Abstract
Objective
Ketosis prone diabetes (KPD) is heterogeneous. Longitudinal follow-up revealed that patients with “A-β+” KPD (absent autoantibodies and preserved beta cell function) segregated into two subgroups with distinct evolution of beta cell function and glycemic control. Generalized linear analysis demonstrated that the variable that most significantly differentiated them was presence of a clinically evident precipitating event for the index DKA. Hence we performed a comprehensive analysis of A-β+ KPD patients presenting with “provoked” compared to “unprovoked” DKA.
Methods
Clinical, biochemical and beta cell functional characteristics were compared between provoked and unprovoked A-β+ KPD patients followed prospectively for 1-8 years. HLA class II allele frequencies were compared between these two groups and population controls.
Results
Unprovoked A-β+ KPD patients (n=83) had greater BMI, male preponderance, higher frequency of women with oligo/anovulation, more frequent African-American ethnicity and less frequent family history of diabetes than provoked A-β+ KPD patients (n=64). The provoked group had higher frequencies of the HLA class II type 1 diabetes susceptibility alleles DQB1*0302 (than the unprovoked group or population controls), and DRB1*04 (than the unprovoked group), while the unprovoked group had a higher frequency of the protective allele DQB1*0602. Beta cell secretory reserve and glycemic control improved progressively in the unprovoked group, but declined in the provoked group. The differences persisted in comparisons restricted to patients with new-onset diabetes.
Conclusions
“Unprovoked” A-β+ KPD is a distinct syndrome characterized by reversible beta cell dysfunction with male predominance and increased frequency of DQB1*0602, while “provoked” A-β+ KPD is characterized by progressive loss of beta cell reserve and increased frequency of DQB1*0302 and DRB1*04. Unprovoked DKA predicts long-term beta cell functional reserve, insulin-independence and glycemic control in KPD.
Keywords: autoantibody, type 1 diabetes, type 2 diabetes
Introduction
Ketosis prone diabetes (KPD) is defined by presentation with DKA [1, 2]. The recently validated “Aβ” classification scheme distinguishes four forms of KPD based on autoantibodies and beta cell functional reserve [3]. Two forms are characterized by complete, irreversible loss of beta cell function (“β-” KPD) and insulin dependence [3]. The other two forms comprise patients with substantially preserved beta cell functional reserve shortly after the index DKA (“β+” KPD): they include a group with autoantibodies (A+β+ KPD) and another without autoantibodies (A-β+ KPD). A-β+ KPD is a common and heterogeneous form; about half of these patients develop DKA without an evident precipitating factor, while the remainder have an associated precipitating event such as acute illness or treatment noncompliance [1,2].
We observed that about 35-40% of A-β+ KPD patients had persistent or sustained improvement in beta cell function over 12-24 months, with concomitant ability to attain good glycemic control or discontinue insulin therapy. Conversely, about 60-65% showed little improvement or progressive decline in beta cell function, with inability to discontinue insulin. Generalized linear analysis of data at the 24-month time point, using age, gender, ethnicity and BMI as covariates, demonstrated that the variable that most significantly differentiated the two subgroups with these distinct “natural histories” was whether or not the patient had had a clinically evident precipitating event for the index DKA, i.e., categorization of patients with respect to “provoked” compared to “unprovoked” DKA yielded the most significant difference with respect to linear change across time (P=0.04).
To understand the basis for this difference, we prospectively tracked a large number of patients belonging to these two subgroups over a prolonged period, to delineate their clinical, biochemical and genetic characteristics.
Methods
Patient Identification
The protocol was approved by the Institutional Review Boards for Human Studies of Baylor College of Medicine and the Harris County Hospital District. 584 consecutive adult patients admitted with DKA to Ben Taub General Hospital from June 1, 1999 to October 31, 2006 were interviewed during the admission and offered follow-up in the KPD research clinic. 292 patients gave informed consent. They were classified according to the Aβ scheme [3], based on fasting and stimulated C-peptide levels measured 3-4 weeks after correction of ketoacidosis as well as presence or absence of beta cell autoantibodies, into four categories described previously [1,3,4]. 147 patients were classified as having A-β+ KPD (i.e., lacking autoantibodies and with beta cell functional reserve). These patients were followed prospectively for 1 to 8 years.
For the reasons described above, these longitudinally followed A-β+ KPD patients were classified as “provoked” if they had a clinically evident precipitant of the index DKA, or “unprovoked” if they lacked a precipitant. The DKA-precipitating factors were either acute medical conditions in 55.7% of the provoked patients (infections, pancreatitis, steroid use, hypertriglyceridemia, seizures, upper gastrointestinal bleeding), or discontinuation of insulin or oral agents in persons previously known to have diabetes, in 44.3%. The physicians who admitted and treated the patients during the index DKA episode performed the diagnosis and documentation of the acute medical conditions or of non-compliance with prior anti-diabetic therapy. The investigators, who did not participate in the admission or inpatient management of patients at the time of the index DKA episode, reviewed post-discharge hospital charts (laboratory test results, microbiological and radiological data) to confirm the primary inpatient physicians' diagnoses.
Following the index episode of DKA, all patients were followed in a dedicated research clinic with a standard outpatient management protocol [3, 4]. Patients were placed on twice-daily NPH insulin with or without pre-meal rapid-acting insulin, and self-monitoring of capillary blood glucose levels. If glucose values during the following 2-week period attained ADA-defined goals [5], the insulin dose was reduced by 50% and the patient reassessed one week later. If the mean blood glucose values remained at ADA goals at 2 consecutive clinic visits, insulin was discontinued, and the patient was monitored closely. Conversely, if the patient developed ketosis upon decreasing the insulin dose, the regimen was intensified and no further attempts made to discontinue insulin.
