Abstract
The duration of immunity of the dual-subtype feline immunodeficiency virus (FIV) vaccine, Fel-O-Vax FIV, for protection against subtype-B FIV was assessed in this study. Vaccinated cats along with controls were challenged with FIVFC1, a subtype-B FIV strain, 54 weeks after the final vaccination, and monitored for 46–48 weeks for provirus and viral RNA in peripheral blood, provirus in lymphoid organs, and CD4:CD8 ratios. Results of provirus detection in peripheral blood and lymphoid organs and plasma viral RNA loads showed that 10/14 vaccinated cats were fully protected for 48 weeks against infection with FIVFC1 whereas 5/5 controls were persistently infected with FIVFC1. CD4:CD8 inversions were noted in association with FIV infection and viral loads were not significantly different between FIV infected controls and the unprotected vaccinated animals.
Feline immunodeficiency virus (FIV) is a lentivirus morphologically similar but antigenically distinct from other lentiviruses such as human immunodeficiency virus. FIV infects cats and causes progressive impairment of the host immune system, eventually leading to acquired immunodeficiency syndrome or AIDS-like disease in infected cats. One hallmark of FIV infection is depletion of CD4+ T cells and reduction in the CD4:CD8 lymphocyte ratio. 1 Serological surveys of domestic cats revealed that the FIV prevalence rate is generally 2–5% for healthy cats, and as high as 12–14% for sick cats in North America and much of Europe. Higher prevalence rates have been reported for other geographic locations such as Japan and Italy. 2,3
FIV isolates from domestic cats have been classified into five subtypes based on the env or gag amino acid or nucleotide sequences. 1,4,5 There are generally 7–27% nucleic acid difference between subtypes and 2–17% nucleic acid difference within a subtype. 1 The large intra- and inter-subtype genetic heterogeneity makes the development of an efficacious vaccine difficult. A variety of experimental vaccines have been evaluated for efficacy, and these include inactivated infected cell, inactivated whole virus, protein or vectored subunit, or DNA vaccines. 1,6 Most success achieved in FIV vaccine trials so far has been made using the conventional approach. 1 A dual-subtype FIV vaccine consisting of inactivated whole viruses from subtype A (FIVPet) and subtype D (FIVShi) strains was found to be effective against homologous FIV (ie, vaccine strains) and against subtype-B strain, FIVBang. 7,8 A vaccine based on this technology, Fel-O-Vax FIV, was approved for commercial release in the US in 2002. Without a boost dose prior to challenge, 84% of the vaccinated cats were protected against a heterologous FIV challenge of the same subtype 1 year following the vaccination. 9 This vaccine was found to be completely protective against contact challenge with cats infected with one heterologous subtype-B strain (FIVAomori2), 10 and against intravenous challenge with another subtype-B strain (FIVFC1). 11 In both studies, vaccinated cats were subjected to challenge exposure at 2–3 weeks following the administration of the final vaccine dose. The duration of immunity of the vaccine for subtype-B FIV was not addressed in either study. The purpose of the present study was to determine whether 1-year duration of immunity in cats can be attainable with Fel-O-Vax FIV for subtype-B FIV.
In this study, cats were held for 12 months after completion of the primary vaccination, and then challenged with an in vivo-derived challenge inoculum of FIVFC1 along with unvaccinated control cats of the same age. Following challenge, all cats were monitored for 46–48 weeks for FIV provirus and viral RNA in peripheral blood, FIV provirus in various lymphoid organs. In addition, the CD4:CD8 lymphocyte ratio was monitored.
Material and methods
Experimental animals
Nineteen specific pathogen-free (SPF) kittens born in the same week were obtained from Liberty Research (New York) and randomly assigned to two groups: the vaccinate group (six males and eight females) and the control group (two males and three females). Vaccinated cats and controls were housed together in isolation pens at Fort Dodge Animal Health animal facilities during the entire study period. Care was provided by staff of the Veterinary Services group of Fort Dodge Animal Health. All the animal experiments described in this paper were reviewed and approved by the Institutional Animal Care and Use Committee of Fort Dodge Animal Health. To reduce respiratory infection during the long study period, all cats in this study were also vaccinated with Fel-O-Vax PCT (Fort Dodge Animal Health, USA), a vaccine that protects cats against diseases caused by feline panleukopenia virus, feline calicivirus and feline rhinotracheitis virus. Fel-O-Vax PCT was administered to each cat via the subcutaneous route at 14 and 28 days after the third vaccination with Fel-O-Vax FIV. All cats were monitored daily for general heath and found to be clinically healthy throughout the study.
