Abstract
Rationale: AERAS-402 is a novel tuberculosis vaccine designed to boost immunity primed by bacillus Calmette-Guérin (BCG), the only licensed vaccine.
Objectives: We investigated the safety and immunogenicity of AERAS-402 in healthy Mycobacterium tuberculosis–uninfected BCG-vaccinated adults from a tuberculosis-endemic region of South Africa.
Methods: Escalating doses of AERAS-402 vaccine were administered intramuscularly to each of three groups of healthy South African BCG-vaccinated adults, and a fourth group received two injections of the maximal dose. Participants were monitored for 6 months, with all adverse effects documented. Vaccine-induced CD4+ and CD8+ T-cell immunity was characterized by an intracellular cytokine staining assay of whole blood and peripheral blood mononuclear cells.
Measurements and Main Results: AERAS-402 was well tolerated, and no vaccine-related serious adverse events were recorded. The vaccine induced a robust CD4+ T-cell response dominated by cells coexpressing IFN-γ, tumor necrosis factor-α, and IL-2 (“polyfunctional” cells). AERAS-402 also induced a potent CD8+ T-cell response, characterized by cells expressing IFN-γ and/or tumor necrosis factor-α, which persisted for the duration of the study.
Conclusions: Vaccination with AERAS-402 is safe and immunogenic in healthy adults. The immunity induced by the vaccine appears promising: polyfunctional T cells are thought to be important for protection against intracellular pathogens such as Mycobacterium tuberculosis, and evidence is accumulating that CD8+ T cells are also important. AERAS-402 induced a robust and durable CD8+ T-cell response, which appears extremely promising.
Clinical trial registered with www.sanctr.gov.za (NHREC no. 1381).
Keywords: tuberculosis, vaccine, immunity, CD4, CD8
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Effective tuberculosis vaccines are urgently needed to boost bacillus Calmette-Guérin–induced immunity, especially in tuberculosis (TB)-endemic countries. This is the first clinical report of an Ad35-vectored vaccine given to humans in a heterologous prime–boost strategy. The AERAS-402 vaccine comprises a recombinant, replication-deficient Ad35, which expresses the mycobacterial antigens Ag85A, Ag85B, and TB10.4. The findings in this study strongly support further clinical trials assessing the efficacy of AERAS-402 as a boosting vaccine.
What This Study Adds to the Field
AERAS-402 vaccination was safe and immunogenic in healthy Mycobacterium tuberculosis–uninfected BCG-vaccinated adults, and induced a robust polyfunctional CD4+ T-cell response. It also induced a robust and durable CD8+ T-cell response.
One third of the world's population is infected with Mycobacterium tuberculosis (Mtb), and every year 1.8 million people die from tuberculosis (TB) (1). Effective vaccination strategies may constitute the most sustainable interventions. The only current TB vaccine, bacillus Calmette-Guérin (BCG), reliably protects infants against miliary disease and meningitis (2, 3). However, the vaccine's efficacy in protecting against lung TB is highly variable (4). A concerted effort has been made toward strategies in which a heterologous vaccine would boost immunity primed by BCG or a recombinant BCG, in an effort to ultimately better protect against pulmonary disease (5–14). Here, we investigated one such candidate boost vaccine, AERAS-402.
The AERAS-402 vaccine comprises a recombinant, replication-deficient adenovirus, serotype 35 (Ad35), which expresses a fusion protein created from the sequences of the mycobacterial antigens Ag85A, Ag85B, and TB10.4. The antigens are fused contiguously as a one-piece fusion polyprotein that should be expressed on immunization with the Ad35 vaccine (AERAS-402) (15). In animal models, recombinant adenoviral vectors have been used to deliver vaccine antigens in combination with BCG, poxvirus-vectored vaccines, and DNA-based vaccines (8, 16–20). In these studies, heterologous prime–boost strategies have demonstrated enhanced immunogenicity and protective immunity against malaria (18, 21) and Mtb (22). Recombinant human adenovirus serotype 5 (Ad5) vaccines have been well tolerated, and have shown good safety profiles, in phase 1 trials. However, the prevalence of neutralizing antibody titers against Ad5 of up to 90% in sub-Saharan Africa (23), with associated limitations of the usefulness of this vector (16, 20, 24), has prompted exploration of alternative adenoviral vectors such as Ad35. The seroprevalence, and levels of neutralizing antibody titer, to Ad35 are lower than those of Ad5 worldwide including sub-Saharan Africa (20 vs. 90%, respectively), with significant levels of neutralizing titers (>200) in less than 5% of persons in sub-Saharan Africa (23).
Protective immunity against TB disease has yet to be fully elucidated. T-cell immunity, comprising CD4+ and CD8+ cells, is thought to be important for effective prevention of disease after Mtb infection (25). Induction of a durable Mtb-specific T-cell response is therefore an objective of novel vaccine strategies. Several T-cell effector molecules may play critical roles in Mtb control, including helper T-cell type 1 (Th1) cytokines IFN-γ (26–28), tumor necrosis factor (TNF)-α (29–31), and IL-2 (32). IL-2 promotes secondary expansion of memory T cells, and maintenance of a stable pool of these cells (33). Moreover, vaccination-induced “polyfunctional” T cells, which coexpress IFN-γ, TNF-α, and IL-2, have been associated with efficient control of murine Leishmania major (34) and Mtb infection (35) on virulent challenge.
In this phase 1 study we evaluated the safety and immunogenicity of AERAS-402 in healthy Mtb-uninfected, BCG-vaccinated South African adults. Escalating doses of AERAS-402 were administered intramuscularly to each of three groups, whereas a fourth group received two injections of the maximal dose. The vaccine was safe and immunogenic. This is the first clinical report of an Ad35-vectored vaccine given to humans in a heterologous prime–boost strategy.
METHODS
Study Design
This study was a phase 1 double-blind, randomized, placebo-controlled dose escalation study in four groups of healthy, Mtb-uninfected adults, previously vaccinated with BCG. The Medicines Control Council of South Africa and the research ethics committees of the University of Cape Town (Cape Town, South Africa) approved the protocol and subsequent amendments. Written, informed consent was obtained from all participants. The trial was conducted according to International Conference on Harmonization/Good Clinical Practice (ICH-GCP) guidelines and Guidelines for Good Clinical Practice in the Conduct of Clinical Trials in Human Participants in South Africa.
