Abstract
AQPs are water channel proteins. In particular, AQP1 was demonstrated to be involved in cell migration. According to the model proposed by Verkman and collaborators, AQP drives water influx, facilitating lamellipodia extension and cell migration. Investigating the possible connection between AQP1 and cytoskeleton, our group showed that such a water channel through Lin7/β-catenin affects the organization of the cytoskeleton and proposed a model.
All together, these data appear particularly intriguing since the use of AQP1 as target might be useful to modulate angiogenesis/vasculogenic mimicry.
Key words: AQP, cytoskeleton, Lin7, β-catenin, motility, molecular adhesion
Aquaporins were discovered by Peter Agre, who won the Nobel Prize in Chemistry in 2003. They are a family of water-specific, membrane-channel proteins expressed in diverse tissues. Two functional groups of mammalian aquaporins are now recognized: aquaporins (AQP1, AQP2, AQP4, AQP5 and AQP8) which are primarily water selective and aquaglyceroporins (AQP3, AQP7, AQP9 and AQP10) which are permeable to small uncharged solutes such as lactate, glycerol and urea in addition to water.1 The characterization of the organization of aquaporin genes and identification of their position within the human and mouse genomes have established a primary role for some aquaporins in clinical disorders such as congenital cataracts and nephrogenic diabetes insipidus.2 More recently, in the control of fat accumulation, aquaporins were demonstrated to play an important role.3–6 A characterization of AQPs was recently carried out in neuronal stem cells.7 More interesting, an impairment of endothelial cell migration, without altering their proliferation or adhesion, was shown by AQP1 null mice.8 Based on findings of slowed lamellipodial dynamics in AQP deficiency and AQP polarization to the leading edge of migrating cells, a mechanism of AQP-facilitated cell migration was proposed by Verkman and collaborators.9 According to this model, actin cleavage and ion uptake at the tip of lamellipodium creates local osmotic gradients and drives water influx, facilitating lamellipodial extension and cell migration.9 AQP-facilitated cell migration has also been found in brain astroglial cells,10,11 kidney proximal tube cells12 and skin cells.13 In this connection, AQP1 has been proposed as a novel promoter of tumor angiogenesis.14 It is still unclear, however, how actin is cleaved. On the other hand, according to Verkman’s model, AQP1 is the water channel that drives water influx.
We have recently proposed a new model. In a recent paper published in PLoS ONE Journal, we have investigated the possi-ble relationship between AQP1 and the cytoskeleton in endothelial and melanoma cells (both expressing AQP1), focusing on the possible involvement of Lin proteins.15 The latter are plasma membrane-associated proteins containing one or several PDZ domains16 and are required for the organization of the cytoskeleton. A scaffold complex common for epithelial and neuronal cells is the heterotrimeric complex consisting of the CASK/Lin-2, Lin-7 and Lin-10 PDZ proteins.17–20 In mammals, Lin-7 can recruit cell adhesion molecules, receptors, ion channels and signaling proteins.17–20 Therefore, heterotrimeric PDZ complex plays a role in regulating the localization of interacting proteins. The novelties of our paper are the following: firstly, AQP1 plays the same role in human melanoma and endothelial cells, suggesting that this water channel has a global physiological role. Secondly, AQP1 interacts, at least, with Lin-7/β-catenin. Another interesting aspect is that the knock down of AQP1 induced the proteolytic degradation of Lin7/β-catenin through proteasoma complex. In the model proposed in PLoS ONE Journal, AQP1 is not only a water channel but a critical scaffold for plasma-membrane associated multiprotein-complex important for cytoskeleton build-up, adhesion and motility.15 Our data show, actually, that AQP1 plays a role in stabilizing the cytoskeleton affecting the migration capacity.21 Considering both Verkman’s model and our findings, I suggest that, in presence of local osmotic gradients like as at the tip of lamelllipodium, water is driven inside through AQP(s), leading to the disruption of scaffold proteins which are degraded through proteasoma (Lin7/β-catenin). The effect on the cell is the cleavage of actin.
These findings corroborate the analysis of manifold cellular functions of AQPs in normal cells and in diseases and the possi-bility to consider aquaporins as specific therapeutic targets for various pathophysiological conditions.22 In particular, AQP1 might be an interesting target for tumors. In fact, AQP1 is expressed both by tumor and endothelial cells and a targeted inhibition or silencing of such a protein might affect both the migratory and the angiogenesis/vasculogenic mimicry capacity.
