Summary
The spatio-temporal patterns of ion and metabolite levels in living cells are important in understanding signal transduction and metabolite flux. Imaging approaches using genetically encoded sensors are ideal for detecting such molecule dynamics, which are hard to capture otherwise. Recent years have seen iterative improvements and evaluations of sensors, which in turn are starting to make applications in more challenging experimental settings possible. In this review, we will introduce recent progress made in the variety and properties of biosensors, and how biosensors are used for measurement of metabolite and ion in live cells. The emerging field of applications, such as parallel imaging of two separate molecules, high-resolution transport studies and high-throughput screening using biosensors, will be discussed.
1. Introduction
The challenge we face in the post genome era is the daunting task of integrating many layers of information (genomic modification, control of transcript and protein levels, post-transcriptional modification, metabolite and ion levels) and understanding how the regulations of these layers ensure the function of the system as a whole. Without a doubt, intricate intra- and intercellular communication is required for the proper function of the higher order units such as tissues and organs. For example, the behavior of a neuronal cell is controlled by the fine balance between excitatory and inhibitory inputs dictated by the network within which the cell is placed and cannot be reproduced in an isolated cell. Therefore, methods to extract information at different levels of regulations from a single cell in its original context are especially relevant in systems biology.
The advancement of cell separation techniques such as Fluorescence Activated Cell Sorting (FACS) and laser dissection, as well as the improvement of amplification and analytical techniques, made it possible to investigate levels of transcripts [1–3] and proteins [4,5] at the cellular level. These studies revealed that even seemingly identical cells could differ in transcriptional and protein profiles, underscoring the importance of high-resolution studies [6,7]. Analyses of metabolites and ions at higher resolution, on the other hand, present a unique challenge. Because these molecules are subject to rapid metabolism and/or transport, accurate determination of concentrations in vivo using lengthy fractionation methods is, in many cases, not appropriate. Rapid sampling and analytical techniques as represented in capillary electrophoretic separation techniques in combination with laser-induced fluorescence (CE-LIF) or mass spectrometric detection (CE-MS) enable detection in very small sample volumes (low nanomolar range for CE-MS) [8]. They are, therefore, promising methodologies for high spatial resolution metabolome analyses. However, while these methods provide an overview of many metabolites, they are not practical for high-resolution time course experiments. Short-lived, temporal modulations of metabolite and ion levels play crucial roles in signal transduction, often involving concerted, sequential modulation of messenger molecules (e.g., neurotransmittor, calcium ion, inositol phosphates, cAMP). Because these transient changes are very short-lived (the typical peak of a neurotransmittor in the synaptic cleft is in the 10 millisecond range) yet physiologically relevant, there is great interest in methods that allow measurements of real-time concentrations in vivo.
Selective chemical dyes for ions and pH that appropriate real-time monitoring of cellular concentrations offer excellent spatial and temporal resolutions, and hence have proven to be revolutionizing tools to study the roles of specific molecules in various cellular events [9]. While an imaging approach to studying the in vivo roles of other cellular molecules with higher spatial and temporal resolution is highly desirable, for the majority of metabolites such specific dyes are not available. A real breakthrough in in vivo-compatible sensors for metabolites came with the availability of Fluorescent Proteins (FPs) derived from organisms in phylum Cnidaria, such as Aequorea jellyfish and corals, and proteins that derive from them [10–17]. FPs have a number of advantageous properties as reporters of cellular events. First, they can be genetically introduced into cells or organisms to function as a fluorescent reporter, offering a large advantage when compared to reporters that need to be externally loaded into the cell. Second, they can be engineered so that a conformational distortion that leads to changes in spectroscopic property is caused under certain conditions, allowing them to report changes in their environment. Finally, it has been proven that two FPs which serve as a Föster Resonance Energy Transfer (FRET) donor and acceptor pair (see below) can function as a reporter of biochemical events in a resolution beyond the limit of optical microscopy. Taking advantage of these properties, it is now possible to use FP-based sensors to observe a number of events in living cells (protein trafficking, ligand-receptor binding, voltage dependent conformational change, protein-protein interaction, enzymatic reactions, and ligand binding to proteins). Here, we review recent advances in metabolite and ion imaging using fluorescence-based sensor proteins. Because of the space limitation, only those types of genetically sensors that detect the concentration of small molecules and ions through fluorescence intensity or spectroscopic properties will be discussed. For other types of sensors that report functions of cellular proteins through protein-protein interactions, protein trafficking and enzymatic activities, and the subtype of metabolite sensors that respond to ligands by changing subcellular localizations, readers are referred to recent excellent reviews [18–22]. New areas of application and perspectives will be discussed.
2. Types of genetically encoded FRET sensors for metabolites and ions
Currently, a wide variety of genetically encoded sensors that recognize metabolites and ions are available. Here, we discuss two subtypes of sensors that report cellular metabolites and ions through their fluorescent spectroscopic properties and intensity. The list of currently available sensors is found in Supplemental Table 1.
2-1 FRET-based fluorescent sensors
FRET is a quantum mechanical effect observed when two chromophores are located in near-field (usually less than 10nm). FRET efficiency is sensitive to both distance and relative dipole-dipole orientation between the donor and acceptor fluorophores, and therefore is an excellent reporter of local protein configuration changes induced by molecular interactions (i.e. protein-ligand binding, Fig. 1). The theoretical details of FRET measurement using fluorescent microscope have been reviewed extensively [20,21] and will not be discussed in this review.
Fig. 1. Various protein modules used for FP-based biosensors.