Enrollment and Vaccination
The aim was to enroll 40 participants, who would be assigned to one of four study groups. Healthy adult volunteers, aged 21–45 years, were recruited from the Worcester region of Western Cape Province of South Africa. Inclusion and exclusion are defined in the online supplement. In each of groups 1–3, seven participants would be assigned to receive a single dose of 1 ml of the vaccine, AERAS-402. The 1 ml contained 3 × 108 viral particles (VP) for study group 1, 3 × 109 VP for group 2, and 3 × 1010 VP for group 3. In each of these groups, three participants would receive 1 ml of placebo, consisting of the AERAS-402 vaccine diluents (sterile buffer containing Tris, MgCl2, NaCl, sucrose polysorbate 80, and water). Vaccine or placebo would be administered in a double-blind fashion (shown in Table 1). Participants in group 4 would receive two doses of vaccine or placebo, on Study Days 0 and 56. In this group, eight participants would be assigned to receive study vaccine (3 × 1010 VP), and two to receive placebo (shown in Table 1). In every case, vaccine or placebo was administered into the deltoid muscle on the contralateral side to the BCG vaccine, and the second dose (group 4) was administered in the opposite arm, that is, ipsilateral to the BCG vaccine.
TABLE 1.
TREATMENT ALLOCATION BY STUDY GROUP AND DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS ENROLLED*
| Group 1 | Group 2 | Group 3 | Group 4 | Total (%) | |
|---|---|---|---|---|---|
| AERAS-402 treatment, n | 7 | 7 | 7 | 8* | 29 (72.5%) |
| Vaccine doses | 1 | 1 | 1 | 2 | |
| Vaccine dose (viral particles) | 3 × 108 | 3 × 109 | 3 × 1010 | 3 × 1010 | |
| Placebo treatment, n | 3 | 3 | 3 | 2 | 11 (27.5%) |
| Total treatment, n | 10 | 10 | 10 | 10* | 40 (100%) |
| Male, n | 4 (40%) | 6 (60%) | 4 (40%) | 3 (30%) | 17 (42.5%) |
| Age, median (min–max, yr) | 24.0 (22.0–38.0) | 26.0 (22.0–38.0) | 27.5 (22.0–39.0) | 27.0 (21.0–38.0) | 26.5 (21.0–39.0) |
| Ethnic group, n | |||||
| Black | 0 | 1 | 2 | 4 | 7 (17.5%) |
| Colored | 7 | 9 | 7 | 3 | 26 (65%) |
| White | 3 | 0 | 1 | 3 | 7 (16.5%) |
| BMI, mean (SD), kg/m2 | 24.8 ± 2.9 | 23.4 ± 3.4 | 24.1 ± 3.5 | 24.4 ± 2.9 | 24.2 ± 3.1 |
Definition of abbreviation: BMI = body mass index.
Two of eight participants did not meet eligibility criteria for revaccination.
Follow-up and Safety Evaluation
Participants in groups 1–3 were evaluated on Days 0, 2, 7, 14, 28, 42, 84, and 182 after vaccination, whereas participants from group 4 were evaluated on Days 0, 7, 14, 28, 56, 58, 63, 70, 84, and 182 after vaccination. Blood for safety evaluation, which included biochemistry and hematology tests, was collected prevaccination and on Days 0, 7, and 28. In group 4, these tests were also done on Days 56, 63, and 84. Adverse events were recorded during the first 28 days after vaccination in all groups, and additionally from Days 56 to 84 in group 4. Participants received a daily diary card to record adverse events themselves for the first 7 days after vaccination. Assessment and classification of adverse events are provided in the online supplement.
Peripheral Blood Mononuclear Cell–based Intracellular Cytokine Staining Assay
The frequency of antigen-specific cytokine responses in peripheral blood mononuclear cell (PBMC)-derived T cells was determined as previously described (8), and the antibody panel is described in the online supplement.
Whole Blood Intracellular Cytokine Staining Assay
The frequency and pattern of antigen-specific cytokine–producing T cells in whole blood were determined as previously described (36), and the antibody panel is described in the online supplement.
Neutralizing Antibody Titers against Ad35 Viruses
Serum was isolated from blood obtained from the 20 study subjects in groups 3 and 4 before initial injection of AERAS-402 or placebo on Study Day 0, and again 6 months after initial injection on Day 182. These serum specimens were analyzed for the presence of neutralizing activity against Ad35, using a validated assay at Crucell (Leiden, The Netherlands) (37).
Data Analysis
Basic descriptive analysis was performed to examine adverse events. Comparisons of immunogenicity results between different time points were performed with Mann-Whitney U tests, using Prism 4.03 (GraphPad, San Diego, CA). Analysis of data is described in the online supplement.
RESULTS
Participants, Vaccination, and Follow-up
Three-hundred and ninety-six adults were screened between April and October 2007, to enroll 40 healthy adults into this trial—a screening-to-enrollment ratio of approximately 10:1. The main reason for the high screening failure rate was latent infection with Mtb, as demonstrated by a positive QuantiFERON-TB Gold In-Tube test (QFT; Cellestis, Victoria, Australia) and/or tuberculin skin test (≥15 mm) (Table 2, which also lists other reasons for screening failures).
TABLE 2.
REASONS FOR EXCLUSION OF INDIVIDUALS* WHO UNDERWENT SCREENING
| Reason | N (%)† |
|---|---|
| Screening failures | 356 (89.9) |
| Breastfeeding | 3 (0.8) |
| Quantiferon positive | 247 (62.4) |
| TST ≥15 mm | 143 (36.1) |
| Abnormal ECG | 10 (2.5) |
| Abnormal biochemistry | 20 (5.1) |
| Abnormal hematology | 22 (5.6) |
| Abnormal urine dipstick results | 2 (0.5) |
| Abnormal chest radiograph | 2 (0.5) |
| Chronic illness: hypertensive (n = 2); chronic corneal herpes infection (n = 1) | 3 (0.8) |
| Loss to follow-up during screening process | 3 (0.8) |
| Pregnant | 4 (1.0) |
| Withdrew consent during screening process | 9 (2.3) |
| Age outside range | 3 (0.8) |
| BMI outside range of 18–30 | 5 (1.3) |
| Smoker (>3 d/wk) | 4 (1.0) |
| Hepatitis B surface antigen positive | 19 (4.8) |
| Hepatitis C surface antigen positive | 2 (0.5) |
| HIV positive | 22 (5.5) |
Definition of abbreviations: BMI = body mass index; ECG = electrocardiogram; TST = tuberculin skin test.
Some subjects were excluded for multiple reasons.
Total individuals screened: 396 (100%).
The groups enrolled and vaccinated are described in Table 1. In group 4, two of eight participants did not meet eligibility criteria for revaccination with the study vaccine, one because of abnormal urine analysis, and one because of an elevated white cell count. Because these two participants received only a single dose, for purposes of analyzing immune responses, they were therefore moved into group 3 for analysis purposes only. All vaccine recipients, including the latter two participants, completed all study procedures and attended all visits.
