Abstract
Purpose
Much current research on lower urinary tract physiology focuses on afferent mechanisms. The main goals are to define and control the signaling pathways by which afferent information is generated and conveyed to the central nervous system. We summarize recent research on bladder afferent mechanisms.
Materials and Methods
We systematically reviewed the literature by searching PubMed® up to June 2009 with focus on the last 5 years.
Results
At least 2 signaling pathways can be identified, including the urothelial and the myogenic pathway. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells and afferent nerves in the lamina propria. Signaling occurs via muscle-mucosal mechanoreceptors, mucosal mechanoreceptors and chemoreceptors. The myogenic pathway is activated via in-series mechanoreceptors responding to distention and via spontaneous contractile activity in units of myocytes generating afferent noise.
Conclusions
To control dysfunctional micturition we must know more about all components involved in normal micturition control, including how afferent information is handled by the central nervous system.
Keywords: urinary bladder, afferent pathways, muscle contraction, urothelium, urination disorders
In the last decade research in the field of LUT physiology/pharmacology has provided much new information and the emergence of several new concepts of central and peripheral nervous control of voiding, and voiding dysfunction etiology.1 The search for new therapy for voiding disorders has been intensive and new targets for drugs aimed at micturition control have been defined, such as urothelial signaling mechanisms.2 Although our understanding of bladder function has increased in recent years, the detrusor muscle and its functional regulation still provide considerable challenges for future basic, clinical and translational research. The detrusor muscle has for many years been a target of drug treatment. However, depression of detrusor contractility, resulting in decreased ability to empty the bladder, has not produced any success in treating voiding dysfunction.
Voiding contraction is a result of coordination of the contractile units of the detrusor,3 made possible by the concerted action of bladder motor nerves and ICC. It is the goal of treatment, eg for DO and OAB symptoms, not to interfere with emptying ability but rather to eliminate involuntary bladder contractions. In this context the difference between whole bladder contraction and the contractile activity of its individual myocytes should be emphasized. The detrusor muscle is not quiet during the filling phase. Myocytes and contractile units show uncoordinated spontaneous activity that can produce firing in afferent nerves, contributing to uncontrolled micturition reflex activation and sensations, eg of urgency.
Much current research on LUT physiology focuses on afferent mechanisms. Currently we are learning how to control 2 identified signaling pathways by which afferent information is generated and conveyed to the central nervous system, that is the urothelial and the myogenic pathway. We focus on these pathways with particular reference to their role in the pathophysiology of DO and OAB syndrome, and discuss future directions of research aimed at controlling bladder activity.
AFFERENT PATHWAYS
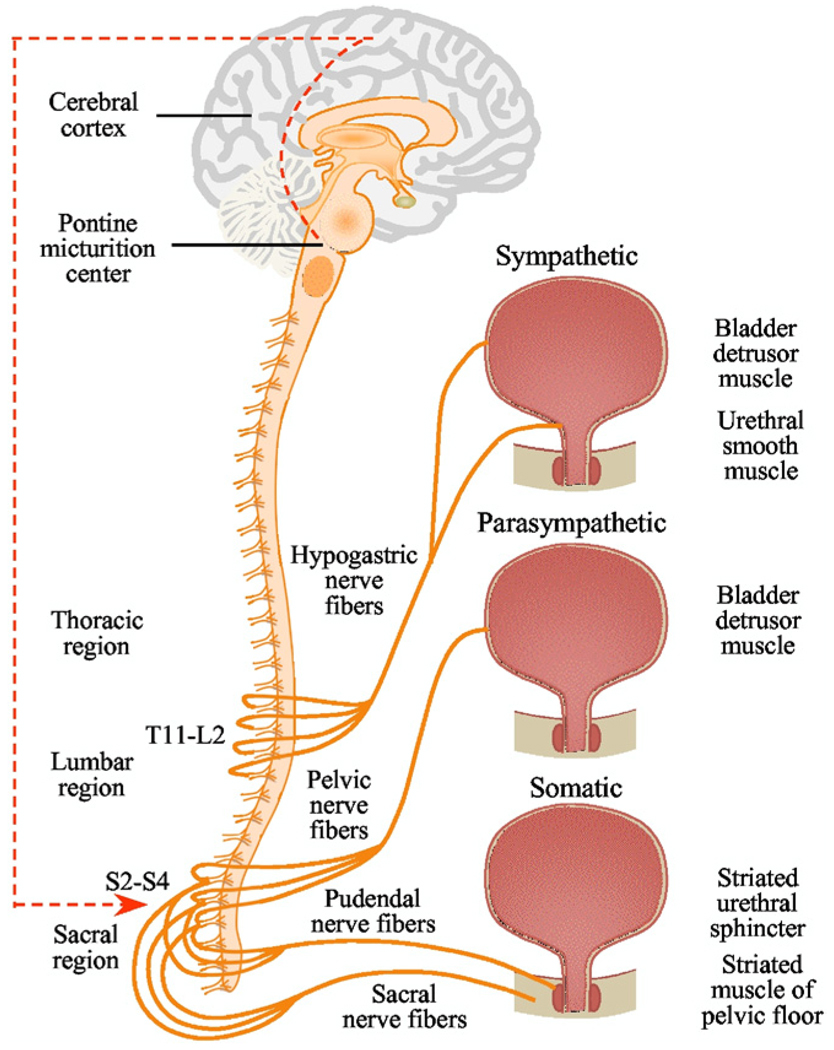
The pelvic, hypogastric and pudendal nerves carry sensory information in afferent fibers from the LUT to the lumbosacral spinal cord.4 In humans the somata of the pelvic and pudendal afferent nerves are located in DRG at sacral segments S2-S4 and the somata of the hypogastric nerve in DRG are at thoracolumbar segments T11-L2 (fig. 1). After entering the spinal cord the primary afferent fibers of the pelvic and pudendal nerves travel rostral in Lissauer’s tract. Sensory information is transmitted to second order neurons in the spinal cord. Pelvic nerve afferents monitor bladder volume during the storage phase and the amplitude of bladder contractions during voiding. Thus, sensory nerves initiate the micturition reflex and reinforce the drive that maintains bladder contraction.