FRET-based sensors based on different types of ligand-recognition modules. i) Sensors based on a chimeric peptide consisting of ligand recognition domain (green) and a peptide sequence that binds to the ligand-bound form of the recognition domain (blue). ii) Sensors whose binding domain consists of a single protein that binds to the ligand and changes its conformation. B. Single-FP based sensors. i) Variants of FPs that change fluorescent intensity and/or spectra in the presence of specific ions. ii) Circularly permutated FPs fused to an external recognition module similar to the ones in FRET-based sensors.
The first of the FP-based FRET sensors for calcium ions were developed by two laboratories [23,24]. In these prototype sensors, a Calmodulin (CaM)-binding peptide was placed in between FRET donor and acceptor, and subsequent binding of Ca2+/CaM to the sensory domain lead to change in FRET efficiency. Their seminal work clearly demonstrated that protein-ligand binding that induces a significant conformational change could be visualized in living cells using FRET, paving the way for a large number of FRET-based sensors.
Typically, an FP-based FRET sensor consists of donor and acceptor fluorophores attached to a naturally occurring ligand recognition module that binds to the substrate of interest, and in many cases, another module that binds to the ligand-bound form of the recognition module. Conformational change caused by ligand binding to this domain induces change in FRET efficiency between the donor and acceptor pair (Fig.1A). Many types of protein modules (e.g. enzymes, membrane receptors, ligand-binding proteins) have successfully been used as substrate recognition modules. Metabolites and ions that function as signaling molecules were major targets for FRET sensor development. Sensors for Zn2+ [25–27], glutamate [28,29], cAMP [30–32], cGMP [33–36], phosphoinositides [37], inositol 1,4,5-triphosphate (IP3) [38,39], diacylglycerol[40], and bacterial quorum-sensing signaling molecules [41] have been developed, and in many cases these sensors were successfully used to achieve sub-second temporal resolution.
Another class of molecules for which many FRET sensors have been developed includes central metabolites such as sugar and amino acids. A family of sensors (FLIP series) pioneered by the Frommer laboratory takes advantage of periplasmic binding proteins (PBPs) from gram-negative bacteria that undergo a Venus-flytrap-like conformational change that is well conserved among this protein family [42,43]. Based on the basic design in which PBPs are sandwiched between FRET donor and acceptor FPs, a number of FRET-based sensors for sugars (maltose [44], ribose [45], glucose/galactose [46], arabinose [47], sucrose [48]), amino acids (glutamate [29]) and ions (phosphate [49]) have been developed. A series of tryptophan sensors that utilizes the dimerization of TrpR, an E.coli Trp operon repressor, has also been developed [50].
Notably, in some configurations of these sensors, conformational change in the binding domain is not likely to lead to distance change between two fluorophores [29,49,51]. Although the exact working mechanisms of this class of FRET sensors are not understood, allosterical hindrance between protein modules created by the conformational change and/or changes in dipole-dipole orientation could explain such phenomena. These cases demonstrate that designs that do not seem to induce large distance change can still function as FRET sensors, making the success rate of this approach surprisingly high thus far.
2-2 Single FP molecule sensors
The chromophore of FP is located in the center of an 11-strand β-barrel structure and usually protected from bulk solvents, making the fluorescence property of FPs stable in a wide range of environments. Conditions that allow solvents to enter the β-barrel and interact with the chromophore, or change the protonation status of the chromphore, however, can change the fluorescence intensity and/or spectroscopic property (Fig. 1B). For example, YFP has several cavities in the vicinity of the chromophore that allow the access of solvent, making the intensity of this fluorophore susceptible to pH and halides [52]. A few mutations in YFP that further increase affinity to halides have also been identified [53,54]. These FPs, either by themselves or as ratiometric sensors in combination with a halide-insensitive FP, can be used as Cl- sensors in vivo [53–56]. Similar single-FP based sensors can be engineered by mutations that allow conditional solvent access to the chromophore. GFP variants that function as sensors for pH [57–59], Hg2+ [60], redox status [61,62], and reactive oxygen species (hydrogen peroxide and super oxide) [63], have been developed so far.
Another class of single-FP sensor has a sensory module attached or inserted into the FP sequence. In these cases, ligand-binding-induced conformational changes result in a change in solvent accessibility to the chromophore, shifting the fluorescence intensity and/or excitation and emission spectra [64] (Fig. 1B). The Ca2+ binding module, similar to the ones used in FRET-based Ca2+ indicators, was successfully used to create single-FP based Ca2+ sensors [65–68]. This category includes sensors for ATP:ADP ratio [69] and cGMP [70].
3 Engineering efforts to improve genetically encoded sensors
While the initial success rate in converting a metabolite or ion binding module into a sensor protein was surprisingly high, performing quantitative in vivo imaging requires a higher dynamic range than what is required for in vitro experiments because of factors such as background fluorescence. Therefore, a great deal of research effort has been invested in improving the dynamic range and signal/noise ratio of genetically encoded sensors.
Although algorithms to predict the efficiency of a particular construct have been developed [71], generally it is difficult to predict the dynamic range of a sensor from the structures of FPs and binding modules. The steric effects of conformational change of the binding module on conformation of fluorophore(s) are often unpredictable. This is exemplified by cases where a single mutation in binding modules changes the direction of response (i.e. increase or decrease of FRET efficiency) induced by ligand binding [49]. Therefore, the strategies employed so far mostly relied on empirical exhaustion of possible configurations between the binding domain and FPs. Testing multiple sensory modules as scaffolds [48,49], systematic adjustment of the linker sequence between the binding module and FPs [28,51], replacing FPs with FRET-optimized fluorophore pair [72], and introducing circular permutation into FPs [73,74] and binding modules [75] have proven to be effective strategies for improving the dynamic range.
