Abstract
BACKGROUND
An inverse association between adult body mass index (BMI) and risk of endometriosis has frequently been reported. However, the association between body size during childhood and early adulthood and endometriosis is not as well documented.
METHODS
Using data collected from the Nurses' Health Study II, a prospective cohort study of premenopausal US nurses, that began in 1989, we have attempted to clarify this relationship. Data are updated every 2 years with follow-up for these analyses through 2001. In 1989 women recalled their body size at ages 5, 10 and 20 years using a validated 9-level figure drawing.
RESULTS
During 831 910 person-years of follow-up, 1817 cases of self-reported laparoscopically-confirmed endometriosis were observed among women with no past infertility. After adjusting for age, birthweight, age at menarche, parity, oral contraceptive use and adult BMI, we observed a significant reduction in the incidence of endometriosis with increasing body size for all time periods. The relative risks (RRs) comparing the smallest and largest figure sizes to the middle category during childhood (ages 5–10) were 1.18 (95% confidence interval 1.02–1.36) and 0.82 (0.66–1.02), P-trend = 0.0002. At age 20, the RRs for the same comparisons were 1.32 (1.06–1.65) and 0.87 (0.74–1.03), P-trend = 0.04. Additional adjustment by menstrual cycle length and regularity yielded similar associations. The associations were stronger among nulliparous women than among parous women, although not all differences were statistically significant.
CONCLUSION
In this large cohort of premenopausal women, there was evidence of a persistent inverse association between childhood and early adulthood body size and incidence of laparoscopically confirmed endometriosis, independent of adult BMI and menstrual cycle characteristics.
Keywords: endometriosis, childhood, anthropometry, body mass index
Introduction
Endometriosis, the third leading cause of gynecological hospitalization in the USA, is defined as the presence of endometrial tissue (glands and stroma) external to the uterus (Eskenazi and Warner, 1997). Despite the high morbidity and health-care cost associated with endometriosis, its etiology has not been fully delineated. The pathophysiology likely includes hormonal, anatomic, genetic and immune factors. Risk may be associated with factors that increase the volume, frequency and duration of retrograde menstruation and promote implantation and growth of endometrial plaques (Oral and Arici, 1997). There is also strong circumstantial evidence that endometriosis is influenced by steroid hormones (Witz and Schenken, 1997; Barbieri, 1998; Witz, 1999). Signs and symptoms arise from cyclic bleeding into the surrounding tissues, resulting in inflammation and formation of scarring and adhesions.
While earlier studies of the association between body mass index at the time of diagnosis and endometriosis have shown only weak associations (Cramer et al., 1986; Darrow et al., 1993; Signorello et al., 1997), more recent studies have found stronger significant inverse associations (Hemmings et al., 2004; Missmer et al., 2004a; Ferrero et al., 2005; Hediger et al., 2005; Matalliotakis et al., 2007). However, body size during childhood and adolescence, around the time of menarche, may be a more relevant exposure than body size at the time of diagnosis, and this relation has not been as thoroughly explored. Two case–control studies have examined this relationship. A small study of 32 cases and 52 controls found that controls had larger body sizes at ages 15–24 than did cases (Hediger et al., 2005). A larger study capturing exposure as relative weight (underweight, average, overweight) found a significant increase in risk for women overweight at age 10 and underweight at age 16 (Nagle et al., 2009). In the prospective cohort study used for the present analysis, birthweight and body mass index at 18 were inversely associated with endometriosis (Missmer et al., 2004a, b). Both associations were significant when using all endometriosis cases and when the analysis was restricted to only those with concurrent infertility.
Using data collected from the Nurses' Health Study II (NHSII), an ongoing, prospective cohort study of premenopausal US nurses that began in 1989, we have attempted to clarify the relation between childhood and early adulthood body size and laparoscopically confirmed endometriosis.
Materials and Methods
Study population and data collection
Data for these analyses were collected in the NHSII cohort from September 1989 to June 2001. Questionnaires requesting information on incidence of diseases and demographic, biological, environmental and lifestyle risk factors are updated and mailed biennially. A total of 116 608 female registered nurses, ranging in age from 25 to 42 and residing in one of 14 states in the USA, completed the baseline questionnaire. Follow-up of this cohort in each 2-year interval has been consistently ≥90%. This research was approved by the Institutional Review Board of the Harvard School of Public Health.
Case ascertainment and analytic definition
In 1993, the women were first asked if they had ‘ever had physician-diagnosed endometriosis.’ If ‘yes,’ they were asked to report when the diagnosis had occurred (before September 1989, September 1989–May 1991 and June 1991–May 1993, which correspond to the follow-up periods) and if it had been confirmed by laparoscopy—a standard surgical method for diagnosing endometriosis (Duleba, 1997; Pardanani and Barbieri, 1998). These questions were asked again in each subsequent questionnaire.
As described previously (Missmer et al., 2004a), in March 1994 we conducted a study to validate self-reported endometriosis diagnosis within the NHSII prospective cohort. Supplementary questionnaires were mailed to 200 women who were randomly selected from the then 1766 cases who had reported incident diagnosis. Among those who reported laparoscopic confirmation and for whom records were received and reviewed (n = 105), a laparoscopic diagnosis of endometriosis was confirmed in 96%. However, among those women without laparoscopic confirmation (n = 26), evidence of clinical diagnosis was found in only 54% of the records. Severity data (defined by the staging system outlined by the American Society for Reproductive Medicine) suggested that the majority of laparoscopically confirmed cases (61%) had minimal or mild disease. Requests for permission to review medical records were also sent to any woman who indicated that she had had a hysterectomy during the 2-year interval of reported endometriosis diagnosis. A diagnosis of endometriosis at the time of surgical procedure was confirmed in 80% (n = 144/181) of the records received. However, endometriosis was the primary indication for hysterectomy in only 6% (n = 9/163) of women for whom indication information was available.
