Abstract
The parathyroid hormone receptor (PTH1R) is a class B G protein-coupled receptor that is activated by parathyroid hormone (PTH) and PTH-related protein (PTHrP). Little is known about the oligomeric state of the receptor and its regulation by hormone. The crystal structure of the ligand-free PTH1R extracellular domain (ECD) reveals an unexpected dimer in which the C-terminal segment of both ECD protomers forms an α-helix that mimics PTH/PTHrP by occupying the peptide binding groove of the opposing protomer. ECD-mediated oligomerization of intact PTH1R was confirmed in living cells by bioluminescence and fluorescence resonance energy transfer experiments. As predicted by the structure, PTH binding disrupted receptor oligomerization. A receptor rendered monomeric by mutations in the ECD retained wild-type PTH binding and cAMP signaling ability. Our results are consistent with the hypothesis that PTH1R forms constitutive dimers that are dissociated by ligand binding and that monomeric PTH1R is capable of activating G protein.
Keywords: G Protein-coupled Receptors (GPCR), Membrane Proteins, Peptide Hormones, Peptide Interactions, Protein Structure, Osteoporosis
Introduction
G protein-coupled receptors (GPCRs)3 constitute the largest family of cell-surface signal transduction proteins in the human genome. They function as plasma membrane-embedded receptors for ligands such as biogenic amines, peptides, and protein hormones. The receptor transduces the hormone binding signals across the membrane to heterotrimeric G proteins. The oligomeric state of the receptor and the stoichiometry of the receptor-G protein complex is a topic of considerable debate (1–4). Many GPCRs from each of three major classes (A, B, and C) have been shown to homo-oligomerize, and hetero-oligomerization of structurally related members of the same class has also been demonstrated (2, 3, 5). In many cases the specific oligomeric state of the receptor is unclear. It often is assumed to be the dimer, although evidence for higher order arrays of receptors also exists (6–8). Ligand effects on oligomerization can vary from no effect (9) to ligand-induced oligomerization (10) or ligand-induced disruption of oligomerization (11). The receptor domains responsible for oligomerization can vary from individual helices of the 7-transmembrane (7-TM) helical domain (12) to extracellular (13) or intracellular domains (14). The most convincing argument for a functional role for oligomerization comes from studies of the class C γ-aminobutyric acid, type B receptor (14–16) and the sweet and umami taste receptors (17), which function as obligate heterodimers. In addition, the class A leukotriene B4 and dopamine D2 receptors have been shown to couple to G protein as dimers (18, 19). These studies suggested that the pentameric complex of a GPCR dimer and a single G protein heterotrimer is the minimal functional signaling unit. However, several rigorous biochemical studies have shown that the β2-adrenergic receptor and rhodopsin, both class A receptors, are capable of functionally coupling to G proteins as monomers (20–23) even though oligomerization of these receptors is well documented (6, 8, 24). Perhaps the only recurring theme to emerge from these studies is that, whereas GPCRs oligomerize, the receptor domains that mediate oligomerization, the ligand effects on oligomerization, and the functional consequences of oligomerization vary depending on the receptor family or even on the individual subtype of the receptor.
The class B or secretin family GPCRs constitute a small peptide binding family that includes the receptors for secretin, parathyroid hormone, calcitonin, glucagon, corticotropin-releasing factor, and several other clinically important peptides. Several class B GPCRs have been shown to homo-oligomerize, including the secretin receptor (SecR) (12, 25), vasoactive intestinal polypeptide receptors (9), calcitonin receptor (26), corticotropin releasing factor receptor (27), and the pituitary adenylate cyclase activating polypeptide receptor (28); several of the members can also form hetero-oligomers (29). Class B GPCRs consist of an N-terminal extracellular domain (ECD) of roughly 160 amino acids in addition to the 7-TM domain. Ligand binding is characterized by a two-domain model whereby the C-terminal portion of the peptide interacts with the ECD to impart high affinity receptor binding and the N-terminal portion interacts with the 7-TM domain and is responsible for receptor activation (30). Class B GPCRs primarily signal through the stimulatory Gs protein to increase intracellular cAMP, but activation of other pathways can also occur. Of the class B receptors, SecR oligomerization has been most extensively characterized using bioluminescence (BRET) and fluorescence resonance energy transfer (FRET) techniques that enable the study of receptor oligomerization in living cells. The SecR forms homodimers (31) that are mediated by the lipid-exposed face of transmembrane helix IV (12) and secretin binding does not affect the dimeric status (9). Competitive disruption of SecR dimerization by a synthetic helix IV peptide reduced secretin-stimulated cAMP accumulation, suggesting that the dimer activates Gs better than the monomer (12). It is unclear if the SecR findings will be consistent for other members of the family, but if GPCRs from class A provide any guide, it seems likely that diverse mechanisms of receptor oligomerization will be observed in the class B GPCRs.
The parathyroid hormone receptor (PTH1R) is a class B GPCR that mediates the actions of parathyroid hormone (PTH) and PTH-related protein (PTHrP), two peptides that have distinct biological actions (32, 33). PTH is an endocrine hormone that is produced by the parathyroid glands and secreted into the circulation in response to low calcium levels; it maintains calcium and phosphate homeostasis by activating PTH1R expressed in bone and kidney tissues (34). Sustained elevation of PTH in the circulation restores calcium levels by stimulating catabolic bone resorption and reabsorption of calcium, inhibition of phosphate reabsorption, and synthesis of 1,25-dihydroxyvitamin D3 in the kidneys. Paradoxically, PTH also has anabolic effects on bone via a direct effect on osteoblasts when administered intermittently to osteoporosis patients, providing the basis for its clinical use to treat osteoporosis (35). PTHrP was originally isolated as the factor secreted by various tumors that caused humoral hypercalcemia of malignancy (36) and was subsequently shown to be a locally produced paracrine factor that is a critical regulator of bone development (37, 38). PTHrP also has anabolic effects on bone when administered to osteoporosis patients (39). A detailed structural and mechanistic understanding of the receptor activation and G protein-coupling mechanisms is critical for elucidating how PTH and PTHrP elicit their distinct functions via a common receptor and for aiding the rational development of optimized therapeutics targeting PTH1R. The oligomeric state of PTH1R, the effects of PTH/PTHrP on receptor oligomeric state, and the stoichiometry of the PTH1R-G protein complex remain poorly understood.
We previously devised a method that allowed us to purify the PTH1R ECD and to crystallize and determine structures of the ECD bound to synthetic fragments of PTH and PTHrP (40, 41). These studies each revealed a monomeric PTH1R ECD in a 1:1 complex with the ligand. Structures of several other class B GPCR ECD-ligand complexes have been reported, and in all cases the complexes are monomeric (42–44). Here we present the crystal structure of the PTH1R ECD in the absence of bound ligand. Surprisingly, the ligand-free PTH1R ECD dimerizes by a structural mimicry of endogenous ligand binding mechanism; the ECD C-terminal segment (which connects the ECD core domain to the first transmembrane helix) forms an α-helix that occupies the peptide binding groove of the opposing subunit, mimicking PTH/PTHrP binding. We confirmed ECD-mediated oligomerization of intact PTH1R in living cells by BRET and FRET experiments and we show that, as predicted by the structure, PTH disrupted the oligomerization of PTH1R. Guided by the structure, we made specific amino acid substitutions in the ECD designed to disrupt the dimer, and we show that one of these altered receptors failed to oligomerize but retained wild-type PTH binding and cAMP signaling ability, suggesting that a PTH1R monomer is capable of functionally coupling to Gs.