In a number of cases, decreases in the dynamic range of the sensors were observed in vivo compared to the value obtained from purified sensor proteins ([73,74], Okumoto unpublished results). While in most cases the reason for such a decrease in the dynamic range is unknown, it is reasonable to speculate that complexity in the cellular milieu could restrict the motion of sensor proteins and hence influence the dynamic range. Therefore, at this point the optimization of genetically encoded sensors requires systemic and rigorous tests in the system for which the sensors are intended (i.e. living cells) [28,35].
4. In vivo measurement of metabolite and ion levels
4-1 Steady-state level of substrates in a subcellular compartment
Metabolism in eukaryotic cells is highly compartmentalized; steady-state metabolite concentrations in each compartment are difficult to measure using classic biochemical approaches because of potential cross-contamination. Moreover, cellular and sub-cellular metabolite levels in transient states (e.g. elevated metabolite level in the environment) are near impossible to capture since the turnover rates of these molecules are extremely high. Consequently, a surprisingly small number of studies have reported absolute concentration of metabolites in cellular compartments.
Genetically encoded sensors allow real-time measurement of a substrate at subcellular resolution by targeting sensor proteins to the compartment of interest, offering potential to fill the current knowledge gap. To investigate concentration of a substrate of interest using genetically encoded sensors, a sensor that matches the range of concentration in the tested condition has to be expressed in the compartment of interest. Estimating substrate concentration in a given compartment is generally difficult, since currently there are few technologies that allow such measurement. Therefore it is advisable to simply introduce an array of sensors with different affinities for the cell type of interest. This process assures that the full range of substrate fluctuation is covered, especially for substrates with larger concentration range than the detection range of one sensor (Fig. 2A). In addition, sensors that are out of their dynamic range (i.e. either saturated or do not bind to the substrate at the given concentration, see Fig. 2B) serve as excellent negative controls for a given experimental condition. Since in most cases affinity mutants are created by small number of mutations in the binding domain, artifacts caused by experimental conditions (i.e. fluorescent intensities of FPs) can be examined using these “out-of-range” sensors. Using this principle, Deuschle et al. demonstrated that the steady-state concentration of glucose in epidermal cells and root cells from Arabidopsis plants are drastically different [76].
Fig. 2. Conceptual concentration substrate level change in the cytosol.
A. Two model cases where the steady-state cytosolic levels are altered. The box above the trace indicates the time period when the substrate was externally supplied. The three shaded areas represent the working ranges of sensors with different affinities. Note that the sensors with higher affinity have the smaller absolute working range. B. The response of cytosolic sensors at higher (upper panel) and lower (lower panel) steady-state substrate concentration.
Typically, to monitor concentration of a specific substrate in a cellular compartment, cells expressing sensor proteins in the desired compartments are perfused in media with incremental increase in the concentration of the substrate (Fig. 3A). FRET efficiency change at a given concentration relative to the maximal FRET efficiency change can be calculated according to Equation 2.
| (Eq.1) |
where S is saturation, ΔEx is the FRET efficiency change at the given concentration, ΔEapo is the FRET efficiency change without the external substrate, and ΔEsat is the FRET efficiency change in saturating concentration of the substrate.
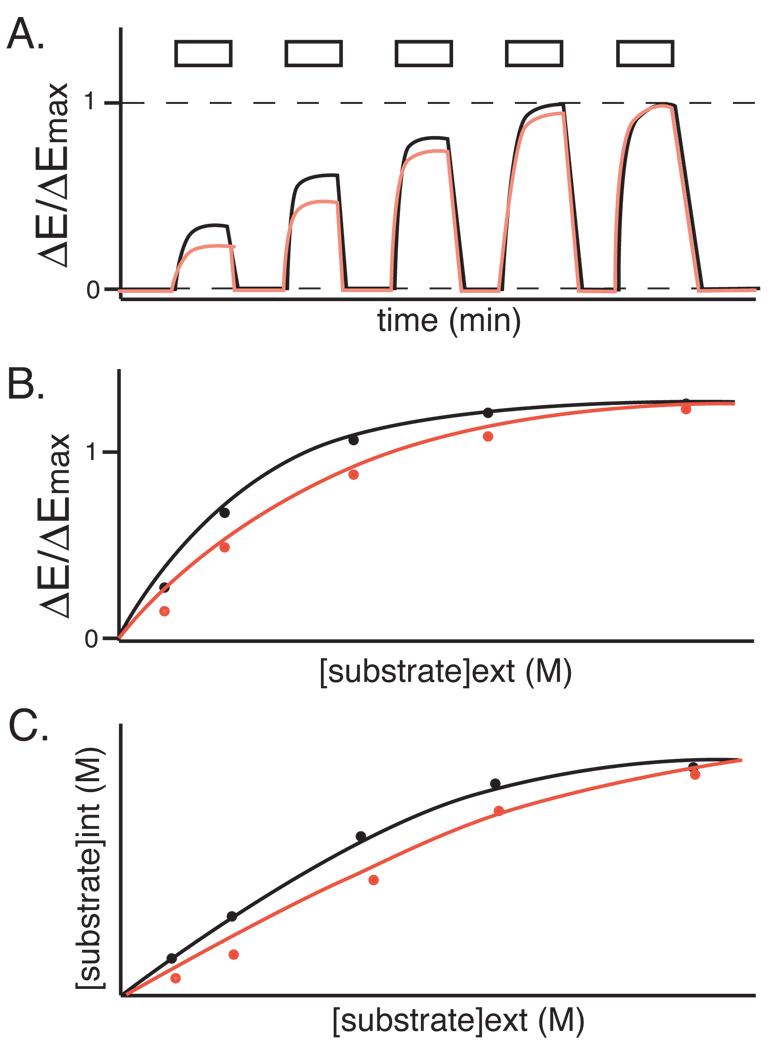
Fig. 3. Determining cellular concentration using FRET sensors.