Figure 1.
Human LUT innervation. Coordination between bladder and outlet (bladder neck, urethra and urethral sphincters) is mediated by sympathetic (hypogastric), parasympathetic (pelvic) and somatic (pudendal) nerves. Primary cell bodies of Aδ and C-fiber afferents of pelvic and pudendal nerves are contained in lower lumbar and sacral DRG, and afferent innervation in hypogastric nerve arises in rostral lumbar DRG.
Afferents comprise myelinated Aδ fibers and unmyelinated C fibers. Aδ fibers, located primarily in the detrusor smooth muscle layer, respond to detrusor stretching during bladder filling and convey fullness sensations. Unmyelinated sensory C fibers are more widespread and reside in the detrusor muscle, close to the urothelium in the lamina propria and directly adjacent to urothelial cells.5 There is also good evidence that C fibers carry information on bladder volume changes. For example, in the rat many C fibers (conduction velocity less than 1.3 m second−1) respond to slow distention with physiological volume but do not respond to bladder contraction.6 Approximately 23% of Aδ fibers and 64% of C fibers studied during ventral root evoked bladder contraction behaved in this fashion. This finding suggests that volume receptor afferents are mainly C fibers that discharge during normal bladder distention but with higher thresholds than Aδ in-series tension receptors7 and can be activated by various neurotransmitters and chemical mediators released by the detrusor and the urothelium, as described. C fibers with their peripheral nerve endings in the urothelium may be volume afferents, and perhaps chemosensitive and thermosensitive afferents, given their proximity to the intravascular milieu. Approximately 9% of afferents innervating the mouse bladder are found in the urothelium.8
AFFERENT NERVE LOCALIZATION IN BLADDER WALL
Sensory neurons have become an attractive target for novel pharmacological treatments for DO/OAB.9 It is still unclear exactly how many functional classes of distention sensitive afferents innervate the bladder. There are at least 2 types of C-fiber afferents distinguished by central projections and by the presence of neuropeptides. The first type is neuropeptidergic, projects primarily to spinal lamina I and outer lamina II, and is positive for calcitonin gene-related peptide and substance P. The second type is nonpeptidergic, projects to the inner lamina II of the spinal dorsal horn and binds isolectin B4.10
Several groups have characterized bladder mechanoreceptors in terms of their adequate mechanical stimuli, chemosensitivity, receptive field site and electrophysiological parameters. In in vivo studies bladder afferents have generally been divided into distention sensitive mechanoreceptors, distention insensitive chemoreceptors and silent afferents. Of distention sensitive bladder afferents low and high threshold afferents have been identified that conduct in small myelinated Aδ and unmyelinated C-fiber ranges.11 Low threshold fibers are considered mainly involved in micturition control and high threshold afferents (threshold greater than 20 mm Hg) are associated with painful sensation generation.12 In several in vivo studies low threshold stretch sensitive afferents fired in proportion to intravesical pressure, behaving as in-series tension receptors.11 Several recent investigations generally confirmed previous data but also added interesting detailed information on bladder afferents.8,13
Zagorodnyuk et al examined the effects of various stimuli, including stretch, von Frey hair compression, receptive field stroking and application of chemical stimuli to the mucosa, on electrophysiological recordings from guinea pig bladder afferents in vitro.14 Several functionally distinct classes of bladder sensory neurons were distinguished, including stretch sensitive afferents (muscle mechanoreceptors and muscle-mucosal mechanoreceptors). Stretch insensitive afferents (mucosal mechanoreceptors and chemoreceptors) were also recorded.