EXPERIMENTAL PROCEDURES
Protein Purification and Peptide Synthesis
The maltose-binding protein (MBP)-PTH1R ECD-His6 fusion protein, containing residues 29–187 of human PTH1R fused to the C terminus of bacterial MBP, was previously described (41). The protein is not glycosylated because it is expressed in Escherichia coli. Peptides were custom-synthesized and high performance liquid chromatography-purified by SynBioSci (Livermore, CA).
Crystallization, Data Collection, Structure Solution, and Refinement
MBP-PTH1R ECD-H6 in 10 mm Tris-HCl, pH 7.5, 1 mm maltose, and 1 mm EDTA was spin-concentrated to 18.7 mg/ml (about 310 μm) for crystallization. Rectangular, rod-shaped crystals were grown by the hanging drop vapor diffusion method at 20 °C with drops containing equal volumes of the protein sample and a reservoir solution of 2 m ammonium sulfate, 0.1 m sodium HEPES, pH 7.5, 20 mm sodium acetate, 2% polyethylene glycol 400, and 3% cadaverine dihydrochloride. The crystals were transferred into a cryoprotectant solution of 3 m sodium malonate, pH 7.0, 0.1 m ammonium sulfate, 2% polyethylene glycol 400, and 0.7% cadaverine dihydrochloride by quick soak and then were flash-frozen in liquid nitrogen. A data set was collected from a single crystal at LS-CAT beamline 21-ID-F of the Advanced Photon Source (Argonne, IL) at a wavelength of 0.9785 Å and a temperature of 100 K. The HKL2000 package (45) was used to process the data, and the Scalepack intensities were converted to structure factor amplitudes with the CCP4 suite (46). The structure was solved by molecular replacement with Phaser (47) using separate search models for MBP and the PTH1R ECD from PDB coordinate file 3C4M (41). There are two MBP-PTH1R ECD molecules in the asymmetric unit that compose the dimer. Manual rebuilding in O (48) and restrained refinement with Refmac5 (49) were used to complete the structure. Tight NCS restraints were maintained throughout the refinement. TLS refinement (50) was included using one TLS group corresponding to the contents of the asymmetric unit. Structure validation with Procheck (51) indicated that 92.1% of the residues were in the most favored region of the Ramachandran plot, 7.7% were in the additional allowed region, 0.2% were in the generously allowed region, and no residues were in the disallowed region. The data collection and refinement statistics are listed in Table 1. Analysis of solvent-accessible surface area was performed within the CCP4 suite. Structure figures were prepared with PyMol (52).
TABLE 1.
Data collection and refinement statistics
| MBP-PTH1R ECDa | |
|---|---|
| Data collection | |
| Space group | C2221 |
| Cell dimensions | |
| a, b, c (Å) | 136.70, 182.33, 97.17 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| Resolution (Å) | 50.00-3.24 (3.36-3.24)b |
| Rmerge | 0.110 (0.493) |
| I /σI | 12.59 (1.94) |
| Completeness (%) | 99.8 (98.5) |
| Redundancy | 4.0 (3.5) |
| Refinement | |
| Resolution (Å) | 50.00-3.24 |
| No. reflections | 18,632 |
| Rwork/Rfree | 22.5/25.8 |
| No. atoms | |
| Protein | 7652 |
| Ligand/ion | 46 |
| Water | 0 |
| Mean B-factors | |
| MBP MOL A | 82.2 |
| MBP MOL B | 84.7 |
| PTH1R ECD MOL A | 67.7 |
| PTH1R ECD MOL B | 66.5 |
| Maltose | 67.9 |
| Root mean square deviations | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.078 |
aA single crystal was used.
b Values in parentheses are for the highest resolution shell.
Renilla Luciferase (Rlu)-, Yellow Fluorescent Protein (YFP)-, and Cyan Fluorescent Protein (CFP)-tagged PTH1R Constructs
The expression plasmids were constructed starting with a pcDNA3.1 plasmid encoding human PTH1R obtained from the Missouri S&T cDNA Resource Center. PTH1R constructs fused in-frame at their C terminus with Rlu, YFP, or CFP were prepared by cloning the respective Rlu, YFP, or CFP fragment into the Nhe1/Xba1 restriction sites before the TGA stop codon. Site-directed mutagenesis was performed using a QuikChange kit (Stratagene) according to the manufacturer's instructions. All constructs were verified by automated DNA sequencing.
Cell Culture
COS-1 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum in a 37 °C, 5% CO2 incubator. For transient transfections, 500,000 cells were seeded in a 10-cm dish and transfected the following morning by the DEAE-dextran method with 3 μg of the indicated PTH1R expression construct.
BRET Assays
Bioluminescence and fluorescence measurements were performed with ∼25,000 receptor-bearing COS cells in suspension as described previously (12). In brief, 48 h after transfection, the receptor-bearing COS cells were lifted using nonenzymatic cell dissociation solution (Sigma) and washed with Krebs-Ringers-HEPES buffer (25 mm HEPES, pH 7.4, 104 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm KH2PO4, 1.2 mm MgSO4) before the assay. The BRET assay was started by adding 5 μm coelenterazine h, the cell-permeant Renilla luciferase-specific substrate, to the cell suspension in a 96-well white Optiplate. The BRET signal was acquired by using the dedicated BRET protocol (emission filter sets for luminescence (460 nm, bandwidth 25 nm) and for fluorescence (535 nm, bandwidth 25 nm) with a mirror (<700 nm)) in the 2103 Envision fluorescence plate reader (PerkinElmer Life Sciences). The BRET ratio was calculated based on the ratio of emission as described previously (12). For saturation BRET assays, COS cells were transiently co-transfected with a fixed amount of Rlu-tagged wild-type or mutant receptor constructs (1.0 μg DNA/dish) and with increasing amounts of YFP-tagged wild-type or mutant receptor constructs (0.3–6 μg of DNA/dish). Forty-eight hours after transfection, cells were detached using cell dissociation medium and were used in BRET assays, as described above. Curves were fit to these data and evaluated for quality of fit based on R2 values using Prism 3.0.
Morphological FRET
FRET microscopy was performed as described previously (12). COS cells were transfected with CFP- and YFP-tagged PTH1 receptor constructs. Imaging was performed with an Axiovert 200M inverted epifluorescence microscope (Carl Zeiss, Thornwood, NY) equipped with fixed filter sets for CFP (excitation, 436/20 nm; dichroic mirror, 455 dichroic long pass; emission, 480/40 nm), YFP (excitation, 500/20 nm; dichroic mirror, 515 dichroic long pass; emission, 535/30 nm), and FRET (excitation, 436/20 nm; dichroic mirror, 455 dichroic long pass; emission, 535/30 nm) (Chroma Technology Corp., Brattleboro, VT). Images were collected after fixed-length exposures with an ORCA-12ER CCD camera (Hamamatsu, Bridgewater, NJ) and QED-InVivo 2.039 software (Media Cybernetics, Inc., Silver Springs, MD). The FRET analysis was performed using the sensitized emission method in Metamorph Version 6.32 (Molecular Devices, Sunnyvale, CA) after correcting for donor and acceptor bleed-through into the FRET channel, as described previously (12). Publication images were prepared using Adobe Photoshop Version 7.0 (Adobe Systems, Mountain View, CA).