Black and red traces represent data from two independent cell types expressing the same sensor. A. Typical time-course of FRET efficiency with step-wise increase in concentration of the substrate. Boxes on top represent the period when the substrate was externally supplied. B. Relative FRET efficiency change (ΔEx-ΔEmin)/(ΔEmax-ΔEmin) follows a hyperbolic saturation curve. C. The internal concentration in given external concentration can be calculated from the Eq. 3.
The relative FRET efficiency change at a given concentration follows a hyperbolic curve, reflecting the saturation curve of the sensor (Fig. 3B). Therefore, the internal concentration can be calculated from the FRET efficiency by fitting the saturation ΔEx/ΔEmax with the saturation curve of the sensor;
| (Eq.2) |
where Kd is the half-saturation point of the sensor calculated from the in vitro titration. The internal concentration at a given extracellular substrate concentration can be calculated accordingly (Fig. 3C).
To perform the calculation above, it is essential to determine the maximal response of the sensor (ΔEmax). For substrates that are rapidly transported into cellular compartments, typically this step is easily achieved since the range of substrate exceeds the dynamic range of the sensor. When the substrate concentration change in the given compartment is smaller than the dynamic range of the sensor protein, absolute concentrations of metabolites can be estimated by in situ titration using drugs that permeate the metabolite of interest [39]. Care must be taken when using such an approach, because the changes caused by drugs can directly affect properties of FPs.
4-2 Characterization of cellular transport mechanisms
For many biologically important metabolites and ions, a single cell expresses multiple transporter proteins that control the accumulation and elimination in a given subcellular compartment. Except for in a limited number of cases, the dominant transporter in a particular subcellular compartment is not known, and even in organisms with sequenced genomes, there are a large number of orphan transporters that could also contribute to the transport processes. Metabolite sensors, in combination with methods that allow inhibition of specific gene product(s) (e.g. chemical inhibitors and RNAi), can be used to examine the contribution of individual transporters to the collective flux of the substrate. For example, investigation of glucose transport kinetics in Arabidopsis roots using FRET glucose sensors demonstrated that the kinetics of cytosolic glucose in roots exposed to external glucose do not rely on a pH gradient across the membrane, suggesting the existence of novel mechanisms for glucose transport that do not rely on H+-symport [77]. In other experiments, a combination of chemical inhibitor and RNAi revealed a predominant transporter (i.e. GLUT1) in HepG2 cell line, demonstrating that these sensors can be used as a mean of analyzing transporter activities in a specific cell type [78,79]. Importantly, the substrate elimination process from a cellular compartment, which otherwise requires pre-loading of labeled compounds, can easily be traced using sensors, using either sensor proteins expressed in the compartment of interest [50] or supplied exterior to the cells of interest [80]. Compared to metabolite import into cells, export processes and their components are largely unexplored. Combined with a high-throughput imaging approach, biosensors for metabolites might serve as platforms for discovery of molecular mechanisms for cellular metabolite export, as well as the chemical inhibitors of such processes [79].
Ultimately, transporters and enzymes that are characterized should be integrated into a model that explains the behavior of cells as a whole. Metabolite concentration measured using a biosensor in a given compartment is the summation of uptake, biosynthesis, catabolism and export, and therefore provides a means to examine whether a given model explains the flux of metabolite. The dynamics of metabolite level in the subcellular pool can be predicted using a set of differential equations describing transporter and enzyme reaction kinetics, which can be fitted to the experimental data [81]. Construction of such a model requires that all fluxes around the substrate of interest are identified at the subcellular level, and therefore is not feasible in many cases. Nevertheless, using this approach, Fehr et al. predicted bi-directional glucose transport mechanism on the ER membrane of hepatoma cell line HepG2 [78].
Although the activities of some metabolite transporters are rapidly regulated at the post-transcriptional level, typically cellular metabolite levels change in the course of minutes [46,49,50,77,78]. The temporal-resolutions of genetically encoded sensors are in the order of ten to one hundred milliseconds, and therefore are sufficient in resolving such relatively slow changes. In stark contrast, the temporal signatures of signaling molecules are in the order of tens of milliseconds. Hence the reaction kinetics of detection methods is an important consideration when interpreting the data. Improvement in temporal resolution of genetically encoded sensors has been reported, but even the responses of the fastest sensors reported are still slower than synthetic dyes [82,83]. In situations where head-to-head comparison between genetically encoded sensors and synthetic dyes are possible, an algorithm to resolve a single peak can be developed [84]. Obviously such a comparison is not possible for molecules to which specific dyes are not available, hence the on and off rates of sensors have to be kept in mind when interpreting transients below mid millisecond range.
5. Emerging application of genetically encoded sensors
Due to rigorous improvements in signal/noise ratio and the dynamic range of biosensors, at least for a handful of molecules it is now possible to ask biological questions that require faster and more complex detection systems. Research areas that have witnessed particular progress are summarized as follows. Even though these advanced imaging techniques have been reported for a limited number of metabolites and ions, we predict that these techniques will become applicable for an increasing number of molecules as the repertoire and properties of biosensors improve.