Measures
Age, age at diagnosis of diabetes, gender, ethnicity (by patient self-identification), duration of diabetes, family history of diabetes, height, weight, body mass index (BMI), and treatment before and after DKA were recorded. A comprehensive reproductive history was obtained from all women, including age at menarche and menopause, menstrual frequency, and use of estrogen/progestin for contraception or replacement. Polycystic ovary syndrome (PCOS) was defined by two of the following: oligo- or anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries by ultrasound examination [6].
Laboratory values measured during the hospital stay for the index DKA were fasting serum glucose levels, HbA1c, total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol. C-peptide concentration was measured in a fasting serum sample obtained 24 hours after complete correction of ketoacidosis and before the morning dose of subcutaneous insulin. Within 2-3 weeks after resolution of DKA, C-peptide response to glucagon stimulation was performed as described previously [1]. C-peptide concentrations were determined using a human C-peptide RIA kit (Linco Research Inc, St. Louis, MO). Serum samples were analyzed for the presence of autoantibodies to the 65 and 67 kDa isoforms of glutamic acid decarboxylase, (GAD65 Ab, GAD67 Ab), tyrosine-phosphatase like protein IA-2 (IA-2 Ab) and Zn-T8 (Zn-T8 Ab to all three isotypes: ZnT8R, ZnT8W, and ZnT8G) by highly sensitive and specific quantitative radioligand binding assay methods [1, 22]. Beta cell function and insulin resistance indices were calculated using the HOMA2 model [7].
HbA1c was measured every 3 months, using high performance liquid chromatography. Fasting serum C-peptide and glucose levels (to calculate HOMA2-IR and HOMA2-%B) were measured 6 and 12 months after the index episode of DKA, and yearly thereafter.
Analysis
We compared baseline and follow-up characteristics between the two subgroups using the JMP 6.0.2 statistical package (SAS, 2006). Demographic, clinical, and biochemical data were analysed using Student's t-test, ANOVA, and χ2 tests. P values were calculated using the likelihood ratio method, with significance at P<0.05. A subanalysis of patients presenting with DKA as the first manifestation of diabetes was also carried out, comparing the 16 provoked patients with new-onset diabetes to new-onset unprovoked patients in two ways: a) to all 83 new-onset unprovoked patients, and b) to a subgroup of 18 representative new-onset unprovoked patients group selected on the basis of their age, gender, waist circumference, total cholesterol, triglycerides, BMI and initial HbA1c being within 1.5 SD of the mean from the complete set of 83. Both the complete set of 83 new-onset unprovoked patients and the representative subgroup of 18 patients satisfied the Shapiro-Wilks test for normality.
The four forms of KPD differ in their frequencies of HLA class II alleles known to confer susceptibility or resistance to autoimmune type 1 diabetes [8]. Hence we compared the frequencies of key alleles between A-β+ KPD patients who presented with and without precipitating factors for DKA as well as to those of a large control population of a similarly heterogeneous ethnic composition in Texas [9]. Genotyping was performed as described previously [8]. Fisher's exact test was used to compare allele frequencies between A-β+ KPD subgroups and normal control subjects.
Results
83 patients (56.5%) had A-β+ KPD with unprovoked DKA, while 64 (43.5%) had A-β+ KPD with provoked DKA. DKA precipitants that defined the latter group included acute illness in 35 patients and non-compliance with diabetes therapy in 29. All 83 unprovoked A-β+ KPD patients were “new-onset”, and none developed a repeat episode of DKA during the follow-up period of this study. Of the 64 patients with provoked A-β+ KPD, 16 had new-onset diabetes at presentation with DKA (with a concomitant acute illness), while 48 had previously diagnosed diabetes (1 managed with diet/exercise, 21 on oral antidiabetic medications, 10 on insulin and oral medications, and 16 on insulin alone).
Clinical and metabolic differences
Baseline
Mean duration of diabetes was 0 years in the unprovoked A-β+ KPD subgroup compared to 5.2 ± 6.8 years in the provoked A-β+ KPD subgroup (P<0.0001) (Table 1). Ethnic distribution in the unprovoked subgroup was 51% African-American, 41% Hispanic, 8.0% Caucasian and 0% Asian, compared to 28%, 62%, 7% and 3% respectively among the provoked subgroup (P=0.01). 58 (70%) of the unprovoked patients had a family history of diabetes compared to 59 (92%) of the provoked patients (P=0.0007). Patients of the unprovoked subgroup had greater mean BMI (33.2 ± 8.3 compared to 31.1 ± 8.9 kg/m2, P=0.07) and a higher frequency of obesity (66.3% [95% CI: 56.4% to 75.2%] compared to 39.1% [95% CI: 28.5% to 50.9%], P=0.04).
