Abstract
The liver is a critically important organ that has numerous functions including the production of bile, metabolism of ingested nutrients, elimination of many waste products, glycogen storage, and plasma protein synthesis. The liver is often incidentally irradiated during radiation therapy (RT) for tumors in the upper- abdomen, right lower lung, distal esophagus, or during whole abdomen or whole body RT. This article describes the endpoints, time-course, and dose-volume effect of radiation on the liver.
Keywords: Liver, normal tissue toxicity, radiation induced liver disease
1. Clinical Significance
The liver is an important organ with numerous functions including bile production, metabolism of ingested nutrients, elimination of waste products, glycogen storage, and protein synthesis. The liver is often incidentally irradiated during radiation therapy (RT) for tumors in the upper- abdomen, right lower lung, distal esophagus, or whole abdomen or whole body RT.
2. Endpoints
The Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0, defines grades 2, 3, 4, and 5 liver dysfunction as jaundice, asterixis, encephalopathy or coma, and death, respectively. These serious adverse events are rare following RT. Acute post-RT changes in liver function tests are far more common and occur during and following RT, presumably related to self-limited liver inflammation. Such liver enzyme abnormalities are classified under the CTCAE metabolic/laboratory category. Grades 2, 3, and 4 elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) include levels that are >2.5-5.0, >5.0-20, and >20 times the upper limit of normal, respectively. Radiographically, clinically insignificant transient declines in CT-defined tissue density can be seen 2-3 months after fractionated RT. This observation by itself should not be confused with tumor progression or irreversible liver injury(1).
The Child-Pugh scoring system assesses liver dysfunction based on clinical and laboratory parameters (Table 1). It can be used to characterize baseline liver function and post-treatment changes in liver function.
Table 1.
Child-Pugh Scoring System to Assess Severity of Liver Disease.
| Criterion | 1 point | 2 points | 3 points |
| Bilirubin (total) | <2 mg/dL | 2-3 mg/dL | >3 mg/dL |
| Serum albumin | >3.5 g/dL | 2.8-3.5 g/dL | <2.8 g/dL |
| INR | <1.7 | 1.71-2.20 | > 2.20 |
| Ascites | None | controlled with medication | Refractory |
| Hepatic encephalopathy | None | CTCAE Grade I-II (or controlled with medication) | CTCAE Grade III-IV (or refractory) |
Legend: INR = international normalized ratio; CTCAE = Common Terminology Criteria for Adverse Events. Patients are grouped into Child-Pugh Class A if the total score is 5-6, Class B if the score is 7-9, and Class C if the score is 10 or higher.
RT-induced liver disease (RILD) is separated into “classic” and “non-classic” RILD. Classic RILD involves anicteric hepatomegaly and ascites, typically occurring between 2 weeks to 3 months after therapy(2). Classic RILD also involves elevated alkaline phosphatase (more than twice the upper limit of normal or baseline value). This endpoint can occur in patients who have otherwise fairly well-functioning pre-treatment livers. Pathologically, there is occlusion and obliteration of the central veins of the hepatic lobules, retrograde congestion and secondary hepatocyte necrosis. Treatment options for RILD are limited, and liver failure and death can result.
Non-classic RILD, typically occuring between 1 week and 3 months after therapy, involves elevated liver transaminases more than five times the upper limit of normal or CTCAE grade 4 levels in patients with baseline values more than 5 times the upper limit of normal within 3 months after completion of RT, or a decline in liver function (measured by a worsening of Child-Pugh score by 2 or more), in the absence of classic RILD. This endpoint has been described in HCC patients who have poor liver function (hepatitis B infection, Child-Pugh Classes B and C) (3-5). CTCAE, while not as useful for classic RILD, is most useful for scoring non-classic RILD. The underlying pathology of non-classic RILD is unclear.
A confounder of RILD, especially in populations with pre-existing liver dysfunction, is the baseline rate of morbidity within this population due to their preexisting liver disease. In a recent randomized trial in unresectable HCC, there was a 54% rate of serious adverse events among the placebo group due to progression of cirrhosis and/or HCC(6). Another described endpoint is hepatitis B reactivation(7), which can contribute to liver function abnormalities. Patients at risk for HBV should have appropriate serum testing, and prophylactic antiretroviral therapy has been associated with a lower rate of post-RT re-activation of HBV or exacerbation.