Whole Cell Radioligand Binding Assay
Approximately 50,000 transfected cells were seeded in each well of a 24-well plate 24 h post-transfection. At 72 h post-transfection the cells were rinsed twice with binding buffer (50 mm Tris-HCl, pH 7.7, 100 mm NaCl, 5 mm KCl, 2 mm CaCl2, 5% (v/v) heat-inactivated horse serum, 0.5% (v/v) heat-inactivated fetal bovine serum, 0.01% soybean trypsin inhibitor). 125I-[Nle8,18,Tyr34]hPTH-(1–34)OH (PerkinElmer Life Sciences) was added to ∼20,000 cpm per well (∼20 pm) in the absence or presence of varying amounts of PTH-(1–34)NH2 competitor in binding buffer and incubated for 1 h at room temperature. The binding mixture was removed by aspiration, and the cells were rinsed twice with ice-cold binding buffer and then lysed with 0.5 ml of 0.5 m NaOH. Radioactivity in the cell lysates was quantified by liquid scintillation counting in UltimaGold mixture (PerkinElmer Life Sciences) with a Microbeta Trilux LSC. Curve-fitting was performed with Prism 5.0 software (Graph Pad Software, San Diego, CA) using a one-site competitive binding equation to fit the log IC50. Bound radioactivity was typically less than 10% of the total counts added.
cAMP Assay
Approximately 40,000 transfected cells were seeded in each well of a 96-well plate 24 h post-transfection. At 48 h post-transfection the cells were rinsed twice with phosphate-buffered saline, pH 7.4, and stimulated for 30 min at 37 °C with PTH-(1–34)NH2 in Krebs-Ringers-HEPES buffer supplemented with 0.2% bovine serum albumin, 0.01% soybean trypsin inhibitor, 0.1% bacitracin, and 2 mm 3-isobutyl-1-methylxanthine. The reactions were stopped with ice-cold 6% perchloric acid, and the cell lysates were neutralized with KHCO3. cAMP was measured with a LANCE cAMP kit (PerkinElmer Life Sciences) according to the manufacturer's instructions. Curve-fitting was performed with Prism 5.0 software using a fixed-slope dose-response stimulation equation.
RESULTS
The PTH1R ECD Crystallizes as a Dimer in the Absence of Ligand
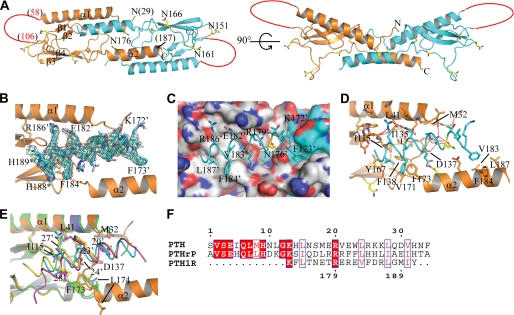
The human PTH1R ECD (residues 29–187) was prepared as a fusion protein with an N-terminal MBP tag and a C-terminal His6 tag. The fusion protein was crystallized in the absence of ligand in the C2221 space group, and the structure was solved by molecular replacement and refined to a Rwork value of 22.5% (Rfree of 25.8%) at 3.24 Å resolution (Table 1). The crystallographic refinement was aided by the availability of the high resolution MBP and PTH1R ECD models used for molecular replacement. The low B-factors (Table 1) and excellent Ramachandran plot statistics (“Experimental Procedures”) attest to the high quality of the refined model. Surprisingly, the structure revealed an ECD dimer in which the C-terminal segment (residues 173–187) forms an α-helix (α2) that occupies the peptide binding site of the opposing subunit (Fig. 1A). In our previous structures of the ECD in complex with PTH or PTHrP, the ECD was monomeric, and residues 176–187 were disordered (40, 41). In the current ligand-free structure, clear electron density was observed for the C-terminal α-helix and the first two residues of the His6 tag (Fig. 1B). The remainder of the ECD exhibits the same tertiary fold observed in our previous structures, including the long, N-terminal α-helix (α1), four β-strands, and three conserved disulfide bonds.
FIGURE 1.
Structure of the PTH1R ECD in the absence of ligand and comparison with PTH- and PTHrP-bound ECD structures. A, two views of the “ligand-free” PTH1R ECD dimer are shown. Subunit A of the dimer is colored orange, and subunit B is colored cyan. The four consensus N-linked glycosylation sites are shown in stick representation and colored yellow. The red lines represent the disordered loop (residues 59–105) that connect α1 and β1. MBP is not shown for clarity. B, omit electron density maps were calculated with the C-terminal α-helix (α2) of the ECD removed from the model. The 2Fo − Fc map is shown as a blue mesh contoured at 1 σ, and the Fo − Fc map is shown as a green mesh contoured at 3 σ. Subunit B α2 is shown in stick representation. The last two residues modeled are the first two histidine residues of the His6 tag, denoted H188* and H189*. C, shown is the molecular surface of subunit A showing the hydrophobic groove that forms the base of the peptide binding site. Carbon atoms are colored gray, oxygen atoms are red, and nitrogen atoms are blue. Subunit B α2 is shown as a cyan coil, and the remainder of subunit B is shown as a molecular surface with carbon atoms colored cyan. D, shown is a detailed view of the interaction between subunit B α2 and the subunit A ECD. α2 is shown as a cyan coil with selected side chains shown as sticks. Hydrogen bonds are depicted as red dashes. E, shown is structural alignment of the subunit A ECD-subunit B α2 complex from the ligand-free dimer structure and the ECD-PTH (PDB code 3C4M) and ECD-PTHrP (PDB code 3H3G) complexes. Subunit B α2 of the ECD dimer is shown as a cyan coil, PTH as a yellow coil, and PTHrP as a magenta coil. The subunit A ECD of the dimer is shown in orange, the PTH-bound ECD is green, and the PTHrP-bound ECD is slate blue. Selected side chains are shown in stick representation. F, amino acid sequence alignment of residues 172–191 of human PTH1R and the 1–34 fragments of human PTH and PTHrP is shown.
The extensive dimer interface buries ∼3440 Å2 of solvent-accessible surface area (ASA) including ∼20% of the total ASA of each subunit. The exceptional quality of the electron density allowed us to confidently discern the molecular interactions responsible for dimerization. The dimerization is primarily mediated by the binding of the C-terminal α2-helix to the peptide binding groove of the opposing subunit (Fig. 1, C and D), but intersubunit interactions involving the N terminus of the ECD and the β3-β4 loop also occur (supplemental Fig. S1). MBP and the His6 tag do not appear to contribute to the dimerization. The C-terminal α2-helix mimics PTH/PTHrP binding to the ECD (Fig. 1, D and E), enabled by sufficient amino acid sequence similarity to the C-terminal, ECD binding portions of PTH and PTHrP (Fig. 1F). Receptor interactions formed by Arg-179 ′ of the C-terminal α2-helix are identical to those formed by Arg-20′ of PTH and PTHrP, including a salt bridge with Asp-137 and a hydrogen bond to the backbone carbonyl of Met-32 (ECD α2, PTH, and PTHrP residues are denoted with a prime to distinguish them from the ECD residues with which they interact). Arg-179′ also forms hydrogen bonds with Asn-176′. Val-183′, and Leu-187′ form hydrophobic interactions with the base of the peptide binding groove, similar to the hydrophobic residues at positions 24′ and 28′ of PTH and PTHrP, and the aliphatic portion of the Arg-186′ side chain makes hydrophobic contact with Ile-115, much like Lys-27′ and Leu-27′ of PTH and PTHrP, respectively (Fig. 1E and supplemental Fig. S2A).