Table 1. A. Demographic, clinical and biochemical characteristics of “unprovoked” and “provoked” A-β+ KPD patients at baseline and after 12 months of treatment.
| Unprovoked (N=83) |
Provoked (N=64) |
P | ||
|---|---|---|---|---|
| Age | 41.6 ± 12.6 | 41.9 ± 12.8 | 0.9 | |
| Age at diagnosis of diabetes | 41.5 ± 12.5 | 37.5 ± 13.6 | 0.06 | |
| Years with diabetes | 0 ± 0 | 5.2 ± 6.8 | <0.0001 | |
| Index DKA was the first episode of DKA | 83 (100%) | 46 (67%) | <0.0001 | |
| Male:Female ratio | 2.8:1 | 0.9:1 | <0.0001 | |
| Female with normal menstrual cycles at time of diagnosis of diabetes | 3 (14.3%) | 22 (57.9%) | 0.0007 | |
| Ethnicity (% by column) | 0.01 | |||
| African-American | 42 (51%) | 19 (28%) | ||
| Hispanic | 34 (41%) | 42 (62%) | ||
| Caucasian | 7 (8.0%) | 5 (7%) | ||
| Asian | 0 | 2 (3%) | ||
| Family history of diabetes | 58 (70%) | 59 (92%) | 0.0007 | |
| BMI (kg/m2) | 33.2 ± 8.3 | 31.1 ± 8.9 | 0.07 | |
| Weight category (% by column) | 0.04 | |||
| Lean (BMI <25 kg/m2) | 17 (20.5%) | 18 (28.1%) | ||
| Overweight (BMI ≥ 25 and < 30 kg/m2) | 11 (13.3%) | 21 (32.8%) | ||
| Obese (BMI ≥ 30 kg/m2) | 55 (66.3%) | 25 (39.1%) | ||
| Serum glucose at admission for index DKA | 509.4 ± 187.3 | 418.6 ± 141.2 | 0.001 | |
| HbA1c at baseline (%) | 13.9 ± 2.3 | 13.2 ± 2.4 | 0.1 | |
| HbA1c after 12 months (%) | 7.6 ± 2.7 | 8.8 ± 2.6 | 0. 01 | |
| HbA1c range at 12 months after index DKA | (n=81) | (n=61) | <0.001 | |
| HbA1c ≤ 7.0% | 52 (64.2%) | 18 (29.5%) | ||
| HbA1c > 7.0% and < 9.0% | 19 (23.5%) | 20 (32.8%) | ||
| HbA1c ≥ 9.0% | 10 (12.3%) | 23 (37.8%) | ||
| Fasting C-peptide at baseline (ng/dL) | 1.9 ± 1.7 | 1.9 ± 1.5 | 0.6 | |
| Fasting C-peptide at 12 months (ng/dL) | 3.3 ± 1.6 | 2.6 ± 1.6 | 0.07 | |
| GST-AUC C-peptide at baseline (ng/dL/10 min) | 25.1 ± 17.3 | 27.9 ± 21.2 | 0.3 | |
| GST-AUC C-peptide at 12 months (ng/dL/10 min) | 43.6 ± 19.4 | 31.4 ± 15.4 | 0.04 | |
| HOMA2-%β at baseline | 35.2 ± 34.7 | 34.2 ± 39.7 | 0.8 | |
| HOMA2-%β at 12 months | 128.9 ± 67.2 | 66.3 ± 47.1 | <0.0001 | |
| Change from baseline in HOMA2-%β | 89.7 ± 74.8 | 30.1 ± 58.2 | <0.0001 | |
| HOMA2-IR at baseline | 1.9 ± 1.8 | 1.9 ± 2.0 | 0.8 | |
| HOMA2-IR at 12 months | 2.6 ± 1.7 | 2.7 ± 2.5 | 0.8 | |
| Insulin discontinued | 37 (44.6%) | 15 (23.4%) | 0.008 | |
| Diabetes treatment at 12 months | 0.001 | |||
| Diet and Exercise only | 9 (10.8%) | 0 | ||
| Oral antidiabetic medications only | 25 (30.1%) | 12 (18.8%) | ||
| Insulin only | 29 (34.9%) | 33 (51.6%) | ||
| Both insulin and oral antidiabetic medications | 20 (24.1%) | 19 (29.7%) | ||
| B. HLA Class II allele frequencies in unprovoked A-β+ KPD, provoked A-β+ KPD, and population controls. | ||||
| HLA allele | Population controls |
Unprovoked A-β+ KPD |
Provoked A-β+ KPD |
|
| N = 561 | N = 53 | N = 43 | ||
| Susceptibility | DRB1*03 | 97 (17) | 7 (13) | 8 (19) |
| DRB1*04 | 202 (36) | 14 (26) | 19 (44) † | |
| DRB1*07 | 104 (19) | 8 (15) | 6 (14) | |
| DQB1*02 | 154 (27) | 15 (28) | 9 (21) | |
| DQB1*0302 | 96 (17) | 10 (19) | 18 (42)*† | |
| Resistance | DRB1*15 | 137 (24) | 11 (21) | 6 (14) |
| DQB1*0602 | 77 (14) | 14 (26)* | 7 (16) | |
Data are N(%).
P<0.05 (A-β+ KPD subset compared to population controls).
P<0.05 (unprovoked A-β+ KPD compared to provoked A-β+ KPD).