No established therapies for classic RILD exist, though the use of anticoagulants and steroids have been suggested. Treatment of RILD is primarily supportive, and diuretics are often used for the ascites. While a few patients may recover, a substantial fraction will die of liver failure.
3. Challenges Defining Volumes
The liver is relatively easy to identify on CT images. If intravenous and oral contrast are not used, the left border can be indistinct against the heart or stomach. Ideally, the liver parenchyma (minus the biliary duct system and vasculature) should be distinguished as the “functional” component. Literature concerned with modeling liver tolerance to RT typically defines normal liver volume as the total liver minus gross tumor volume(5, 8), presuming that minimal function remains in liver tumors themselves.
Extensive work involving fluoroscopy, 4D-CT, and cine-MRI has described liver motion due to breathing and the effect of this motion on delivered RT dose. Regular breathing can result in liver tumor displacement ≥ 2cm. Strategies to manage this motion include abdominal compression, shallow breathing, breath holding, deformation modeling, gated treatments, and real-time tumor tracking(9-13). Attempts to assess/compensate for liver motion are essential for stereotactic RT and are advisable in other circumstances, especially when the normal liver volume irradiated poses a substantial risk of RILD.
4. Review of Dose/Volume Data
The liver parenchyma is composed of numerous functional subunits. This parallel architecture allows the liver to tolerate substantial focal injury prior to any clinical sequelae. In non-cirrhotic patients, surgical resection that leaves only a 20-25% liver remnant has been shown to be well tolerated(14). Because of this redundant capacity, partial liver irradiation to high doses is possible if adequate normal liver parenchyma can be spared. Pre-existing liver dysfunction secondary to co-morbid conditions such as hepatitis B/C infection and cirrhosis may render patients more susceptible to RT-induced liver injury.
Whole liver RT
The classic paper by Ingold (1965) is the first report of a dose-complication relationship for whole liver RT(15). RILD occurred in 1/8 patients who received 30-35 Gy over 3-4 weeks and 12/27 (44%) patients who received >35 Gy. In the 1991 report by Emami, the TD 5/5 for whole liver radiation was estimated to be 30 Gy in 2 Gy fractions(16). More recent experiences include the RTOG 84-05 dose escalation study of accelerated hyperfractionation in which it was observed that 0/122 patients who received 27-30 Gy in twice daily 1.5 Gy fractions of whole liver radiation therapy experienced RILD, whereas 5/51 (9.8%) who received 33 Gy in 1.5 Gy fractions developed RILD(17).
Partial liver RT
Table 2 summarizes the toxicity reported after partial liver RT for primary liver cancer and small volume metastatic disease. In most, the key factor predicting RILD was baseline liver condition. Two studies noted a dosimetric parameter associated with increased toxicity risk: mean dose and V30 (volume receiving ≥30 Gy). In each series where mean normal liver dose was reported, patients with RILD had a higher mean dose than those without RILD.
Table 2.
Series of fractionated partial liver irradiation and rates of RILD.
| Study Group | N | Diagnosis | Baseline Child Pugh Score | Prescription dose fractionation | Crude percent RILD | Mean normal liver dose in patients with vs without RILD | factors associated with RILD |
|---|---|---|---|---|---|---|---|
| Michigan [8,23] | 203* | PLC+LMC | 203 A | 1.5 Gy bid | 9.4% (19/203) | 37 Gy vs 31.3 Gy | PLC vs LMC mean liver dose |
| Taipei [20] | 89** | HCC | 68 A 21 B |
1.8-3.0 Gy | 19% (17/89) | 23 Gy vs. 19 Gy | HBV, liver cirrhosis |
| Shanghai [3, 18] | 109** | PLC | 93 A 16 B |
4-6 Gy | 15.6% (17/109) | 24.9 Gy vs 19.9 Gy | Liver cirrhosis |
| Guangdong [20] | 94** | HCC | 43 A 51 B |
4-8 Gy | 17% (16/94) note: 4 fatal |
Not stated | Liver cirrhosis |
| S Korea (Seong, Park) [21] | 158** | HCC | 117 A 41 B |
1.8 Gy | 7% (11/158) | Not stated | dose |
| S Korea (Kim)[4] | 105** | HCC | 85 A 20 B |
2.0 Gy | 12.3% (13/105) | 25.4 Gy vs. 19.1 Gy | Total liver volume receiving 30 Gy or more above 60% |
Patients also received FUdR or BUdR; in this series the mean normal liver dose was calculated as corrected for 1.5 Gy bid equivalent dose, and the comparison of patients with vs without RILD refers to the median value of mean normal liver dose, whereas for other series the comparison is between the average (mean) of mean normal liver dose in each group.