Based upon these validation results, self-reported physician-diagnosed endometriosis without laparoscopic confirmation may be misclassified substantially. In addition, allowing women who report endometriosis and a hysterectomy in the same follow-up period to be cases might yield spurious results because it would be unclear if the associated risk factors are related to endometriosis or to the pathology for which the hysterectomy was performed. Therefore, to reduce the magnitude of misclassification and prevent confounding by indication for hysterectomy, analyses of incident diagnosis of endometriosis were restricted to those women who reported laparoscopic confirmation of their diagnosis.
Within this restricted case definition, the relation between endometriosis and infertility status is complex. At baseline, the prevalence of infertility (defined as attempting to become pregnant for more than 1 year without success) was greater among women with laparoscopic confirmation (20%) than among those who were clinically diagnosed without laparoscopic confirmation (4%) Approximately 20% of all infertile women are found to have endometriosis (Tanahatoe et al., 2003). While pelvic pain information is not available in the NHSII cohort, we might assume that cases with infertility are more likely to be asymptomatic in terms of their pelvic pain because they likely underwent an exploratory laparoscopy to identify the cause of their infertility rather than the cause of pelvic pain. Had these women not attempted to become pregnant, a large proportion may never have received a laparoscopic diagnosis of endometriosis. We may also assume that cases with no infertility who have had a laparoscopic diagnosis are more likely to have experienced pelvic pain symptoms, otherwise an invasive surgical evaluation would not have been conducted. Under these assumptions, we believe that endometriosis with infertility may be indicative of asymptomatic disease secondary to other primary causes of infertility and the risk factors for endometriosis with infertility could differ from those for endometriosis without concurrent infertility. Hence, we looked at risk factors separately by these two ‘subtypes’ of endometriosis—(i) cases with neither past nor concurrent infertility, and (ii) cases with concurrent infertility. Within this cohort, self-reported infertility was validated in a study of 100 randomly selected women who reported ovulatory infertility; 95% of the self-reports were confirmed through medical record review (Rich-Edwards et al., 1994).
Assessment of exposure
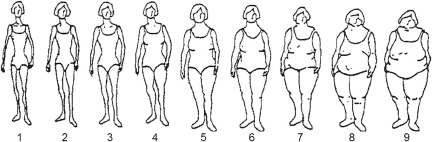
In 1989 participants recalled their body size at ages 5, 10 and 20 years using a 9-level figure drawing where the first category represents the most lean body shape and the ninth represents the most obese. These drawings were originally developed by Stunkard and colleagues (Stunkard et al., 1983) and are shown in Figure 1. To obtain an estimate of childhood body size, we averaged each participant's figures at ages 5 and 10 years; the goal of this approach was to reduce the effects of random error in the assessment of body size. Changes in body size between ages 5 and 10 years, between ages 10 and 20 years, and between ages 5 and 20 years were also calculated by subtracting each participant's figure (levels 1 though 9) at the younger age from that at the older age.
Figure 1.
Figure drawing used to assess body size at ages 5, 10 and 20 years among Nurses' Health Study II participants. Reproduced with permission of Lippincott, Williams and Willkins, Philadelphia, USA.
Must and colleagues (Must et al., 1993) evaluated the validity of remote recall of body size among 181 participants in the Third Harvard Growth Study, a longitudinal study of physical and mental growth in children that was conducted between 1922 and 1935 in the Boston area. Height and weight were measured as part of annual examinations during childhood and adolescence and were used to calculate body mass index in kilograms per square-meter. In 1988 and 1989, when participants were between ages 71 and 76 years, they were interviewed again and asked to recall their body size at ages 5, 10, 15 and 20 years, using the same 9-level figure drawing as that on the 1989 NHSII questionnaire. Pearson correlations between recalled body size and body mass index at approximately the same ages were 0.60 for age 5 years, 0.70 for age 10 years, 0.75 for age 15 years and 0.66 for age 20 years. Other studies have yielded similar findings (Munoz et al., 1996; Koprowski et al., 2001; Tehard et al., 2002; Must et al., 2002) indicating that these figure drawings can provide a reasonably accurate assessment of body size at young ages. Additionally, this assessment of body size has been strongly associated with breast cancer risk in both NHS and NHSII analyses (Berkey et al., 1999; Baer et al., 2005).
Statistical analysis
Exclusion criteria
Participants who reported the diagnosis of endometriosis or a history of infertility prior to September 1989 were excluded from all analyses. Analyses were also restricted to those who were premenopausal and had intact uteri, because the occurrence of endometriosis after hysterectomy or in post-menopausal women is rare. Women with prior cancer diagnoses, other than non-melanoma skin cancer, and participants who did not report their body size at age 5, 10 or 20 years were also excluded.
Person-time calculation
Person-months at risk were calculated from entry into the cohort until independently confirmed death or cancer diagnosis (other than non-melanoma skin cancer), or self-reported laparoscopically confirmed endometriosis diagnosis, hysterectomy or the onset of menopause. Women who reported physician-diagnosed endometriosis with no laparoscopic confirmation were censored at the time of that report, but were allowed to re-enter the analysis population if they reported laparoscopic confirmation on a subsequent questionnaire. In addition, because infertility is so strongly correlated with the diagnosis of endometriosis via laparoscopy, we censored at self-report of infertility. Therefore, in all analyses our comparison group consists of women with neither diagnosed endometriosis nor infertility, allowing for a more homogeneous comparison group as we have previously described in detail (Missmer et al., 2004a).