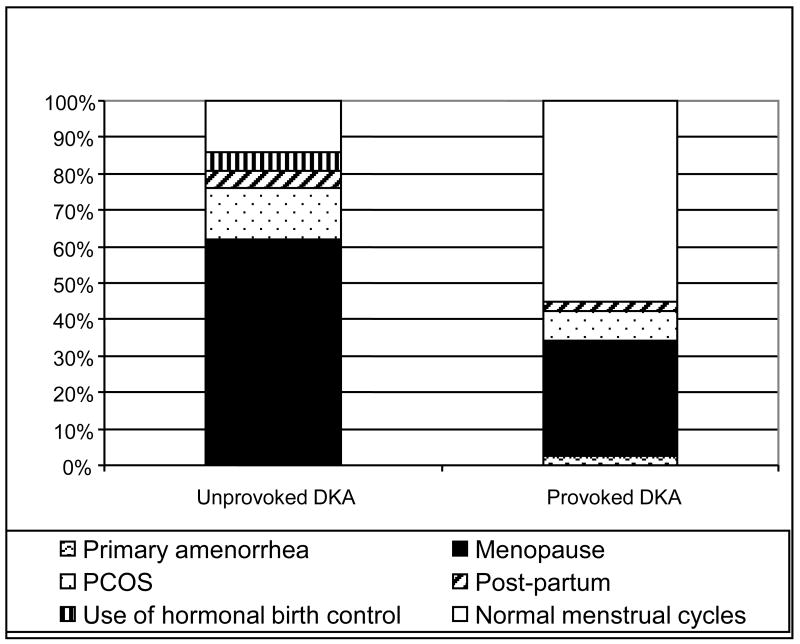
Male predominance was apparent in the unprovoked A-β+ KPD subgroup, with a male:female ratio of 2.8:1 compared to 0.9:1 in the provoked subgroup (P<0.0001). Among the 21 women in the unprovoked subgroup, only 3 (14.3%) had experienced regular menses during the previous year, while 22 of the 38 women (57.9%) in the provoked subgroup had experienced regular menses (P=0.0007). Analysis of menstrual history at the time of the index DKA revealed that 61.9% of unprovoked A-β+ KPD women had attained menopause and 14.3% had PCOS, compared to 31.6% and 7.9% respectively of provoked patients (P=0.02) (Figure 1).
Figure 1. Reproductive / menstrual status of women with “unprovoked” compared to “provoked A-β+ KPD, at the time of index DKA.
The different designs in each bar represent percentage of women with the indicated reproductive / menstrual status.
Patients of both A-β+ KPD subgroups had evidence of chronic hyperglycemia at the time of the index DKA, but unprovoked patients had higher serum glucose concentrations and HbA1c levels. Baseline measures of beta cell functional reserve showed no group differences: mean fasting C-peptide levels measured 2-3 weeks after resolution of DKA were similar in unprovoked and provoked groups (1.9 ± 1.7 compared to 1.9 ± 1.5 ng/ml, P=0.6), as were mean areas under the curve (AUC) for C-peptide concentration during the initial glucagon stimulation test (25.1 ± 17.3 compared to 27.9 ± 21.2 ng/ml over 10 minutes, P=0.3) and HOMA2-%B (35.2 ± 34.7 compared to 34.2 ± 39.7 (P=0.8) (Table 1). Baseline HOMA2-IR was also similar in the two subgroups.
Follow-up
At 12 months of follow-up, HbA1c was obtained in 137 patients. Unprovoked patients (n=76) had significantly lower mean HbA1c than provoked patients (n=61) (7.6 ± 2.7 %compared to 8.8 ± 2.6%, P=0.0001) (Table 1). The unprovoked subgroup also included a higher proportion of patients with HbA1c <7% (64.2% [95% CI: 53.1 to 73.2%] compared to 29.5% [95% CI:20.6 to 43.5%], P<0.001). Insulin treatment was discontinued in a higher proportion of unprovoked patients (44.6% [95% CI: 34.4 to 55.7%] compared to 23.4% [95% CI: 15.9 to 36.4%], P=0.01). The distribution of diabetes treatment modalities at 12 months was 10.8% diet/exercise, 30.1% oral medications only, 24.1 % insulin plus oral medications and 34.9% insulin only in the unprovoked group, compared to 0%, 18.8%, 29.7% and 51.6% respectively in the provoked group (P=0.001).
Despite similar levels of beta cell functional reserve at baseline, there were subgroup differences in these measures after 12 months. The unprovoked group showed greater improvement than the provoked group: mean serum fasting C-peptide 3.3 ± 1.6 compared to 2.6 ± 1.6 ng/ml, P=0.07; mean AUC for C-peptide during the GST 43.6 ± 19.4 compared to 31.4 ± 15.4 ng/ml over 10 minutes, P=0.04; HOMA2-%β 128.9 ± 67.2 compared to 66.3± 47.1%, P<0.0001.
Clinical and metabolic differences between new-onset patients of the two A-β+ KPD subgroups
Since duration of diabetes can affect beta cell functional reserve and glycemic control, we performed a subgroup comparison restricted to new-onset patients (Table 2). This comparison revealed the same differences between new-onset provoked and unprovoked patients as there were between all provoked and unprovoked patients. With regard to beta cell functional and glycemic parameters at 12 months, new-onset unprovoked patients had significantly higher fasting C-peptide values (3.3 ± 1.6 compared to 2.5 ± 1.2 ng/ml, P=0.04), AUC-GST C-peptide levels (43.6 ± 19.4 compared to 28.4 ± 9.7 ng/ml over 10 minutes, P=0.04), and HOMA2-%B scores (128.9 ± 67.2 compared to 79.8 ± 53.2 %, P=0.01), together with lower HbA1c (7.6 ± 2.7 compared to 8.7 ± 3.5 %, P=0.04) than new-onset provoked patients. Since there was asymmetry in the number of new onset patients in each subgroup (16 provoked and 83 unprovoked), we performed another comparison between the 16 new-onset provoked patients and 18 representative new onset unprovoked patients selected as described in Methods. This comparison yielded similar results, and showed that after 12 months of follow-up these 18 representative new-onset unprovoked patients had significantly higher fasting C-peptide values (3.3 ± 1.1 compared to 2.5 ± 1.2 ng/ml, P=0.04), AUC-GST C-peptide levels (44.3 ± 14.7 compared to 28.4 ± 9.7 ng/ml over 10 minutes, P=0.03), and HOMA2-%B scores (109.7 ± 54.9 compared to 79.8 ± 53.2 %,, P=0.04) and lower HbA1c (7.2 ± 2.3 compared to 8.7 ± 3.5 %, P=0.05) than the 16 new-onset provoked patients.