AERAS-402 Displays an Acceptable Safety Profile in Healthy Mtb-uninfected Adults
A total of 158 adverse events were recorded across the study groups; 129 in vaccine recipients and 29 in placebo recipients. Eighty (50.6%) adverse events were related to vaccination, of which 69 (43.7%) were considered related to the study vaccine (Table 3). The adverse events related to placebo administration include the following: injection site pain (n = 2), malaise (n = 3), myalgia (n = 1), sore throat (n = 1), upper respiratory tract infection (n = 1), headache (n = 2), and nausea (n = 1).
TABLE 3.
SUMMARY OF ADVERSE EVENTS
| Group | Placebo | Group 1 | Group 2 | Group 3 | Group 4 | Total |
|---|---|---|---|---|---|---|
| Severity | ||||||
| Mild | 23 | 29 | 16 | 26 | 23 | 117 (74.0%) |
| Moderate | 4 | 4 | 10 | 11 | 1 | 30 (19.0%) |
| Severe | 2 | 3 | 2 | 0 | 4 | 11 (7.0%) |
| Total AEs | 29 | 36 | 28 | 37 | 28 | 158 (100%) |
| AEs related to the vaccine | 11 (37.9%) | 20 (55.6%) | 17 (60.7%) | 14 (37.8%) | 18 (64.3%) | 80 (50.6%) |
Definition of abbreviation: AE = adverse event.
The majority of events were considered mild (74%) or moderate (19%) in severity (Table 3). Three serious adverse events were recorded during the study. A vaccine recipient reported pain in the ipsilateral arm 51 days after vaccination, which required hospitalization and extensive investigations, but no specific diagnosis was made. The other two serious adverse events were an attempted suicide and HIV seroconversion, neither of which was considered to be a vaccine-related event.
Of the adverse events recorded, 11 were graded severe according to the parameters of the U.S. Food and Drug Administration (FDA) toxicity tables for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (April 2005; http://www.fda.gov/cber/gdlns/toxvac.htm) or the investigator's evaluation of their impact on normal daily activities. Three of these were solicited adverse events as recorded on the diary cards, namely fever and malaise (presumed to be related to the study vaccine), and sore throat (thought not to be related). Two cases of fever were reported during the study: one participant had a maximal temperature of 39.2°C on the day of vaccination, which returned to 37°C on Day 2 postvaccination, whereas the second case of fever was classified as mild (38°C to 38.4°C).
Among the eight unsolicited severe adverse events, two were recorded in the placebo group and six were recorded in the AERAS-402 groups. Of these eight, three were serious adverse events considered not related to the study vaccine and are described above and the remaining five were laboratory test abnormalities, for which the deviation from normal met the criteria for a grade 3 or 4 event according to the FDA toxicity tables. Two of the abnormal blood tests were considered possibly related to study vaccine, namely an increased creatinine phosphokinase and a decrease in hemoglobin. The remaining six in this group of severe, unsolicited adverse events were considered unlikely to be related, or not related, to the vaccine.
There appeared to be a relationship between the incidence of injection site pain and dosage level (Table 4), albeit not statistically significant. There did not appear to be an increase in incidence of solicited or unsolicited adverse events after the second dose of vaccine, compared with administration of a single dose only (Tables 3 and 4).
TABLE 4.
SOLICITED AND UNSOLICITED EVENTS
| Placebo (n = 11) n (%) | Group 1 (n = 7) n (%) | Group 2 (n = 7) n (%) | Group 3 (n = 9) n (%) | Group 4 (n = 6) n (%) | Total (n = 40) n (%) | |
|---|---|---|---|---|---|---|
| Subjects with at least one adverse event | 9 (81.8) | 7 (100%) | 6 (85.7%) | 8 (88.9%) | 5 (83.3%) | 35 (100%) |
| Solicited events | ||||||
| Arthralgia | — | 2 (28.6) | — | 1 (11.1) | — | 3 (8.6) 33 |
| Conjunctivitis | — | — | — | 1 (11.1) | — | 1 (2.9) 3 |
| Diarrhea | — | 3 (42.9) | — | — | — | 3 (8.6) |
| Dysuria | 1 (9.1) | — | — | 2 (22.2) | — | 3 (8.6) |
| Injection site erythema | — | — | 1 (14.3) | 1 (11.1) | — | 2 (5.7) |
| Injection site pain | 2 (18.2) | 1 (14.3) | 1 (14.3) | 5 (55.5) | 4 (66.7) | 13 (37.1) |
| Injection site swelling | — | — | 2 (28.6) | — | — | 2 (5.7) |
| Malaise | 3 (27.3) | 2 (28.6) | 2 (28.6) | 4 (44.4) | 1 (16.7) | 12 (34.3) |
| Myalgia | 1 (9.1) | 2 (28.6) | 1 (14.3) | 3 (33.3) | 2 (33.4) | 9 (25.7) |
| Fever | — | — | 1 (14.3) | 1 (11.1) | — | 2 (5.7) |
| Rash | 1 (9.1) | — | — | — | — | 1 (2.9) |
| Sore throat | 1 (9.1) | 2 (28.6) | 2 (28.6) | — | 3 (50) | 8 (22.9) |