At least 77% of patients in these series also received trans-arterial chemoembolization (TACE)
Abbreviations: HCC, hepatocellular carcinoma; PLC, primary liver cancer; HBV, hepatitis B viral infection
The University of Michigan (UM) has extensively investigated RT dose escalation of primary and metastatic liver cancers since 1987. Using CT-based RT planning, the parameter effective volume (Veff) of normal liver irradiated was defined as the normal liver volume, which, if irradiated to the prescribed dose, would be associated with the same normal tissue complication probability (NTCP) as the non-uniform dose delivered. An analysis of 203 patients treated with 3-D conformal RT, and concurrent hepatic arterial chemotherapy, demonstrated that small portions of the liver can be irradiated to a very high dose (up to 90 Gy) if the Veff was low(8). Mean liver dose was also a strong predictor of RILD in the UM series (see section 6).
Dose/Volume limit recommendations are discussed in Section 8 below. Regarding risk, in general, the risks reported in the studies cited within this review are realistic estimates, as the follow-up durations in the studies are greater than the 3-4 months within which RILD typically occurs.
5. Factors Affecting Risk
Pre-existing liver dysfunction may render patients more susceptible to RT-induced liver injury (Table 2). Patients with Child-Pugh B or C scores have a higher risk of RT-related problems than those with Child-Pugh A scores(3, 18-20). Additional factors reportedly associated with a higher risk of RILD include hepatitis B carrier status(21), prior transcatheter arterial chemoembolization (TACE)(18), concurrent chemotherapy(8), portal vein tumor thrombosis(4, 18, 22), tumor stage(18), male sex(8), and Cancer of the Liver Italian Program (CLIP) staging system(18, 22).
While it is likely that the risk of liver injury relates to the dose per fraction received by portions of the liver, it is difficult to characterize the magnitude of any effect since most series include patients treated within a narrow range of dose per fraction. Furthermore, with any size fraction given to the tumor, the adjacent normal liver receives a broad range of doses due to beam entrance/exit zones and penumbra, further complicating the analysis. The topic of dose modeling is discussed further in Section 6, and the topic of hypofractionation is discussed further in Section 7.
6. Mathematical/Biological Models
The Lyman NTCP model has been applied by numerous groups. From the series referenced in table 2, the range of estimates of the parameters generated among patients with Child Pugh A or better liver function and no hepatitis B virus (HBV) infection are as follows: n, 0.86-1.1; m, 0.12-0.31; and TD50, 39.8-46.1 Gy(8, 21). For patients with HBV or Child Pugh B dysfunction, the ranges are: n = 0.26-0.7, m = 0.4-0.43, TD50 = 23-50 Gy(3, 21). These patients with worse liver dysfunction likely have lower TD50 values within the previous range, though this needs to be clarified in future studies.