Vaccination
Fel-O-Vax FIV containing the minimal release potency was used in this study. Cats in the vaccinate group were vaccinated by the subcutaneous route with three 1.0 ml doses given 3 weeks apart. The controls were not administered the test vaccine or a placebo injection. All cats were 7–8 weeks of age (52–57 days of age) at the time of first vaccination.
Serological tests
Serum samples were collected from jugular veins of cats under anesthetization with Ketaset (Fort Dodge Animal Health, USA) on the day of each vaccination, 7 and 21 days after the third vaccination, 1, 3, 6, 9 and 12 months after the third vaccination, and 8, 16 and 40-weeks post challenge (wpc). The levels of FIV-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA) using recombinant FIV core antigen p24 protein or transmembrane (TM) peptide containing an immunodominant epitope of the transmembrane portion of the FIV envelope glycoprotein (KVEAMEKFLYTAFAMQELGCNQNQFFCKIPLE) as coating antigen. The FIV p24 ELISA was performed as previously described, 9 with the exception that plates were read kinetically using the VersaMax microplate reader, and the Vmax values were calculated using SoftMax Pro software (Molecular Devices, USA). The TM ELISA was similarly performed as for p24 ELISA except that the TM peptide was used as coating antigen.
Challenge procedure and sample collection
All vaccinated cats were held for 54 weeks following the final vaccination and then challenged along with age-matched unvaccinated controls with FIVFC1, a virulent subtype-B FIV strain described previously. 11 The challenge inoculum consisted of pooled peripheral blood mononuclear cells (PBMC) directly isolated from two SPF kittens infected with FIVFC1. To prepare the challenge material, two SPF kittens were each inoculated intravenously (IV) with 1 ml of whole blood taken from one cat infected with FIVFC1 and provided by Dr Janet Yamamoto. At 8 weeks following the experimental infection, both cats were exsanguinated for EDTA (ethylenediaminetetraacetic acid) whole blood. PBMC were prepared from the whole blood using discontinuous Percoll gradients as previously described, 12 and stored in single-use aliquots under liquid nitrogen. The titer of the frozen PMBC challenge material was determined in vivo using kittens of 20–30 weeks of age. It was found that 1000 or more PBMC inoculated IV to each kitten caused FIV viremia 6 weeks later in all challenged animals. To evaluate the efficacy of the dual-subtype FIV vaccine, 1000 PMBC of the challenge material was inoculated IV to each cat in both vaccinate and control groups. To detect FIV infection, peripheral blood samples were collected by jugular venepuncture from each cat under anesthetization with Ketaset on the day of challenge, and at 6 wpc, 8 wpc, 10 wpc, 12 wpc, 14 wpc, 16 wpc, 28 wpc and 40 wpc. To detect and quantitate provirus in lymphoid organs, cats were euthanased from 46 wpc to 48 wpc, and bone marrow, spleen, thymus, mesenteric lymph node and popliteal lymph nodes were collected from each cat.
Preparation of mononuclear cells
Bone marrow, spleen, thymus, mesenteric and popliteal lymph nodes were gently homogenized and passed through sterile cell dissociation sieve (Sigma–Aldrich, USA). The collected cell suspension of each sample was used to purify mononuclear cells using discontinuous Percoll gradients as previously described. 12
Detection and quantitation of FIV provirus
To detect or quantitate FIV provirus in various samples, PCR-ready DNA was prepared from whole blood or purified mononuclear cells using MagAttract DNA mini M48 kit (Qiagen, USA) on Qiagen M48 BioRobot following the manufacturer's recommendations. For each sample, 200 μl of peripheral blood or 1–2×10 6 purified mononuclear cells were processed, and DNA was eluted in 100 μl elution buffer and stored at −20°C if not freshly tested.