| Upper respiratory tract infection | 2 (18.2) | 3 (42.9) | 1 (14.3) | 1 (11.1) | 1 (16.7) | 8 (22.9) |
| Unsolicited events | ||||||
| Abdominal pain | — | — | 1 (14.3) | — | — | 1 (2.9) |
| Activated partial thromboplastin time prolonged | — | — | 1 (14.3) | — | 1 (16.7) | 2 (5.7) |
| Acute HIV infection | — | — | 1 (14.3) | — | — | 1 (2.9) |
| Alanine aminotransferase increased | — | 1 (14.3) | — | — | — | 1 (2.9) |
| Aspartate aminotransferase increased | — | 1 (14.3) | — | — | — | 1 (2.9) |
| Blood creatine phosphokinase increased | 4 (36.4) | 2 (28.6) | 3 (42.9) | 2 (22.2) | 2 (33.4) | 13 (37.1) |
| Blood pressure systolic increased | 1 (9.1) | — | — | 1 (11.1) | 1 (16.7) | 3 (8.6) |
| Bradycardia | — | 1 (14.3) | 1 (14.3) | — | — | 2 (5.7) |
| Cystitis | 1 (9.1) | — | — | — | — | 1 (2.9) |
| Dysmenorrhea | 1 (9.1) | 1 (14.3) | — | — | — | 2 (5.7) |
| Dyspepsia | — | — | — | 1 (11.1) | — | 1 (2.9) |
| Escherichia urinary tract infection | — | — | — | 1 (11.1) | 1 (16.7) | 2 (5.7) |
| γ-Glutamyltransferase increased | 1 (9.1) | — | — | — | 1 (16.7) | 2 (5.7) |
| Hematuria | — | — | — | 2 (22.2) | 2 (33.4) | 4 (11.4) |
| Hemoglobin decreased | 3 (27.3) | 4 (57.1) | 2 (28.6) | 3 (33.3) | 4 (66.7) | 16 (45.7) |
| Headache | 2 (18.2) | 4 (57.1) | 1 (14.3) | 2 (22.2) | 1 (16.7) | 10 (28.6) |
| Hypertension | 1 (9.1) | — | — | — | — | 1 (2.9) |
| Lymphocyte count decreased | 1 (9.1) | 1 (14.3) | — | — | — | 2 (5.7) |
| Nausea | 1 (9.1) | — | 2 (28.6) | — | — | 3 (8.6) |
| Neutrophil count decreased | — | — | — | 2 (22.2) | — | 2 (5.7) |
| Ocular hyperemia | — | 1 (14.3) | — | — | — | 1 (2.9) |
| Pain in extremity | 1 (9.1) | 1 (14.3) | — | — | — | 2 (5.7) |
| Proteinuria | — | — | 1 (14.3) | 3 (33.3) | 1 (16.7) | 5 (14.3) |
| Prothrombin time prolonged | — | 1 (14.3) | 1 (14.3) | — | — | 2 (5.7) |
| Schistosomiasis | — | — | — | 1 (11.1) | — | 1 (2.9) |
| Sinusitis | — | 1 (14.3) | — | — | — | 1 (2.9) |
| Suicide attempt | — | — | 1 (14.3) | — | — | 1 (2.9) |
| Tachycardia | — | — | 2 (28.6) | — | — | 2 (5.7) |
| Tonsillitis | — | — | — | 1 (11.1) | — | 1 (2.9) |
| Toothache | — | 1 (14.3) | — | — | — | 1 (2.9) |
| Vaginal infection | 1 (9.1) | — | — | — | — | 1 (2.9) |
| White blood cell count increased | — | 1 (14.3) | 1 (14.3) | — | 1 (16.7) | 3 (8.6) |
When vaccine and placebo recipients were compared, the overall incidence of adverse events was similar regardless of dose of AERAS-402 (Table 3).
Proportion and Kinetics of Vaccine Responses, Measured by PBMC-based and Whole Blood–based Flow Cytometric Assays
PBMCs, cryopreserved from each time point, were later thawed and incubated with peptide pools, to measure T-cell–specific expression of IFN-γ, TNF-α, and IL-2 by flow cytometry. In the PBMC assay, all three cytokines were measured in a single flow cytometric channel. Whole blood from vaccine recipients was also incubated with the peptide pools of the vaccine antigens, to detect expression of four cytokines and three surface markers individually, by multiparameter flow cytometry. The gating strategy for the latter analysis is shown in Figure E1 in the online supplement. The whole blood analysis was completed in groups 3 and 4, for participants who received the highest dose of the vaccine. The whole blood assay was more sensitive than the PBMC-based assay when comparing groups 3 and 4: for example, 56 and 33% of vaccine recipients in groups 3 and 4 showed responses to Ag85A/B detectable with the PBMC assay, whereas 100 and 83% showed responses detectable with the whole blood assay (defined here as any cytokine response), respectively (see Table E1). Of note, PBMCs were stimulated for 6 hours, whereas whole blood was stimulated for 12 hours. It is possible that the extended period of stimulation of the whole blood analysis could account for the increased sensitivity observed.
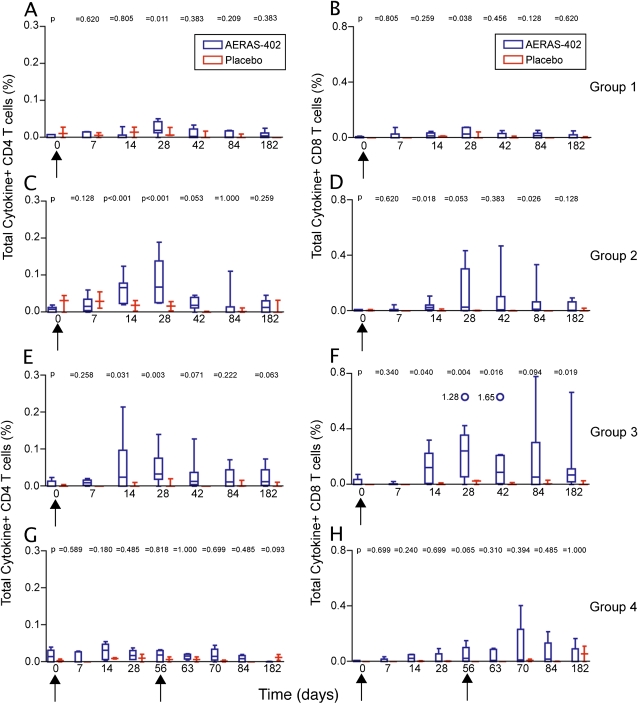
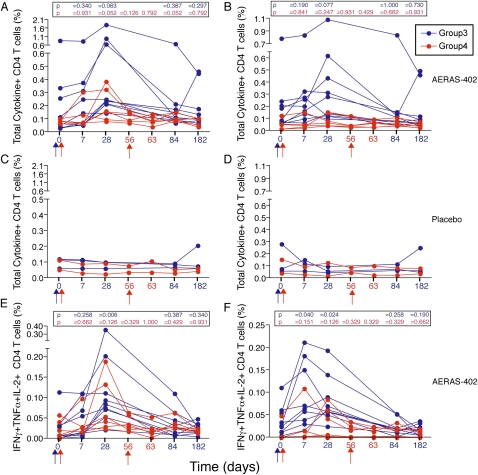
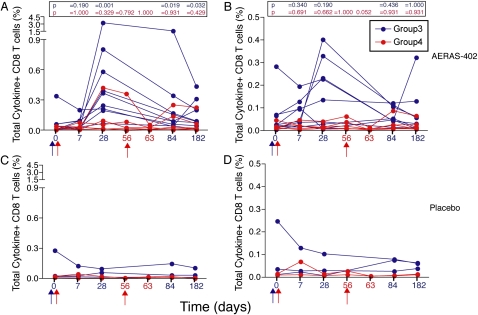
Both the PBMC-based assay (Figure 1; and Figure E3) and the whole blood assay (Figures 2A and 3A) showed that the Ag85-specific CD4+ and CD8+ T-cell response peaked at 28 days postvaccination. The TB10.4-specific CD4+ T-cell response often peaked earlier (see Figures E2, E4, and Figure 2B), whereas the CD8+ T-cell response to this antigen peaked on Day 28 (Figures E2, E4, and Figure 3B). The Ag85-specific CD8+ T-cell response persisted significantly over baseline for the duration of the study (Figure 3A). Overall, placebo recipients did not show an increase in specific CD4+ and CD8+ T cells above baseline (Figures 1–3 and Figures E2–E4).
