Abstract
The platelet activating factor (PAF) signaling cascade evolved as a component of the repertoire of innate host defenses, but is also an effector pathway in inflammatory and thrombotic diseases. This review focuses on the PAF signaling cascade in systemic inflammatory responses and, specifically, explores its activities in experimental and clinical sepsis and anaphylaxis in the context of the basic biochemistry and biology of signaling via this lipid mediator system.
Keywords: platelet activating factor, sepsis, anaphylaxis, inflammation, lipid mediators
Introduction
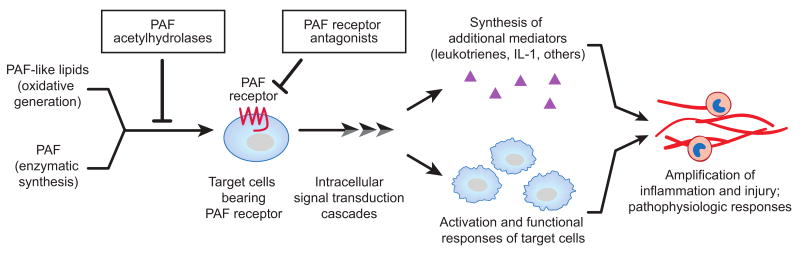
The platelet-activating factor (PAF) signaling cascade consists of PAF and PAF-like lipids (PAF-LL) that are specific, structurally-defined ligands for a G-protein coupled receptor, the PAF receptor (PAFR), which has restricted expression on key target cells of the inflammatory, immune, and hemostatic systems. Engagement of the PAFR by PAF or PAF-LL triggers a variety of intracellular signaling cascades and, via these biochemical mechanisms, induces functional responses of PAFR-bearing cells that then initiate or amplify inflammatory and thrombotic events (Figure 1). Under physiologic circumstances, receptor desensitization and other regulatory mechanisms control responses of cells activated by binding of phospholipid ligands to the PAFR. In addition, the synthesis of PAF is tightly regulated, and a family of intracellular and extracellular phospholipases A2 termed PAF acetylhydrolases (PAF AH) control and terminate signals in this cascade by selectively degrading PAF and PAF-LL, thereby determining their concentrations and half lives and regulating engagement of the PAFR (Figure 1). Molecular and biochemical features of the PAF signaling cascade, and its known roles in health and disease, have been extensively reviewed [1-7].
Figure 1. The PAF Signaling Cascade.
The PAF signaling system includes PAF and PAF-like lipids, which are phospholipid ligands, and a G-protein-coupled receptor, the PAFR, that has restricted distribution on target inflammatory, immune, and hemostatic cells. Engagement of the receptor triggers cellular activation and, via intracellular signaling cascades, alterations in cellular phenotype and function. A variety of regulatory mechanisms have evolved to control the PAF signaling system including PAFR downregulation and desensitization, intracellular biochemical modulation, and activities of a family of enzymes – the PAF acetylhydrolases – that selectively degrade PAF and PAF-LL. Plasma PAF AH, a secreted form, is constitutively present in blood under basal conditions and limits the half life of circulating PAF to minutes. Recombinant PAF AH and competitive PAFR antagonists have been studied as candidate therapies for sepsis in experimental models and clinical trials. Modified from [80] with permission.
This focused review will emphasize features of the PAF signaling cascade in systemic inflammatory responses. It will draw heavily on translational studies that link basic inquiries into the biochemistry and biology of PAF signaling to clinical trials and analysis of activities of the PAF signaling cascade in human cells and surrogate models of systemic inflammatory syndromes1.
Inflammation and hemostasis can be localized, occurring at restricted anatomic sites, or systemic, with manifestations in multiple tissues and organ systems. Systemic inflammatory responses are frequently pathologic and uncontrolled, and often are lethal in humans and experimental animals. Sepsis and anaphylaxis are important examples of systemic inflammatory responses. In each case, there is extensive clinical and investigational evidence indicating that the PAF signaling cascade is involved. While this article examines some of these pathologic issues, it should be remembered that the PAF signaling system (Figure 1) likely evolved as a protective and physiologic cascade that is part of the intricate and extensive innate host defense repertoire [8].
The PAF Signaling Cascade in Sepsis
Sepsis is a syndrome of pathologic systemic inflammation and dysregulated hemostasis that involves activation of myeloid leukocytes, platelets, and endothelial cells, microvascular injury and thrombosis, and damage or dysfunction of many organs, including the lungs [9-11]. Sepsis is induced by intravascular or extravascular microbial invasion, or challenge to the host with microbial toxins and products, and is common and frequently lethal. A systemic inflammatory response with pathophysiologic features similar to those in sepsis can also be induced in inflammatory syndromes and in conditions of tissue injury or neoplasia in the apparent absence of microbes and their toxins [11-14].