Muscle mechanoreceptors, which behaved as in-series tension receptors, were silent and had a low threshold to stretch. Firing in these receptors was not affected by removing the superficial mucosal layer. It was concluded that the receptive fields were probably located in the muscle layer without involvement of the lamina propria or urothelium. Muscle-mucosal mechanoreceptors could be activated by stretch, mucosal stroking with light von Frey hair (0.1 to 2 mN) and hypertonic solutions applied locally to the receptive mucosal fields. Some of these afferents showed occasional bursting activity associated with spontaneous muscle contractions that were sensitive to nicardipine. When the urothelium and lamina propria were removed, stretch induced firing and contractile responses decreased.
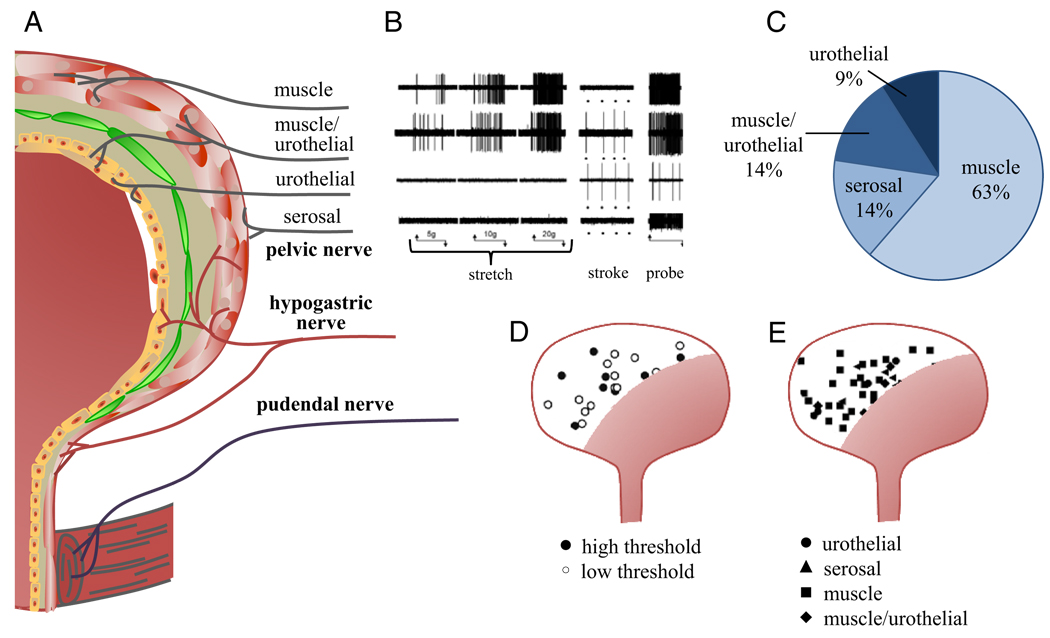
In the mouse bladder Xu and Gebhart recorded mechanosensitive primary afferents from the lumbar splanchnic and pelvic nerves, and distinguished them based on their response to receptive field stimulation with different mechanical stimuli, such as probing, stretch and stroking of the urothelium.8 Four afferent classes were distinguished, including serosal, muscle, muscle/urothelial and urothelial (fig. 2). Lumbar splanchnic nerves contained principally serosal and muscle afferents (97% of the total sample) but all 4 afferent classes were present in pelvic nerves, of which 63% were muscle afferents.
Figure 2.
LUT afferent nerve classes and distribution. A, fiber classes in bladder wall and urethra. B, in pelvic nerve 4 types of mechanosensitive fibers were identified by stretch, stroke and probe. C, proportion of afferent fiber types recorded in pelvic nerve. D, low and high threshold receptive fields of pelvic nerve muscle fibers based on response to stretch. E, receptive fields of 4 pelvic nerve fiber classes. B to E, adapted from Xu and Gebhart.8
Of bladder afferents 18% to 28% that do not respond to any level of distention have been called silent afferents.11 Some may become loosely mech-anosensitive during acute inflammation.15 However, volume receptors may also exist that sense bladder distention regardless of pressure.6 The putative receptors for this function are not well characterized. Some afferents, eg muscle-mucosal receptors, may be associated with spontaneous contractions and involved in the generation of afferent noise during bladder filling.