Figure 1.
Frequency of Ag85A/B-specific T cells induced by AERAS-402, as measured by flow cytometry after incubation of peripheral blood mononuclear cells with a peptide pool of the antigens. CD4+ T-cell (left) and CD8+ T-cell (right) responses, in AERAS-402–vaccinated (blue boxes) and placebo-vaccinated (red boxes) participants are shown. Participants from (A and B) group 1, (C and D) group 2, and (E and F) group 3 received a single, escalating dose of AERAS-402 on Day 0 (indicated by the black arrow under the x axis). (G and H) Group 4 participants received two doses of AERAS-402 on Days 0 and 56 (indicated by the black arrows under the x axis), and were bled additionally on Days 56, 63, and 70. Total cytokine-positive frequencies denote any T cell that expresses IFN-γ, tumor necrosis factor-α, or IL-2 alone or in combination. Background values (unstimulated) were subtracted for each condition from each individual. For each plot, the median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The open circles and accompanying numbers represent high responders that exceed the maximal value on the scale. The P values indicated were derived by comparing responses with those at baseline, using the Mann-Whitney U test.
Figure 2.
Frequency of (A, C, and E) Ag85A/B-specific and (B, D, and F) TB10.4-specific CD4+ T cells induced by AERAS-402, as measured by flow cytometry after incubation of whole blood with a peptide pool of the antigens. Vaccinations for groups 3 and 4 are indicated with blue and red arrows, respectively, under the x axis. (A–D) Total (any) cytokine-expressing and (E and F) polyfunctional IFN-γ+IL-2+TNF-α+ CD4+ T-cell responses are shown for group 3 (single high dose, administered on Day 0, blue arrow) and group 4 (two doses of the vaccine, administered on Days 0 and 56, red arrows). Each line displayed represents a vaccine participant. Background values (unstimulated) were subtracted for each condition from each individual. The P values indicated were derived by comparing responses with those at baseline, using the Mann-Whitney U test.
Figure 3.
Frequency of (A and C) Ag85A/B-specific and (B and D) TB10.4-specific CD8+ T cells induced by AERAS-402, as measured by flow cytometry after incubation of whole blood with a peptide pool of the antigens. Vaccinations for groups 3 and 4 are indicated with blue and red arrows, respectively, under the x axis. Total (any) cytokine-expressing CD8+ T-cell responses are shown, for group 3 (single high dose, administered on Day 0, blue arrow) and group 4 (2 doses of the vaccine, administered on Days 0 and 56, red arrows). Each line displayed represents a vaccine participant. Background values (unstimulated) were subtracted for each condition from each individual. The P values indicated were derived from comparing responses with those at baseline, using the Mann-Whitney U test.
Double vaccination (group 4) did not result in significant boosting of T-cell responses (Figures 1–3 and Figures E2–E4).
AERAS-402 Induces Polyfunctional CD4+ T Cells That Coexpress Th1 Cytokines, But Does Not Induce IL-17–expressing CD4+ T Cells
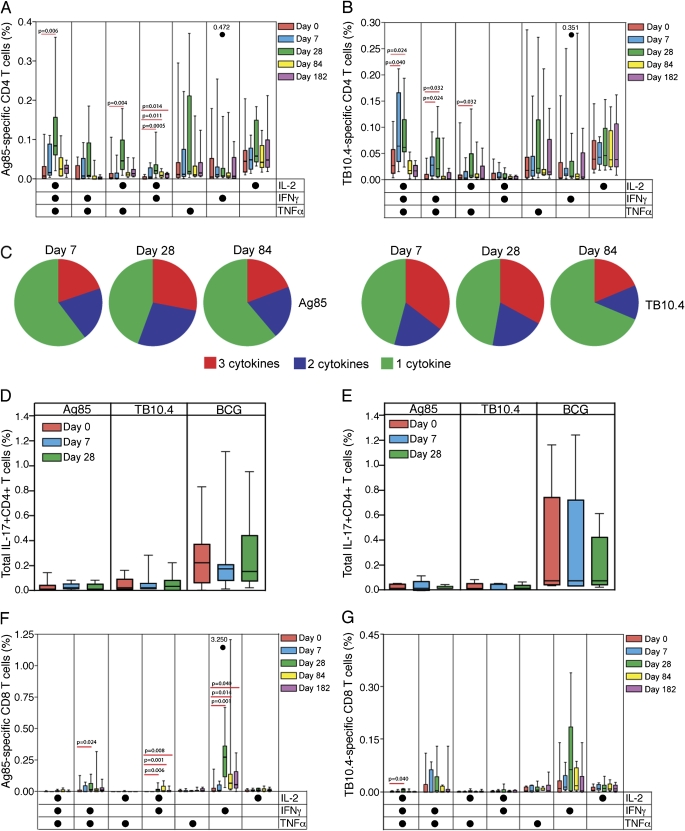
To assess the potential functional characteristics of CD4+ T cells induced by AERAS-402 more comprehensively, we analyzed coexpression patterns of cytokines, as detected with the whole blood assay. We were particularly interested in coexpression of cytokines in so-called multifunctional or polyfunctional cells, which may be associated with more optimal protection (see Introduction).
Seven subsets of vaccine-induced CD4+ T cells could be delineated, based on expression of IFN-γ, TNF-α, and IL-2, alone or in combination (Figures 4A and 4B). The peak Ag85-specific and TB10.4-specific CD4+ T-cell responses were dominated by a polyfunctional IFN-γ+IL-2+TNF-α+ subset (Figures 4A and 4B). The only Ag85-specific CD4+ T-cell subset frequency that was significantly higher on Day 182, compared with baseline, expressed IFN-γ and IL-2 together (Figure 4A).
Figure 4.
Detailed analysis of cytokine expression patterns of specific CD4+ and CD8+ T cells induced by AERAS-402, as measured by flow cytometry after incubation of whole blood with a peptide pool of the antigens. Patterns of single or combined expression of the helper T-cell type 1 cytokines in (A) Ag85A/B-specific and (B) TB10.4-specific CD4+ T cells of participants vaccinated with a single high dose of AERAS-402 (group 3) are shown, as frequencies of specific CD4+ T cells. (C) Among these participants, pie charts represent the mean proportions of cells producing three cytokines (red), two cytokines (blue), and one cytokine only (green), of the total cytokine CD4+ T-cell response, on Days 7, 28, and 84 postvaccination. (D and E) Again among these participants, the frequency of specific IL-17–expressing CD4+ T cells, after AERAS-402 vaccination, is shown for (D) AERAS-402–vaccinated and (E) placebo-vaccinated participants. Bacillus Calmette-Guérin was used a positive control. (F and G) Patterns of single or combined expression of the cytokines in (F) Ag85A/B-specific and (G) TB10.4-specific CD8+ T cells of participants vaccinated with a single high dose of AERAS-402 (group 3) are shown, as frequencies of specific CD8+ T cells. Background values (unstimulated) were subtracted for each condition from each individual. The open circles and accompanying numbers represent high responders that exceed the maximal value on the scale. For each plot, the median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. Differences between prevaccination and postvaccination responses were evaluated with the Mann-Whitney U test: P values less than 0.05 are shown.