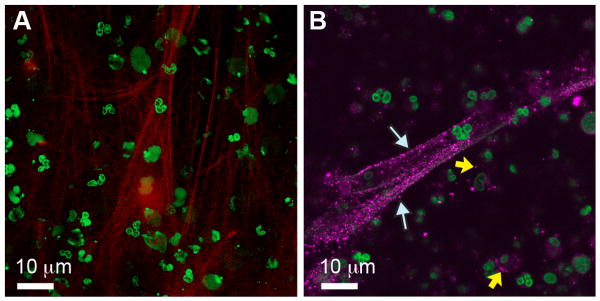
Evidence that PAF signaling is involved and, in some cases, may be a critical determinant of septic syndromes has been reviewed in detail [9-11, 15-17]. These studies span the spectrum from basic investigations in experimental models to clinical observations and trials. Studies in human cell models have provided informative basic observations. Early reports demonstrated that the PAF signaling activates human neutrophils (PMNs), monocytes, and platelets and mediates endothelial-leukocyte, platelet-leukocyte, and platelet-endothelial cell interactions [9, 11]. Each of these responses is a feature of the cellular pathogenesis of sepsis [18]. More recent observations and discoveries also identify previously-unrecognized cellular activities relevant to sepsis that are induced by PAF signaling. As an example, activation of human PMNs by PAF induces extrusion of neutrophil extracellular traps (NETs) (Figure 2), which mediate extracellular capture and killing of bacteria [19]. Impaired NET formation is a novel feature of neonatal neutrophil dysfunction that may contribute to sepsis and other infectious complications [19]. NETs are also implicated in microvascular injury in sepsis [20]. As a second example, PAF induces human platelets to synthesize interleukin 1 [21] and express tissue factor mRNA (Rondina, Schwertz, et al manuscript submitted) by activating novel post-transcriptional gene expression pathways that include a previously-unrecognized extranuclear pre-mRNA splicing mechanism [11, 22]. Activation of post-transcriptional mechanisms complements known activities of PAF signaling in induction of transcriptionally-regulated gene expression [1, 23]. Thus, new responses of human “target” cells in the PAF signaling cascade (Figure 1) continued to be discovered. Primary human cell models also provide new insights into how PAF may be synthesized and distributed in sepsis [24]. In addition to primary human cell models, in vitro systems employing isolated cells from experimental animals also demonstrate that the PAF receptor and PAF signaling induce changes in cellular function and phenotype relevant to sepsis [15, 16, 25, 26]. A PAF AH ortholog has also been identified and studied in yeast [27], providing yet another informative experimental system.
Figure 2. PAF induces NET formation by human neutrophils.
Neutrophils from healthy adults were activated with nanomolar concentrations of PAF for 60 min. Left panel: NETs were examined using live cell imaging by confocal microscopy after incubation with a cell-impermeable DNA dye (red) or a cell permeable DNA dye (green). Red staining indicates extracellular NET formation, which was further documented by election microscopy (not shown). Right panel: NETs were stained for elastase (magenta fluorescence, white arrows), an intracellular and secreted anti-bacterial enzyme that associates with NETs and is a marker for their formation [19]. Yellow arrows indicate intracellular elastase in neutrophil granules. Reproduced from [19] with permission.
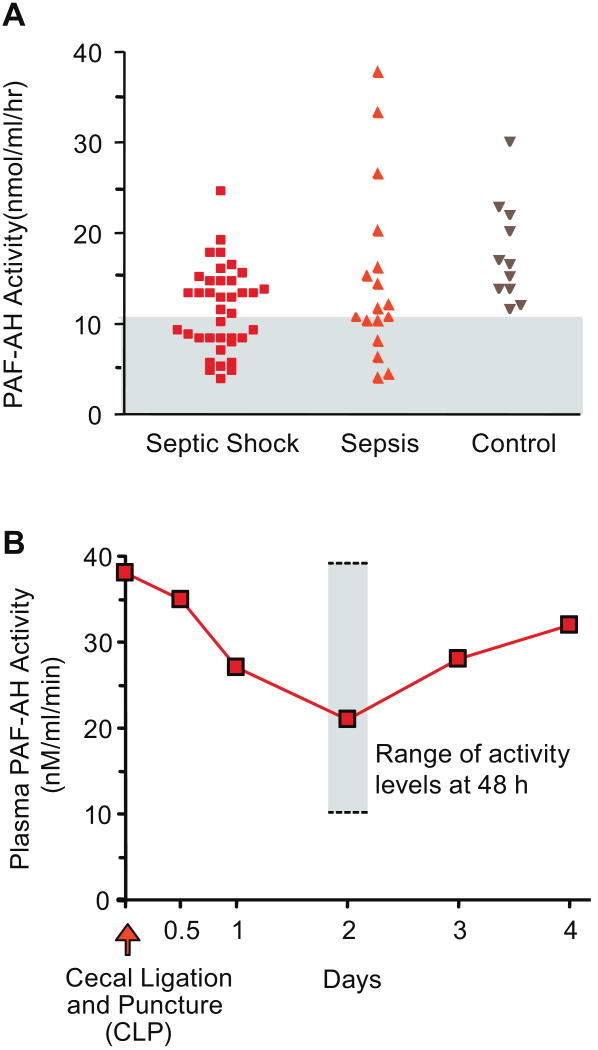
Similarly, pre-clinical animal models contribute evidence indicating that the PAF signaling system is activated in sepsis and that its dysregulation is a feature of the pathobiology of lethal septic syndromes. In early studies, administration of PAFR antagonists [28] or genetic deletion of the PAFR altered leukocyte accumulation and physiologic dysfunction and improved mortality in animal models of sepsis; in contrast, overexpression of the PAFR increased mortality in animals challenged with lipopolysaccharide [29] (reviewed in [2, 15, 16]). Several observations indicate that additive or synergistic activities of PAF and cytokines may have key pathologic effects in sepsis and septic acute lung injury (ALI), and suggest that interactions between PAF, tumor necrosis factor α (TNFα), and IL-1 signaling cascades are particularly important [9, 30]. Subsequently, interactions of the PAF signaling system and toll-like receptor (TLR) signaling have been detected in models of lung inflammation [31]. Combinatorial activities of PAF, LPS and other TLR agonists, and cytokines may be complex, depending on the experimental model and protocol [32]. Recent studies in animal models also extend early observations demonstrating that PAF signaling mediates altered vasoreactivity and increased vascular permeability [33-36], which are cardinal features of septic