Relative risk estimation
Incidence rates for each exposure category were computed as the number of incident cases divided by the person-time accumulated. Time-varying Cox proportional hazards models treating age in months and the 2-year questionnaire period as the time scale were used to estimate multivariate (MV) incidence rate ratios (RR) and to calculate 95% confidence intervals (CI), after adjusting simultaneously for confounding variables. Tests for trend in ordinal categorical exposures were calculated by creating an ordinal variable in which the median value or midpoint of each category was assigned to all participants in that group. Tests for heterogeneity comparing the effect estimates among cases having no past or current infertility with effect estimates among cases having concurrent infertility were calculated with a Wald statistic referred to a chi-squared distribution with 1 degree of freedom (Prentice et al., 1978). To evaluate if the body size and endometriosis associations varied by levels of other risk factors, stratified analyses were conducted, and likelihood ratio tests comparing the models with both the main effects and the interaction terms to the models with the main effects only were calculated.
Results
After baseline exclusions, a total of 87 603 women contributed 831 910 person-years of follow-up; 1817 incident cases of laparoscopically confirmed endometriosis with no past infertility were reported. These included 1428 cases with no past or current infertility and 371 cases who reported an infertility evaluation during the same follow-up period as laparoscopic confirmation of endometriosis.
Women who reported greater body size at age 10 were more likely to have a heavier birthweight and a larger body mass index, both at age 18 and as an adult (Table I). An age at menarche before age 12 was strongly associated with greater body size at age 10. Participants who reported greater body size were somewhat less likely to have used oral contraceptives or to have regular or shorter menstrual cycles in late adolescence. Nulliparous participants were more likely to report larger age 10 figures, but among parous women, the number of births and age at first birth were not associated with body size.
Table I.
Endometriosis risk factors at baseline according to body size at age 10 years among the 87 603 premenopausal women studied.
| Figure at age 10 years |
|||||
|---|---|---|---|---|---|
| 1 (n = 15 999) | 2 (n = 27 025) | 3 (n = 20 008) | 4 (n = 13 936) | ≥5 (n = 10 635) | |
| Age in 1989 (mean years) | 34.2 | 33.6 | 33.7 | 34.0 | 34.3 |
| Birthweight ≥8.5 lbs (%) | 10.6 | 11.7 | 14.4 | 15.9 | 17.3 |
| Body mass index at age 18 (mean kg/m2) | 19.4 | 20.2 | 21.6 | 22.9 | 24.4 |
| Adult body mass index (mean kg/m2) | 21.8 | 22.6 | 24.3 | 26.0 | 27.0 |
| Age at menarche <12 years (%) | 15.2 | 19.0 | 25.7 | 31.4 | 35.4 |
| Age at menarche (mean years) | 12.8 | 12.6 | 12.3 | 12.1 | 12.0 |
| Oral contraceptive user (%) | 84.2 | 83.0 | 81.6 | 81.4 | 80.1 |
| Nulliparous (%) | 30.8 | 28.8 | 29.5 | 32.4 | 37.6 |
| Parity (mean births)a | 2.1 | 2.1 | 2.1 | 2.1 | 2.0 |
| Age at first birth (mean years) | 25.2 | 25.4 | 25.4 | 25.4 | 25.3 |
| Regular menstrual cycles (ages 18–22) (%) | 77.9 | 78.9 | 79.1 | 77.7 | 76.1 |
| Short cycles (ages 18–22) (%)b | 13.8 | 11.9 | 10.8 | 11.3 | 11.4 |
aAmong parous women only.
bShort cycles defined as <26 days.
Body size at ages 5, 10 and 20 were all inversely associated with laparoscopically confirmed endometriosis (Table II). To maintain an adequate number of cases in the largest body size category, we grouped figures 5–9 together. After adjusting for age, birthweight, age at menarche, parity, OC use and adult body mass index, compared with the middle category of body size at age 5 (figure level 3) there was a 23% increased risk for endometriosis among the most lean (figure level 1) (RR = 1.23, 95% CI = 1.08–1.40) and a 10% decreased risk for the most overweight (figure level ≥5) (RR = 0.90, 95% CI = 0.73, 1.11), P-value test for trend <0.0001). The associations at age 10, averaged over childhood, and at age 20 were similar. When cases were restricted to only those with concurrent infertility, the associations were slightly weaker, although there was no significant heterogeneity. Additional adjustment by menstrual cycle length and cycle regularity between ages 18 and 22 yielded similar inverse associations (data not shown).
Table II.
Relative risks of endometriosis by body size at ages 5, 10 and 20 years and during childhood and early adulthood among 87 603 premenopausal Nurses' Health Study II participants (1989–2001).