AFFERENT SIGNALING AND SENSITIZATION
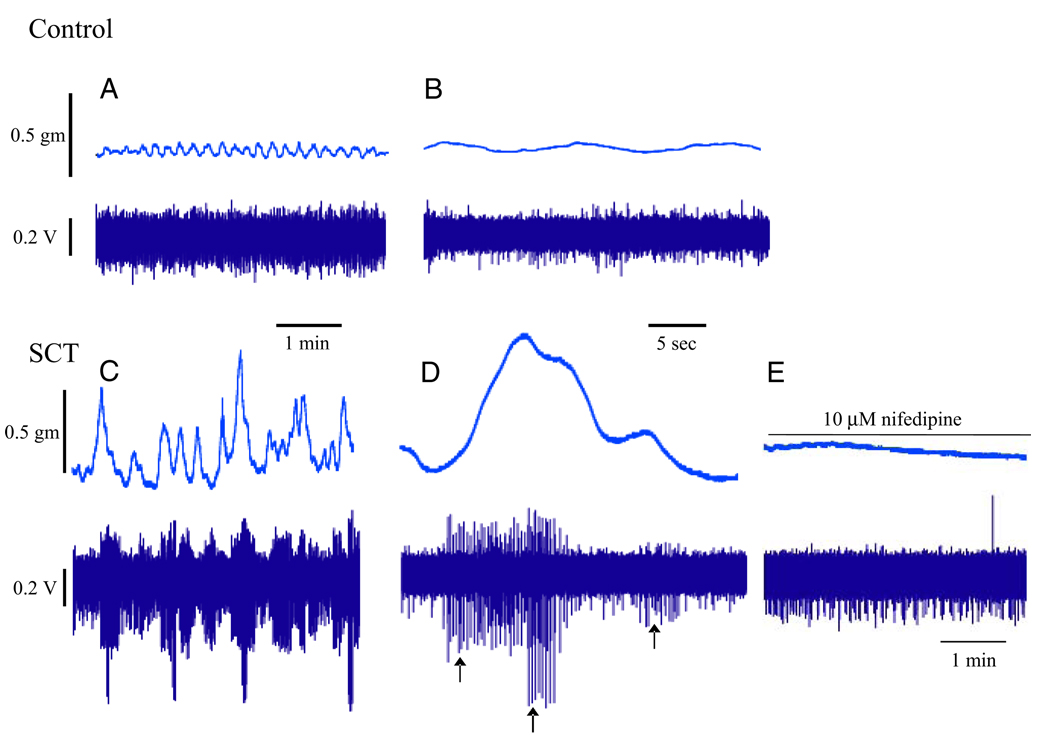
A characteristic of the bladder is its ability to fill with only a marginal increase in intravesical pressure. There is normally no parasympathetic outflow from the spinal cord during filling12 but the bladder maintains tone and shows nonsynchronized local contractions and relaxations.1,16 This is believed to be caused by myogenic contractile activity, which may be reinforced by mediator release from nonneuronal (urothelium, lamina propria and other structures) and neuronal sources, as described. The spontaneous contractile, phasic activity of detrusor smooth muscle during filling may generate afferent input (afferent noise) and in pathological conditions, eg OAB/DO, may contribute to these disorders.17 However, the relations between the contractile activity of individual myocytes, the concerted action of several myocytes (postulated functional units of smooth muscle cells),3 and the generation of afferent nerve activity in normal and diseased bladders remain largely to be established. McCarthy et al provided new information on the correlation between afferent nerve firing and corresponding spontaneous bladder contractions.13 Using optical mapping of Ca2+ transients, tension measurement and single unit afferent fiber recording they noted high frequency, low amplitude spontaneous contractions emanating from multiple sites in normal bladders. These small contractions did not generate any distinct afferent activity. In contrast, in the bladder of spinal cord transected animals large amplitude, spontaneous contractions evoked afferent nerve activity that was sensitive to nifedipine (fig. 3).
Figure 3.
Simultaneous recordings show pathological bladder spontaneous contractile activity and corresponding afferent firing.13 A, normal adult. B, normal adult expanded time base. C, T8-T9 level spinal cord transected (SCT) mouse bladder. D, SCT mouse bladder expanded time base. Arrows indicate action potential of different amplitudes recorded from 3 fibers that responded to spontaneous contraction. E, effect of 10 µM nifedipine on spontaneous contractions and afferent firing in SCT mouse bladder. Reprinted with permission from Elsevier.