organ dysfunction and septic shock [13, 18]. Experiments in murine models of sepsis, including cecal ligation and puncture (CLP), also demonstrate that endogenous levels of plasma PAF AH are altered in septic challenge (Figure 3) and that administration of recombinant PAF AH reduces mortality in experimental sepsis [37]. Dissection of events in sublethal compared to lethal CLP in PAF receptor-deficient mice and mice treated with receptor antagonists provided additional important insights [38]. Similar approaches in a murine model of dengue virus infection, which induces systemic inflammation, indicate previously-unrecognized roles of the PAF signaling system in this common and dangerous syndrome [39].
Figure 3. Plasma PAF AH is depressed in some, but not all, patients with sepsis and is dynamically regulated in an experimental model of sepsis.
A. Plasma PAF AH activity levels were measured in samples from patients with septic shock or sepsis without shock identified by consensus criteria, and in samples from healthy control subjects. The gray area indicates activity levels below the lowest value in the range of activities in samples from control subjects. B. Mice were subjected to cecal ligation and puncture and plasma PAF AH activity levels were measured in serial fashion in surviving animals. These figures were modified from [37] with permission.
Many clinical observations are consistent with conclusions drawn from in vitro primary human cell models and surrogate animal experiments (reviewed in [11]), [17]). Activity consistent with PAF and/or PAF-LL has been detected in some, but not all, samples collected from septic patients; several biologic and technical variables make measurements of PAF and PAF-LL challenging in clinical studies (reviewed in [9, 17]). PAFR occupancy was detected on platelets from septic patients [40], consistent with circulation of PAF and/or PAF-LL and their binding to the receptor. In parallel, the activity of endogenous plasma PAF AH is decreased in some subjects with sepsis [37, 41-44] (Figure 3). This establishes the potential for pathologic imbalance resulting from generation of PAF and/or PAF-LL and, in parallel, depression of plasma PAF AH, a key enzyme that degrades these lipids and thereby terminates their signaling capacity (Figure 1) (reviewed in [4, 11, 17]). In a recent study of inter-individual variation in plasma PAF AH activity and PAF AH genotype in subjects with ALI and acute respiratory distress syndrome – many of whom had sepsis as an underlying cause – plasma PAF AH activities were significantly lower in non-survivors compared to survivors in a seven day analysis [45].
Local PAF-mediated events may cause tissue injury and disruption of host defenses that then predispose to bacteremia or endotoxemia and systemic manifestations of sepsis. PAF is a key agonist in neonatal necrotizing enterocolitis (NEC), an inflammatory enteropathy of newborn infants that is particularly common in premature neonates [46, 47]. NEC disrupts gut mucosal barrier integrity, perhaps in part because of apoptotic activities [25], leading to endotoxemia and circulation of proinflammatory cytokines [48]. Thus, PAF may contribute to pathologic events that initiate sepsis, in addition to its systemic manifestations. Plasma PAF AH is expressed in a developmentally-regulated fashion in human infants, and some premature neonates have low activity levels [46]. This potentially contributes to a proinflammatory imbalance in neonatal sepsis and other inflammatory conditions (see above).
Two strategies to interrupt proinflammatory cellular activation mediated by dysregulation of the PAF signaling cascade have been examined in clinical trials. The first is straightforward, and employs competitive blockade of the PAFR with receptor antagonists (Figure 1). Pooled data from several studies involving over 1,000 patients with sepsis [49-54] yielded a small reduction in mortality (3.1%) that was not statistically significant, but several additional analyses also indicated subgroup effects [10]. Variables that may account for inconsistent efficacy of competitive PAFR antagonists in sepsis have been reviewed [9]. Subsequent experiments in pre-clinical animal models suggest that protocols involving PAFR antagonists in combination with blockade of the receptor for leukotriene B4 or, potentially, receptors for other proinflammatory mediators may be useful in syndromes of dysregulated inflammation [55].