Table 2. Comparison of all 18 new-onset “provoked” to new-onset “unprovoked” A-β+ KPD patients, at baseline and after 12 months of treatment.
| New-Onset Provoked (N=16) |
All New-Onset Unprovoked (N=83) |
Representative New-Onset Unprovoked (N=18) |
P New-onset Provoked (N=16) vs. All New-onset Unprovoked (N=83) |
P New-onset Provoked (N=16) vs. Representative New-onset Unprovoked (N=18) |
|
|---|---|---|---|---|---|
| Age | 37.1 ± 12.7 | 41.6 ± 12.6 | 41.9 ± 9.6 | 0.2 | 0.2 |
| Age at diagnosis of diabetes | 37.1 ± 12.3 | 41.5 ± 12.5 | 41.9 ± 9.6 | 0.2 | 0.2 |
| Years with diabetes | 0 ± 0 | 0 ± 0 | 0 ± 0 | N/A | N/A |
| Male:Female ratio | 1.25:1 | 2.8:1 | 3.50:1 | 0.1 | 0.2 |
| Ethnicity (% by column) | 0.01 | 0.04 | |||
| African-American | 2 (12.5%) | 42 (51%) | 11 (61.1%) | ||
| Hispanic | 10 (62.5%) | 34 (41%) | 5 (27.8%) | ||
| Caucasian | 2 (12.5%) | 7 (8.0%) | 2 (11.1%) | ||
| Asian | 2 (12.5%) | 0 | 0 | ||
| Family History of diabetes | 13 (81.2%) | 58 (70%) | 14 (77.8 %) | 0.2 | 0.4 |
| BMI (kg/m2) | 31.4 ± 8.9 | 33.2 ± 8.3 | 32.6 ± 5.9 | 0.5 | 0.6 |
| Weight category (% by column) | |||||
| Lean (BMI <25 kg/m2) | 3 (18.8%) | 17 (20.5%) | 2 (11.1%) | 0.03 | 0.05 |
| Overweight (BMI ≥ 25 and < 30 kg/m2) | 8 (50%) | 11 (13.3%) | 2 (11.1%) | ||
| Obese (BMI ≥ 30 kg/m2) | 5 (31.2%) | 55 (66.3%) | 14 (77.8%) | ||
| Serum glucose at index DKA | 438.3 ± 179.1 | 509.4 ± 187.3 | 559.2 ± 181.9 | 0.1 | 0.04 |
| HbA1c at baseline (%) | 12.7 ± 2.4 | 13.9 ± 2.3 | 14.9 ± 1.9 | 0.08 | 0.02 |
| HbA1c at 12 months (%) | 8.7 ± 3.5 | 7.6 ± 2.7 | 7.2 ± 2.3 | 0.04 | 0.05 |
| Fasting C-peptide at baseline (ng/dL) | 1.8 ± 1.3 | 1.9 ± 1.7 | 1.8 ± 0.6 | 0.7 | 0.9 |
| Fasting C-peptide at 12 months (ng/dL) | 2.5 ± 1.2 | 3.3 ± 1.6 | 3.3 ± 1.1 | 0.04 | 0.04 |
| GST-AUC C-peptide at baseline (ng/dL/10 min) | 29.8 ± 16.1 | 25.1 ± 17.3 | 23.8 ± 11.8 | 0.2 | 0.3 |
| GST-AUC C-peptide at 12 months (ng/dL/10 min) | 28.4 ± 9.7 | 43.6 ± 19.4 | 44.3 ± 14.7 | 0.04 | 0.03 |
| HOMA2-%β at baseline | 39.2 ± 31.7 | 35.2 ± 34.7 | 32.9 ± 12.4 | 0.2 | 0.3 |
| HOMA2-%β at 12 months | 79.8 ± 53.2 | 128.9 ± 67.2 | 109.7 ± 54.9 | 0.01 | 0.04 |
| HOMA IR at baseline | 2.0 ± 1.8 | 1.9 ± 1.8 | 2.0 ± 1.8 | 0.5 | 0.6 |
| HOMA IR at 12 months | 2.6 ± 2.3 | 2.6 ± 1.7 | 2.7 ± 1.9 | 0.7 | 0.8 |
Note: “Representative new-onset unprovoked” group refers to 18 patients of the new-onset unprovoked group selected on the basis of their mean values for key phenotypic characteristics being within 1.5 SD of the means in the complete set of 88 new-onset unprovoked patients. See text for details
Long-term evolution of beta cell function
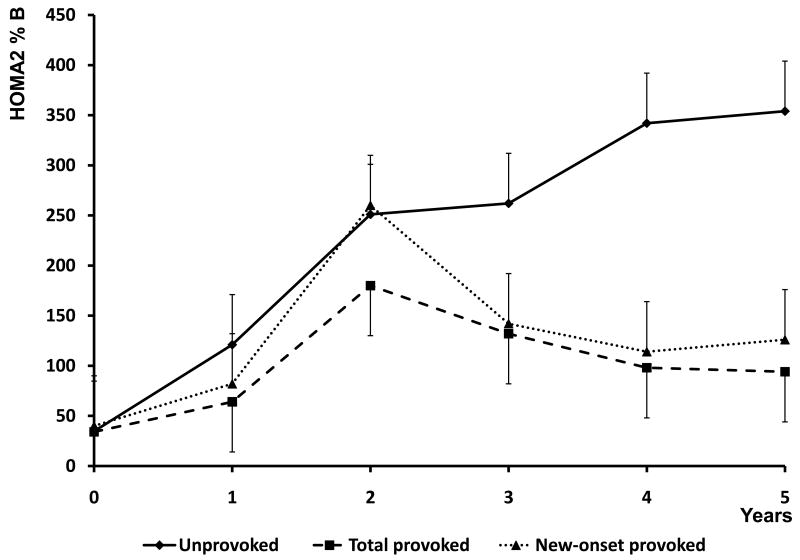
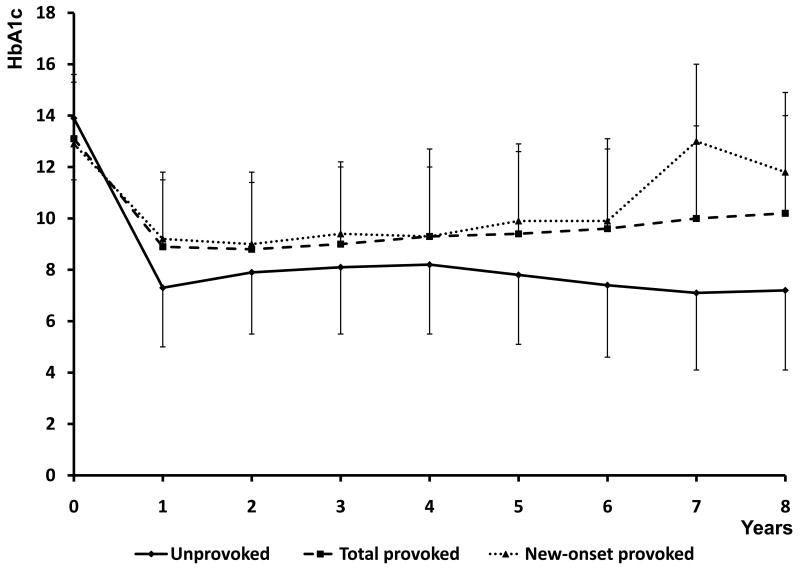