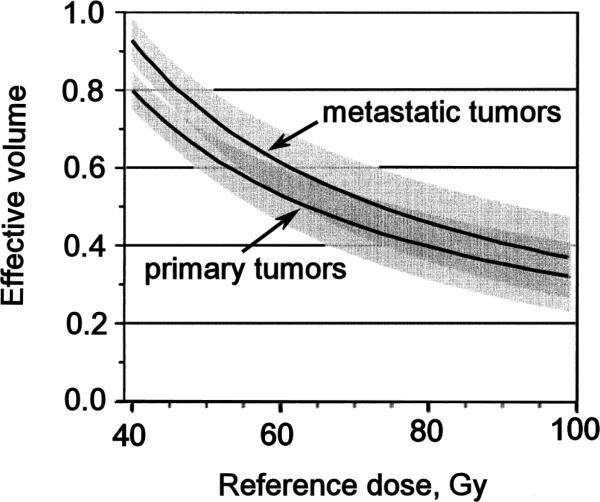
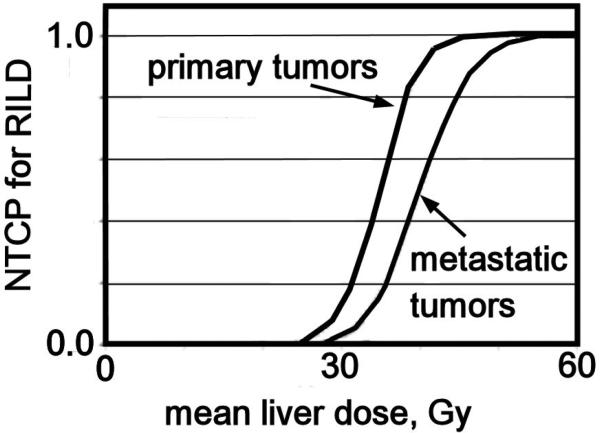
Analysis of UM patients treated for primary hepatobiliary cancer or 98 metastases with concurrent continuous hepatic arterial Floxuridine (FUdR) or bromodeoxyuridine (BUdR) and RT in twice daily 1.5 Gy fractions revealed a strong correlation of RILD with the mean liver dose. No classic RILD was observed when the mean liver dose was <31 Gy, with or without chemotherapy. For patients treated with FUdR, the mean liver doses associated with 5% risk of classic RILD was 28 Gy for primary and 32 Gy for metastatic liver cancer (corrected to 2 Gy fraction equivalent doses using the linear-quadratic (LQ) model, assuming an α/β = 2 Gy)). Based upon these observations and derived Lyman model parameter estimates, the partial volume tolerance of the liver for a defined allowable risk of RILD can be graphed (Figure 1) for a 5% risk of RILD. With “n” of approximately 1 in the Lyman NTCP model, a large volume effect is seen and a strong correlation of NTCP with mean liver dose is revealed. Again based on the UM data, Figure 2 demonstrates the relationship between mean liver dose and NTCP for RILD for patients with primary or metastatic liver tumors.
Figure 1.
Reference dose vs Effective Volume for 5% Isotoxicity curve for classic RILD after conformal radiation therapy, delivered in 1.5 Gy BID fractionation, for primary or metastatic tumors. Redrawn from [8]. The shaded areas around each curve represent the 80% confidence limits, which overlap above a reference dose of approximately 45 Gy.
Figure 2.
Mean liver dose, corrected with LQ modeling for 2.0 Gy fractions, versus Lyman normal tissue complication probability (NTCP) of classic radiation-induced liver disease (RILD) for primary and metastatic liver cancer, redrawn from reference [24].
When a similar analysis was conducted on different populations from Taiwan(21) and China(18), with a majority of patients having hepatitis B viral infections, the tolerance of the liver to radiation was less predictable, and the most common effect consisted of elevation of transaminases rather than RILD. HCC patients who were HBV carriers or had Child-Pugh B cirrhosis had a greater susceptibility to RILD and had a smaller volume effect on normal liver response according to Lyman modeling. For patients with HBV treated in Taiwan, Lyman NTCP parameters for classic and non-classic RILD are: n 0.26, m 0.4 TD50 50 Gy(21).
The Shanghai group likewise noted differences in Lyman model parameter estimates based on baseline liver dysfunction, with less volume effect for Child Pugh B relative to Child Pugh A(3, 18); in this series fraction sizes were 4 – 6 Gy (see Table 2). For the patients with Child Pugh B liver dysfunction treated with 4- 6 Gy per fraction, Lyman parameters for classic or non-classic RILD are n 0.7, m 0.43, TD50 23 Gy(3). From these patients treated with 4 – 6 Gy per fraction, the mean liver doses associated with a 5% risk of liver toxicity were estimated to be 23 Gy and 6 Gy for Child-Pugh A and B patients respectively.
One report of damage injury model parameterization of the early UM data led to local damage parameters of D50=42 Gy, k=2; and fraction of liver injury required for RILD parameters of F50=0.5, σ = 0.05(23). A subsequent analysis of 203 UM patients led to a lower threshold and a shallower slope for the population cumulative functional reserve: F50=0.4, σ = 0.08, however the confidence limits on these parameters were very large(24). In another analysis from the National Taiwan University (NTU) group, including patients with HCC and gastric cancer patients, valid fits were only obtained for the non-HBV carriers with local damage parameters of D50=25 Gy, k=60; and fraction of liver injury required for RILD parameters of F50=0.59, σ = 0.12(25), but these parameters have high uncertainty.