The second strategy aimed at terminating PAF signaling is based on noncompetitive interruption of the cascade, and employs recombinant PAF AH (rPAF AH) to hydrolyze PAF and PAF-LL “upstream” from receptor occupancy (Figure 1) (reviewed in [9, 17]). This approach could be particularly useful when large quantities of PAF-LL are generated by unregulated oxidant attack on membrane phospholipids [1, 56, 57] in sepsis or other inflammatory conditions, yielding very high local concentrations of ligands for the PAFR that would be difficult to block with competitive inhibitors [11]. In addition, this strategy would address depression in levels of endogenous plasma PAF AH that occurs in some patients (see above) (Figure 3). In early studies rPAF AH hydrolyzed PAF and oxidatively-modified PAF-LL and eliminated their biologic activities in in vitro and in vivo experiments (reviewed in [1, 17]). More recently, rPAF AH was shown to improve survival in pre-clinical models of sepsis involving LPS challenge and CLP [37]. In clinical studies, administration of rPAF AH to 127 patients with severe sepsis in a multicenter phase II trial resulted in significant improvement in 28 day all cause mortality (from 44 to 21%, p=0.03) and a trend toward reduction in multiple organ dysfunction [58]. Although this was extremely encouraging, a subsequent multicenter phase III trial in patients with severe sepsis was terminated after an interim analysis indicated apparent lack of efficacy [10, 59]. Unfortunately, detailed subgroup analysis was not reported in the phase III study.
Several variables may account for different outcomes of studies evaluating rPAF AH in pre-clinical models relevant to sepsis and in the phase II versus the phase III clinical trials [11, 59, 60]. One is dynamic alterations in levels of endogenous plasma PAF AH, which may account for variable levels of activity in patients with sepsis and other inflammatory syndromes and was not considered in the phase III trial [59, 61]. Dynamic variation in endogenous PAF AH levels, with changing activity over time, occurs in experimental sepsis [37] (Figure 3) and in critically-ill patients with sepsis [62]. It is possible that administration of rPAF AH to patients in the phase III trial whose endogenous levels were normal or, potentially, elevated (Figure 3) may have been futile [10], whereas administration of the recombinant enzyme to patients with depressed PAF AH levels (Figure 3) may have been of benefit [11, 61]. Study subjects were not stratified in this fashion, however [59].
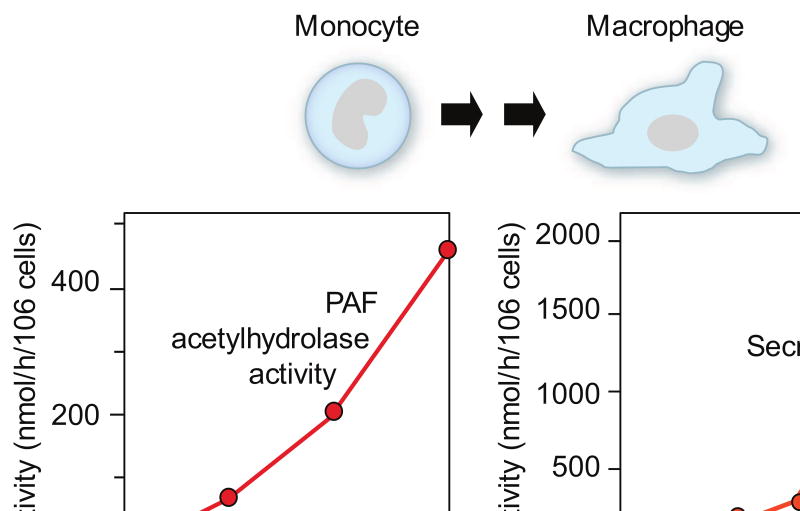
Mechanisms that account for decreased activity of endogenous PAF AH in septic patients (Figure 3) may include inhibition of its synthesis by macrophages mediated by LPS or cytokines, and oxidative inactivation of its activity in the inflammatory milieu [1, 7, 63]. LPS and cytokines can have variable effects on plasma PAF AH expression depending on the model system studied [7, 64]. The factors that contribute to increasing and/or frankly elevated levels of endogenous PAF AH in sepsis (Figure 3) and other inflammatory conditions are not completely defined. Human cell models [65, 66] and other experimental systems indicate that expression of the plasma PAF AH gene during macrophage differentiation (Figure 4) is one potential mechanism (reviewed in [7]). In critically-ill patients plasma PAF AH activity was correlated with levels of neopterin, a macrophage marker [62], and was detected in alveolar macrophages [67], consistent with these in vitro observations. PAF AH was also detected in lung macrophages in a porcine model of ALI [68]. In vitro and in vivo experiments indicate that plasma PAF AH levels may be increased by synthetic glucocorticoids [7], which are commonly given to critically-ill patients and in systemic inflammatory syndromes, potentially by altering macrophage synthesis. Thus, both biologic and iatrogenic variables may influence activity of PAF AH in clinical sepsis and other syndromes. There is evidence that plasma PAF AH is also expressed by dendritic cells, platelets, and model human megakaryocytes [69-71]. Macrophages, dendritic cells, and megakaryocytes each respond to inflammatory and hemostatic signaling factors [11], and may increase expression and synthesis of plasma PAF AH in sepsis and related conditions. Together, these observations suggest that endogenous plasma PAF AH is a circulating marker of inflammation that is temporally regulated and differentially expressed. It has also been suggested that the level of plasma PAF AH is a marker of disease activity in atherosclerosis [72], a syndrome in which macrophages and inflammation are central in the pathogenesis. Other forms of PAF AH [7] may also be temporally and differentially regulated in response to inflammatory signals in vivo. In clinical sepsis, individual variation in the duration of depressed endogenous PAF AH levels (Figure 3) due to biologic and genetic factors [7, 45] may provide information on the natural history of the inflammatory response, and may identify specific patient subsets and temporal windows for further evaluation of candidate therapeutic agents that interrupt the PAF signaling cascade (receptor antagonists, rPAF AH) [60, 61].