Oligonucleotide primers specific for the gag gene of FIVFC1 strain were employed in real-time TaqMan polymerase chain reaction (PCR). The forward primer has the sequence 5′-CTAGACCGCTGCCCTATTTCA-3′. The reverse primer has the sequence 5′-GCCAACCTTCCTAGTGCTTCA-3′. This set of primers amplifies a 135-bp fragment. The TaqMan probe has the sequence 5′-FAM-TCAAGAACAACAAGCGGAGCCCAGA-TAMRA-3′. For each reaction, 10 μl of PCR-ready DNA, 10 ρmol of each primer and 5 ρmol of the probe were added to Platinum PCR mix (Invitrogen, USA) in a total volume of 50 μl. The reaction was run with an initial cycle of 95°C for 5 min followed by 45 cycles with each cycle consisting of 95°C for 30 s and 60°C for 30 s. Amplification, data acquisition and data analysis were carried out in a DNA Engine Opticon (Bio-Rad, USA). The detection limit of the TaqMan PCR assay was determined to be two copies of FIV proviral DNA per reaction.
The proviral loads expressed as copy number of FIV provirus in blood and lymphoid tissues were calculated by the Opticon Monitor Analysis Software (Bio-Rad, USA) using standard curves generated with a plasmid containing a 611-bp FIVFC1 gag DNA fragment, and were normalized for 106 mononuclear cells. The detection limit was estimated to be 20 copies of FIV provirus per 106 mononuclear cells.
Quantitation of FIV plasma viral RNA
Viral RNA was extracted from plasma samples using MagAttract Viral RNA M48 kit (Qiagen, USA) on Qiagen M48 BioRobot following the manufacturer's directions. For each sample, 300 μl of blood plasma was processed, and viral RNA was eluted in 100 μl of the supplied buffer and stored at −70°C if not freshly tested.
Plasma viral RNA loads were determined using real-time reverse transcription (RT) PCR with the same set of primers and probe as described for TaqMan PCR. For each reaction, 10 μl of RNA sample, 10 ρmol of each primer and 5 ρmol of the TaqMan probe were added to QuantiTect Probe RT-PCR Kit (Qiagen, USA) in a total volume of 50 μl. RT was performed at 50°C for 30 min followed by incubation at 95°C for 15 min to inactivate the reverse transcriptase and activate the Taq polymerase. The cDNA was amplified by cycling for 45 cycles between 95°C for 15 s and 60°C for 30 s. Amplification, data acquisition and data analysis were carried out in the DNA Engine Opticon. The viral RNA loads expressed as copy numbers of FIV viral RNA in plasma were calculated by the Opticon Monitor Analysis Software using standard curves generated with a FIVFC1 gag RNA fragment that was generated using in vitro transcription, and normalized for 1 ml of plasma. The detection limit of this RT-PCR assay was determined to be one copy of FIV RNA per reaction, and 20 copies of FIV RNA in a 300 μl sample.
Determination of CD4 and CD8 lymphocyte subsets
EDTA whole blood was collected from jugular veins of cats prior to challenge, at 31 wpc and 40 wpc. Whole blood was labelled with anti-feline CD4-fluorescein isothiocyanate (FITC) and anti-feline CD8β-phycoerythrin (PE) (Southern Biotechnology Associates, USA), and then subjected to whole-blood lysis using OptiLyse B reagent (Immunotech, France). The cells were washed and analyzed on a BD FACS Canto flow cytometer (BD Biosciences, USA). For each sample, 10,000–20,000 events were collected for CD4 and CD8 lymphocyte subset analysis.
Statistical analysis
Data for proviral loads, plasma viral loads and CD4:CD8 ratios were found to be normally distributed, and analyzed using one-way analysis of variance (ANOVA). The statistical analysis was performed using StatView 5.0.1 (SAS Institute Inc). The level of significance was set at <0.05. The preventable fraction (PF), a measure of vaccine efficacy, was calculated as follows: PF=1−Pv/Pc while Pv is the proportion of vaccinates that become infected with FIV and Pc is the proportion of controls that become infected with FIV following challenge.