Limited data about the utility of V30 exist from studies of mostly HCC patients, with both classic and non-classic RILD combined together. V30 was found to be useful in segregating higher risk patients from lower risk patients in some studies at cutoff levels of 28-60%(4, 18, 26); however, the effect of V30 is not uniformly observed(5). Other studies suggest the importance of V20-V40(4) and V5-V40(18), but only for Child-Pugh Grade A patients in the latter study. The critical volume model is discussed in section 7.
7. Special Situations
Most clinical data published involves analyses of conventionally fractionated or hyperfractionated treatment involving daily prescription doses to the tumor in the range of 2 Gy or less. Consequently, the daily doses received by surrounding normal liver parenchyma are even lower. Current interest in the use of stereotactic body radiation therapy (SBRT) raises questions about the extent to which observations made using low dose per fraction are applicable to the setting of SBRT, where the daily prescription dose to the tumor is on the order of 10 Gy or higher, and portions of the normal liver will receive doses in that range. Use of the models discussed in Section 6 should be done with caution, as the LQ conversion for larger fraction sizes will likely be inadequate.
SBRT produces transient hypodensity on CT scan that appears within months after treatment and then resolve(27). RILD after SBRT occurs in fewer than 5% of cases with careful patient selection and technique. Mendez-Romero (median follow-up 12.9 months) observed 1 classic and 1 non-classic case of RILD among 8 patients with HCC treated with SBRT for liver tumors; another patient with baseline Child-Pugh B liver dysfunction and HCC experienced portal hypertension and concomitant non-hepatic infection and died two weeks after treatment. No grade 4 or 5 toxicity occurred among the 17 patients with liver metastases, suggesting that patients with HCC are more susceptible to SBRT-related toxicity, especially if there is underlying liver dysfunction(28). In a phase II study of 61 patients treated with SBRT for colorectal metastases treated with 15 Gy x 3 within 5-8 days, Hoyer(median follow-up 4.3 years) observed severe toxicity in one patient that was possibly related to SBRT (60% of liver received ≥10 Gy, median dose 14.4 Gy in 3 fractions). This patient died of hepatic failure 7 weeks post-RT, but the exact cause was unclear(29). In a Princess Margaret Hospital study of 41 patients treated with SBRT, using an NTCP estimate for dose in six fractions (median 36.0 Gy, range 24.0 to 54.0 Gy) and with a median follow-up 17.6 months, for HCC or intrahepatic cholangiocarcinoma, 17% experienced progression from Child-Pugh A to B within 3 months after RT (median mean liver dose 17.5 Gy, range 5.2 – 25.2 Gy, in 6 fractions) (30). In contrast. in 68 patients with liver metastases treated with SBRT (28 – 60 Gy, in 6 fractions), the risk of any serious liver toxicity within three months was very low (95% confidence interval 0 – 5.3%), despite similar doses delivered to the liver (median mean liver dose 16.9 Gy, range 3 – 22 Gy, in 6 fractions)(31).
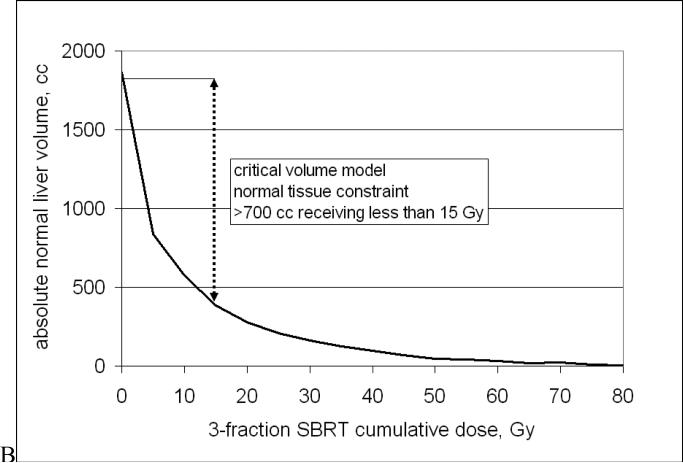
In the University of Colorado (UC) trial of SBRT for liver metastases (median follow-up 12.9 months), a modification of the critical volume model(32) was applied. For liver SBRT, the fundamental premise is that to preserve adequate liver function, a minimum volume of normal liver must be spared from receiving a dose that might render it non-functional. This minimum “critical volume” was estimated from partial hepatectomy series to be 700 cc; the maximum dose allowed to this critical volume was estimated to be 15 Gy in 3 fractions (based on LQ conversion, α/β = 3 Gy)(33). No RILD or other severe toxicity has been observed to date after SBRT given according to these constraints(34).