| Case definition |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| All women (no past infertility) |
No past or concurrent infertilitya |
Concurrent infertilitya |
|||||||
| Cases | Person-years | Age-adjusted RRb | MV RR (95% CI)c | Cases | MV RR (95% CI)c | Cases | MV RR (95% CI)c | pd | |
| Figure at age 5 | |||||||||
| 1 | 504 | 196 367 | 1.23 | 1.23 (1.08, 1.40) | 397 | 1.27 (1.10, 1.47) | 102 | 1.09 (0.82, 1.45) | 0.84 |
| 2 | 564 | 268 816 | 0.98 | 0.99 (0.87, 1.13) | 446 | 1.03 (0.89, 1.19) | 116 | 0.88 (0.67, 1.16) | |
| 3 | 428 | 202 963 | 1.00 | 1.00 | 332 | 1.00 | 92 | 1.00 | |
| 4 | 206 | 107 550 | 0.91 | 0.88 (0.74, 1.04) | 165 | 0.90 (0.74, 1.08) | 37 | 0.79 (0.54, 1.16) | |
| ≥5 | 115 | 56 214 | 0.98 | 0.90 (0.73, 1.11) | 88 | 0.88 (0.70, 1.12) | 24 | 0.90 (0.57, 1.42) | |
| Ptrend < 0.0001 | Ptrend < 0.0001 | Ptrend = 0.24 | |||||||
| Figure at age 10 | |||||||||
| 1 | 360 | 148 773 | 1.12 | 1.14 (0.99, 1.32) | 284 | 1.16 (0.99, 1.37) | 74 | 1.09 (0.79, 1.50) | 0.77 |
| 2 | 572 | 258 668 | 1.00 | 1.02 (0.90, 1.16) | 443 | 1.02 (0.88, 1.17) | 124 | 1.04 (0.78, 1.38) | |
| 3 | 422 | 192 175 | 1.00 | 1.00 | 335 | 1.00 | 85 | 1.00 | |
| 4 | 259 | 132 966 | 0.89 | 0.86 (0.74, 1.01) | 211 | 0.87 (0.73, 1.03) | 44 | 0.80 (0.55, 1.15) | |
| ≥5 | 204 | 99 328 | 0.95 | 0.88 (0.74, 1.04) | 155 | 0.83 (0.68, 1.01) | 44 | 1.01 (0.69, 1.46) | |
| Ptrend = 0.0004 | Ptrend = 0.0004 | Ptrend = 0.30 | |||||||
| Average childhood figure (ages 5–10 years) | |||||||||
| 1 | 333 | 137 283 | 1.15 | 1.18 (1.02, 1.36) | 263 | 1.19 (1.01, 1.40) | 68 | 1.18 (0.86, 1.64) | 0.70 |
| 1.5–2 | 583 | 258 173 | 1.05 | 1.08 (0.95, 1.22) | 454 | 1.07 (0.93, 1.23) | 125 | 1.14 (0.86, 1.50) | |
| 2.5–3 | 454 | 212 703 | 1.00 | 1.00 | 362 | 1.00 | 88 | 1.00 | |
| 3.5–4.5 | 350 | 172 605 | 0.96 | 0.93 (0.80, 1.07) | 271 | 0.88 (0.75, 1.04) | 74 | 1.11 (0.81, 1.52) | |
| ≥5 | 97 | 51 145 | 0.90 | 0.82 (0.66, 1.02) | 78 | 0.82 (0.64, 1.04) | 16 | 0.77 (0.45, 1.31) | |
| Ptrend = 0.0002 | Ptrend = 0.0002 | Ptrend = 0.19 | |||||||
| Figure at age 20 | |||||||||
| 1 | 90 | 33 663 | 1.36 | 1.32 (1.06, 1.65) | 71 | 1.28 (1.00, 1.65) | 18 | 1.55 (0.93, 2.57) | 0.36 |
| 2 | 456 | 211 910 | 1.04 | 1.04 (0.92, 1.18) | 345 | 0.99 (0.86, 1.14) | 103 | 1.23 (0.94, 1.16) | |
| 3 | 672 | 319 455 | 1.00 | 1.00 | 542 | 1.00 | 123 | 1.00 | |
| 4 | 406 | 176 226 | 1.09 | 1.05 (0.93, 1.19) | 313 | 1.01 (0.88, 1.16) | 92 | 1.29 (0.98, 1.70) | |
| ≥5 | 193 | 90 655 | 1.01 | 0.87 (0.74, 1.03) | 157 | 0.91 (0.76, 1.10) | 35 | 0.85 (0.57, 1.26) | |
| Ptrend = 0.04 | Ptrend = 0.21 | Ptrend = 0.20 | |||||||
RR, rate ratio; MV, multivariate; CI, confidence interval.
aInfertility is defined as attempting to become pregnant for >1 year without success. Cases with ‘no past or concurrent infertility’ are women who never reported infertility. Cases with ‘concurrent infertility’ are women who reported an infertility evaluation in the same follow-up cycle as laparoscopic confirmation of endometriosis.
bAdjusted for current age (continuous months) and calendar time (2-year questionnaire period).
cAdjusted for current age (continuous months), calendar time (2-year questionnaire period), birthweight (not full-term, <5.5, 5.5–6.9, 7.0–8.4, >8.4 pounds), age at menarche (<10, 10, 11, 12, 13, 14, 15, >15 years), parity (0, 1+), oral contraceptive use (never, past, current) and adult body mass index (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, ≥30 kg/m2).
dP-value test for heterogeneity comparing the effect of body size among women with no past or current infertility with those having concurrent infertility.
To see if the inverse association with body size continued to decrease within the largest figure sizes (figure levels >5), we ran models using additional indicators for body sizes 5, 6 and 7, although the number of cases in these upper categories was small. Compared with category 3, the RRs (95% CIs) for age 5 body sizes 5, 6, and 7 were 0.94 (0.74, 1.17), 0.82 (0.52, 1.29) and 0.57 (0.18, 1.78), respectively. At age 10, the RRs were 0.90 (0.75, 1.08), 0.82 (0.60, 1.14) and 0.69 (0.32, 1.46) and at age 20 they were 0.83 (0.68, 1.01), 0.91 (0.67, 1.23) and 1.14 (0.74, 1.77).
We examined potential effect modification by OC use (ever, never), breast or pelvic examination by a physician in the past 2 years (a proxy for frequency of use of the medical system that may be correlated with the likelihood of undergoing laparoscopy in the presence of disease symptoms), adult body mass index (<25, ≥25 kg/m2) and nulliparity. The associations between body size and change in body size were similar across the levels of OC use, breast/pelvic examination and body mass index (data not shown). However, we observed a stronger inverse association among nulliparous women than among parous women (Table III). This trend was seen for all ages, though the difference between groups was only statistically significant for body size at age 20. Among nulliparous women, comparing the smallest and largest body sizes at age 20 to the middle category, we observed a 41% increase in risk (RR = 1.41, 95% CI 1.00–2.00) and a 20% reduction in risk (RR = 0.80, 95% CI 0.62, 1.04), respectively. Among parous women, the RRs for the most lean and most overweight women were 1.24 (95% CI 0.91, 1.68) and 0.96 (95% CI 0.75, 1.22) (P-value test for heterogeneity =0.03).