On Day 7 postvaccination, about 40–50% of specific CD4+ T cells expressed two or three cytokines, whereas on Day 28 postvaccination, more than 50% of specific CD4+ T cells expressed two or three cytokines simultaneously (Figure 4C). At 84 days, single-cytokine–expressing cells predominated (Figure 4C).
AERAS-402 did not induce specific expression of the proinflammatory cytokine IL-17 (Figure 4D). In contrast, mycobacteria-specific IL-17+CD4+ T-cell subsets were detectable on BCG stimulation, as we have shown previously (38) (Figures 4D and 4E).
Vaccine-specific CD8+ T Cell Response to AERAS-402 Is Dominated by IFN-γ–expressing Cells
The Ag85-specific CD8+ T-cell response was dominated by a subset expressing only IFN-γ. This response was long-lived (Figure 4F). Smaller subsets, coexpressing IFN-γ and IL-2 or IFN-γ and TNF-α, were also induced, and the former population persisted for the duration of the study (Figure 4F). The TB10.4-specific CD8+ T-cell response was also dominated by IFN-γ expression; however, significant increases over baseline could not be detected (Figure 4G). This may be due to the small number of participants, and high prevaccination responses to TB10.4 (Figure 4G). A small number of polyfunctional (IFN-γ+TNF-α+IL-2+) TB10.4-specific CD8+ T cells was induced by the vaccine, as detected 28 days after vaccination (Figure 4G); however, this population did not persist.
High Neutralizing Antibody Levels against the Ad35 Vector in Participants Receiving Two Doses of Vaccine
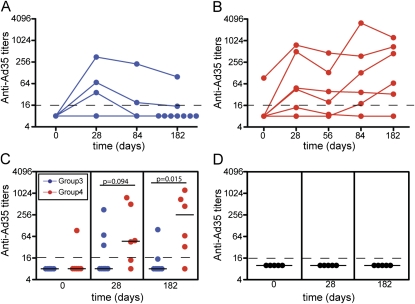
Although it is known that antibody levels against Ad35 are relatively low in African populations (23), neutralizing activity by preexisting and by vaccination-induced antibodies was anticipated to impact the T-cell response to vaccine antigens. Ad35-specific neutralizing activity was therefore determined in serum obtained on Days 0, 28, 84, and 182 for both groups, as well as before the second boost (Day 56) for group 4 participants. Among 20 subjects analyzed from groups 3 and 4, only 1 (5%) had quantifiable levels of Ad35-neutralizing activity on Day 0 (Figures 5A−5C). Of nine subjects who were vaccinated with AERAS-402 in group 3, three subjects (33.3%) had an increase in Ad35 titer from Days 0 to 28 (Figure 5A), whereas five of six subjects in group 4 had an increase (83.3%; Figures 5B and 5C). In group 4 participants before revaccination (Day 56), four of six subjects had measureable titers (66.7%), which further increased in three of these participants (50%) postboosting (Figure 5B). At the end of the study (Day 182), only one of nine participants in group 3 (11.1%; Figures 5A and 5C) had a significant titer, compared with five of six participants (83.3%) in group 4 (Figures 5B and 5C). No increase in antibody levels was shown in placebo recipients (Figure 5D).
Figure 5.
Ad35-neutralizing antibody titers induced by AERAS-402 vaccination of group 3 and 4 participants. (A and B) Longitudinal analysis of Ad35-neutralizing antibody titers on Days 0, 28, 84, and 182 postvaccination in serum from (A) group 3 participants (single high dose) and on Days 0, 28, 56, 84, and 182 postvaccination in serum from (B) group 4 participants (two high doses). (C and D) Comparison of Ad35-neutralizing antibody titers on Days 0, 28, and 182 postvaccination in serum from (C) group 3 and 4 participants and from (D) placebo recipients.
DISCUSSION
In this study we demonstrated that AERAS-402 displayed an acceptable safety profile and was immunogenic in healthy Mycobacterium tuberculosis–uninfected adults, previously vaccinated with BCG. This is the first reported clinical trial in humans in which adenovirus serotype 35 has been used as a vaccine vector in a heterologous prime–boost strategy. There were no serious adverse events that were related to the vaccine, and increasing vaccine dose did not result in an increase in adverse events.
We regard the vaccine candidate assessed in this study as safe because the adverse events were mostly mild to moderate, of short duration, and resolved without sequelae. As a comparison, almost 100% of recipients of BCG demonstrate local adverse events, some even ulceration at the site of the vaccine administration; however, BCG is regarded as one of the safest vaccines ever used.
Because there was a protracted period between priming with BCG and boosting with AERAS-402, it cannot be excluded that further priming of the immune response could have occurred via exposure to environmental mycobacteria. Regardless of the priming mechanism, immunity induced by the vaccine was impressive. Interestingly, analysis of baseline responses revealed that the majority of individuals had preexisting responses to the vaccine antigens, which may be a consequence either of memory responses to BCG vaccination at birth, or from exposure to environmental (nontuberculous) mycobacteria later in life.
The most striking immunogenicity results were that the vaccine induced a robust CD8+ T-cell response, against both Ag85A/b and TB10.4. This response was persistent up to the last measurement of the induced immune response. IFN-γ–producing T cells predominated, although other, smaller subsets, expressing combinations of cytokines, were also detected. It may be important to note that our assays did not specifically assess cytotoxic activity, which is a major functional characteristic of CD8+ T cells. Regardless, to date, most new TB vaccines have been reported to induce reasonable CD4+ T-cell responses, but relatively negligible CD8+ T-cell responses. CD8+ T-cell responses were induced and measureable directly ex vivo after administration of MVA85A only at high antigen dose, but not after any other vaccines (39). Whelan and colleagues reported that vaccination of individuals with a lower dose of MVA85 led to an Ag85-specific CD8+ T-cell response, which was detectable when dendritic cells were used as antigen-presenting cells in assays to expand specific CD8+ T cells (40). Strong evidence is emerging that CD8+ T cells mediate important roles in protective immunity against TB (41–45); we therefore hypothesize that the induction of robust vaccine-specific CD4+ and CD8+ T-cell responses after AERAS-402 would correlate with a more efficacious outcome.