Results
Serological antibody responses
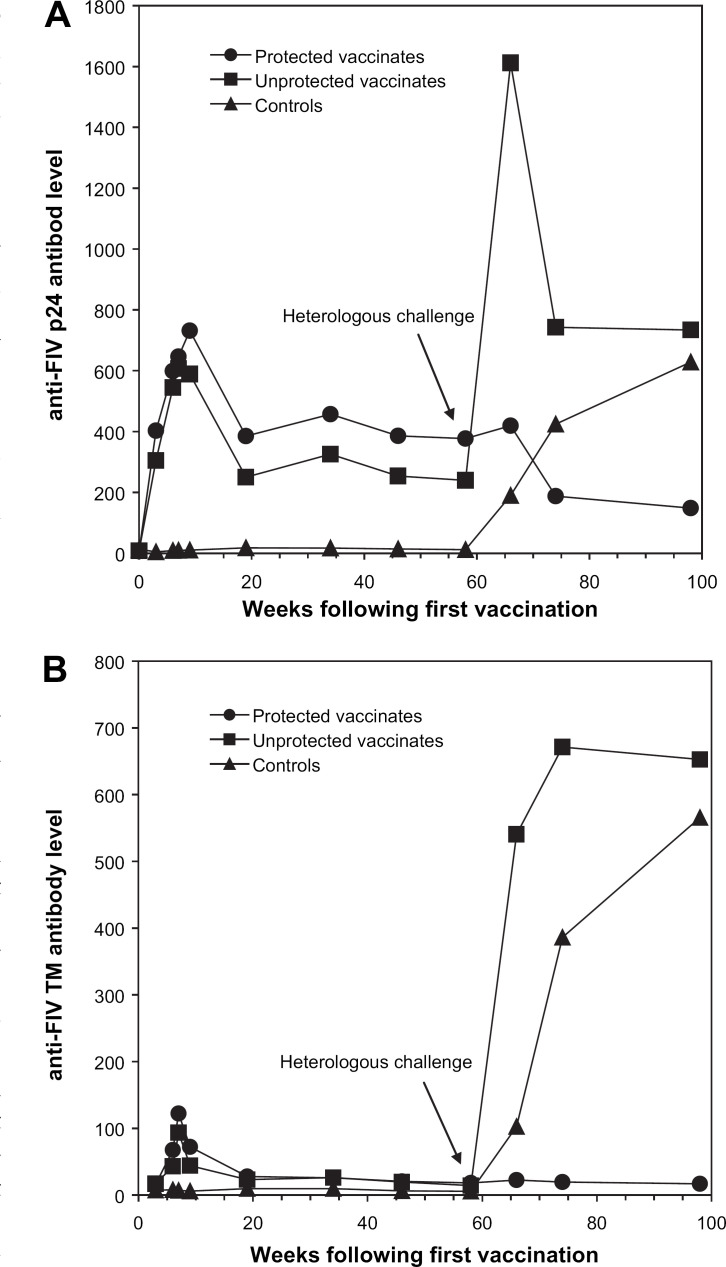
All cats used in this study were negative for FIV infection on the day of the first vaccination, as confirmed by a lack of FIV p24 or TM-specific antibodies in the serum samples. After one vaccination, all vaccinated cats developed FIV p24 binding antibodies with a mean Vmax of 375. The antibody levels peaked from 1–3 weeks post the third vaccination (wpv3) with a mean Vmax of 635 at 1 wpv3 and 691 at 3 wpv3. The antibody levels decreased at 3 months following the third dose, and maintained steady through the 12th month for most vaccinates (Fig. 1, panel A). A similar pattern was observed for TM-specific antibodies (Fig. 1, panel B).
Fig 1.
Kinetics of the humoral immune responses in vaccinate and control groups. ELISA was performed at a serum dilution of 1:200, and results are expressed as mean normalized Vmax values for various groups. The protected vaccinates (n=10) were FIV provirus negative whereas the unprotected vaccinates (n=4) and controls (n=5) were FIV provirus positive following challenge with FIVFC1. Panel A represents the kinetics of anti-FIV p24 antibody responses. Panel B represent the kinetics of anti-FIV TM antibody responses.
Following challenge with FIVFC1, 4/14 vaccinated cats and all five unvaccinated controls were found to be infected with FIV whereas 10/14 vaccinated cats were protected from FIV infection (see next section). All controls developed FIV p24 or TM-specific antibodies following challenge (Fig. 1, panels A and B). All unprotected vaccinates displayed a strong anamnestic response as evidenced by >6-fold increase in anti-FIV core antibody levels at 8 wpc, and >40-fold increase in anti-FIV envelope levels at 8 wpc and later time points. However, for the protected vaccinates, the anti-FIV core antibody levels remained essentially unchanged at 8 wpc and decreased thereafter, and the anti-FIV envelope antibody levels remained unchanged following challenge.