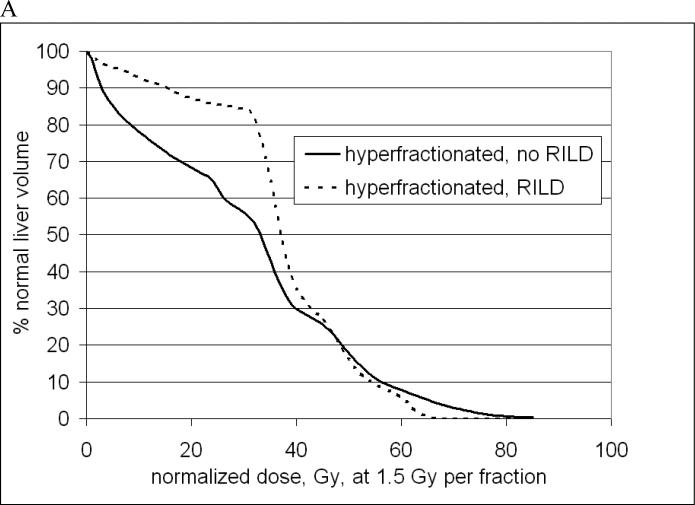
Comparisons between SBRT and conventional fractionation or hyperfractionation must be approached cautiously, given uncertainties in the models used to calculate biological equivalence. Recently Tai combined LQ and Lyman modeling to generate parameters based on clinical data that may be used to estimate equivalent doses based on differing fraction size(35), but Park has noted problems in the application of LQ modeling for SBRT and offered an alternative survival curve formulation(36). For the purpose of offering a visual example of the typical DVHs used in those settings, Figure 3 includes mean DVHs from the UM hyperfractionated experience and the UC SBRT experience, but these should not be interpreted as an ideal DVH for these situations. Regarding the UM data, the doses shown in Figure 3A have been corrected to 1.5 Gy per fraction (using LQ model, α/β = 2.5 Gy) and radiation was given with hepatic arterial FUdR(37). The UC data represent DVHs from the first 18 patients treated(33).
Figure 3.
Characteristic normal liver (minus GTV) DVHs for low (A) or high (B) dose-per fraction. A. Mean normal liver DVHs from the University of Michigan for 204 patients who did or did not experience RILD. B. Mean normal liver DVHs from the University of Colorado SBRT Phase I trial with no radiation-induced liver disease (RILD) observed. See text for additional details.
One additional potential concern related to the use of high dose per fraction treatment is the observation of extrahepatic portal vein occlusion (EHPO) following high dose intraoperative radiation therapy (IORT). Mitsunaga and colleagues observed 12 cases of EHPO among 53 patients who underwent pancreaticoduodenectomy for periampullary disease followed by 20 Gy IORT to the resection bed(38).