Despite the similarities between the α2-ECD and PTH/PTHrP-ECD complexes, notable differences exist. Trp-23′ and Phe-23′ of PTH and PTHrP, respectively, provide hydrophobic contacts to the ECD that significantly contribute to affinity (40, 41), but α2 has the polar, negatively charged Glu-182′ at the equivalent position (Fig. 1E and supplemental Fig. S2A). The Glu-182′ carboxylate points away from the peptide binding site and forms a hydrogen bond with the guanidino group of Arg-186′, which in turn forms a hydrogen bond with the backbone carbonyl of His-114 (Fig. 1D). Another significant difference is the shifted position of residues 172–174 in the dimer structure. Phe-173 occupies roughly the same position as Leu-174 in the PTH- and PTHrP-bound structures (Fig. 1E and supplemental Fig. S2B), causing the hydrophobic binding groove in the ECD to adopt a slightly different surface topology (Fig. 1C) and enabling hydrophobic contact with Phe-184′ (Fig. 1, C and D). Overall, the differences result in a roughly 1–1.5 Å lateral shift of the α2 helix relative to PTH and PTHrP (Fig. 1E and supplemental Fig. S2, A and B).
PTH1R Forms an ECD-mediated Oligomer in Living Cells That Is Disrupted by Ligand Binding, and Monomeric PTH1R Is Sufficient for G Protein Activation
The structure suggested that ligand-free PTH1R can dimerize via ECD-ECD interactions and predicted that ligand binding can dissociate the dimer. However, it is unclear if the full-length receptor in its native membrane environment could dimerize by the mechanism observed in the structure, given the anti-parallel arrangement of the α2 helices and the constraints it places on possible orientations of the ECD dimer with respect to the membrane. Moreover, the recombinant protein is not glycosylated, and one of the four putative N-linked glycosylation sites, Asn-176′, is buried at the dimer interface (Fig. 1, A, C, and D). This raises the question of whether or not the dimeric structure is an artifact due to crystallization of recombinant ECD in the absence of the 7-TM domain. We, therefore, employed BRET technology to examine oligomerization of full-length PTH1R transiently expressed in COS cells. If PTH1R dimerizes in cells by the mechanism observed in the crystal structure, we predicted that 1) mutations in the ECD α2 helix or in the ECD peptide binding site that are designed to disrupt the α2-ECD interaction should prevent dimerization, and 2) binding of peptide hormone to the ECD should dissociate the dimer.
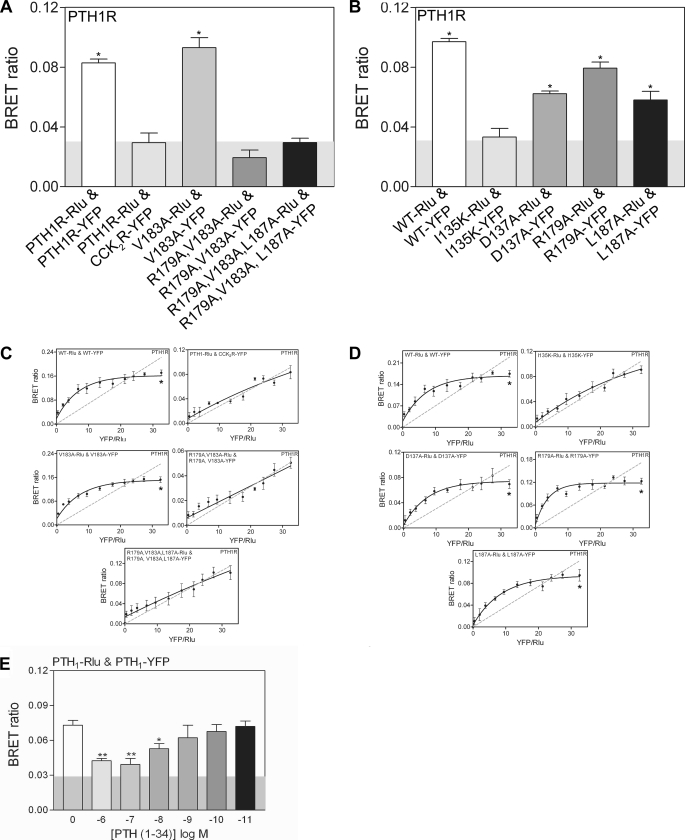
PTH1R tagged with Rlu or YFP at the C terminus exhibited essentially wild-type PTH binding (supplemental Fig. S3A) and cAMP signaling ability (supplemental Fig. S3B), indicating that the tagged constructs were expressed at the cell surface and were functional. Co-expression of PTH1R-Rlu and PTH1R-YFP yielded a BRET ratio significantly higher than the control ratio obtained with PTH1R-Rlu and a structurally unrelated class A GPCR, YFP-tagged cholecystokinin 2 receptor (CCK2R-YFP), indicating homo-oligomerization of PTH1R in living cells (Fig. 2A). To test if an intact ECD α2 helix was required for oligomerization, we assessed the BRET ratio of receptors altered at residues Arg-179, Val-183, and Leu-187, which form the face of the helix that sits in the peptide binding site of the opposing subunit (Fig. 1C). The R179A/V183A/L187A triple mutant and R179A/V183A double mutant receptors elicited significantly reduced BRET signals, not different from the control (background), suggesting the absence of oligomerization. The single mutant V183A, R179A, and L187A receptors retained the ability to oligomerize (Fig. 2, A and B). These results suggested that the face of the ECD α2 helix that mimics PTH/PTHrP binding was required for PTH1R oligomerization in cells, consistent with the crystal structure.
FIGURE 2.
BRET analysis of PTH1R oligomerization in COS-1 cells. A and B, static BRET ratios obtained from cells co-expressing the indicated Rlu- and YFP- tagged PTH receptor constructs. The shaded area represents the nonspecific BRET signal (∼0.03) obtained between Rlu-tagged PTH1 receptor and YFP-tagged CCK2 receptor (CCK2R-YFP). Levels of significance: **, p < 0.01 from wild-type receptor; and data are the means ± S.E. of 4–5 experiments performed in duplicate. C and D, shown are saturation BRET curves for the indicated receptors. E, BRET signals derived from the effect of PTH ligand on the PTH1 receptor homodimer are shown. Data are the means ± S.E. of 4–5 experiments performed in duplicate.