Evolution of beta cell functional reserve in the two A-β+ KPD subgroups was assessed over follow-up periods from 1 to 8 years, and of glycemic control from 1 to 5 years (Figure 2). Profiles of fasting C-peptide levels and HOMA2-%B scores were significantly different between the subgroups by repeated measures ANOVA analysis (P=0.027 and 0.035, respectively). The unprovoked subgroup demonstrated greater improvement after 12 months, followed by stability or further improvement over years 2-8, while the provoked subgroup demonstrated less improvement over years 1-2, followed by a decline in function. Concomitantly, unprovoked patients maintained better, sustained glycemic control over time (P=0.005). The longitudinal patterns of beta cell function in the 16 provoked A-β+ KPD patients with new-onset diabetes were similar to those of all provoked patients taken together, except at the year 2 time point, when they were similar to the unprovoked A-β+ KPD patients.
Figure 2. Longitudinal measures of beta cell function (HOMA2%B) and glycemic control (HbA1c) for the unprovoked A-β+ KPD (all new-onset), total provoked A-β+ KPD, and subgroup of new-onset provoked A-β+ KPD patients.
Data points are mean ± SD. Diamonds / solid line = unprovoked A-β+ KPD; squares / dashed line = total provoked A-β+ KPD; triangle / dotted line = new-onset provoked A-β+ KPD. By repeated measures ANOVA, P=0.035 for differences in HOMA2%B, and P = 0.005 for group differences in HbA1c.
HLA class II alleles
Frequency of the classic type 1 diabetes susceptibility allele DQB1*0302 was significantly higher in the provoked A-β+ KPD subgroup than either the unprovoked subgroup (42% compared to 19%, P<0.05) or population controls (42% compared 17%, P<0.05), with no difference between the latter two groups. Frequency of the classic type 1 diabetes susceptibility allele DRB1*04 was significantly higher in the provoked A-β+ KPD subgroup than the unprovoked subgroup (44% compared to 26%, P<0.05). Conversely, frequency of the type 1 diabetes resistance allele DQB1*0602 was significantly higher in the unprovoked A-β+ KPD subgroup than the provoked subgroup (26% compared to 14%, P<0.05), while the latter had a similar frequency of this allele as the population controls. Analysis of ethnic-specific allele frequencies, as performed previously [23], was not attempted here due to the small sample sizes in the A-β+ KPD subgroups when they were further divided by ethnicity.
Discussion
These data demonstrate that A-β+ KPD comprises two groups of patients distinguishable by whether the index DKA event was provoked by a clinically evident precipitating event. Patients with unprovoked A-β+ KPD have better recovery and sustained stability of beta cell function, with better long-term glycemic control and increased likelihood of becoming insulin-independent, than those with provoked A-β+ KPD. There is male predominance in the unprovoked group, but no gender predilection in the provoked group. Unprovoked A-β+ KPD patients frequently present with DKA as the initial manifestation of diabetes, while provoked patients often have a pre-existing diagnosis of type 2 diabetes; however, duration of diabetes does not appear to affect subgroup differences in recovery and evolution of beta cell functional reserve. These distinctions persist over a prolonged period, and point to different pathophysiologic bases for beta cell dysfunction in the two subgroups of A-β+ KPD.