Table III.
Relative risks of endometriosis by body size at ages 5, 10 and 20 years among 87 603 premenopausal Nurses' Health Study II participants (1989–2001) stratified by parity.
| Nulliparous |
Parous |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-years | Age-adjusted RRa | MV RR (95% CI)b | Cases | Person-years | Age-adjusted RRa | MV RR (95% CI)b | P-valuec | |
| Figure at age 5 | |||||||||
| 1 | 212 | 41 647 | 1.35 | 1.31 (1.06, 1.62) | 277 | 148,041 | 1.15 | 1.18 (0.99, 1.41) | 0.20 |
| 2 | 212 | 55 081 | 1.00 | 0.98 (0.80, 1.20) | 339 | 205,448 | 1.00 | 1.02 (0.86, 1.20) | |
| 3 | 164 | 42 995 | 1.00 | 1.00 | 252 | 154,222 | 1.00 | 1.00 | |
| 4 | 87 | 25 546 | 0.90 | 0.93 (0.71, 1.21) | 112 | 78 955 | 0.87 | 0.86 (0.68, 1.07) | |
| ≥5 | 52 | 16 306 | 0.85 | 0.89 (0.64, 1.22) | 62 | 38 137 | 1.00 | 0.96 (0.73, 1.27) | |
| Ptrend = 0.003 | Ptrend = 0.009 | ||||||||
| Figure at age 10 | |||||||||
| 1 | 147 | 31 414 | 1.13 | 1.11 (0.88, 1.40) | 202 | 112,297 | 1.10 | 1.17 (0.96, 1.42) | 0.08 |
| 2 | 223 | 51 446 | 1.02 | 1.00 (0.81, 1.22) | 337 | 199,095 | 1.02 | 1.07 (0.90, 1.26) | |
| 3 | 168 | 40 008 | 1.00 | 1.00 | 240 | 146,597 | 1.00 | 1.00 | |
| 4 | 101 | 31 087 | 0.78 | 0.80 (0.62, 1.03) | 151 | 98 023 | 0.94 | 0.92 (0.75, 1.13) | |
| ≥5 | 88 | 27 621 | 0.77 | 0.80 (0.61, 1.04) | 112 | 68 791 | 1.00 | 0.96 (0.77, 1.21) | |
| Ptrend = 0.008 | Ptrend = 0.03 | ||||||||
| Figure at age 20 | |||||||||
| 1 | 39 | 7233 | 1.44 | 1.41 (1.00, 2.00) | 48 | 25 400 | 1.17 | 1.24 (0.91, 1.68) | 0.03 |
| 2 | 179 | 39 714 | 1.12 | 1.10 (0.90, 1.35) | 271 | 165,243 | 1.00 | 1.03 (0.88, 1.21) | |
| 3 | 240 | 58 634 | 1.00 | 1.00 | 418 | 251,309 | 1.00 | 1.00 | |
| 4 | 170 | 41 354 | 1.01 | 1.06 (0.86, 1.29) | 219 | 129,648 | 1.02 | 1.00 (0.85, 1.18) | |
| ≥5 | 99 | 34 641 | 0.71 | 0.80 (0.62, 1.04) | 86 | 53 203 | 0.99 | 0.96 (0.75, 1.22) | |
| Ptrend = 0.02 | Ptrend = 0.30 | ||||||||
RR, rate ratio; MV, multivariate; CI, confidence interval.
aAdjusted for current age (continuous months) and calendar time (2-year questionnaire period).
bAdjusted for current age (continuous months), calendar time (2-year questionnaire period), birthweight (not full-term, <5.5, 5.5–6.9, 7.0–8.4, >8.4 pounds), age at menarche (<10, 10, 11, 12, 13, 14, 15, >15 years), parity (0, 1+), oral contraceptive use (never, past, current), and adult body mass index (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, ≥30 kg/m2).
cP-value test for heterogeneity comparing the effect of body size among nulliparous women with the effect among parous women.
Since there was an inverse association between body size and risk of endometriosis at all ages, we examined how a change in body size from one age to another was associated with endometriosis, hypothesizing that gaining weight would be associated with decreased risk and losing weight would be associated with increased risk. In models adjusted for age, birthweight, age at menarche, parity, OC use, adult body mass index and body size in the earlier time period, we found that decreases in size from ages 10–20 and from ages 5–20 were associated with increased risk (Table IV). From ages 5–20 and 10–20, the RRs for a decrease in size were 1.21 (95% CI 1.02, 1.43) and 1.17 (95% CI 1.00–1.37), respectively. In the fully adjusted models, increases in body size at any age were not significantly associated with endometriosis.
Table IV.