We tested if an intact ECD peptide binding groove was required for oligomerization by assessing the BRET ratio of mutant receptors containing the single D137A or I135K mutations that alter residues at the base of the peptide binding groove (Fig. 1D). The D137A receptor retained oligomerization ability, whereas the I135K receptor gave a BRET ratio similar to the control, suggesting that it failed to oligomerize (Fig. 2B). Hyperbolic saturation BRET curves for the WT, V183A, R179A, L187A, and D137A receptors indicated that their oligomerization was due to a saturable molecular interaction (Fig. 2, C and D). Importantly, saturation BRET experiments with the R179A/V183A/L187A, R179A/V183A, and I135K receptors yielded a straight line similar to the control with cholecystokinin 2 receptor, strongly indicating that these mutants failed to oligomerize (Fig. 2, C and D). All of the altered receptors retained the ability to traffic to the cell surface as qualitatively assessed by fluorescence microscopy of cells expressing the YFP-tagged constructs (supplemental Fig. S4), indicating that the BRET results were not due to variable expression levels. PTH binding decreased the BRET signal of WT PTH1R in a dose-dependent manner (Fig. 2E), suggesting disruption of the oligomerization of PTH1R as predicted by the structure. Taken together, the BRET results presented in Fig. 2 are consistent with the hypothesis that the oligomerization of PTH1R in cells is dimerization that occurs by the PTH/PTHrP mimicry mechanism observed in the crystal structure.
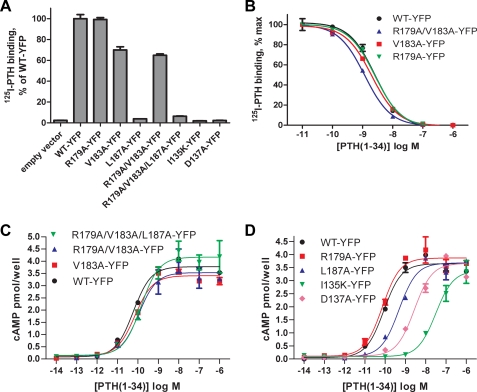
The PTH binding and cAMP signaling ability of the YFP-tagged mutant receptors was assessed to determine functional effects of the mutations. In a whole cell radioligand binding assay, the WT, R179A, V183A, and R179A/V183A receptors exhibited detectable 125I-PTH binding, whereas the L187A, R179A/V183A/L187A, I135K, and D137A receptors did not (Fig. 3A). These results are in agreement with a previous report from Gardella and co-workers (53) that showed that the L187A mutation diminished PTH binding, whereas the V183A mutation did not. The R179A, V183A, and R179A/V183A receptors retained wild-type affinity for PTH as measured by displacement binding curves (Fig. 3B). PTH stimulation of the mutant receptors elicited cAMP levels similar to that of the wild-type receptor, but diminished PTH potency was observed for the D137A, I135K, L187A, and R179A/V183A/L187A receptors (Fig. 3, C and D). The D137A and I135K receptors were the most severely affected, consistent with their decreased PTH binding due to mutation of the ECD peptide binding site. PTH potency was not diminished at the R179A, V183A, and R179A/V183A receptors. Untagged versions of the V183A, R179A/V183A, and R179A/V183A/L187A receptors gave the same results as the YFP-tagged receptors (supplemental Fig. S5A). The R179A/V183A double mutant was the most informative receptor because it did not oligomerize, but retained wild-type PTH binding (Fig. 3B) and wild-type cAMP signaling ability, as measured both by dose-response assay (Fig. 3C and supplemental Fig. S5A) and time course of cAMP accumulation (supplemental Fig. S5B). These results suggest that monomeric PTH1R is sufficient to couple to Gs and activate signaling, consistent with a model where ligand binding dissociates the dimeric receptor.
FIGURE 3.
PTH binding and cAMP signaling ability of wild-type and mutant PTH receptors with alterations in the ECD α2 helix or ECD peptide binding site. A, shown is a whole cell radioligand binding assay assessing the ability of COS-1 cells expressing the indicated constructs to bind 125I-PTH-(1–34). The data represent the mean ± S.E. of triplicate samples. B, shown is displacement binding with cold PTH-(1–34) competitor for the indicated constructs. The data represent the mean ± S.E. of triplicate samples. C and D, shown is a dose-response assay for cAMP accumulation. COS cells expressing the indicated constructs were stimulated with PTH for 30 min at 37 °C. The data represent the mean ± S.E. of duplicate samples. The EC50 values for WT, R179A/V183A/L187A, R179A/V183A, and V183A receptors in C were 60, 144, 109, and 72 pm, respectively. The EC50 values for WT, R179A, L187A, I135K, and D137A receptors in D were 86 pm, 73 pm, 482 pm, 31 nm, and 3 nm, respectively.
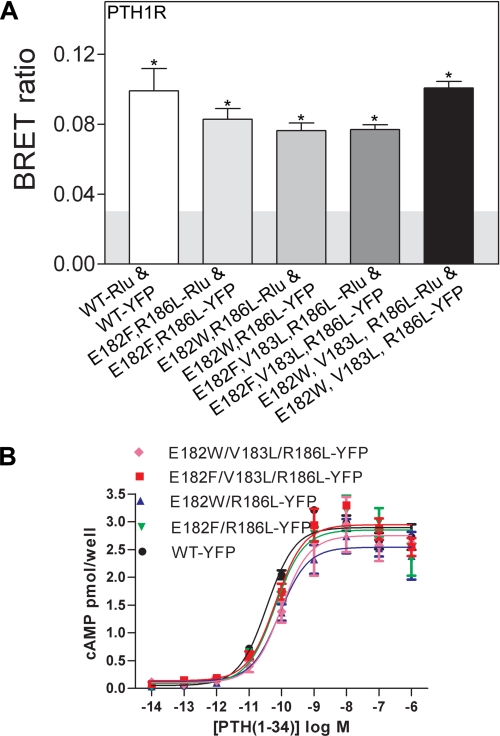
If the oligomerization of PTH1R in cells is truly dependent on the ability of α2 to mimic PTH/PTHrP binding to the ECD, but with lower affinity, then we reasoned that it might be possible to engineer a more stable PTH1R dimer by introducing mutations into the α2 helix that render it more like PTH or PTHrP (Fig. 1F). To this end we introduced alterations in α2 that substituted Phe or Trp for Glu at position 182, Leu for Val at 183, and Leu for Arg at 186 in the four combinations shown in Fig. 4. All four mutant receptors were cell surface-expressed (supplemental Fig. S4) and retained the ability to oligomerize (Fig. 4A). The cAMP assay indicated that they retained signaling ability with slightly reduced PTH potencies (Fig. 4B). Although it is unclear if these mutations stabilized the dimer, the fact that such dramatic alterations in α2 did not prevent oligomerization is consistent with the mechanism of dimerization observed in our crystal structure and provides an additional control indicating that only specific mutations in α2 prevent oligomerization.
FIGURE 4.
Oligomerization and cAMP signaling ability of mutant PTH1 receptors with alterations in the ECD α2 helix designed to stabilize the dimer by more closely mimicking PTH/PTHrP binding. A, static BRET ratios for the indicated receptors expressed in COS-1 cells are shown. B, dose-response assay for cAMP accumulation in COS cells expressing the indicated receptors is shown. The data represent the mean ± S.E. of duplicate samples. The EC50 values for WT, E182F/R186L, E182W/R186L, E182F/V183L/R186L, and E182W/V183L/R186L receptors were 36, 64, 86, 66, and 98 pm, respectively.