Yu and de Groat studied the influence of spontaneous contractions on afferent excitability in normal and cyclophosphamide treated animals in an in vitro whole rat bladder pelvic nerve preparation and multiunit recording.18 In preparations from normal animals bladder distention resulted in phasic and tonic afferent activity. Phasic firing mirrored spontaneous contractions while tonic activity increased as a function of stretch, a consequence of fiber recruitment in multiunit recordings. Cyclophosphamide induced cystitis increased bladder afferent nerve activity, which mimicked the sensitizing effect of purinergic agonists. These effects were also decreased by the purinergic receptor antagonists (2′-(or 3′)-O-(trinitrophyl)-ATP and pyridoxalphosphate-6-azophenyl-2′4′-disulfonic acid, suggesting that purinergic mechanisms contribute to the afferent sensitization induced by chemical cystitis.
The normal stimulus for micturition reflex activation is considered bladder distention, initiating activity in in-series coupled low threshold mechano-receptive afferents. However, several functionally distinct classes of bladder sensory neurons can be identified,14 including muscle-mucosal mechanoreceptors, mucosal mechanoreceptors and chemoreceptors, via which stimuli applied to the urothelium can initiate afferent signals.
Evidence from ice water cystometry, which elicits a C-fiber dependent spinal micturition reflex, suggests considerable C-fiber up-regulation in symptomatic patients with neurogenic DO19 and bladder outlet obstruction.20 Of patients with bladder outlet obstruction and a positive ice water test the DO rate was significantly greater in those who reported nocturia 3 times or more than in those who reported fewer episodes.20 The contribution of afferent hyper-excitability to the emergence of DO and OAB symptoms was also identified in clinical studies using neurotoxins such as botulinum toxin and RTX. For example, suppression of bladder afferent activity with botulinum toxin, which in essence produces chemical denervation, effectively treats DO and mitigates urgency in neurogenic and idiopathic DO cases while sustained therapy decreases expression of the capsaicin receptor TRPV1 and the ATP receptor P2X3 in C fibers.21
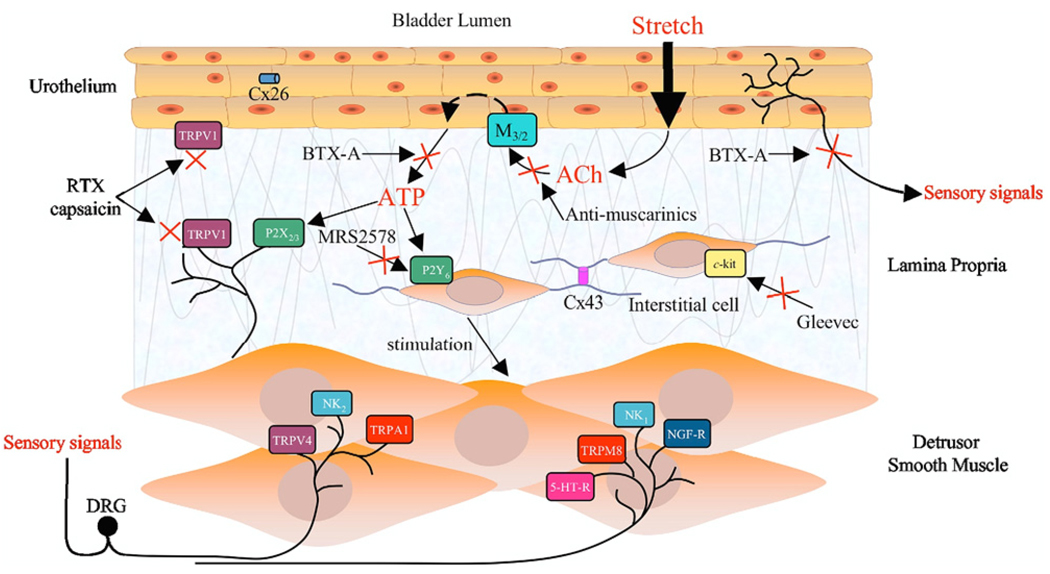
In patients with spinal cord injury induced DO the clinical response to intravesical therapy with the C-fiber toxin RTX led to a marked decrease in nerve fibers positively stained for the neuronal marker PGP9.5 and TRPV1 with improvement on cystometry and other parameters.22 Spinal injured patients who did not respond to RTX showed no decrease in the nerve fiber population, similar to controls. Also, intravesical RTX administered to patients with idiopathic DO delayed or suppressed involuntary detrusor contractions during filling cystometry. Mean time to the first involuntary contraction more than doubled vs baseline at 30 and 90 days, mean maximal cystometric capacity increased, the mean number of urinary incontinence episodes daily decreased to fewer than 1 and mean daily frequency also decreased significantly.23 Overall the hyperexcitability of bladder afferent pathways, especially the C-fiber population, is likely to contribute to the emergence of OAB symptoms. Thus, therapy targeting receptors/ion channels expressed in C fibers could be effective to decrease symptoms in DO/OAB cases (fig. 4).