Figure 4. Plasma PAF AH is expressed as human monocytes differentiate into macrophages in vitro.
Left: Total PAF AH activity [65]. Right: Secreted and intracellular PAF AH activities [66]. The figures illustrate results from separate experiments using different macrophage cultures, accounting for the differences in total PAF AH activity in the two panels. Additional studies documented expression and synthesis of plasma PAF AH by primary and model human macrophages and by rodent macrophages (reviewed in [7]).
The PAF Signaling System in Anaphylaxis
Anaphylaxis is a complex hypersensitivity reaction characterized by systemic hypotension, vascular leakage, and laryngeal and airway obstruction that is rapid and potentially fatal [73]. Mast cells, which are pivotal cells in inflammatory regulation, and systemically-released mediators including histamine and PAF are key factors in the pathogenesis of human and experimental anaphylactic syndromes [73, 74]. Interestingly, histamine is a potent agonist for PAF synthesis by human endothelial cells, where it mediates juxtacrine signaling of leukocytes [1, 75]. Early and more recent studies demonstrated that activation of the PAF signaling system is a key event in rodent models of anaphylaxis [74, 76, 77], and identified new aspects of PAF-mediated vascular leak relevant to anaphylaxis [36]. Targeted deletion of the PAFR impaired anaphylactic responses in genetically-altered mice [78]. Recent studies demonstrated that recombinant PAF AH is protective and reduces mortality in murine models of anaphylaxis [79], (F Bozza et al, personal communication), consistent with key roles for the PAF signaling cascade in anaphylactic syndromes [74].
In a study of children and adults, serum PAF levels were significantly elevated in patients with acute allergic reactions, and levels of PAF were higher in samples from patients with severe anaphylaxis compared to those with milder manifestations of the syndrome based on a severity scoring system [73]. In addition, the severity of anaphylaxis correlated with decreased PAF AH activity in serum samples, and the concentration of PAF was inversely correlated with PAF AH activity. Serum PAF AH activity was significantly lower in subjects with fatal peanut anaphylaxis compared to activity in samples from control subjects [73]. These clinical observations are consistent with dysregulation of the PAF signaling cascade as a major mechanism in human anaphylaxis, as well as in experimental anaphylaxis as outlined above, and may provide new avenues for therapy of this systemic syndrome (Figure 1).
Acknowledgments
The authors thank Jenny Pierce for preparation of the manuscript, Diana Lim for creating the figures, and our colleagues, students, and technical associates for many contributions to work cited. Studies mentioned in this review were supported by NIH K08 HD049699 (CYY), individual grants from the NIH to ASW and GAZ, and an NIH Special Center of Research in ARDS.