Detection of FIV proviral DNA in peripheral blood
Results of FIV proviral DNA detection from peripheral blood using TaqMan PCR are shown in Table 1. Four of five unvaccinated controls became positive for FIV provirus by 6 wpc. All five controls became infected by 8 wpc and remained infected through 46–48 wpc. As for the vaccinate group, 4/14 cats were positive for FIV provirus at 6 wpc and they remained positive through 46–48 wpc while 10 other vaccinated cats remained negative for FIV provirus at all time points. The PF for the vaccine was 71%.
Table 1.
Detection of FIV proviral DNA from peripheral blood by TaqMan PCR.
| Treatment | Cat ID | Sex | wpc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 8 | 10 | 12 | 14 | 16 | 28 | 40 | 46–48 | |||
| Vaccinated | 05AFZ4 | F | − | − | − | − | − | − | − | − | − | − |
| 05AGB1 | M | − | − | − | − | − | − | − | − | − | − | |
| 05AGB4 | M | − | − | − | − | − | − | − | − | − | − | |
| 05AGB5 | F | − | − | − | − | − | − | − | − | − | − | |
| 05AGB6 | F | − | − | − | − | − | − | − | − | − | − | |
| 05AGC6 | F | − | − | − | − | − | − | − | − | − | − | |
| 05AGD2 | M | − | − | − | − | − | − | − | − | − | − | |
| 05AGD3 | M | − | − | − | − | − | − | − | − | − | − | |
| 05AGD4 | M | − | + | + | + | + | + | + | + | + | + | |
| 05AGD7 | F | − | − | − | − | − | − | − | − | − | − | |
| 05AGG9 | F | − | + | + | + | + | + | + | + | + | + | |
| 05AGH2 | M | − | + | + | + | + | + | + | + | + | + | |
| 05AGI2 | F | − | − | − | − | − | − | − | − | − | − | |
| 05AGI4 | F | − | + | + | + | + | + | + | + | + | + | |
| Control | 05AGC4 | F | − | − | + | + | + | + | + | + | + | + |
| 05AGD1 | M | − | + | + | + | + | + | + | + | + | + | |
| 05AGG6 | M | − | + | + | + | + | + | + | + | + | + | |
| 05AGH7 | F | − | + | + | + | + | + | + | + | + | + | |
| 05AGJ5 | F | − | + | + | + | + | + | + | + | + | + | |
Peripheral blood samples taken at indicated times following challenge with FIVFC1 were examined for proviral DNA using TaqMan PCR. +=PCR positive; −=PCR negative; M=male; F=female; ND=not done.
Detection of FIV viral RNA in peripheral blood
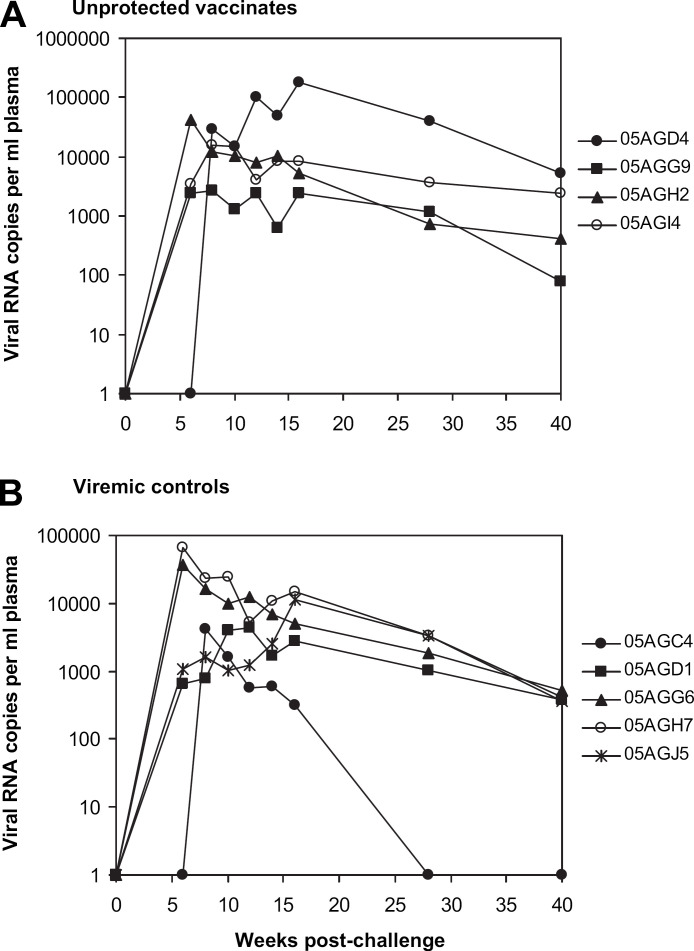