Figure 4.
Multiple pharmacological targets in bladder may be selected to modulate afferent activity, including receptors on urothelium, interstitial cells and afferent terminals. For example, stretch mediated release of transmitters from urothelium can be inhibited by desensitizing TRPV1 channels using capsaicin or RTX. Interstitial cell activity can be inhibited by blocking P2Y6 receptor activation, eg selective P2Y6 antagonist MRS2578 or c-kit, eg c-kit tyrosine kinase inhibitor Gleevec™. Afferent nerves can be modulated directly by targeting numerous receptors, such as those shown. Clinical studies also showed efficacy of compounds such as botulinum toxin A (BTX-A) on sensory symptoms indicating an action on afferent nerves.
SPONTANEOUS BLADDER CONTRACTIONS AND AFFERENT FIRING
It is well established that in the bladder of many species, including humans, there is rhythmic activity during the filling phase. Such activity is generated in the bladder wall and described as spontaneous or autonomous activity.16 Spontaneous bladder contractions decrease in frequency but increase in magnitude in a number of lower urinary tract pathologies, including outlet obstruction24 and spinal cord injury.25 Activity is believed to involve contraction waves and localized bladder wall stretch, and may be influenced by several factors, including the myocytes.
Myogenic activity can be defined as the ability of a smooth muscle cell to generate mechanical activity independent of external stimuli.1 In the individual myocyte contractile activity is preceded and initiated by an action potential that is calcium driven.26 The detrusor muscle may be arranged into units (modules), which are circumscribed muscle areas.3 These modules show contractile activity during the filling phase of the micturition cycle and may be controlled by several factors, including a peripheral myovesical plexus consisting of intramural ganglia and ICC.16 Intercellular connections may contribute to module control but also locally generated mediators. Detrusor smooth muscle cells are electrically coupled via gap junctions.27 The human detrusor expresses connexin 45 rather than connexin 43 and in, eg bladder outlet obstruction, connexin 45 protein expression patterns were focally altered.28 Kinder and Mundy found that spontaneous contractile activity developed more often in muscle strips from overactive than normal bladders,29 a finding underlined by Brading24 and confirmed by Mills et al.30 Brading discussed patchy denervation in DO cases with subsequent changes in smooth muscle cells, eg supersensitivity to acetylcholine.24 Such increased sensitivity was noted in smooth muscle preparations from patients with idiopathic and neurogenic DO.31 Suburothelial ICC respond to purinergic stimulation by firing Ca2+ transients.32 These suburothelial ICC may affect detrusor myocyte activity.33
The incidence of spontaneous rhythmic contractions in isolated detrusor smooth muscle preparations seems to vary among species and probably also depends on experimental factors.1,16 Characteristically these contractions are resistant to the Na channel blocker tetrodotoxin and cannot be blocked by hexamethonium, atropine, α or β-adrenoceptor blockers, or suramin, apparently excluding direct involvement by nerves and nerve released transmitters.1 Contractions can be effectively inhibited by L-type Ca2+ channel blockers, K+ channel openers and agents that decrease K+ permeability, supporting the important role of L-type Ca2+ channels for the activity.
UROTHELIUM GENERATED SIGNALS
Much evidence suggests that urothelium together with suburothelial ICC (myofibroblasts) may serve as a mechanosensor.34 Urothelial cells respond to distention, low pH, high K+, increased osmolality and chemical irritation by generating and releasing agents. These agents can directly or indirectly act via ICC to influence activity in afferent nerves and, thus, initiation of the micturition reflex.2 Released agents include acetylcholine, which acts via nicotinic and muscarinic receptors; ATP, which acts via receptors for P2X and P2Y; noradrenaline, which acts via α and β-adrenoceptors; and bradykinin.2
Urothelium also contains Na+ channels and a number of TRP channels, eg TRPA1, TRPV1, 2 and 4, and TRPM8.2,35 For example, intravesical ATP induces DO in conscious rats,36 as does removal of urothelial nitric oxide by intravesical oxyhemoglobin.37 Mice lacking P2X3 receptor have a hypoactive bladder.38 Liu et al noted P2X3 receptors on suburothelial myofibroblasts in the human bladder but not on any suburothelial afferent nerve fibers containing calcitonin gene related peptide, substance P and isolectin B4.39 They speculated that suburothelial myofibroblasts may be an important intermediate for processing ATP mediated activation of afferent nerves. TRPV1 knockout mice showed decreased nitric oxide and ATP release from urothelial cells,40 and decreased bladder overactivity in response to bladder irritation.41
There also seem to be other urothelial factors, unidentified to date, that may influence bladder function.42 Fovaeus et al found a previously unrecognized nonadrenergic, nonnitrergic, nonprostanoid inhibitory mediator that is released from the rat bladder by muscarinic receptor stimulation.43 However, it was not clear whether this factor came from the detrusor muscle or from the bladder and the urothelium. Hawthorn et al reported data suggesting the presence of a diffusible, urothelium derived inhibitory factor that could not be identified.44 To our knowledge the identity and possible physiological role of this unknown factor remains to be established and should offer an interesting field for further research.