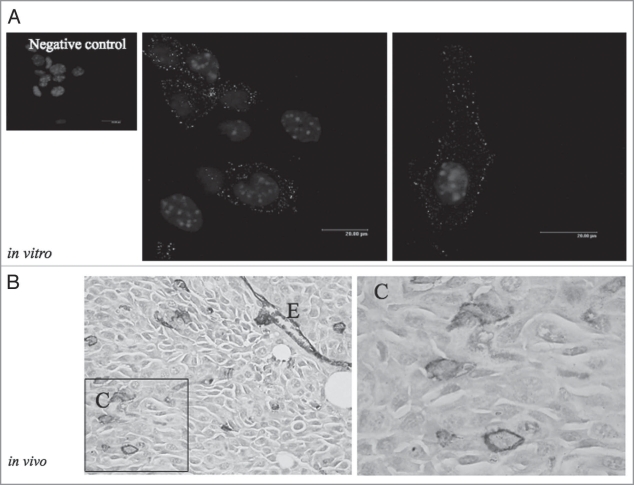
Vasculogenic mimicry was described for the first time by the unique ability of aggressive melanoma cells to express an endothelial phenotype and to form vessel-like networks in three dimensional cultures, “mimicking” the pattern of embryonic vascular networks and recapitulating the patterned networks seen in patients with aggressive tumors correlated with poor prognosis (reviewed in ref. 23). In fact, the word “vasculogenic” was selected to indicate the generation of the pathway de novo and “mimicry” was used because the tumor uses cell pathways for transporting fluid in tissues that were clearly not blood vessels. Additional studies have reported vasculogenic mimicry in several other tumor types (reviewed in ref. 23). As shown in Figure 1, human melanoma cell line WM115 expresses AQP1 at the plasma membrane in vitro and only a few cells express such a water channel in tumor xenograft according to the low/undetectable number of initiating/cancer stem cells found in tumor xenograft and melanoma biopsies.24
Figure 1.
(A) Immunofluorescence of AQP1 in WM115 cells. Subconfluent cells were grown on 400 mm2 glass cover and fixed in 4% paraformaldeide for 20 min. Thus, the cells were incubated with the same buffer containing 0.5% TRITON X-100 for 5 min and incubated in 1% BSA-PBS for 20 min and with the primary antibody (anti-AQP-1) overnight at 4°C. A secondary antibody conjugated with TRIC was used. Nuclei were stained with DAPI. (B) 5 × 104 living WM115 cells were injected subcutaneous in SCID mice and the tumor mass was collected 19 days after and processed for immunohistochemistry. The slides (4–5 mm) deparaffinized were incubated overnight with anti-AQP1 (1:100) and the colour was developed using VIP. Magnification 200X. E, endothelial cells AQP1-positive. (C) Shows a higher magnification of a part of figure shown in (B) where few melanoma cells are AQP1-positive (1,000X).
Finally, there are no AQP inhibitors reported that are suitable candidates for clinical development. An interesting new way might be to study the possibility of functionally significant AQP polymorphisms. In this connection, AQP4 polymorphisms was found to be associated with increased severity of brain edema.25 It may be worthwhile to investigate polymorphisms of AQP1 or other AQPs in cancer and endothelial associated tumor.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10949
References
- 1.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2007;118:3222–3225. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 2.Verkman AS. Aquaporins: translating bench research to human disease. J Exp Biol. 2009;212:1707–1715. doi: 10.1242/jeb.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King LS, Kozovo D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2002;5:687–688. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 4.Oshio K, Binder DK, Liang Y, Bollen A, Feuerstein B, Berger MS, et al. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery. 2005;56:371–375. doi: 10.1227/01.neu.0000148904.57841.6b. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez A, Catalán V, Gómez-Ambrosi J, Frühbeck G. Role of aquaporin-7 in the pathophysiological control of fat accumulation in mice. FEBS Lett 2. 2006;580:4771–4776. doi: 10.1016/j.febslet.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 6.Agre P. The aquaporin water channels. Proc Am Thorac Soc. 2006;3:5–13. doi: 10.1513/pats.200510-109JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavazzin C, Ferrari D, Facchetti F, Russignan A, Vescovi AL, La Porta CAM, et al. Unique expression localization of aquaporin-4 and aquaporin-9 in murine and human neural stem cells and in their glial progeny. Glia. 2007;53:167–181. doi: 10.1002/glia.20256. [DOI] [PubMed] [Google Scholar]
- 8.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pfugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvment of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Science. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 11.Auguste KL, Jn S, Uchida K, Yan D, Manley GT, Papadopoulos MC, et al. Greatly impaired migration of impaired aquaporin-4-deficient atroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 12.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epihelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 13.Hara-Chituma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferatio during wound healing. J Mol Med. 2008;96:523–529. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 14.Clapp C, Martínez de la Escalera G. Aquaporin-1: a novel promoter of tumor angiogenesis. Trends Endocrinol Metab 2. 2006;17:1–2. doi: 10.1016/j.tem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Monzani E, Bazzotti R, Perego C, La Porta CAM. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PloS One. 2009;4:6167. doi: 10.1371/journal.pone.0006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 17.Borg JP, Straight SW, Kaech SM, de Taddéo-Borg M, Kroon DE, Karnak D, et al. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J Biol Chem. 1998;273:31633–31636. doi: 10.1074/jbc.273.48.31633. [DOI] [PubMed] [Google Scholar]
- 18.Butz S, Okamoto M, Südhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 19.Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monzani E, Bazzotti R, Perego C, La Porta CAM. AQP 1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PloS One. 2009;4:6167. doi: 10.1371/journal.pone.0006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landon S, Kozono D, Agre P. From structuring to disease: the evolving tale of aquaporin biology. Nature Reviews Molecular Biology. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 23.Monzani E, La Porta CAM. Targeting cancer stem cells to modulate alternative vascularization mechanisms. Stem Cell Rev. 2008;4:51–56. doi: 10.1007/s12015-008-9009-1. [DOI] [PubMed] [Google Scholar]
- 24.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumorigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Verkman AS. Aquaporins: translating bench research to human disease. J Exp Biol. 2009;212:1707–1718. doi: 10.1242/jeb.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]