8. Recommended Dose/Volume Limits
All dose/volume recommendations are associated with some uncertainty. Nevertheless, the data for RILD estimates have been reasonably well studied and analyzed. Long term liver injury or biliary duct system damage is less well understood, as few patients have been followed for 5 or more years. Broad guidelines for normal liver dose constraints, for 5% or less risk of RILD, are offered as follows:
Palliative whole liver doses
Liver metastases
≤30 Gy, in 2 Gy per fraction
21 Gy in 7 fractions(39)
Primary liver cancers
≤28 Gy, in 2 Gy per fraction
21 Gy in 7 fractions(40)
Therapeutic partial liver RT (standard fractionation)
Mean normal liver dose (liver minus GTV)
< 28 Gy in 2 Gy fractions for primary liver cancer
< 32 Gy in 2 Gy fractions for liver metastases
Non-uniform liver recommendations (SBRT, 3-6 fractions)
Mean normal liver dose (liver minus GTV)
< 13 Gy for primary liver cancer, in 3 fractions
< 18 Gy for primary liver cancer, in 6 fractions
< 15 Gy for liver metastases, in 3 fractions
< 20 Gy for liver metastases, in 6 fractions
< 6 Gy for primary liver cancer, Child-Pugh B, in 4-6 Gy per fraction (for classic or non-classic RILD)
Critical volume model-based
≥700 cc of normal liver receives ≤ 15 Gy in 3-5 fractions
9. Future Toxicity Studies
Prospective studies with dose-volume data and serial long-term clinical/objective outcomes are needed. Differences in dose per fraction should also be considered.
The impact of clinical variables (e.g. pre-RT liver function) and other therapies (e.g. chemotherapy) that may impact the liver's functional reserve need to be assessed.
The timeframe for post-RT liver regeneration has not been well characterized.
An improved understanding of the biological pathophysiology of RT-induced liver injury, especially for non-classic RILD, is needed, with an emphasis on identifying opportunities for injury mitigation by modulation of key signaling pathways (e.g. transforming growth factor-β(41)). In this setting, pre-treatment pathologic evaluation of the non-malignant liver could potentially be useful for predicting the NTCP and may be a subject for future research.
RT effects on non-parenchymal structures within the liver e.g. biliary duct tolerance
Spatial variation in radiation sensitivity
10. Toxicity Scoring
Studies of RT-induced liver injury should separately record the incidence of classic and non-classic RILD. Pre-existing liver dysfunction, as measured by the Child-Pugh score, should be recorded, as well as any change in status of Child-Pugh score following treatment. The use of CTCAE criteria for elevations of AST, ALT, alkaline phosphatase, platelet count, bilirubin, albumin, prothrombin time is advisable to promote consistency of reporting. Screening for and treatment of hepatitis B and/or C prior to radiotherapy is recommended.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors have no conflict of interest
I, Charlie Pan, M.D. and all other authors, declare that I have no proprietary, financial, professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Radiation-Associated Liver Injury”.
References
- 1.Yamasaki SA, Marn CS, Francis IR, et al. High-dose localized radiation therapy for treatment of hepatic malignant tumors: CT findings and their relation to radiation hepatitis. AJR American Journal of Roentgenology. 1995;165:79–84. doi: 10.2214/ajr.165.1.7785638. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. International Journal of Radiation Oncology*Biology*Physics. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z-Y, Liang S-X, Zhu J, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. International Journal of Radiation Oncology, Biology, Physics. 2006;65:189–195. doi: 10.1016/j.ijrobp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Kim TH, Kim DY, Park J-W, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. International Journal of Radiation Oncology*Biology*Physics. 2007;67:225–231. doi: 10.1016/j.ijrobp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JC-H, Wu J-K, Huang C-M, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. International Journal of Radiation Oncology*Biology*Physics. 2002;54:156–162. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JRS, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S, Poulin-Costello M, Moscovici M, Voliotis D, Bruix J. Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): Results of a Phase III randomized placebo-controlled trial (SHARP trial) 2007:25. [Google Scholar]
- 7.Kim JH, Park JW, Kim TH, et al. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:813–819. doi: 10.1016/j.ijrobp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. International Journal of Radiation Oncology*Biology*Physics. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 9.Shirato H, Seppenwoolde Y, Kitamura K, et al. Intrafractional tumor motion: lung and liver. Semin Radiat Oncol. 2004;14:10–18. doi: 10.1053/j.semradonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Brock KK, McShan DL, Ten Haken RK, et al. Inclusion of organ deformation in dose calculations. Med Phys. 2003;30:290–295. doi: 10.1118/1.1539039. [DOI] [PubMed] [Google Scholar]