All 5 muscarinic (M1 through M5) receptor subtypes and various nicotinic receptor subunits have been identified in rat,45 mouse46 and human47 urothelium. While there is some species diversity in nicotinic receptor subunits, the net effect of nicotinic stimulation on rat bladder cystometry was to increase the intercontractile interval by the release of yet to be determined factors from the urothelium. The role of urothelium was verified by its disruption with protamine sulfate, which abolished the decrease in the intercontractile interval induced by nicotine. The factors released may act on afferents to decrease excitability and/or on smooth muscle to promote relaxation. Thus, they may offer potential therapeutic targets for treating detrusor overactivity.
As mentioned previously, a network of ICC extensively linked by connexin 43 containing gap junctions is beneath the urothelium in the bladder.33,48 This cellular network was suggested to operate as a functional syncytium connected by gap junctions (connexin 43). These cells are believed to integrate signals and responses in the bladder wall. Increased connexin 43 expression was found in suburothelial myofibroblasts in patients with DO. Adherens junctions (also referred to as zonulae adherens), multiprotein complexes formed by cadherins and catenins were also noted in the normal human bladder.49 Cadherin-catenin coupling is required for complete interaction between cadherins and the actin based cytoskeleton, and there is evidence of cadherin mediated suburothelial myofibro-blast cell-cell interaction.49 Supporting an important role in bladder pathophysiology, Roosen et al found that cadherin-11 was up-regulated in suburothelial myofibroblasts in patients with overactive bladder.50 They suggested that this may be significant in DO/OAB pathogenesis.
CONCLUSIONS
Current research on LUT physiology focusing on afferent mechanisms initiated by urothelial and myogenic pathways has revealed a number of peripheral mechanisms involved in the regulation of normal and dysfunctional micturition. Some mechanisms may be realistic targets for drugs but signals in preclinical models should be critically analyzed, keeping in mind the large number of steps between a preclinical signal and a drug candidate.
Abbreviations And Acronyms
- ATP
adenosine triphosphate
- DO
detrusor overactivity
- DRG
dorsal root ganglia
- ICC
interstitial cells of Cajal
- LUT
lower urinary tract
- OAB
overactive bladder
- RTX
resiniferatoxin
- TRP
transient receptor potential
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 2.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake MJ, Mills IW, Gillespie JI. Model of peripheral autonomous modules and a myovesical plexus in normal and overactive bladder function. Lancet. 2001;358:401. doi: 10.1016/s0140-6736(01)05549-0. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura N, Kaiho Y, Miyazato M, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmied Arch Pharmacol. 2008;377:437. doi: 10.1007/s00210-007-0209-z. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi Y, Tomoyoshi T, Fujimiya M, et al. Substance P-containing axon terminals in the mucosa of the human urinary bladder: pre-embedding immunohistochemistry using cryostat sections for electron microscopy. Histochemistry. 1993;100:401. doi: 10.1007/BF00267819. [DOI] [PubMed] [Google Scholar]
- 6.Morrison J. The activation of bladder wall afferent nerves. Exp Physiol. 1999;84:131. doi: 10.1111/j.1469-445x.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 7.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson KE. LUTS treatment: future treatment options. Neurourol Urodyn. 2007;26:934. doi: 10.1002/nau.20500. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura N, Seki S, Erickson KA, et al. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci. 2003;23:4355. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea VK, Cai R, Crepps B, et al. Sensory fibers of the pelvic nerve innervating the Rat’s urinary bladder. J Neurophysiol. 2000;84:1924. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 12.de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50:36. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy CJ, Zabbarova IV, Brumovsky PR, et al. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J Urol. 2009;181:1459. doi: 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zagorodnyuk VP, Gibbins IL, Costa M, et al. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janig W, Koltzenburg M. Pain arising from the urogenital tract. In: Chur C Maggi., editor. Nervous Control of the Urogenital System. Switzerland: Harwood Academic; 1993. pp. 525–578. [Google Scholar]