AERAS-402 induced a robust and highly complex vaccine-specific CD4+ T-cell response, which was dominated in both the Ag85A/B and TB10.4 responses by a polyfunctional IFN-γ+TNF-α+IL-2+ subset, which did not persist beyond 28 days postvaccination, and a prominent population expressing TNF-α and IL-2 together. The Ag85A/B response was further characterized by an IFN-γ+IL-2+ subset that persisted for the duration of the study. The induction of polyfunctional CD4+ T cells may be important, as data suggest that stable long-lived populations of polyfunctional T cells correlate well with protection against subsequent challenge with intracellular pathogens (34, 35). In animal models of vaccination against Leishmania major (34) and against Mtb (35), vaccination strategies that induce the highest frequency of polyfunctional antigen-specific CD4+ T cells are associated with the best outcome, especially when detected in the primary site of the infection. Specifically, it was demonstrated that the magnitude and quality of the immune response measured in the lung, but not in the spleen or blood, correlated well with host protection after aerosol challenge with Mtb in the mouse (35).
In a study by Aagaard and colleagues it was revealed that vaccination of mice and guinea pigs with Ag85B and TB10.4 in IC31H adjuvant resulted in the induction of polyfunctional CD4+ T cells that were associated with protection against subsequent challenge with Mtb (46). Interestingly, it was demonstrated that the magnitude and quality of the vaccine induced T-cell response, as well as the protective efficacy, were highly dependent on the antigen dose (46).
Importantly, elite controllers of HIV infection have been shown to have high frequencies of polyfunctional HIV-specific T cells, whereas a rapid onset of disease has been associated with diminishing levels of polyfunctional T cells (47–49).
In contrast to MVA85A, AERAS-402 did not induce any IL-17–expressing CD4+ T cells. Mycobacteria-specific IL-17–expressing T cells have been detected both in mice and humans (6, 38, 50). At this stage, we cannot say whether induction of Th17 cells, after novel vaccination, would result in more or lesser optimal immunity. On the one hand, IL-17 may be needed for an optimal Th1 response, although the persistence of the Th1 response shown in this study would argue to the contrary. Also, too much inflammation, induced by IL-17, may not necessarily be optimal.
Preexisting neutralizing antibodies against Ad5 has been shown to inhibit the immunogenicity of rAd5 vaccines in both preclinical studies (16, 20, 24), and in clinical trials (51). This is a major concern, because up to 90% of individuals in sub-Saharan Africa have detectable anti-Ad5 antibodies (23). AERAS-402 incorporates Ad35, which has been shown to be prevalent in only 20% of individuals in sub-Saharan Africa (23) with neutralizing titers greater than 200 in less than 5% of individuals; this vector is therefore more attractive for antigen delivery. Anti-Ad35–neutralizing antibodies were present in only 5% of participants in this trial, before vaccination, which is lower than reported for this region. As shown in the results, AERAS-402 did indeed induce anti-Ad35 antibodies: 22% of participants who received a single dose, and 83.3% of participants who received two doses, had detectable anti-Ad35 titers at the end of the trial. Assessment of anti-Ad35–neutralizing titers immediately before the second dose of the vaccine in group 4, revealed that two-thirds of individuals had significant titers, which likely led to suppression of immunogenicity, and subsequently suboptimal boosting of the T-cell response induced by second immunization with AERAS-402. This is in agreement with a preclinical study by Thorner and colleagues, which demonstrated reduced immunogenicity to Ad35 vaccination if high anti-Ad35 titers were already present (20). All Ad35 titers on Day 182 were within the range of titers seen in a previous study of naturally occurring neutralizing activity to adenovirus (23).
Other known mechanisms of immune regulation, such as the induction of regulatory T cells and immune-suppressive factors such as IL-10 and transforming growth factor-β, might further explain the negligible boost observed in group 4; however, these were not assessed in the current study. The aim of this study was to measure vaccine take and not immune mechanisms; therefore, these possibilities were not assessed.
This is the first study showing the safety and immunogenicity of AERAS-402 in a heterologous prime–boost strategy in human vaccinees. AERAS-402 administration was found to be safe and immunogenic in healthy Mycobacterium tuberculosis–uninfected adults previously vaccinated with BCG. AERAS-402 induces a robust CD8+ T-cell response as well as a polyfunctional CD4+ T-cell response, and supports further clinical trials assessing the efficacy of AERAS-402 as a boosting vaccine.
Supplementary Material
Acknowledgments
The authors thank all those who participated in this trial, and K. Radosevic for critically reviewing the manuscript.
Supported by the Aeras Global Tuberculosis Vaccine Foundation and by Crucell NV. B.A. is supported by an NRF Innovation Postdoctoral Fellowship, and W.A.H. is supported by the NIH (RO1-AI065653 and NO1-AI70022).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200910-1484OC on February 18, 2010
Conflict of Interest Statement: B.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.T. has participated as a speaker at vaccinology courses organized and sponsored by GlaxoSmithKline (GSK) in 2008 and 2009. N.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.G. is employed by Aeras Global TB Vaccine Foundation, which has a relationship with Crucell, which manufactures and codevelops the investigational product. J.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.V.D.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.H. has been employed full time by Aeras (the sponsor) since January 2008. From July 2007 to December 2007, A.H. was a sponsored trainee at Aeras Global TB Vaccine Foundation in Rockville, Maryland. his current position with Aeras is Head, Africa Office. A.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. is employed by the University of Cape Town as a coinvestigator on clinical trials sponsored by Aeras Global TB Vaccine Foundation. G.S. is an employee of Crucell and holds options in the company. M.G.P. is an employee of Crucell, holds options, and is an inventor on patents filed by Crucell. J.H. is an employee of Crucell and holds options in the company. G.J.W. is a Crucell employee and owns Crucell options. J.G. is an employee of Crucell. D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.B.M.'s institution, Aeras Global TB Vaccine Foundation, is a partner with Crucell NV (Leiden, The Netherlands) on the AERAS-402 tuberculosis vaccine. M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.G. is an employee of Aeras Global TB Vaccine Foundation. H.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.D.H.'s institution received unrestricted educational grants from GSK and Sanofi Aventis. J.C.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.A.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.World Health Organization. World Health Organization report: global tuberculosis control—epidemiology, strategy, financing. WHO/htm/TB/2009.411. Geneva, Switzerland: World Health Organization; 2009.