Relative risks of endometriosis by changes in body size between ages 5, 10 and 20 years among 87 603 premenopausal Nurses' Health Study II participants (1989–2001).
| Cases | Person-years | Age-adjusted RRa | MV RR (95% CI)b | MV RR (95% CI)c + starting figure | |
|---|---|---|---|---|---|
| Change from ages 5–10 years | |||||
| Decreased | 100 | 48 041 | 0.99 | 0.97 (0.79, 1.19) | 1.08 (0.88, 1.34) |
| No change | 1223 | 574 263 | 1.00 | 1.00 | 1.00 |
| Increased 1 level | 371 | 155 297 | 1.12 | 1.09 (0.97, 1.23) | 1.07 (0.95, 1.21) |
| Increased 2 or more levels | 123 | 54 308 | 1.07 | 1.02 (0.85, 1.23) | 0.98 (0.81, 1.18) |
| Change from ages 10–20 years | |||||
| Decreased | 312 | 146 908 | 1.07 | 1.05 (0.91, 1.20) | 1.17 (1.00, 1.37) |
| No change | 556 | 280 438 | 1.00 | 1.00 | 1.00 |
| Increased 1 level | 697 | 310 055 | 1.10 | 1.12 (1.00, 1.25) | 1.05 (0.94, 1.18) |
| Increased 2 or more levels | 252 | 94 508 | 1.29 | 1.22 (1.05, 1.42) | 1.13 (0.96, 1.32) |
| Change from ages 5–20 years | |||||
| Decreased | 228 | 107 618 | 1.09 | 1.07 (0.91, 1.25) | 1.21 (1.02, 1.43) |
| No change | 492 | 255 098 | 1.00 | 1.00 | 1.00 |
| Increased 1 level | 690 | 306 368 | 1.14 | 1.14 (1.02, 1.28) | 1.07 (0.94, 1.21) |
| Increased 2 or more levels | 407 | 162 826 | 1.25 | 1.19 (1.04, 1.36) | 1.06 (0.92, 1.23) |
RR, rate ratio; MV, multivariate; CI, confidence interval.
aAdjusted for current age (continuous months) and calendar time (2-year questionnaire period).
bAdjusted for current age (continuous months), calendar time (2-year questionnaire period), birthweight (not full-term, <5.5, 5.5–6.9, 7.0–8.4, >8.4 pounds), age at menarche (<10, 10, 11, 12, 13, 14, 15, >15 years), parity (0, 1+), oral contraceptive use (never, past, current) and adult body mass index (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, ≥30 kg/m2).
cAdjusted for the same factors as above, plus figure at age 5 years (for change from ages 5 to 10 years and from ages 5 to 20 years) or at age 10 (for change from ages 10 to 20 years), each modeled as an ordinal variable.
We repeated our main analyses without censoring on first report of infertility without endometriosis diagnosis to assess what effect our censoring scheme had on the results. When we allowed these women to continue contributing person-time, we observed 829 additional cases of laparoscopically confirmed endometriosis. These cases had almost the exact same distribution of age 5, 10 and 20 body sizes as did cases without a history of infertility. The associations between body sizes and endometriosis were essentially unchanged when these cases were allowed into the analysis. Additionally, we also repeated our analyses allowing women who reported endometriosis without laparoscopic confirmation to be included as cases. When these 1170 women were included, we found that the analyses were unchanged. For example, the RRs (95% CIs) for age 5 body sizes 1, 2, 4 and ≥5 compared with category 3 were 1.21 (1.06–1.38), 1.00 (0.88–1.14), 0.86 (0.73–1.02) and 0.90 (0.73–1.11), respectively. These associations are almost identical to those shown in Table II.
Discussion
In this prospective study among premenopausal women, we observed significant inverse associations between incidence of laparoscopically confirmed endometriosis and body size during childhood and early adulthood. There was a significant 18% increase in risk comparing the smallest figure size during childhood to the middle category and a borderline significant 18% reduction in risk comparing the largest size to the referent. The associations at age 20 were similar. While there was no significant heterogeneity by case definition, the associations appeared stronger among cases with no past or concurrent infertility. There was some evidence of effect modification by parity. We found that decreases in body size from ages 5–20 and 10–20 were associated with increases in risk, but increases in size were not associated with endometriosis risk. The inverse associations were independent of adult body mass index, age at menarche and menstrual cycle characteristics, suggesting that these variables may not be part of the biological mechanism by which childhood and early adulthood body size could influence risk of endometriosis.
To date, three studies have examined the association between childhood and adolescent body size and endometriosis. In a prospective analysis within the same cohort as the present analysis, a significant inverse association was found using BMI at age 18 (Missmer et al., 2004a). Two case–control studies have also examined the relationship. Hediger et al. (2005) used the same 9-level figure drawing in a study of 32 surgically confirmed cases and 52 controls who had no diagnosis of endometriosis after a laparoscopy for another benign condition. These subjects reported their perceived body sizes during 5-year increments from ages 15 to 45. Within each 5-year age range, the cases reported smaller body sizes than did the controls, on average. In a recent study with 268 surgically confirmed cases and 244 controls, the authors examined relative weight at ages 10 and 16 (Nagle et al., 2009). The controls were identified through a twin registry and one twin per twin pair was randomly selected and frequency matched to cases. Cases and controls were asked to report their weight as underweight, average weight or overweight. When available, the subjects' mothers also reported relative weight. Associations were examined using self-report, mothers' report and concordant reports between the participants and their mothers. At age 10, the authors found a significant association with endometriosis when comparing self-reported overweight to average weight (OR = 2.8, 95% CI 1.1, 7.5) and no associations with underweight. At age 16, there was no association of endometriosis with being underweight or overweight by self-report, but significant increases in risk for being underweight were seen when using mothers' report and concordant mother–daughter reports.
Body size in adulthood has also been associated with endometriosis in previous studies. In a 2004 analysis in the present study cohort, body mass index was inversely associated with laparoscopically confirmed endometriosis among cases with concurrent infertility at the time of diagnosis, but not among all women or cases with no history of infertility (Missmer et al., 2004a). Among infertile women, compared with women with a body mass index of 19–20.4, women with body mass index ≥30 had a 60% reduction in risk of endometriosis (RR = 0.4, 95% CI 0.2–0.7). Additionally, lower body mass index has been significantly associated with increased endometriosis risk in case–control studies (Hemmings et al., 2004; Ferrero et al., 2005; Hediger et al., 2005; Matalliotakis et al., 2007).