Footnotes
Because this is an abbreviated review, many important primary reports and review articles are not cited. This is simply because of the limitations of space and the expectations for a focused summary of the literature, and not because these articles do not contain interesting and substantive information. Many informative primary articles are referenced in the cited reviews and articles.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 2.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002;68-69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Koizumi H, Aoki J, Inoue K. Platelet-activating factor acetylhydrolase (PAF-AH) J Biochem. 2002;131:635–640. doi: 10.1093/oxfordjournals.jbchem.a003145. [DOI] [PubMed] [Google Scholar]
- 4.Castro Faria Neto HC, Stafforini DM, Prescott SM, Zimmerman GA. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem Inst Oswaldo Cruz 100 Suppl. 2005;1:83–91. doi: 10.1590/s0074-02762005000900014. [DOI] [PubMed] [Google Scholar]
- 5.Karasawa K. Clinical aspects of plasma platelet-activating factor-acetylhydrolase. Biochim Biophys Acta. 2006;1761:1359–1372. doi: 10.1016/j.bbalip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 7.Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2) Cardiovasc Drugs Ther. 2009;23:73–83. doi: 10.1007/s10557-008-6133-8. [DOI] [PubMed] [Google Scholar]
- 8.Munford RS, Pugin J. The crucial role of systemic responses in the innate (non-adaptive) host defense. J Endotoxin Res. 2001;7:327–332. [PubMed] [Google Scholar]
- 9.Zimmerman GA, Albertine KH, McIntyre TM. Pathogenesis of sepsis and septic-induced lung injury. In: Matthay MA, editor. Acute Respiratory Distress Syndrome. Marcel Dekker, Inc.; New York Basel: 2003. pp. 245–287. [Google Scholar]
- 10.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 11.Harris ES, Rondina MT, Schwertz H, Weyrich AS, Zimmerman GA. Pathogenesis of sepsis and sepsis-induced lung injury. In: Choi AMK, editor. Acute Respiratory Distress Syndrome. Informa Healthcare USA, Inc; New York: 2009. [Google Scholar]
- 12.Alberti C, Brun-Buisson C, Chevret S, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med. 2005;171:461–468. doi: 10.1164/rccm.200403-324OC. [DOI] [PubMed] [Google Scholar]
- 13.Baron RM, Baron MJ, Perrella MA. Pathobiology of sepsis: are we still asking the same questions? Am J Respir Cell Mol Biol. 2006;34:129–134. doi: 10.1165/rcmb.F308. [DOI] [PubMed] [Google Scholar]
- 14.Munford RS. Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu Rev Pathol. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 15.Mathiak G, Szewczyk D, Abdullah F, Ovadia P, Rabinovici R. Platelet-activating factor (PAF) in experimental and clinical sepsis. Shock. 1997;7:391–404. doi: 10.1097/00024382-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kuijpers T, van der Poll T. The role of platelet-activating factor in endotoxin-related disease. In: Brade H, Opal S, Vogel S, Morrison D, editors. Endotoxin in Health and Disease. Marcel Dekker, Inc.; New York Basel: 1999. pp. 449–462. [Google Scholar]
- 17.Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 19.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 21.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008;28:s17–24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kravchenko VV, Pan Z, Han J, Herbert JM, Ulevitch RJ, Ye RD. Platelet-activating factor induces NF-kappa B activation through a G protein-coupled pathway. J Biol Chem. 1995;270:14928–14934. doi: 10.1074/jbc.270.25.14928. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe J, Marathe GK, Neilsen PO, et al. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem. 2003;278:33161–33168. doi: 10.1074/jbc.M305321200. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Caplan MS, Saraf AP, et al. Platelet-activating factor-induced apoptosis is blocked by Bcl-2 in rat intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G340–350. doi: 10.1152/ajpgi.00182.2003. [DOI] [PubMed] [Google Scholar]
- 26.Han SH, Kim JH, Seo HS, et al. Lipoteichoic acid-induced nitric oxide production depends on the activation of platelet-activating factor receptor and Jak2. J Immunol. 2006;176:573–579. doi: 10.4049/jimmunol.176.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foulks JM, Weyrich AS, Zimmerman GA, McIntyre TM. A yeast PAF acetylhydrolase ortholog suppresses oxidative death. Free Radic Biol Med. 2008;45:434–442. doi: 10.1016/j.freeradbiomed.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlan AF, Hau H, Dunlop LC, Berndt MC, Hancock WW. P-selectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J Exp Med. 1994;179:329–334. doi: 10.1084/jem.179.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii S, Nagase T, Tashiro F, et al. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J. 1997;16:133–142. doi: 10.1093/emboj/16.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han SJ, Ko HM, Choi JH, et al. Molecular mechanisms for lipopolysaccharide-induced biphasic activation of nuclear factor-kappa B (NF-kappa B) J Biol Chem. 2002;277:44715–44721. doi: 10.1074/jbc.M202524200. [DOI] [PubMed] [Google Scholar]