- 2.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993;22:1154–1158. [DOI] [PubMed] [Google Scholar]
- 3.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006;367:1173–1180. [DOI] [PubMed] [Google Scholar]
- 4.Fine PA, Milstein J, Clements C. Issues relating to the use of BCG in immunization programmes: a discussion document. 1999. WHO/V&B/99.23. Available from: http://www.who.int/vaccines-documents/DocsPDF99www9943.pdf
- 5.Brookes RH, Hill PC, Owiafe PK, Ibanga HB, Jeffries DJ, Donkor SA, Fletcher HA, Hammond AS, Lienhardt C, Adegbola RA, et al. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS ONE 2008;3:e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe L, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 2008;198:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess J, Miko D, Catic A, Lehmensiek V, Russell DG, Kaufmann SH. Mycobacterium bovis bacille Calmette-Guerin strains secreting listeriolysin of Listeria monocytogenes. Proc Natl Acad Sci USA 1998;95:5299–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magalhaes I, Sizemore DR, Ahmed RK, Mueller S, Wehlin L, Scanga C, Weichold F, Schirru G, Pau MG, Goudsmit J, et al. rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime–boost setting with an adenovirus 35 tuberculosis vaccine vector. PLoS ONE 2008;3:e3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime–boost vaccination regimen for murine tuberculosis. Infect Immun 2001;69:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004;10:1240–1244. [DOI] [PubMed] [Google Scholar]
- 11.Radosevic K, Wieland CW, Rodriguez A, Weverling GJ, Mintardjo R, Gillissen G, Vogels R, Skeiky YA, Hone DM, Sadoff JC, et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of γ interferon. Infect Immun 2007;75:4105–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol 2006;4:469–476. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 2004;173:6357–6365. [DOI] [PubMed] [Google Scholar]
- 14.Xing Z, Santosuosso M, McCormick S, Yang TC, Millar J, Hitt M, Wan Y, Bramson J, Vordermeier HM. Recent advances in the development of adenovirus- and poxvirus-vectored tuberculosis vaccines. Curr Gene Ther 2005;5:485–492. [DOI] [PubMed] [Google Scholar]
- 15.Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, Weichold F, Damen I, Kaspers J, Lemckert A, et al. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J Gen Virol 2006;87:2135–2143. [DOI] [PubMed] [Google Scholar]
- 16.Lemckert AA, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, Nanda A, Ewald BA, Gorgone DA, Lifton MA, et al. Immunogenicity of heterologous prime–boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-Ad5 immunity. J Virol 2005;79:9694–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol 2007;81:6594–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ophorst OJ, Radosevic K, Havenga MJ, Pau MG, Holterman L, Berkhout B, Goudsmit J, Tsuji M. Immunogenicity and protection of a recombinant human adenovirus serotype 35–based malaria vaccine against Plasmodium yoelii in mice. Infect Immun 2006;74:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shott JP, McGrath SM, Pau MG, Custers JH, Ophorst O, Demoitie MA, Dubois MC, Komisar J, Cobb M, Kester KE, et al. Adenovirus 5 and 35 vectors expressing Plasmodium falciparum circumsporozoite surface protein elicit potent antigen-specific cellular IFN-γ and antibody responses in mice. Vaccine 2008;26:2818–2823. [DOI] [PubMed] [Google Scholar]
- 20.Thorner AR, Lemckert AA, Goudsmit J, Lynch DM, Ewald BA, Denholtz M, Havenga MJ, Barouch DH. Immunogenicity of heterologous recombinant adenovirus prime–boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J Virol 2006;80:12009–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, Cobb M, Burge JR, Larson D, Ware LA, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun 2007;75:2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radosevic K, Rodriguez A, Lemckert A, Goudsmit J. Heterologous prime–boost vaccinations for poverty-related diseases: advantages and future prospects. Expert Rev Vaccines 2009;8:577–592. [DOI] [PubMed] [Google Scholar]
- 23.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M, et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 2004;18:1213–1216. [DOI] [PubMed] [Google Scholar]
- 24.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol 2004;172:6290–6297. [DOI] [PubMed] [Google Scholar]
- 25.Winslow GM, Cooper A, Reiley W, Chatterjee M, Woodland DL. Early T-cell responses in tuberculosis immunity. Immunol Rev 2008;225:284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993;178:2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol 1993;151:518–525. [PubMed] [Google Scholar]
- 28.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today 1998;19:491–494. [DOI] [PubMed] [Google Scholar]
- 29.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene–targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol 1999;162:3504–3511. [PubMed] [Google Scholar]
- 30.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995;2:561–572. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs M, Togbe D, Fremond C, Samarina A, Allie N, Botha T, Carlos D, Parida SK, Grivennikov S, Nedospasov S, et al. Tumor necrosis factor is critical to control tuberculosis infection. Microbes Infect 2007;9:623–628. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BJ, Bekker LG, Rickman R, Brown S, Lesser M, Ress S, Willcox P, Steyn L, Kaplan G. rHuIL-2 adjunctive therapy in multidrug resistant tuberculosis: a comparison of two treatment regimens and placebo. Tuber Lung Dis 1997;78:195–203. [DOI] [PubMed] [Google Scholar]
- 33.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006;441:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional Th1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007;13:843–850. [DOI] [PubMed] [Google Scholar]
- 35.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008;181:4955–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods 2004;291:185–195. [DOI] [PubMed] [Google Scholar]
- 37.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol 2003;41:5046–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17– and IL-22–producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 2008;180:1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beveridge NE, Fletcher HA, Hughes J, Pathan AA, Scriba TJ, Minassian A, Sander CR, Whelan KT, Dockrell HM, Hill AV, et al. A comparison of IFNγ detection methods used in tuberculosis vaccine trials. Tuberculosis (Edinb) 2008;88:631–640. [DOI] [PubMed] [Google Scholar]
- 40.Whelan KT, Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Hill AV, McShane H. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS ONE 2009;4:e5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittrucker HW, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SH. Poor correlation between BCG vaccination–induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA 2007;104:12434–12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol 2000;30:3689–3698. [DOI] [PubMed] [Google Scholar]
- 43.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 2006;24:105–117. [DOI] [PubMed] [Google Scholar]
- 44.Woodworth JS, Behar SM. Mycobacterium tuberculosis–specific CD8+ T cells and their role in immunity. Crit Rev Immunol 2006;26:317–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog 2009;5:e1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 2009;4:e5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 2007;204:2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, Pollard RB, Yee HF Jr, Martin JN, Deeks SG, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 2009;113:3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, et al. IL-17–mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 2007;178:3786–3796. [DOI] [PubMed] [Google Scholar]
- 51.Harro CD, Robertson MN, Lally MA, O'Neill LD, Edupuganti S, Goepfert PA, Mulligan MJ, Priddy FH, Dubey SA, Kierstead LS, et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res Hum Retroviruses 2009;25:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.