We previously reported population-based and ethnic-specific differences in HLA class II allele frequencies between the four forms of KPD [8], including a higher-than-population frequency of the type 1 diabetes susceptibility allele DQB1*0302 in A-β+ KPD. The present data show that this high frequency of DQB1*0302 is largely accounted for by patients with provoked A-β+ KPD, while unprovoked A-β+ patients have a higher frequency of the protective allele DQB1*0602. The provoked A-β+ KPD subgroup also has a higher frequency of the HLA class II susceptibility allele DRB1*04 compared to the unprovoked subgroup. The finding of these HLA class II allele differences between clinically distinct subgroups of an apparently “non-autoimmune” mediated form of KPD has pathophysiologic implications. The high prevalence of DQB1*0302 and DRB1*04 in the provoked patients suggests that specific immunologic factors may contribute to their inexorable decline in beta cell function. Conversely, reversibility of the beta cell functional defect in the unprovoked subgroup may be due in part to the protective effects of DQB1*0602. Li et al have reported previously that persons with type 2 diabetes and a “mixed” family history of both type 1 and type 2 diabetes have a frequency of the DQB1*0302 allele that is higher than among those with a family history of only type 2 diabetes, but lower than among patients with type 1 diabetes; patients with type 2 diabetes who possess the DQB1*0302 allele have decreased insulin responses to oral glucose challenge compared to those who do not [10]. Although we lack details regarding “type” of diabetes in the family histories of our provoked A-β+ KPD patients, it is possible that they have an accelerated form of the beta cell dysfunction that develops progressively in patients with type 2 diabetes and “mixed” family history as described by Li et al. Immunologic studies are ongoing to identify mechanisms such as altered T-lymphocyte reactivity to islet antigens that may underlie the different autoantibody-negative forms of KPD.
Other investigators have reported male preponderance in cohorts of West African [11, 12] and African-American [13] patients with a phenotype of A-β+ KPD. The former study was confined to patients who presented with unprovoked DKA or ketosis, while the latter did not distinguish provoked from unprovoked presentation, and neither distinguished new-onset from previously known diabetes. The male:female ratios in these cohorts were 3.12 and 1.63 respectively. In our cohort of A-β+ KPD patients of mixed ethnicity (African-American, Hispanic and White), the separation of these two subgroups slightly increased the ratio in favor of men and restricted it to the unprovoked subgroup. Hence “unprovoked, new-onset A-β+ KPD” further refines the phenotype of a unique, gender-associated syndrome, making it more amenable to accurate genetic and metabolic characterization. The potential gender effect in the etiology of unprovoked A-β+ KPD is heightened by the finding that 86% of women in this subgroup had abnormal menstrual cycles or amenorrhea, compared to only 40% in the provoked subgroup. Louet et al. have investigated the role of male gender / anovulation and variations in the neurogenin-3 gene in the pathogenesis of KPD, and have demonstrated that the former was associated with diminished insulin secretion and the latter with lack of glycemic control [12]. Collectively, the human data point to an intrinsic beta cell mechanism of dysfunction in the syndrome of male-predominant, unprovoked A-β+ KPD. These findings add significance to evidence from transgenic mice that altered sex hormone action due to altered tissue specific signaling through their estrogen or androgen receptors may affect beta cell function or proneness to ketosis [14].
Unprovoked A-β+ KPD patients had a greater mean BMI and higher frequency of obesity, together with a lower frequency of family history of diabetes than the provoked patients. Unprovoked A-β+ KPD may be influenced by specific metabolic factors related to increased adiposity or glycemic levels, or to dietary or environmental factors that may affect beta cell function or ketogenesis, while provoked A-β+ KPD may be influenced more by genetic factors related to the vulnerability of beta cells to damage. These differences could play a role in the reversibility of beta cell dysfunction in the former, and lack thereof in the latter.
In our multiethnic KPD cohort, African-Americans were most highly represented in the unprovoked A-β+ subgroup, and Hispanics most highly represented in the provoked subgroup. The increased association of African ancestry with unprovoked A-β+ KPD is consistent with reports of similar phenotypes of KPD among West Africans [11] and African-Americans [15, 16] – however, this syndrome is by no means restricted to such populations [15, 17, 18]. About 40% of our unprovoked A-β+ patients were of Hispanic decent and 8% were Caucasian. Although not precisely specified, syndromes resembling unprovoked A-β+ KPD have been reported in persons of such diverse ethnicities as Asians [19], Apache Indians [20] and Caucasians [21].
Mauvais-Jarvis et al reported long-term clinical evolution in patients of Sub-Saharan African origin with “KPD-non-insulin dependent” (KPD-NID) [11], a condition defined very similarly to “unprovoked A-β+ KPD”. The evolution of beta cell function in KPD-NID patients paralleled that of our cohort of unprovoked A-β+ KPD patients. 40% of the KPD-NID patients were insulin-independent after long-term follow-up, consistent with the 45% rate of insulin discontinuation among the unprovoked A-β+ KPD patients in the present study.
These data indicate that A-β+ KPD comprises two subgroups of patients with distinct etiologies. The unprovoked subgroup represents a syndrome of severe but partially reversible beta cell dysfunction, with potential gender-related influences and effects of HLA-DQB1*0602 on its pathophysiology and clinical course. The provoked subgroup has a more irreversible and gender-independent trajectory of beta cell dysfunction, with potential contributions from the effects of HLA-DQB1*0302 and DRB1*04. Future studies should investigate metabolic and other genetic factors responsible for the distinct mechanisms of beta cell dysfunction and proneness to ketosis in these well-characterized KPD patients.