There is strong circumstantial evidence that endometriosis is dependent on circulating steroid hormones. The disease has only rarely been reported in pre-pubertal girls (Marsh and Laufer, 2005) and the rare cases in post-menopausal women have only been in those who were exposed to hormone replacement therapy (Houston, 1984). Thus factors that may alter estrogen status such as age at menarche (Apter et al., 1989; MacMahon et al., 1982) and body mass index (De Ridder et al., 1990; De Ridder et al., 1992; Wabitsch et al., 1995; Van Hooff et al., 2000) may influence the incidence of endometriosis. It is not clear whether endogenous hormone levels influence the volume of retrograde menstruation or if they are involved in the promotion of extra-uterine implant survival. Hormone levels in childhood and adolescence may be the most relevant exposure if there is something about the early menstrual cycles that is especially significant in the pathogenesis of endometriosis. MacMahon et al. (1982) suggested that ‘menarche initiates a short-term period during which dramatic shifts in hormonal milieu act to initiate neoplasia and other abnormal growth, however the milieu returns to a more typical level by mid-age or perhaps as early as the end of the teen years once cyclicity is established.’
Overweight girls experience earlier menarche than normal weight girls (Meyer et al., 1990; Moisan et al., 1990a; Moisan et al., 1990b; Maclure et al., 1991; Moisan et al., 1991; Merzenich et al., 1993; Cooper et al., 1996; Koprowski et al., 1999; Berkey et al., 2000; Freedman et al., 2002; Koziel and Jankowska, 2002) and an earlier age at menarche is associated with increased risk of endometriosis (Cramer et al., 1986; Signorello et al., 1997; Moen and Schei, 1997; Missmer et al., 2004c). For this reason, we might have expected to see an increase in risk with larger body sizes, but in fact we saw the opposite association. The significant inverse association remained robust after adjustment for age at menarche. A possible explanation for this may be that the effect occurs through a hormonal pathway. Insulin resistance and hyperinsulinemia are present in obese pre-adolescent and adolescent girls (Caprio et al., 1995, 1996) and high circulating levels of insulin can impair oocyte maturation, stimulate androgen production in the ovary and lead to anovulation (Stoll et al., 1994; Stoll, 1998a, b). A recent prospective study of girls found that greater body mass index was associated with lower levels of sex hormone-binding globulin (SHBG) in both premenarcheal and postmenarcheal girls, and there was some evidence of decreased estradiol and testosterone in premenarcheal girls with high body mass index (Baer et al., 2007).
High levels of androgens in adolescent girls are associated with metabolic features of polycystic ovarian syndrome (Apter et al., 1995), a greater frequency of anovulatory cycles (Stoll, 1998a, b), and reduced fertility later in life (Apter and Vihko, 1990). In a previous study conducted among participants in this cohort, higher body mass index at age 18 years was associated with an increased risk of irregular and long menstrual cycles between ages 18 and 22 years as well as an increased risk of ovulatory infertility in adulthood (Rich-Edwards et al., 1994). Though birthweight, body size at ages 5 and 10, and body mass index at age 18 were not associated with sex hormone levels in the mid- to late premenopausal women in this cohort (Tworoger et al., 2006), it may be that adult hormone levels are not related to risk of endometriosis if the critical time for initiation and promotion of endometriotic lesions is during the adolescent years.
The observed inverse associations between childhood and early adulthood body size and endometriosis risk may reflect differences in insulin-like growth factors (IGF). In a cross-sectional analysis in the NHSII, adult plasma levels of IGF-1 were significantly inversely associated with body size at ages 5 and 10 and body mass index at age 18 (Schernhammer et al., 2007). A small study involving paired specimens of eutopic and ectopic endometrial tissue from women with endometriosis and eutopic endometrium from controls found that eutopic tissue from endometriosis cases was significantly less susceptible to spontaneous apoptosis than tissue from controls (Gebel et al., 1998). Additionally, in paired samples from the cases, spontaneous apoptosis of ectopic tissue was always less than that in eutopic tissue. The peritoneal fluid of women with endometriosis has been shown to hydrolyze IGF-binding protein-3 (IGFBP-3), increasing the local bioavailability of IGF-1, which has been shown to be a factor that prevents apoptosis (Koutsilieris et al., 2001) and acts mitogenically on endometrial cells (Koutsilieris et al., 1997). Higher plasma and peritoneal fluid IGF-1 levels have been reported in women with endometriosis, particularly those with more advanced disease (Gurgan et al., 1999; Kim et al., 2000; Druckmann and Rohr, 2002).
This is the first prospective study of childhood and early adulthood body size and endometriosis. The analyses are strengthened by our ability to adjust for a number of early life and adulthood variables, including birthweight, menstrual cycle characteristics, and body mass index in later life. We began our analyses adjusting for factors that we believed to be potential confounders. We then added individually to multivariate models: age at menarche, menstrual cycle length and regularity, and adult body mass index, which are potential intermediates on the causal pathway between body size and endometriosis. We consistently observed a robust significant inverse association when each new variable was added. This implies that the mechanism in which early life body size might influence risk of endometriosis is independent of these potentially intermediate factors. However, we cannot rule out possible confounding by unmeasured variables. Additionally, there may be misclassification of the outcome and exposures. We defined cases as those who self-reported laparoscopically confirmed endometriosis. In a validation study within the NHSII, we found that the diagnosis of endometriosis could be confirmed in 96% of the medical records of women who self-reported laparoscopically confirmed endometriosis. Therefore a small percentage of our cases may be misclassified, but we do not expect that this small number would have affected our results. Although two well-designed validation studies (Must et al., 1993, 2002) demonstrated that long-term recall of body size using this figure drawing has good correlations with BMI at the same ages, no participants in either of those studies recalled their figures as greater than level 7 at young ages; hence, the accuracy of recall at the highest levels of body size could not be assessed. Furthermore, the validation studies showed that women who were obese had a greater tendency to underestimate their body size at young ages than those who were lean, which could exaggerate the observed association for less extreme levels of body size. However, this would not explain the overall association, and the decreasing trend that we observed in age at menarche across all levels of body size at age 10 years is strong evidence of the validity of our assessment. The drawings used depict mature adult body shapes, not childhood shapes and therefore it might have been difficult for participants to select a figure to represent their shape in childhood. However, the drawings typify the undifferentiated obesity seen in childhood (Mueller, 1982). The participants in this study were age 25–42 at baseline and the average age and endometriosis diagnosis was 36. We cannot draw any conclusions regarding early diagnosis of endometriosis from this study.