PTH1R Forms an ECD-mediated Oligomer at the Cell Surface
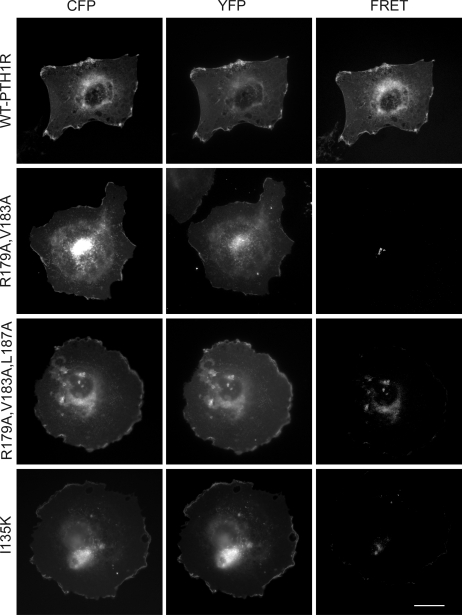
The BRET results presented thus far do not distinguish between cell-surface oligomerization and oligomerization inside the cell. However, the ability of PTH binding to reduce the BRET signal of the WT receptor suggests that most of the BRET signal arises from receptor oligomerization at the cell surface where PTH binding occurs. To further examine PTH1R oligomerization at the cell surface, we employed morphological FRET technology. COS cells co-expressing wild-type PTH1R tagged at the C terminus with CFP or YFP yielded CFP and YFP fluorescence signals at the cell surface as well as a clear FRET signal indicating oligomerization at the cell surface (Fig. 5). The R179A/V183A, R179A/V183A/L187A, and I135K receptors failed to give a FRET signal at the cell surface in agreement with our BRET data. These results indicate that an intact ECD α2 helix and ECD peptide binding site are required for PTH1R oligomerization at the cell surface.
FIGURE 5.
Morphological FRET analysis of PTH1R oligomerization. Shown are representative corrected microscopic images of fixed COS cells expressing CFP- and YFP-tagged constructs as indicated. The images represent background-subtracted CFP, background-subtracted YFP, and corrected FRET signals. The scale bar represents 25 μm.
DISCUSSION
The crystal structure of the ligand-free PTH1R ECD reported here yielded the surprising result of an ECD dimer; the C-terminal segment, which connects the conserved ECD core domain to the first transmembrane helix, forms an α-helix that occupies the peptide binding groove of the opposing subunit, mimicking PTH/PTHrP binding (Fig. 1). This segment of the receptor was previously shown by mutagenesis and cross-linking approaches to provide a contact site for the N-terminal portion of PTH (residues 1–14) in the ligand-bound receptor (53, 54), but its ability to facilitate dimerization of the ligand-free receptor was unexpected. The affinity of the α2-ECD interaction must be quite low in the context of the isolated ECD because the protein purifies as a monomer, and analytical ultracentrifugation showed it to be monomeric at ∼20 μm (55). Attempts to assess the binding of a synthetic α2 peptide (residues 172–191) to the purified PTH1R ECD using the binding assays described previously (40) were unsuccessful because of significant nonspecific effects that occur at high micromolar concentrations of the peptide (data not shown). Although the α2-ECD interaction is clearly weak for the isolated ECD, the affinity is likely to be higher in the intact receptor in the native membrane environment of the cell because the receptor is constrained in two dimensions (56).
The BRET and FRET experiments in COS cells provide strong biophysical evidence that intact PTH1R can oligomerize in cells. To our knowledge this is the first systematic analysis of PTH1R oligomerization. In an elegant study designed to examine the kinetics of PTH1R activation, Vilardaga et al. (57) monitored ligand-induced changes in intramolecular FRET in cells expressing a PTH1R construct tagged with both CFP in the third intracellular loop and YFP at the C terminus. In a control experiment, the authors saw no evidence for intermolecular FRET in cells co-expressing a PTH1R single-tagged with CFP in the third intracellular loop and a PTH1R single-tagged with YFP at the C terminus, but this result does not rule out dimerization and is not directly comparable with our experiments where CFP and YFP are both positioned at the C terminus of the receptor. Our extensive BRET and FRET results clearly show that PTH1R can oligomerize in living cells at the cell surface and are consistent with the hypothesis that the dimer observed in the crystal structure can also form in the intact receptor in its native membrane environment, because mutations in either the α2 helix or the ECD peptide binding site disrupted the oligomerization (Fig. 2, A–D) and oligomerization of the wild-type receptor was disrupted by PTH binding (Fig. 2E).
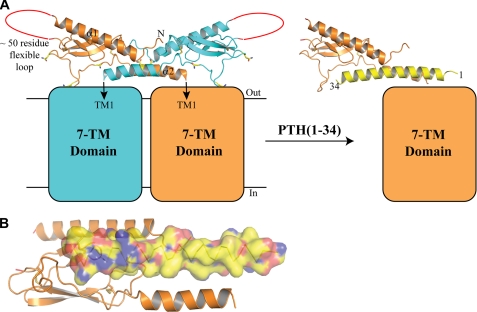
The anti-parallel arrangement of the ECD α2 helices provides significant constraints on possible orientations of the ECD dimer with respect to the attached 7-TM domains in the intact receptor, because α2 extends to residue 187 and the first transmembrane helix is predicted to start at about residue 190. In Fig. 6A we present a model for the intact dimer based on the assumption that the ECD dimer observed in the crystal structure also forms at the cell surface. This arrangement requires an ∼90° turn between α2 and the first transmembrane helix. In support of this model, the NMR structure of a synthetic PTH1R peptide corresponding to residues 168–198 that was determined in dodecylphosphocholine micelles showed that the 180–187 and 189–196 segments formed α-helices that were connected by a 90° turn and that the terminal helix was lipid-embedded (58). The arrangement of the 7-TM domains with respect to the ECD dimer is difficult to predict, because although there are extensive cross-linking data mapping contact sites between PTH/PTHrP and the receptor, there are little data available regarding the domain arrangements in the ligand-free receptor. The PTH1R 7-TM domain and C-terminal tail apparently do not significantly contribute to dimerization, as mutations in the ECD are sufficient to completely disrupt oligomerization; thus, we depict the two 7-TM domains as not contacting each other. Although speculative, PTH disruption of the dimer might occur by PTH binding as the continuous α-helix that was observed in the crystal structure of PTH-(1–34) (59) (Fig. 6A). PTH is an amphipathic peptide that has two distinct hydrophobic faces (one in the 1–14 segment and the other in the 15–34 segment) that are on opposite sides of the helix (Fig. 6B). The model in Fig. 6A would enable the hydrophobic face of the PTH-(1–14) fragment to contact the helical bundle of the 7-TM domain, whereas the hydrophobic face of the PTH-(15–34) fragment contacts the ECD peptide binding groove. Binding of the PTH-(1–14) segment to the 7-TM domain of the receptor with the PTH helical axis parallel to the cell surface and the hydrophobic face pointing toward the core of the 7-TM domain is consistent with a model previously proposed by Gardella and co-workers (60) that incorporated distance restraints from cross-linking studies.
FIGURE 6.