- 31.Knapp S, von Aulock S, Leendertse M, et al. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 32.Jeong YI, Jung ID, Lee CM, et al. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS One. 2009;4:e6503. doi: 10.1371/journal.pone.0006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goggel R, Winoto-Morbach S, Vielhaber G, et al. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155–160. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- 34.Witzenrath M, Gutbier B, Owen JS, et al. Role of platelet-activating factor in pneumolysin-induced acute lung injury. Crit Care Med. 2007;35:1756–1762. doi: 10.1097/01.CCM.0000269212.84709.23. [DOI] [PubMed] [Google Scholar]
- 35.Fainaru O, Adini I, Benny O, et al. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22:3728–3735. doi: 10.1096/fj.08-110494. [DOI] [PubMed] [Google Scholar]
- 36.Camerer E, Regard JB, Cornelissen I, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomes RN, Bozza FA, Amancio RT, et al. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 2006;26:41–49. doi: 10.1097/01.shk.0000209562.00070.1a. [DOI] [PubMed] [Google Scholar]
- 38.Moreno SE, Alves-Filho JC, Rios-Santos F, et al. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J Immunol. 2006;177:1264–1271. doi: 10.4049/jimmunol.177.2.1264. [DOI] [PubMed] [Google Scholar]
- 39.Souza DG, Fagundes CT, Sousa LP, et al. Essential role of platelet-activating factor receptor in the pathogenesis of Dengue virus infection. Proc Natl Acad Sci U S A. 2009;106:14138–14143. doi: 10.1073/pnas.0906467106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez Diez F, Nieto ML, Fernandez-Gallardo S, Gijon MA, Sanchez Crespo M. Occupancy of platelet receptors for platelet-activating factor in patients with septicemia. J Clin Invest. 1989;83:1733–1740. doi: 10.1172/JCI114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham RM, Stephens CJ, Silvester W, Leong LL, Sturm MJ, Taylor RR. Plasma degradation of platelet-activating factor in severely ill patients with clinical sepsis. Crit Care Med. 1994;22:204–212. doi: 10.1097/00003246-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Schlame M, Schmid AB, Haupt R, Rustow B, Kox WJ. Study of platelet-activating factor acetylhydrolase in the perioperative period of patients undergoing cardiac surgery. Shock. 1998;9:313–319. doi: 10.1097/00024382-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Partrick DA, Moore EE, Moore FA, Biffl WL, Barnett CC. Reduced PAF-acetylhydrolase activity is associated with postinjury multiple organ failure. Shock. 1997;7:170–174. doi: 10.1097/00024382-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Trimoreau F, Francois B, Desachy A, Besse A, Vignon P, Denizot Y. Platelet-activating factor acetylhydrolase and haemophagocytosis in the sepsis syndrome. Mediators Inflamm. 2000;9:197–200. doi: 10.1080/09629350020002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Stuart L, Zhang Y, Meduri GU, Umberger R, Yates CR. Inter-individual variability of plasma PAF-acetylhydrolase activity in ARDS patients and PAFAH genotype. J Clin Pharm Ther. 2009;34:447–455. doi: 10.1111/j.1365-2710.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 46.Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J Pediatr. 1990;116:960–964. doi: 10.1016/s0022-3476(05)80661-4. [DOI] [PubMed] [Google Scholar]
- 47.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Sharma R, Tepas JJ, 3rd, Hudak ML, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 49.Dhainaut JF, Tenaillon A, Le Tulzo Y, et al. Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. BN 52021 Sepsis Study Group. Crit Care Med. 1994;22:1720–1728. [PubMed] [Google Scholar]
- 50.Froon AMF, Greve JW, Buurman WA, et al. Treatment with the platelet-activating factor antagonist TCV-309 in patients with severe systemic inflammatory response syndrome: a prospective, multi-center, double-blind, randomized phase II trial. Shock. 1996;5:313–319. doi: 10.1097/00024382-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Dhainaut JF, Tenaillon A, Hemmer M, et al. Confirmatory platelet-activating factor receptor antagonist trial in patients with severe gram-negative bacterial sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. BN 52021 Sepsis Investigator Group. Crit Care Med. 1998;26:1963–1971. doi: 10.1097/00003246-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Poeze M, Froon AH, Ramsay G, Buurman WA, Greve JW. Decreased organ failure in patients with severe SIRS and septic shock treated with the platelet-activating factor antagonist TCV-309: a prospective, multicenter, double-blind, randomized phase II trial. TCV-309 Septic Shock Study Group. Shock. 2000;14:421–428. doi: 10.1097/00024382-200014040-00001. [DOI] [PubMed] [Google Scholar]
- 53.Suputtamongkol Y, Intaranongpai S, Smith MD, et al. A double-blind placebo-controlled study of an infusion of lexipafant (Platelet-activating factor receptor antagonist) in patients with severe sepsis. Antimicrob Agents Chemother. 2000;44:693–696. doi: 10.1128/aac.44.3.693-696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincent JL, Spapen H, Bakker J, Webster NR, Curtis L. Phase II multicenter clinical study of the platelet-activating factor receptor antagonist BB-882 in the treatment of sepsis. Crit Care Med. 2000;28:638–642. doi: 10.1097/00003246-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Belanger C, Elimam H, Lefebvre J, Borgeat P, Marleau S. Involvement of endogenous leukotriene B4 and platelet-activating factor in polymorphonuclear leucocyte recruitment to dermal inflammatory sites in rats. Immunology. 2008;124:295–303. doi: 10.1111/j.1365-2567.2007.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIntyre TM, Zimmerman GA, Prescott SM. Biologically active oxidized phospholipids. J Biol Chem. 1999;274:25189–25192. doi: 10.1074/jbc.274.36.25189. [DOI] [PubMed] [Google Scholar]