The levels of FIV viral RNA in peripheral blood (plasma viral loads) were measured using real-time RT-PCR for a 40-week period after challenge and results for viremic cats are shown in Fig. 2. Plasma viral RNA was detected in all controls by 8 wpc, with viral RNA load peaking between 6 wpc and 16 wpc, ranging from 4160 to 66,170 copies/ml plasma. Plasma viral RNA was detected in the same four vaccinated cats that were positive for proviral DNA, with viral RNA loads peaking at various time points between 6 wpc and 16 wpc, and ranging from 2620 to 177,310 copies/ml plasma. There was no significant difference in plasma viral loads between the unprotected vaccinated cats and viremic controls at all time points. For the 10 protected vaccinated cats, plasma viral RNA was undetectable at all time points.
Fig 2.
Measurement of plasma viral load (copies of viral RNA/ml) by TaqMan RT-PCR in unprotected vaccinate and control groups. Plasma samples taken at indicated times following challenge with FIVFC1 were examined for viral RNA load using real-time RT-PCR. Panel A: unprotected vaccinates. Panel B: viremic controls. Cat identification numbers are shown on the right.
Measurement of proviral loads in lymphoid organs
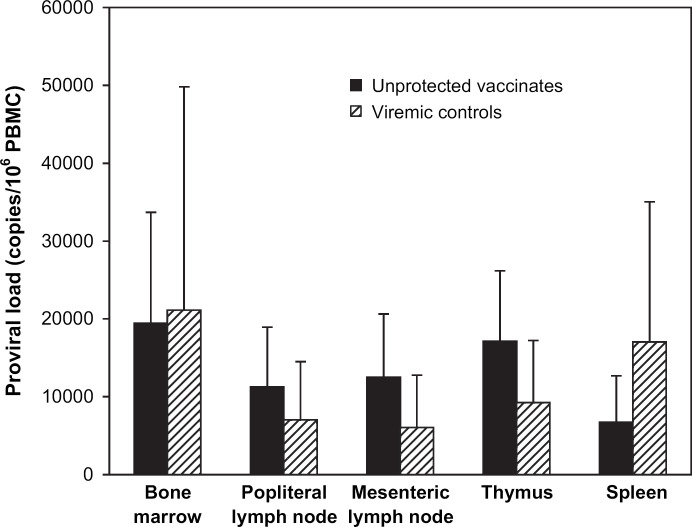
All cats were euthanased at 46–48 weeks after viral challenge. Spleen, thymus, bone marrow, mesenteric and popliteal lymph nodes were collected. Mononuclear cells were isolated from each sample and used for measurement of proviral loads using real-time PCR. For the 10 protected vaccinated cats, the provirus was undetectable in any of the lymphoid organs examined. Results for the unprotected vaccinates and viremic controls are shown in Fig. 3. FIV provirus was detected in all lymphoid organs of each of the five controls and four unprotected viremic vaccinates. There was no significant difference in proviral loads in any lymphoid organ between the unprotected vaccinates and viremic controls. Depending on individual infected animals, highest proviral loads were found in bone marrow, thymus or spleen.
Fig 3.
Measurement of FIV proviral load in lymphoid tissues by TaqMan PCR in unprotected vaccinate and control groups. Various lymphoid tissues were taken at 46–48 wpc and the proviral load measured using real-time PCR. The mean and standard deviation are shown for each lymphoid tissue.