5-1. Dual real-time imaging using genetically encoded sensors
One of the most exciting outcomes of genetically encoded sensors is the possibility to examine correlations between signaling molecules. For example, the signaling pathways of Ca2+ and cAMP are interconnected; the enzymes that catalyze the synthesis and degradation of cAMP can be regulated by Ca2+ and conversely, cAMP can modulate Ca2+ influx through the plasma membrane by modulating the activities of Ca2+ channel proteins [85]. The availability of genetically encoded cAMP sensors that can be spectrally separated from calcium dye enabled simultaneous measurement of two molecules in a single cell. Using this system, researchers revealed a close temporal and causal relationship between the cytosolic Ca2+ and cAMP fluctuation caused by membrane depolarization. In another study, using a FRET based IP3 reporter and Ca2+ dye, the authors demonstrated that the oscillation of IP3 is not essential for the oscillation of Ca2+[39].
Recent expansion of hues in FPs enabled simultaneous recording of two FP-based FRET pairs. ECFP/EYFP and mOrange/mCherry [86], mTFP/Citrine and mAmetrine/tdTomato [87], ECFP/mVenus and TagRFP/mPlum [88], and CFP/YFP and Sapphire/RFP [89] have been successfully used to monitor two simultaneous events in a single cell. Using four FPs inevitably results in larger problems of spectral bleed-through. This problem can be circumvented by detection methods using Fluorescent Lifetime IMaging-FRET (FLIM-FRET) since the method does not require the measurement of acceptor channels [88]. Fluorescence lifetime is also independent of fluorophore concentrations, and therefore provides more accurate measurement in samples with heterologous fluorophore distribution. Since FLIM-FRET experiments require specific setups, and acquisition of lifetime images from the whole cell takes longer than fluorescent-intensity measurement, the recording of genetically encoded FRET sensors using this technique has not been the common method of choice. However, one can expect that the advancement in acquisition of analytical technologies will make this technique accessible to more researchers in the near future.
5-2 High-throughput applications
Recent progress in automated, high-throughput imaging methods has opened up exciting possibilities for genome-wide functional screening [90–92] and image-guided, high-throughput drug discovery assays [93]. These methods generally rely on quantifiable changes (i.e. fluorescence intensity, altered FP-tagged protein localizations etc.) that can be automatically identified among a large collection of images. Although initially such high-throughput studies were typically performed using fixed cells, recent advances in high-throughput microscope systems are starting to make live cell studies more accessible, opening an avenue for cell biology studies at the genome-wide scale.
Since genetically encoded fluorescent sensors can provide quantitative data about a variety of events in living cells, their potential as reporters for high-throughput, image-guided screening was well recognized by researchers, and in some cases demonstrated on a smaller scale [79]. Recently Jiang et al. successfully identified a mitochondrial Ca2+/H+ antiporter through genome-wide screening using a mitochondrial calcium sensor as a reporter [94]. In their study, “pericam”, a genetically encoded sensor that responds to Ca2+ and pH, was expressed in the mitochondria of Drosophila S2 cells to screen for genes that are responsible for calcium uptake into mitochondria using an RNAi library. This example demonstrates that subcellular events can now be reported using genetically encoded sensors with the robustness that is required for high-throughput studies. The signal/noise ratio of genetically encoded sensors and the image resolution, throughput and data analyses of high-throughput imaging systems are rapidly improving. Thus, genetically encoded sensors will serve as indispensable tools for genome-wide analyses of cellular functions in the future.
5-3 Chronic imaging of a specific set of cells in live animals
One of the ultimate goals of studying signaling molecule at the cellular level is to establish the relationship between cellular signaling network and the resulting complex response at the organism level. To answer such questions, it is mandatory to develop a system where cellular responses can be measured directly while responses at the organism level (e.g. movement) take place. To this end, rapid advancement is currently being made in the development of microscopes that can be mounted on live, moving animals [95–97]. Studies using such setups in combination with in vivo whole cell recording demonstrated its usefulness in examining mathematical models as to how hippocampal place cells encode spatiotemporal information [98].
Synthetic calcium indicator dyes have been successfully used in such setups to monitor neuronal activities of a large population of cells in moving animals [95–97]. While approaches using indicator dyes are suitable for short-term experiments, questions that require chronic (long-term) preparation such as learning paradigms are more challenging because bolus loading of calcium indicator disturbs brain physiology. Since genetically encoded sensors can be stably expressed in the same set of cells, they can be used for chronic imaging experiments. Although the use of genetically encoded sensors in imaging of moving animals has been limited by problems such as relatively poor signal-noise-ratio and protein instability, iterative effort to improve the properties is starting to produce genetically encoded sensors that are amenable to such in vivo chronic studies. Recently, two groups reported chronic imaging of calcium transient in live mouse brain using genetically encoed calcium sensors [68,99]. As the varieties and properties of genetically encoded sensors improve, this exciting new area of research will provide insights into the cellular signaling network underlying long-term physiological changes induced by developmental or conditional (e.g. learning) stimuli.
6. Conclusion
Genetically encoded biosensors became indispensable tools to analyze information encoded in the transients of signaling molecules. It also made it possible to analyze cellular activities such as metabolite export in unprecedented temporal resolution, opening up an exciting possibility to uncover previously unknown molecular machinery. Notable results published in the last few years bear evidence of the extensive effort that has been put into the improvement of genetically encoded biosensors. At least for a few molecules, biosensors have now improved to the point where they can be used in more demanding experimental settings such as chronic studies of specific cells placed in their native context (e.g., specific nerve cells in brain), and high-throuput application. Especially in neurobiology, improved biosensors are expected to serve as reporters of neuronal activity network initiated from localized excitation to decipher neuronal connections [100].