Model for the intact, ligand-free PTH1R dimer and PTH disruption of the dimer. A, the model depicts the only orientation of the ECD dimer with respect to the membrane that is possible given the location of the ECD C termini of the two subunits. As modeled, an ∼90° turn between the ECD α2 helix and the first transmembrane helix (TM1) would be required. The four consensus sites of N-linked glycosylation are shown in yellow. The membrane-embedded 7-TM domains are shown as squares that approximate the size of a 7-TM helical bundle relative to the ECDs. The specific arrangement of the individual 7-TM domains with respect to each other and the attached ECD is not clear, but the 7-TM domains apparently do not significantly contribute to dimerization. PTH is shown as a yellow helix modeled by positioning the crystal structure of PTH-(1–34) (PDB code 1ET1) into the peptide binding site of the ECD. B, view of the ECD with docked PTH-(1–34) showing a surface representation of PTH to highlight the hydrophobic face of the 1–14 segment, which is on the opposite side of the helix as the hydrophobic face of the 15–34 segment that contacts the ECD peptide binding groove. Carbon atoms are colored yellow, sulfur atoms are orange, nitrogen atoms are blue, and oxygen atoms are red. As depicted in our model, the hydrophobic face of the 1–14 segment would face toward the membrane and contact the helical bundle of the 7-TM domain.
Dimerization of PTH1R by structural mimicry of PTH/PTHrP binding provides an elegant mechanism for dissociating the dimer by ligand binding, but the function of this putative mechanism remains unclear. Dimerization is not a prerequisite for trafficking to the cell surface, because the monomeric mutant receptors were cell surface-expressed. The monomeric R179A/V183A receptor exhibited wild-type PTH binding and cAMP signaling ability (Fig. 3, B and C, and supplemental Fig. S5), suggesting that a single PTH1R 7-TM domain is sufficient for G protein activation and, hence, a 1:1 stoichiometry for the PTH1R-heterotrimeric G protein complex. The monomeric receptors did not exhibit elevated basal cAMP signaling activity, which argues against an autoinhibition mechanism for dimerization. Moreover, Gardella and co-workers (61) previously showed that PTH(1–14), which interacts primarily with the 7-TM domain, activates wild-type PTH1R and a truncated receptor lacking the ECD to identical extents, suggesting that disruption of an ECD dimer by the 15–34 fragment is not an obligatory event for receptor activation. Thus, dimerization of PTH1R does not appear to affect its primary signaling function of Gs activation. Our results are reminiscent of reports showing that the β2-adrenergic receptor and rhodopsin can activate G proteins as monomers (20–23).
Several other class B GPCRs homo-oligomerize, but the domains involved in oligomerization were mapped only for the SecR. In stark contrast to our findings for PTH1R, SecR dimerization is not mediated by the ECD (25) but, rather, by helix IV of the 7-TM domain (12). In addition, secretin binding did not disrupt the dimer, and the dimer activated G protein better than the monomer. Thus, even within the small family of class B receptors there is great variation in oligomerization mechanisms, in ligand effects on oligomerization, and in functional effects of oligomerization, continuing the trend observed for class A GPCRs. Are there any of the other class B GPCRs likely to dimerize by a similar endogenous ligand structural mimicry mechanism? The glucose-dependent insulinotropic peptide receptor (GIPR) might exhibit enough sequence similarity to its endogenous ligand, GIP, in its ECD C terminus to permit such a mechanism (supplemental Fig. S6, A and B). It will be interesting to see whether this dimerization mechanism is conserved in a small subset of the class B GPCRs or is limited to PTH1R.
Supplementary Material
Acknowledgments
We thank S. Anderson and J. Brunzelle for assistance with data collection at sector 21 (LS-CAT) of the Advanced Photon Source and Mary L. Augustine for assistance with cell culture.
This work was supported, in whole or in part, by National Institutes of Health Grants GM087413 (to H. E. X.) and DK46577 (to L. J. M.). This work was also supported in part by the Jay and Betty Van Andel Foundation (to H. E. X.) and the Fiterman Foundation (to L. J. M.).
The atomic coordinates and structure factors (code 3L2J) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- GPCR
- G protein-coupled receptors
- PTH
- parathyroid hormone
- PTHrP
- PTH-related protein
- PTH1R
- PTH/PTHrP receptor
- BRET
- bioluminescence resonance energy transfer
- FRET
- fluorescence resonance energy transfer
- Rlu
- Renilla luciferase
- YFP
- yellow fluorescent protein
- CFP
- cyan fluorescent protein
- 7-TM
- 7-transmembrane
- ECD
- extracellular domain
- MBP
- maltose-binding protein
- WT
- wild type.
REFERENCES
- 1.Fotiadis D., Jastrzebska B., Philippsen A., Müller D. J., Palczewski K., Engel A. (2006) Curr. Opin. Struct. Biol. 16, 252–259 [DOI] [PubMed] [Google Scholar]
- 2.Gurevich V. V., Gurevich E. V. (2008) Trends Pharmacol. Sci. 29, 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulenger S., Marullo S., Bouvier M. (2005) Trends Pharmacol. Sci. 26, 131–137 [DOI] [PubMed] [Google Scholar]
- 4.Chabre M., le Maire M. (2005) Biochemistry 44, 9395–9403 [DOI] [PubMed] [Google Scholar]
- 5.Rios C. D., Jordan B. A., Gomes I., Devi L. A. (2001) Pharmacol. Ther. 92, 71–87 [DOI] [PubMed] [Google Scholar]
- 6.Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. (2003) Nature 421, 127–128 [DOI] [PubMed] [Google Scholar]
- 7.Guo W., Urizar E., Kralikova M., Mobarec J. C., Shi L., Filizola M., Javitch J. A. (2008) EMBO J. 27, 2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung J. J., Deupi X., Pardo L., Yao X. J., Velez-Ruiz G. A., Devree B. T., Sunahara R. K., Kobilka B. K. (2009) EMBO J. 28, 3315–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harikumar K. G., Morfis M. M., Lisenbee C. S., Sexton P. M., Miller L. J. (2006) Mol. Pharmacol. 69, 363–373 [DOI] [PubMed] [Google Scholar]
- 10.Patel R. C., Kumar U., Lamb D. C., Eid J. S., Rocheville M., Grant M., Rani A., Hazlett T., Patel S. C., Gratton E., Patel Y. C. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3294–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Z. J., Miller L. J. (2001) J. Biol. Chem. 276, 48040–48047 [DOI] [PubMed] [Google Scholar]
- 12.Harikumar K. G., Pinon D. I., Miller L. J. (2007) J. Biol. Chem. 282, 30363–30372 [DOI] [PubMed] [Google Scholar]
- 13.Kunishima N., Shimada Y., Tsuji Y., Sato T., Yamamoto M., Kumasaka T., Nakanishi S., Jingami H., Morikawa K. (2000) Nature 407, 971–977 [DOI] [PubMed] [Google Scholar]
