyama Y, Harashima H, Iga T. Prediction of the volumes of distribution of basic drugs in humans based on data from animals. J Pharmacokinet Biopharm. 1984;12(6):587–596. doi: 10.1007/BF01059554. [DOI] [PubMed] [Google Scholar]
- 106.Sui X, Sun J, Wu X, Li H, Liu J, He Z. Predicting the volume of distribution of drugs in humans. Curr Drug Metab. 2008;9(6):574–580. doi: 10.2174/138920008784892137. [DOI] [PubMed] [Google Scholar]