Supplementary Material
Acknowledgements
I thank Dr. John Todd Holland for discussions and critical reading of the manuscript. This work was supported by the National Institute of Health, National Institute of Neurological Disorders and Stroke (NINDS) 1R21NS064412.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady G. Expression profiling of single mammalian cells--small is beautiful. Yeast. 2000;17:211–217. doi: 10.1002/1097-0061(20000930)17:3<211::AID-YEA26>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawasaki ES. Microarrays and the gene expression profile of a single cell. Ann N Y Acad Sci. 2004;1020:92–100. doi: 10.1196/annals.1310.010. [DOI] [PubMed] [Google Scholar]
- 3.Shai RM. Microarray tools for deciphering complex diseases. Front Biosci. 2006;11:1414–1424. doi: 10.2741/1892. [DOI] [PubMed] [Google Scholar]
- 4.Newman JR, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- 5.Irish JM, Kotecha N, Nolan GP. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat Rev Cancer. 2006;6:146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 6.Le TT, Cheng JX. Single-cell profiling reveals the origin of phenotypic variability in adipogenesis. PLoS One. 2009;4:e5189. doi: 10.1371/journal.pone.0005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene JG, Borges K, Dingledine R. Quantitative transcriptional neuroanatomy of the rat hippocampus: evidence for wide-ranging, pathway-specific heterogeneity among three principal cell layers. Hippocampus. 2009;19:253–264. doi: 10.1002/hipo.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapainis T, Rubakhin SS, Sweedler JV. Capillary electrophoresis with electrospray ionization mass spectrometric detection for single-cell metabolomics. Anal Chem. 2009;81:5858–5864. doi: 10.1021/ac900936g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. Chemical calcium indicators. Methods. 2008;46:143–151. doi: 10.1016/j.ymeth.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 11.Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol. 1999;17:969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- 12.Karasawa S, Araki T, Nagai T, Mizuno H, Miyawaki A. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer. Biochem J. 2004;381:307–312. doi: 10.1042/BJ20040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelmanson IV, Matz MV. Molecular basis and evolutionary origins of color diversity in great star coral Montastraea cavernosa (Scleractinia: Faviida) Mol Biol Evol. 2003;20:1125–1133. doi: 10.1093/molbev/msg130. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Castner EW, Jr, Lawson CL, Falkowski PG. Biophysical characterization of natural and mutant fluorescent proteins cloned from zooxanthellate corals. FEBS Lett. 2004;570:175–183. doi: 10.1016/j.febslet.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Shagin DA, Barsova EV, Yanushevich YG, Fradkov AF, Lukyanov KA, Labas YA, Semenova TN, Ugalde JA, Meyers A, Nunez JM, et al. GFP-like proteins as ubiquitous metazoan superfamily: evolution of functional features and structural complexity. Mol Biol Evol. 2004;21:841–850. doi: 10.1093/molbev/msh079. [DOI] [PubMed] [Google Scholar]
- 16.New Living Colors™ Vectors. Clonechnique. 1998;13:20–24. [Google Scholar]
- 17.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 18.Ananthanarayanan B, Ni Q, Zhang J. Chapter 2: Molecular sensors based on fluorescence resonance energy transfer to visualize cellular dynamics. Methods Cell Biol. 2008;89:37–57. doi: 10.1016/S0091-679X(08)00602-X. [DOI] [PubMed] [Google Scholar]
- 19.Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2:444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- 20.Vogel SS, Thaler C, Koushik SV. Fanciful FRET. Sci STKE 2006. 2006:re2. doi: 10.1126/stke.3312006re2. [DOI] [PubMed] [Google Scholar]
- 21.Wallrabe H, Periasamy A. Imaging protein molecules using FRET and FLIM microscopy. Curr Opin Biotechnol. 2005;16:19–27. doi: 10.1016/j.copbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Frommer WB, Davidson MW, Campbell RE. Genetically encoded biosensors based on engineered fluorescent proteins. Chem Soc Rev. 2009;38:2833–2841. doi: 10.1039/b907749a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 24.Romoser VA, Hinkle PM, Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. J Biol Chem. 1997;272:13270–13274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- 25.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem. 2009;284:16289–16297. doi: 10.1074/jbc.M900501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evers TH, Appelhof MA, Meijer EW, Merkx M. His-tags as Zn(II) binding motifs in a protein-based fluorescent sensor. Protein Eng Des Sel. 2008;21:529–536. doi: 10.1093/protein/gzn029. [DOI] [PubMed] [Google Scholar]
- 27.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hires SA, Zhu Y, Tsien RY. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc Natl Acad Sci U S A. 2008;105:4411–4416. doi: 10.1073/pnas.0712008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci U S A. 2005;102:8740–8745. doi: 10.1073/pnas.0503274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 32.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honda A, Adams SR, Sawyer CL, Lev-Ram V, Tsien RY, Dostmann WR. Spatiotemporal dynamics of guanosine 3',5'-cyclic monophosphate revealed by a genetically encoded, fluorescent indicator. Proc Natl Acad Sci U S A. 2001;98:2437–2442. doi: 10.1073/pnas.051631298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikolaev VO, Gambaryan S, Lohse MJ. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat Methods. 2006;3:23–25. doi: 10.1038/nmeth816. [DOI] [PubMed] [Google Scholar]
- 35.Russwurm M, Mullershausen F, Friebe A, Jager R, Russwurm C, Koesling D. Design of fluorescence resonance energy transfer (FRET)-based cGMP indicators: a systematic approach. Biochem J. 2007;407:69–77. doi: 10.1042/BJ20070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato M, Hida N, Ozawa T, Umezawa Y. Fluorescent indicators for cyclic GMP based on cyclic GMP-dependent protein kinase Ialpha and green fluorescent proteins. Anal Chem. 2000;72:5918–5924. doi: 10.1021/ac0006167. [DOI] [PubMed] [Google Scholar]