Changes in CD4:CD8 lymphocyte ratios
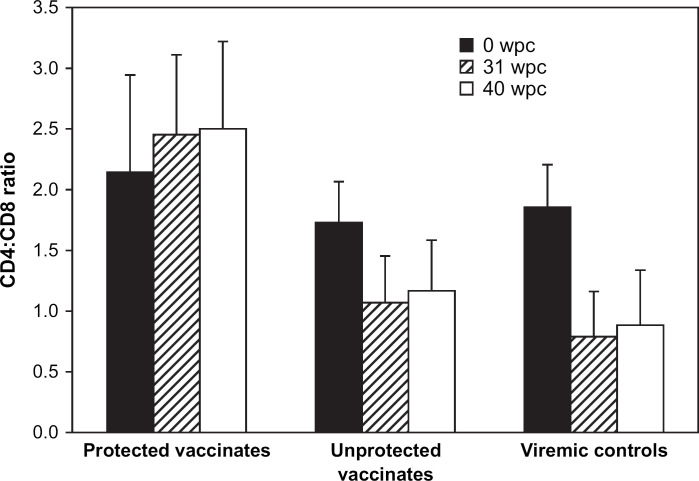
The CD4:CD8 lymphocyte ratios were determined for all cats on the day of challenge (0 dpc), at 31 wpc and 40 wpc, and results are shown in Fig. 4. There was a significant reduction in the CD4:CD8 ratio for the control group when compared to the vaccinate group (P<0.05, repeated measures ANOVA). Moreover, the CD4:CD8 ratio was inverted (<1.0) for 3/5 controls at 31 wpc and for 4/5 controls at 40 wpc. In contrast, the CD4:CD8 ratio was inverted for only 2/15 vaccinates at 31 wpc (05AGG9) or at both 31 wpc and 40 wpc (05AGI4). All affected vaccinated cats were persistently infected with FIVFC1.
Fig 4.
Measurement of CD4:CD8 ratios following challenge with FIVFC1. The CD4:CD8 lymphocyte ratios were determined for all cats at 0 dpc, 31 wpc and 40 wpc. The mean and standard deviation are shown for each group.
Discussion
Data from the present study demonstrated for the first time that duration of protective immunity of 12 months against a subtype-B FIV strain was attained with vaccination using a batch of Fel-O-Vax FIV vaccine containing the minimal release potency. Results from detection and quantitation of provirus in peripheral blood and lymphoid organs, and results for plasma viral loads indicated that 10/14 vaccinated cats were fully protected for at least 48 weeks against infection with FIVFC1, whereas all control cats were persistently infected with FIVFC1. Further evidence of the vaccine-induced protection was provided by the measurement of CD4 and CD8 cell counts as a substantial reduction in the CD4:CD8 ratio was observed with 5/5 controls, and with only 3/15 infected vaccinated cats. The 11 non-viremic vaccinated cats were protected from FIV induced CD4:CD8 ratio depression, and had normal CD4 cell counts and CD4:CD8 ratios. For nearly all affected cats including all five controls, the significant decrease in the CD4:CD8 ratio was accompanied by a significant reduction of CD4 cells. The significant decline in the CD4:CD8 ratio was also accompanied by a corresponding large increase in the CD8 cells for three controls and one vaccinated cat. The large increase in the CD8 cells was primarily due to the dramatic expansion of CD8βlow population (data not shown), which is consistent with findings with other FIV isolates. 5,13
Anti-FIV antibody levels for the vaccinate group were at maximum from 7–21 days following last vaccination. The antibody levels tended to decrease thereafter. However, the antibodies remained detectable 12 months later for all vaccinated cats. This finding suggests that the three-dose vaccination regimen is capable of establishing and maintaining anti-FIV immunological memory for at least 12 months as observed in previous studies. 9 Following challenge, all unprotected vaccinated cats displayed strong anamnestic response as evidenced by drastic increase in anti-FIV antibody levels at 8 wpc. In contrast, for the protected vaccinates, the anti-FIV core antibody levels remained essentially unchanged at 8 wpc and decreased thereafter, and the anti-FIV envelope antibody levels remained unchanged following challenge, suggesting that replication of the challenge virus was either abolished or effectively suppressed by the immune memory response in the protected vaccinates.