Acknowledgments
This study was supported by funds from the Alkek Foundation, RO1-HL73696 and R21-DK082827 (to A.B.). The authors thank the house officers and staff of Ben Taub General Hospital for excellent inpatient care of the patients. The long-term follow-up glycemic data of A-β+ KPD patients were presented in part as a poster at the American Diabetes Association's 68th Scientific Sessions, San Francisco, in June, 2008.
Footnotes
COI Statement: The authors declare no conflict of interest regarding this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maldonado M, Hampe CS, Gaur LK, D'Amico S, Iyer D, Hammerle LP, Bolgiano D, Rodriguez L, Rajan A, Lernmark A, Balasubramanyam A. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab. 2003;88(11):5090–8. doi: 10.1210/jc.2003-030180. [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350–7. doi: 10.7326/0003-4819-144-5-200603070-00011. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanyam A, Garza G, Rodriguez L, Hampe CS, Gaur L, Lernmark A, Maldonado MR. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care. 2006;29(12):2575–9. doi: 10.2337/dc06-0749. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanyam A, Nalini R, Hampe CS, Maldonado M. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29(3):292–302. doi: 10.1210/er.2007-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26 1:S33–50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 6.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 7.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 8.Nalini R, Gaur LK, Maldonado M, Hampe CS, Rodriguez L, Garza G, Lernmark A, Balasubramanyam A. HLA class II alleles specify phenotypes of ketosis-prone diabetes. Diabetes Care. 2008;31:1195–1200. doi: 10.2337/dc07-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormack J, Osowski LD. US normal. In: Gjertson DW, Terasaki PI, editors. HLA 1998. Vol. 232. American Society of Histocompatibility and Immunogenetics; Lenexa, Kansas: 1998. pp. 258–59.pp. 261 [Google Scholar]
- 10.Li H, Lindholm E, Almgren P, Gustafsson A, Forsblom C, Groop L, Tuomi T. Possible human leukocyte antigen-mediated genetic interaction between type 1 and type 2 Diabetes. J Clin Endocrinol Metab. 2001 Feb;86(2):574–82. doi: 10.1210/jcem.86.2.7170. [DOI] [PubMed] [Google Scholar]
- 11.Mauvais-Jarvis F, Sobngwi E, Porcher R, Riveline JP, Kevorkian J, Vaisse C, Charpentier G, Guillausseau PJ, Vexiau P, Gautier JF. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645–53. doi: 10.2337/diabetes.53.3.645. [DOI] [PubMed] [Google Scholar]
- 12.Louet JF, Smith SB, Gautier JF, Molokhia M, Virally ML, Kevorkian JP, Guillausseau PJ, Vexiau P, Charpentier G, German MS, Vaisse C, Urbanek M, Mauvais-Jarvis F. Gender and neurogenin3 influence the pathogenesis of ketosis-prone diabetes. Diabetes Obes Metab. 2008 Sep;10(10):912–20. doi: 10.1111/j.1463-1326.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 13.Pinero-Pilona A, Raskin P. Idiopathic Type 1 diabetes. J Diabetes Complications. 2001;15(6):328–35. doi: 10.1016/s1056-8727(01)00172-6. [DOI] [PubMed] [Google Scholar]
- 14.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103(24):9232–7. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umpierrez GE, Woo W, Hagopian WA, Isaacs SD, Palmer JP, Gaur LK, Nepom GT, Clark WS, Mixon PS, Kitabchi AE. Immunogenetic analysis suggests different pathogenesis for obese and lean African-Americans with diabetic ketoacidosis. Diabetes Care. 1999;22(9):1517–23. doi: 10.2337/diacare.22.9.1517. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Roman MA, Pinero-Pilona A, Adams-Huet B, Raskin P. Comparison of type 1, type 2, and atypical ketosis-prone diabetes at 4 years of diabetes duration. J Diabetes Complications. 2006;20(3):137–44. doi: 10.1016/j.jdiacomp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Sobngwi E, Mauvais-Jarvis F, Vexiau P, Mbanya JC, Gautier JF. Diabetes in Africans. Part 2: Ketosis-prone atypical diabetes mellitus. Diabetes Metab. 2002;28(1):5–12. [PubMed] [Google Scholar]
- 18.Banerji MA, Chaiken RL, Huey H, Tuomi T, Norin AJ, Mackay IR, Rowley MJ, Zimmet PZ, Lebovitz HE. GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes. 1994;43(6):741–5. doi: 10.2337/diab.43.6.741. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa T, Funase Y, Katakura M, Asanuma N, Yamauchi K, Yoshizawa K, Hashizume K. Ketosis-onset diabetes in young adults with subsequent non-insulin-dependency, a link between IDDM and NIDDM? Diabet Med. 1997;14(11):989–91. doi: 10.1002/(SICI)1096-9136(199711)14:11<989::AID-DIA482>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Wilson C, Krakoff J, Gohdes D. Ketoacidosis in Apache Indians with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1997;157(18):2098–100. [PubMed] [Google Scholar]
- 21.Pitteloud N, Philippe J. Characteristics of Caucasian type 2 diabetic patients during ketoacidosis and at follow-up. Schweiz Med Wochenschr. 2000;130(16):576–82. [PubMed] [Google Scholar]
- 22.Sani FV, Oak S, Radtke J, Lernmark Å, Lynch K, Agardh CD, Cilio CM, Lethagen ÅL, Örtqvist E, Landin-Olsson M, Törn C, Hampe CS. ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity. 2009 doi: 10.3109/08916930903555927. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]