- 37.Cicchetti G, Biernacki M, Farquharson J, Allen PG. A ratiometric expressible FRET sensor for phosphoinositides displays a signal change in highly dynamic membrane structures in fibroblasts. Biochemistry. 2004;43:1939–1949. doi: 10.1021/bi035480w. [DOI] [PubMed] [Google Scholar]
- 38.Tanimura A, Nezu A, Morita T, Turner RJ, Tojyo Y. Fluorescent biosensor for quantitative real-time measurements of inositol 1,4,5-trisphosphate in single living cells. J Biol Chem. 2004;279:38095–38098. doi: 10.1074/jbc.C400312200. [DOI] [PubMed] [Google Scholar]
- 39.Tanimura A, Morita T, Nezu A, Shitara A, Hashimoto N, Tojyo Y. Use of Fluorescence Resonance Energy Transfer-based Biosensors for the Quantitative Analysis of Inositol 1,4,5-Trisphosphate Dynamics in Calcium Oscillations. J Biol Chem. 2009;284:8910–8917. doi: 10.1074/jbc.M805865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato M, Ueda Y, Umezawa Y. Imaging diacylglycerol dynamics at organelle membranes. Nat Methods. 2006;3:797–799. doi: 10.1038/nmeth930. [DOI] [PubMed] [Google Scholar]
- 41.Rajamani S, Zhu J, Pei D, Sayre R. A LuxP-FRET-based reporter for the detection and quantification of AI-2 bacterial quorum-sensing signal compounds. Biochemistry. 2007;46:3990–3997. doi: 10.1021/bi602479e. [DOI] [PubMed] [Google Scholar]
- 42.Fukami-Kobayashi K, Tateno Y, Nishikawa K. Domain dislocation: a change of core structure in periplasmic binding proteins in their evolutionary history. J Mol Biol. 1999;286:279–290. doi: 10.1006/jmbi.1998.2454. [DOI] [PubMed] [Google Scholar]
- 43.Fukami-Kobayashi K, Tateno Y, Nishikawa K. Parallel evolution of ligand specificity between LacI/GalR family repressors and periplasmic sugar-binding proteins. Mol Biol Evol. 2003;20:267–277. doi: 10.1093/molbev/msg038. [DOI] [PubMed] [Google Scholar]
- 44.Fehr M, Frommer WB, Lalonde S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc Natl Acad Sci U S A. 2002;99:9846–9851. doi: 10.1073/pnas.142089199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lager I, Fehr M, Frommer WB, Lalonde S. Development of a fluorescent nanosensor for ribose. FEBS Lett. 2003;553:85–89. doi: 10.1016/s0014-5793(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 46.Fehr M, Lalonde S, Lager I, Wolff MW, Frommer WB. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J Biol Chem. 2003;278:19127–19133. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- 47.Kaper T, Lager I, Looger LL, Chermak D, Frommer WB. Fluorescence resonance energy transfer sensors for quantitative monitoring of pentose and disaccharide accumulation in bacteria. Biotechnol Biofuels. 2008;1:11. doi: 10.1186/1754-6834-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lager I, Looger LL, Hilpert M, Lalonde S, Frommer WB. Conversion of a putative Agrobacterium sugar-binding protein into a FRET sensor with high selectivity for sucrose. J Biol Chem. 2006;281:30875–30883. doi: 10.1074/jbc.M605257200. [DOI] [PubMed] [Google Scholar]
- 49.Gu H, Lalonde S, Okumoto S, Looger LL, Scharff-Poulsen AM, Grossman AR, Kossmann J, Jakobsen I, Frommer WB. A novel analytical method for in vivo phosphate tracking. FEBS Lett. 2006;580:5885–5893. doi: 10.1016/j.febslet.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaper T, Looger LL, Takanaga H, Platten M, Steinman L, Frommer WB. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS Biol. 2007;5:e257. doi: 10.1371/journal.pbio.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB. Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci. 2005;14:2304–2314. doi: 10.1110/ps.051508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wachter RM, Yarbrough D, Kallio K, Remington SJ. Crystallographic and energetic analysis of binding of selected anions to the yellow variants of green fluorescent protein. J Mol Biol. 2000;301:157–171. doi: 10.1006/jmbi.2000.3905. [DOI] [PubMed] [Google Scholar]
- 53.Jayaraman S, Haggie P, Wachter RM, Remington SJ, Verkman AS. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J Biol Chem. 2000;275:6047–6050. doi: 10.1074/jbc.275.9.6047. [DOI] [PubMed] [Google Scholar]
- 54.Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001;499:220–224. doi: 10.1016/s0014-5793(01)02561-3. [DOI] [PubMed] [Google Scholar]
- 55.Markova O, Mukhtarov M, Real E, Jacob Y, Bregestovski P. Genetically encoded chloride indicator with improved sensitivity. J Neurosci Methods. 2008;170:67–76. doi: 10.1016/j.jneumeth.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Kuner T, Augustine GJ. A genetically encoded ratiometric indicator for chloride: capturing chloride transients in cultured hippocampal neurons. Neuron. 2000;27:447–459. doi: 10.1016/s0896-6273(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 57.Bizzarri R, Arcangeli C, Arosio D, Ricci F, Faraci P, Cardarelli F, Beltram F. Development of a novel GFP-based ratiometric excitation and emission pH indicator for intracellular studies. Biophys J. 2006;90:3300–3314. doi: 10.1529/biophysj.105.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanson GT, McAnaney TB, Park ES, Rendell ME, Yarbrough DK, Chu S, Xi L, Boxer SG, Montrose MH, Remington SJ. Green fluorescent protein variants as ratiometric dual emission pH sensors. 1. Structural characterization and preliminary application. Biochemistry. 2002;41:15477–15488. doi: 10.1021/bi026609p. [DOI] [PubMed] [Google Scholar]