Though the mechanism of protection has not been firmly established for the dual-subtype FIV vaccine, this vaccine has been shown to be protective against a number of subtype A and subtype-B FIV isolates. 1,8,10,11,14,15 Results from this and previous studies indicate that the protective immunity of the dual-subtype FIV vaccine against certain subtype A or subtype-B FIV isolates can last for at least 12 months.
Acknowledgements
We are very grateful to Dr Janet Yamamoto, University of Florida, for providing FIVFC1. We appreciate the great technical assistance in flow cytometry from Dr Shawn Rigby, Cell Facility of Iowa State University. We wish to thank Nguyet-Thu Nguyen and Jennifer Hess for providing excellent technical support.
References
- 1.Uhl E.W., Martin M., Coleman J.K., Yamamoto J.K. Advances in FIV vaccine technology, Vet Immunol Immunopathol 15, 2008, 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy J.K., Scott H.M., Lachtara J.L., Crawford P.C. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity, J Am Vet Med Assoc 228, 2006, 371–376. [DOI] [PubMed] [Google Scholar]
- 3.Sellon R.K., Hartmann K. Feline immunodeficiency virus infection. Greene C.E. Infectious diseases of the dog and cat, 2nd edn., 2006, Saunders Elsevier: Oxford, 131–143. [Google Scholar]
- 4.Steinrigl A., Klein D. Phylogenetic analysis of feline immunodeficiency virus in Central Europe: a prerequisite for vaccination and molecular diagnostics, J Gen Virol 84, 2003, 1301–1307. [DOI] [PubMed] [Google Scholar]
- 5.Phadke A.P., de la Concha-Bermejillo A., Wolf A.M., Andersen P.R., Baladandayuthapani V., Collisson E.W. Pathogenesis of a Texas feline immunodeficiency virus isolate: an emerging subtype of clade B, Vet Microbiol 115, 2006, 64–76. [DOI] [PubMed] [Google Scholar]
- 6.Klonjkowski B., Klein D., Galea S., et al. Gag-specific immune enhancement of lentiviral infection after vaccination with an adenoviral vector in an animal model of AIDS, Vaccine 27, 2009, 928–939. [DOI] [PubMed] [Google Scholar]
- 7.Hohdatsu T., Okada S., Motokawa K., Aizawa C., Yamamoto J.K., Koyama H. Effect of dual-subtype vaccine against feline immunodeficiency virus infection, Vet Microbiol 58, 1997, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pu R., Coleman J., Omori M., et al. Dual-subtype FIV vaccine protects cats against in vivo swarms of both homologous and heterologous subtype FIV isolates, AIDS 15, 2001, 1225–1237. [DOI] [PubMed] [Google Scholar]
- 9.Huang C., Conlee D., Loop J., Champ D., Gill M., Chu H.J. Efficacy and safety of a feline immunodeficiency virus vaccine, Anim Health Res Rev 5, 2004, 295–300. [DOI] [PubMed] [Google Scholar]
- 10.Kusuhara H., Hohdatsu T., Okumura M., et al. Dual-subtype vaccine (Fel-O-Vax FIV) protects cats against contact challenge with heterologous subtype B FIV infected cats, Vet Microbiol 108, 2005, 155–165. [DOI] [PubMed] [Google Scholar]
- 11.Pu R., Coleman J., Coisman J., et al. Dual-subtype FIV vaccine (Fel-O-Vax FIV) protection against a heterologous subtype B FIV isolate, J Feline Med Surg 7, 2005, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tompkins M.B., Ogilvie G.K., Franklin R.A., Kelley K.W., Tompkins W.A. Induction of IL-2 and lymphokine activated killer cells in the cat, Vet Immunol Immunopathol 16, 1987, 1–10. [DOI] [PubMed] [Google Scholar]
- 13.Willett B.J., Hosie M.J., Callanan J.J., Neil J.C., Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset, Immunol 78, 1993, 1–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Omori M., Pu R., Tanabe T., et al. Cellular immune responses to feline immunodeficiency virus (FIV) induced by dual-subtype FIV vaccine, Vaccine 23, 2004, 386–398. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto J.K., Pu R., Sato E., Hohdatsu T. Dual-subtype FIV vaccine protects cats against in vivo swarms of both homologous and heterologous subtype FIV isolates, AIDS 15, 2007, 1225–1237. [DOI] [PubMed] [Google Scholar]