- 14.White J. H., Wise A., Main M. J., Green A., Fraser N. J., Disney G. H., Barnes A. A., Emson P., Foord S. M., Marshall F. H. (1998) Nature 396, 679–682 [DOI] [PubMed] [Google Scholar]
- 15.Jones K. A., Borowsky B., Tamm J. A., Craig D. A., Durkin M. M., Dai M., Yao W. J., Johnson M., Gunwaldsen C., Huang L. Y., Tang C., Shen Q., Salon J. A., Morse K., Laz T., Smith K. E., Nagarathnam D., Noble S. A., Branchek T. A., Gerald C. (1998) Nature 396, 674–679 [DOI] [PubMed] [Google Scholar]
- 16.Kaupmann K., Malitschek B., Schuler V., Heid J., Froestl W., Beck P., Mosbacher J., Bischoff S., Kulik A., Shigemoto R., Karschin A., Bettler B. (1998) Nature 396, 683–687 [DOI] [PubMed] [Google Scholar]
- 17.Chandrashekar J., Hoon M. A., Ryba N. J., Zuker C. S. (2006) Nature 444, 288–294 [DOI] [PubMed] [Google Scholar]
- 18.Banères J. L., Parello J. (2003) J. Mol. Biol. 329, 815–829 [DOI] [PubMed] [Google Scholar]
- 19.Han Y., Moreira I. S., Urizar E., Weinstein H., Javitch J. A. (2009) Nat. Chem. Biol. 5, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whorton M. R., Jastrzebska B., Park P. S., Fotiadis D., Engel A., Palczewski K., Sunahara R. K. (2008) J. Biol. Chem. 283, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst O. P., Gramse V., Kolbe M., Hofmann K. P., Heck M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10859–10864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayburt T. H., Leitz A. J., Xie G., Oprian D. D., Sligar S. G. (2007) J. Biol. Chem. 282, 14875–14881 [DOI] [PubMed] [Google Scholar]
- 24.Angers S., Salahpour A., Bouvier M. (2001) Life Sci. 68, 2243–2250 [DOI] [PubMed] [Google Scholar]
- 25.Lisenbee C. S., Miller L. J. (2006) Biochemistry 45, 8216–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seck T., Baron R., Horne W. C. (2003) J. Biol. Chem. 278, 23085–23093 [DOI] [PubMed] [Google Scholar]
- 27.Kraetke O., Wiesner B., Eichhorst J., Furkert J., Bienert M., Beyermann M. (2005) J. Recept. Signal Transduct. Res. 25, 251–276 [DOI] [PubMed] [Google Scholar]
- 28.Maurel D., Comps-Agrar L., Brock C., Rives M. L., Bourrier E., Ayoub M. A., Bazin H., Tinel N., Durroux T., Prézeau L., Trinquet E., Pin J. P. (2008) Nat. Methods 5, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harikumar K. G., Morfis M. M., Sexton P. M., Miller L. J. (2008) J. Mol. Neurosci. 36, 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoare S. R. (2005) Drug Discov. Today 10, 417–427 [DOI] [PubMed] [Google Scholar]
- 31.Harikumar K. G., Happs R. M., Miller L. J. (2008) Biochim. Biophys. Acta 1778, 2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardella T. J., Jüppner H. (2000) Rev. Endocr. Metab. Disord. 1, 317–329 [DOI] [PubMed] [Google Scholar]
- 33.Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr., Hock J., Potts J. T., Jr., Kronenberg H. M. (1991) Science 254, 1024–1026 [DOI] [PubMed] [Google Scholar]
- 34.Potts J. T., Gardella T. J. (2007) Ann. N.Y. Acad. Sci. 1117, 196–208 [DOI] [PubMed] [Google Scholar]
- 35.Neer R. M., Arnaud C. D., Zanchetta J. R., Prince R., Gaich G. A., Reginster J. Y., Hodsman A. B., Eriksen E. F., Ish-Shalom S., Genant H. K., Wang O., Mitlak B. H. (2001) N. Engl. J. Med. 344, 1434–1441 [DOI] [PubMed] [Google Scholar]
- 36.Suva L. J., Winslow G. A., Wettenhall R. E., Hammonds R. G., Moseley J. M., Diefenbach-Jagger H., Rodda C. P., Kemp B. E., Rodriguez H., Chen E. Y. (1987) Science 237, 893–896 [DOI] [PubMed] [Google Scholar]
- 37.Karaplis A. C., Luz A., Glowacki J., Bronson R. T., Tybulewicz V. L., Kronenberg H. M., Mulligan R. C. (1994) Genes Dev. 8, 277–289 [DOI] [PubMed] [Google Scholar]
- 38.Kronenberg H. M. (2006) Ann. N.Y. Acad. Sci. 1068, 1–13 [DOI] [PubMed] [Google Scholar]
- 39.Horwitz M. J., Tedesco M. B., Gundberg C., Garcia-Ocana A., Stewart A. F. (2003) J. Clin. Endocrinol. Metab. 88, 569–575 [DOI] [PubMed] [Google Scholar]
- 40.Pioszak A. A., Parker N. R., Gardella T. J., Xu H. E. (2009) J. Biol. Chem. 284, 28382–28391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pioszak A. A., Xu H. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5034–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parthier C., Kleinschmidt M., Neumann P., Rudolph R., Manhart S., Schlenzig D., Fanghänel J., Rahfeld J. U., Demuth H. U., Stubbs M. T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13942–13947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pioszak A. A., Parker N. R., Suino-Powell K., Xu H. E. (2008) J. Biol. Chem. 283, 32900–32912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Runge S., Thøgersen H., Madsen K., Lau J., Rudolph R. (2008) J. Biol. Chem. 283, 11340–11347 [DOI] [PubMed] [Google Scholar]
- 45.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 46.(1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 47.McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. (1991) Acta Crystallogr. A 47, 110–119 [DOI] [PubMed] [Google Scholar]
- 49.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D. Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 50.Winn M. D., Isupov M. N., Murshudov G. N. (2001) Acta Crystallogr. D. Biol. Crystallogr. 57, 122–133 [DOI] [PubMed] [Google Scholar]
- 51.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 52.DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
- 53.Carter P. H., Shimizu M., Luck M. D., Gardella T. J. (1999) J. Biol. Chem. 274, 31955–31960 [DOI] [PubMed] [Google Scholar]
- 54.Zhou A. T., Bessalle R., Bisello A., Nakamoto C., Rosenblatt M., Suva L. J., Chorev M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 3644–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grauschopf U., Lilie H., Honold K., Wozny M., Reusch D., Esswein A., Schäfer W., Rücknagel K. P., Rudolph R. (2000) Biochemistry 39, 8878–8887 [DOI] [PubMed] [Google Scholar]
- 56.Grasberger B., Minton A. P., DeLisi C., Metzger H. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 6258–6262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vilardaga J. P., Bünemann M., Krasel C., Castro M., Lohse M. J. (2003) Nat. Biotechnol. 21, 807–812 [DOI] [PubMed] [Google Scholar]
- 58.Pellegrini M., Bisello A., Rosenblatt M., Chorev M., Mierke D. F. (1998) Biochemistry 37, 12737–12743 [DOI] [PubMed] [Google Scholar]
- 59.Jin L., Briggs S. L., Chandrasekhar S., Chirgadze N. Y., Clawson D. K., Schevitz R. W., Smiley D. L., Tashjian A. H., Zhang F. (2000) J. Biol. Chem. 275, 27238–27244 [DOI] [PubMed] [Google Scholar]
- 60.Gensure R. C., Shimizu N., Tsang J., Gardella T. J. (2003) Mol. Endocrinol. 17, 2647–2658 [DOI] [PubMed] [Google Scholar]
- 61.Luck M. D., Carter P. H., Gardella T. J. (1999) Mol. Endocrinol. 13, 670–680 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.