- 59.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 60.Chapleau RR, Blomberg R, Ford PC, Sagermann M. Design of a highly specific and noninvasive biosensor suitable for real-time in vivo imaging of mercury (II) uptake. Protein Sci. 2008;17:614–622. doi: 10.1110/ps.073358908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 62.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akerboom J, Rivera JD, Guilbe MM, Malave EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci U S A. 1999;96:11241–11246. doi: 10.1073/pnas.96.20.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 67.Souslova EA, Belousov VV, Lock JG, Stromblad S, Kasparov S, Bolshakov AP, Pinelis VG, Labas YA, Lukyanov S, Mayr LM, et al. Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnol. 2007;7:37. doi: 10.1186/1472-6750-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berg J, Hung YP, Yellen G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat Methods. 2009;6:161–166. doi: 10.1038/nmeth.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nausch LW, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc Natl Acad Sci U S A. 2008;105:365–370. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pham E, Chiang J, Li I, Shum W, Truong K. A computational tool for designing FRET protein biosensors by rigid-body sampling of their conformational space. Structure. 2007;15:515–523. doi: 10.1016/j.str.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat Biotechnol. 2005;23:355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- 73.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin- peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Okada S, Ota K, Ito T. Circular permutation of ligand-binding module improves dynamic range of genetically encoded FRET-based nanosensor. Protein Sci. 2009;18:2518–2527. doi: 10.1002/pro.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deuschle K, Chaudhuri B, Okumoto S, Lager I, Lalonde S, Frommer WB. Rapid metabolism of glucose detected with FRET glucose nanosensors in epidermal cells and intact roots of Arabidopsis RNA-silencing mutants. Plant Cell. 2006;18:2314–2325. doi: 10.1105/tpc.106.044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhuri B, Hormann F, Lalonde S, Brady SM, Orlando DA, Benfey P, Frommer WB. Protonophore- and pH-insensitive glucose and sucrose accumulation detected by FRET nanosensors in Arabidopsis root tips. Plant J. 2008;56:948–962. doi: 10.1111/j.1365-313X.2008.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fehr M, Takanaga H, Ehrhardt DW, Frommer WB. Evidence for high-capacity bidirectional glucose transport across the endoplasmic reticulum membrane by genetically encoded fluorescence resonance energy transfer nanosensors. Mol Cell Biol. 2005;25:11102–11112. doi: 10.1128/MCB.25.24.11102-11112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takanaga H, Chaudhuri B, Frommer WB. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim Biophys Acta. 2008;1778:1091–1099. doi: 10.1016/j.bbamem.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dulla C, Tani H, Okumoto S, Frommer WB, Reimer RJ, Huguenard JR. Imaging of glutamate in brain slices using FRET sensors. J Neurosci Methods. 2008;168:306–319. doi: 10.1016/j.jneumeth.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiechert W, Schweissgut O, Takanaga H, Frommer WB. Fluxomics: mass spectrometry versus quantitative imaging. Curr Opin Plant Biol. 2007;10:323–330. doi: 10.1016/j.pbi.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mank M, Reiff DF, Heim N, Friedrich MW, Borst A, Griesbeck O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006;90:1790–1796. doi: 10.1529/biophysj.105.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mao T, O'Connor DH, Scheuss V, Nakai J, Svoboda K. Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators. PLoS One. 2008;3:e1796. doi: 10.1371/journal.pone.0001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tay LH, Griesbeck O, Yue DT. Live-cell transforms between Ca2+ transients and FRET responses for a troponin-C-based Ca2+ sensor. Biophys J. 2007;93:4031–4040. doi: 10.1529/biophysj.107.109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 86.Piljic A, Schultz C. Simultaneous recording of multiple cellular events by FRET. ACS Chem Biol. 2008;3:156–160. doi: 10.1021/cb700247q. [DOI] [PubMed] [Google Scholar]
- 87.Ai HW, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Methods. 2008;5:401–403. doi: 10.1038/nmeth.1207. [DOI] [PubMed] [Google Scholar]
- 88.Grant DM, Zhang W, McGhee EJ, Bunney TD, Talbot CB, Kumar S, Munro I, Dunsby C, Neil MA, Katan M, et al. Multiplexed FRET to image multiple signaling events in live cells. Biophys J. 2008;95:L69–L71. doi: 10.1529/biophysj.108.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Niino Y, Hotta K, Oka K. Simultaneous live cell imaging using dual FRET sensors with a single excitation light. PLoS One. 2009;4:e6036. doi: 10.1371/journal.pone.0006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft- mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 91.Sonnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 92.Starkuviene V, Liebel U, Simpson JC, Erfle H, Poustka A, Wiemann S, Pepperkok R. High-content screening microscopy identifies novel proteins with a putative role in secretory membrane traffic. Genome Res. 2004;14:1948–1956. doi: 10.1101/gr.2658304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolff M, Wiedenmann J, Nienhaus GU, Valler M, Heilker R. Novel fluorescent proteins for high-content screening. Drug Discov Today. 2006;11:1054–1060. doi: 10.1016/j.drudis.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 94.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barretto RP, Messerschmidt B, Schnitzer MJ. In vivo fluorescence imaging with high-resolution microlenses. Nat Methods. 2009;6:511–512. doi: 10.1038/nmeth.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JN. Visually evoked activity in cortical cells imaged in freely moving animals. Proc Natl Acad Sci U S A. 2009;106:19557–19562. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mank M, Santos AF, Direnberger S, Mrsic-Flogel TD, Hofer SB, Stein V, Hendel T, Reiff DF, Levelt C, Borst A, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5:805–811. doi: 10.1038/nmeth.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miesenböck G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.