- 16.Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int. 2004;93:478. doi: 10.1111/j.1464-410x.2003.04667.x. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie JI, van Koeveringe GA, de Wachter SG, et al. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int. 2009;103:1324. doi: 10.1111/j.1464-410X.2009.08377.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder—pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geirsson G, Lindstrom S, Fall M. The bladder cooling reflex and the use of cooling as stimulus to the lower urinary tract. J Urol. 1999;162:1890. doi: 10.1016/S0022-5347(05)68062-7. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama A, Fujimoto K, Matsumoto Y, et al. Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology. 2003;62:909. doi: 10.1016/s0090-4295(03)00588-0. [DOI] [PubMed] [Google Scholar]
- 21.Apostolidis A, Brady CM, Yiangou Y, et al. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Brady CM, Apostolidis AN, Harper M, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93:770. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- 23.Silva C, Ribeiro MJ, Cruz F. The effect of intravesical resiniferatoxin in patients with idiopathic detrusor instability suggests that involuntary detrusor contractions are triggered by C-fiber input. J Urol. 2002;168:575. [PubMed] [Google Scholar]
- 24.Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol Renal Physiol. 2008;295:F454. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John H, Wang X, Wehrli E, et al. Evidence of gap junctions in the stable nonobstructed human bladder. J Urol. 2003;169:745. doi: 10.1097/01.ju.0000045140.86986.a7. [DOI] [PubMed] [Google Scholar]
- 28.John H, Walch M, Lehmann T, et al. Connexin45 expression in the human obstructed detrusor muscle. World J Urol. 2009;27:411. doi: 10.1007/s00345-008-0365-x. [DOI] [PubMed] [Google Scholar]
- 29.Kinder RB, Mundy AR. Pathophysiology of idiopathic detrusor instability and detrusor hyper-reflexia. An in vitro study of human detrusor muscle. Br J Urol. 1987;60:509. doi: 10.1111/j.1464-410x.1987.tb05031.x. [DOI] [PubMed] [Google Scholar]
- 30.Mills IW, Greenland JE, McMurray G, et al. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol. 2000;163:646. doi: 10.1016/s0022-5347(05)67951-7. [DOI] [PubMed] [Google Scholar]
- 31.Stevens LA, Chapple CR, Chess-Williams R. Human idiopathic and neurogenic overactive bladders and the role of M2 muscarinic receptors in contraction. Eur Urol. 2007;52:531. doi: 10.1016/j.eururo.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol. 2004;559:231. doi: 10.1113/jphysiol.2004.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui GP, Wu C, Roosen A, et al. Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol. 2008;295:F688. doi: 10.1152/ajprenal.00133.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry CH, Sui GP, Kanai AJ, et al. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26:914. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- 35.Du S, Araki I, Kobayashi H, et al. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology. 2008;72:450. doi: 10.1016/j.urology.2007.11.127. [DOI] [PubMed] [Google Scholar]
- 36.Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168:1230. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- 37.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder over-activity in conscious, normal rats. J Urol. 2000;164:545. [PubMed] [Google Scholar]
- 38.Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Takahashi N, Yamaguchi O. Expression of P2X3 purinoceptors in suburothelial myofibro-blasts of the normal human urinary bladder. Int J Urol. 2009;16:570. doi: 10.1111/j.1442-2042.2009.02307.x. [DOI] [PubMed] [Google Scholar]
- 40.Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 41.Charrua A, Cruz CD, Cruz F, et al. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 42.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 43.Fovaeus M, Fujiwara M, Hogestatt ED, et al. A non-nitrergic smooth muscle relaxant factor released from rat urinary bladder by muscarinic receptor stimulation. J Urol. 1999;161:649. [PubMed] [Google Scholar]
- 44.Hawthorn MH, Chapple CR, Cock M, et al. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckel JM, Kanai A, Lee SJ, et al. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarghooni S, Wunsch J, Bodenbenner M, et al. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci. 2007;80:2308. doi: 10.1016/j.lfs.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 47.Bschleipfer T, Schukowski K, Weidner W, et al. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci. 2007;80:2303. doi: 10.1016/j.lfs.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 48.Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urinary tract—an assessment of current knowledge. Nat Clin Pract Urol. 2005;2:546. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- 49.Kuijpers KA, Heesakkers JP, Jansen CF, et al. Cadherin-11 is expressed in detrusor smooth muscle cells and myofibroblasts of normal human bladder. Eur Urol. 2007;52:1213. doi: 10.1016/j.eururo.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 50.Roosen A, Apostolidis A, Elneil S, et al. Cadherin-11 up-regulation in overactive bladder suburothelial myofibroblasts. J Urol. 2009;182:190. doi: 10.1016/j.juro.2009.02.148. [DOI] [PubMed] [Google Scholar]