- 57.Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM. Activation of vascular cells by PAF-like lipids in oxidized LDL. Vascul Pharmacol. 2002;38:193–200. doi: 10.1016/s1537-1891(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 58.Schuster DP, Metzler M, Opal S, et al. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: Phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit Care Med. 2003;31:1612–1619. doi: 10.1097/01.CCM.0000063267.79824.DB. [DOI] [PubMed] [Google Scholar]
- 59.Opal S, Laterre PF, Abraham E, et al. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit Care Med. 2004;32:332–341. doi: 10.1097/01.CCM.0000108867.87890.6D. [DOI] [PubMed] [Google Scholar]
- 60.Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Should we continue to target the platelet-activating factor pathway in septic patients? Crit Care Med. 2004;32:585–588. doi: 10.1097/01.CCM.0000110730.38696.9C. [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman GA. Plasma platelet-activating factor acetylhydrolase is a dynamic variable in critical illness: In the end, is change good for you? Crit Care Med. 2005;33:1462–1463. doi: 10.1097/01.ccm.0000166719.65161.d3. [DOI] [PubMed] [Google Scholar]
- 62.Claus RA, Russwurm S, Dohrn B, Bauer M, Losche W. Plasma platelet-activating factor acetylhydrolase activity in critically ill patients. Crit Care Med. 2005;33:1416–1419. doi: 10.1097/01.ccm.0000165807.26485.ed. [DOI] [PubMed] [Google Scholar]
- 63.MacRitchie AN, Gardner AA, Prescott SM, Stafforini DM. Molecular basis for susceptibility of plasma platelet-activating factor acetylhydrolase to oxidative inactivation. FASEB J. 2007;21:1164–1176. doi: 10.1096/fj.06-6743com. [DOI] [PubMed] [Google Scholar]
- 64.Wu X, Zimmerman GA, Prescott SM, Stafforini DM. The p38 MAPK pathway mediates transcriptional activation of the plasma platelet-activating factor acetylhydrolase gene in macrophages stimulated with lipopolysaccharide. J Biol Chem. 2004;279:36158–36165. doi: 10.1074/jbc.M402454200. [DOI] [PubMed] [Google Scholar]
- 65.Elstad MR, Stafforini DM, McIntyre TM, Prescott SM, Zimmerman GA. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J Biol Chem. 1989;264:8467–8470. [PubMed] [Google Scholar]
- 66.Stafforini DM, Elstad MR, McIntyre TM, Zimmerman GA, Prescott SM. Human macrophages secret platelet-activating factor acetylhydrolase. J Biol Chem. 1990;265:9682–9687. [PubMed] [Google Scholar]
- 67.Grissom CK, Orme JF, Jr, Richer LD, McIntyre TM, Zimmerman GA, Elstad MR. Platelet-activating factor acetylhydrolase is increased in lung lavage fluid from patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:770–775. doi: 10.1097/01.CCM.0000053647.82608.29. [DOI] [PubMed] [Google Scholar]
- 68.Salluh JI, Pino AV, Silva AR, et al. Lung production of platelet-activating factor acetylhydrolase in oleic acid-induced acute lung injury. Prostaglandins Leukot Essent Fatty Acids. 2007;77:1–8. doi: 10.1016/j.plefa.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Al-Darmaki S, Schenkein HA, Tew JG, Barbour SE. Differential expression of platelet-activating factor acetylhydrolase in macrophages and monocyte-derived dendritic cells. J Immunol. 2003;170:167–173. doi: 10.4049/jimmunol.170.1.167. [DOI] [PubMed] [Google Scholar]
- 70.Mitsios JV, Vini MP, Stengel D, Ninio E, Tselepis AD. Human platelets secrete the plasma type of platelet-activating factor acetylhydrolase primarily associated with microparticles. Arterioscler Thromb Vasc Biol. 2006;26:1907–1913. doi: 10.1161/01.ATV.0000228821.79588.ef. [DOI] [PubMed] [Google Scholar]
- 71.Foulks JM, Marathe GK, Michetti N, et al. PAF-acetylhydrolase expressed during megakaryocyte differentiation inactivates PAF-like lipids. Blood. 2009;113:6699–6706. doi: 10.1182/blood-2008-11-186312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karabina SA, Ninio E. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim Biophys Acta. 2006;1761:1351–1358. doi: 10.1016/j.bbalip.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 74.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516-507. [DOI] [PubMed] [Google Scholar]
- 75.McIntyre TM, Zimmerman GA, Satoh K, Prescott SM. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985;76:271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vargaftig BB, Braquet PG. PAF-acether today--relevance for acute experimental anaphylaxis. Br Med Bull. 1987;43:312–335. doi: 10.1093/oxfordjournals.bmb.a072185. [DOI] [PubMed] [Google Scholar]
- 77.Terashita Z, Imura Y, Shino A, Nishikawa K. A lethal role of platelet activating factor in anaphylactic shock in mice. J Pharmacol Exp Ther. 1987;243:378–383. [PubMed] [Google Scholar]
- 78.Ishii S, Kuwaki T, Nagase T, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda Y, Kawashima H, Saito K, Inomata N, Matsui M, Nakanishi T. Effect of human plasma-type platelet-activating factor acetylhydrolase in two anaphylactic shock models. Eur J Pharmacol. 2000;390:203–207. doi: 10.1016/s0014-2999(99)00920-6. [DOI] [PubMed] [Google Scholar]
- 80.Zimmerman GA, McIntyre TM. PAF, ceramide and pulmonary edema: alveolar flooding and a flood of questions. Trends Mol Med. 2004;10:245–248. doi: 10.1016/j.molmed.2004.03.009. [DOI] [PubMed] [Google Scholar]