- 11.Rosu M, Dawson LA, Balter JM, et al. Alterations in normal liver doses due to organ motion. International Journal of Radiation Oncology, Biology, Physics. 2003;57:1472–1479. doi: 10.1016/j.ijrobp.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 13.Guckenberger M, Sweeney RA, Wilbert J, et al. Image-guided radiotherapy for liver cancer using respiratory-correlated computed tomography and cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;71:297–304. doi: 10.1016/j.ijrobp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North Am. 2002;82:1075–1090, x-xi. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 15.Ingold JA, Reed GB, Kaplan HS, et al. RADIATION HEPATITIS. American Journal of Roentgenology, Radium Therapy & Nuclear Medicine. 1965;93:200–208. [PubMed] [Google Scholar]
- 16.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 17.Russell AH, Clyde C, Wasserman TH, et al. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. International Journal of Radiation Oncology, Biology, Physics. 1993;27:117–123. doi: 10.1016/0360-3016(93)90428-x. [DOI] [PubMed] [Google Scholar]
- 18.Liang S-X, Zhu X-D, Xu Z-Y, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: The risk factors and hepatic radiation tolerance. International Journal of Radiation Oncology*Biology*Physics. 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma patients with severe cirrhosis. Strahlenther Onkol. 2006;182:713–720. doi: 10.1007/s00066-006-1564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu DH, Liu L, Chen LH. Therapeutic effects and prognostic factors in three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2004;10:2184–2189. doi: 10.3748/wjg.v10.i15.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng JC-H, Wu J-K, Lee PC-T, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. International Journal of Radiation Oncology*Biology*Physics. 2004;60:1502–1509. doi: 10.1016/j.ijrobp.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Seong J, Park HC, Han KH, et al. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. International Journal of Radiation Oncology*Biology*Physics. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 23.Jackson A, Ten Haken RK, Robertson JM, et al. Analysis of clinical complication data for radiation hepatitis using a parallel architecture model. International Journal of Radiation Oncology, Biology, Physics. 1995;31:883–891. doi: 10.1016/0360-3016(94)00471-4. [DOI] [PubMed] [Google Scholar]
- 24.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Seminars in Radiation Oncology. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Cheng JC-H, Liu H-S, Wu J-K, et al. Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. International Journal of Radiation Oncology*Biology*Physics. 2005;62:1150–1156. doi: 10.1016/j.ijrobp.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. International Journal of Radiation Oncology*Biology*Physics. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 27.Herfarth KK, Hof H, Bahner ML, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys. 2003;57:444–451. doi: 10.1016/s0360-3016(03)00586-8. [DOI] [PubMed] [Google Scholar]
- 28.Mendez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 29.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncologica. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 30.Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 31.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 32.Yaes RJ, Kalend A. Local stem cell depletion model for radiation myelitis. Int J Radiat Oncol Biol Phys. 1988;14:1247–1259. doi: 10.1016/0360-3016(88)90404-x. [DOI] [PubMed] [Google Scholar]
- 33.Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. International Journal of Radiation Oncology*Biology*Physics. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 35.Tai A, Erickson B, Khater KA, et al. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int J Radiat Oncol Biol Phys. 2008;70:900–907. doi: 10.1016/j.ijrobp.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 36.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 37.Dawson LA, Biersack M, Lockwood G, et al. Use of principal component analysis to evaluate the partial organ tolerance of normal tissues to radiation. International Journal of Radiation Oncology*Biology*Physics. 2005;62:829–837. doi: 10.1016/j.ijrobp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Mitsunaga S, Kinoshita T, Kawashima M, et al. Extrahepatic portal vein occlusion without recurrence after pancreaticoduodenectomy and intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:730–735. doi: 10.1016/j.ijrobp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Leibel SA, Guse C, Order SE, et al. Accelerated fractionation radiation therapy for liver metastases: selection of an optimal patient population for the evaluation of late hepatic injury in RTOG studies. International Journal of Radiation Oncology, Biology, Physics. 1990;18:523–528. doi: 10.1016/0360-3016(90)90055-o. [DOI] [PubMed] [Google Scholar]
- 40.Abrams RA, Cardinale RM, Enger C, et al. Influence of prognostic groupings and treatment results in the management of unresectable hepatoma: experience with Cisplatinum-based chemoradiotherapy in 76 patients. Int J Radiat Oncol Biol Phys. 1997;39:1077–1085. doi: 10.1016/s0360-3016(97)00389-1. [DOI] [PubMed] [Google Scholar]
- 41.Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor-beta 1 expression in irradiated liver. Radiation Research. 1990;122:77–85. [PubMed] [Google Scholar]