These data indicate that body size during childhood and early adulthood may be inversely related to the incidence of laparoscopically confirmed endometriosis, independent of adult body mass index and menstrual cycle characteristics. The large sample size and prospective design of the NHSII offer a unique opportunity to add to the limited knowledge of childhood and adolescent body size and endometriosis. Further analyses to replicate these findings will help to clarify this relationship.
Authors' roles
A.F.V., H.J.B., S.E.H. and S.A.M. contributed to the concept and design of the analysis. The data analysis was completed by A.F.V. A.F.V. and S.A.M. drafted the manuscript and all authors critically revised the manuscript. S.E.H. and S.A.M. obtained funding for the research.
Funding
This project was supported by NICHD grants HD48544 and HD52473, NIH grant CA50385, and the Eleanor and Miles Shore 50th Anniversary Scholars in Medicine Fellowship.
Acknowledgements
We thank the participants in the NHSII for their continued cooperation and dedication to the study.
References
- Apter D, Vihko R. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab. 1990;71:970–974. doi: 10.1210/jcem-71-4-970. [DOI] [PubMed] [Google Scholar]
- Apter D, Reinila M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783–787. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SS. Metabolic features of polycystic ovary syndrome are found in adolescent girls with hyperandrogenism. J Clin Endocrinol Metab. 1995;80:2966–2973. doi: 10.1210/jcem.80.10.7559882. [DOI] [PubMed] [Google Scholar]
- Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, Willett WC. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7:R314–R325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer HJ, Colditz GA, Willett WC, Dorgan JF. Adiposity and sex hormones in girls. Cancer Epidemiol Biomarkers Prev. 2007;16:1880–1888. doi: 10.1158/1055-9965.EPI-07-0313. [DOI] [PubMed] [Google Scholar]
- Barbieri RL. Endometriosis and the estrogen threshold theory. Relation to surgical and medical treatment. J Reprod Med. 1998;43:287–292. [PubMed] [Google Scholar]
- Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–2409. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Gardner JD, Frazier AL, Colditz GA. Relation of childhood diet and body size to menarche and adolescent growth in girls. Am J Epidemiol. 2000;152:446–452. doi: 10.1093/aje/152.5.446. [DOI] [PubMed] [Google Scholar]
- Caprio S, Hyman LD, Limb C, McCarth S, Lange R, Sherwin RS, Shulman G, Tamborlane WV. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269:E118–E126. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- Caprio S, Bronson M, Sherwin RS, Rife F, Tamborlane WV. Co-existence of severe insulin resistance and hyperinsulinaemia in pre-adolescent obese children. Diabetologia. 1996;39:1489–1497. doi: 10.1007/s001250050603. [DOI] [PubMed] [Google Scholar]
- Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. Br J Obstet Gynaecol. 1996;103:814–817. doi: 10.1111/j.1471-0528.1996.tb09879.x. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–1908. [PubMed] [Google Scholar]
- Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, Selman S. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology. 1993;4:135–142. doi: 10.1097/00001648-199303000-00009. [DOI] [PubMed] [Google Scholar]
- De Ridder CM, Bruning PF, Zonderland ML, Thijssen JH, Bonfrer JM, Blankensein MA, et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J Clin Endocrinol Metab. 1990;70:888–893. doi: 10.1210/jcem-70-4-888. [DOI] [PubMed] [Google Scholar]
- De Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75:442–446. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- Druckmann R, Rohr UD. IGF-1 in gynaecology and obstetrics: update 2002. Maturitas. 2002;41:S65–S83. doi: 10.1016/s0378-5122(02)00016-6. [DOI] [PubMed] [Google Scholar]
- Duleba AJ. Diagnosis of endometriosis. Obstet Gynecol Clin North Am. 1997;24:331–346. doi: 10.1016/s0889-8545(05)70307-7. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121:94–98. doi: 10.1016/j.ejogrb.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042–1047. doi: 10.1016/s0015-0282(98)00073-9. [DOI] [PubMed] [Google Scholar]
- Gurgan T, Bukulmez O, Yarali H, Tanir M, Akyildiz S. Serum and peritoneal fluid levels for IGF I and II and insulinlike growth binding protein-3 in endometriosis. J Reprod Med. 1999;44:450–454. [PubMed] [Google Scholar]
- Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84:1366–1374. doi: 10.1016/j.fertnstert.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings R, Rivard M, Olive DL, Poliquin-Fleury J, Gagne D, Hugo P, Gosselin D. Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81:1513–1521. doi: 10.1016/j.fertnstert.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Houston DE. Evidence for the risk of pelvic endometriosis by age, race and socioeconomic status. Epidemiol Rev. 1984;6:167–191. doi: 10.1093/oxfordjournals.epirev.a036270. [DOI] [PubMed] [Google Scholar]
- Kim JG, Suh CS, Kim SH, Choi YM, Moon SY, Lee JY. Insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP-3 protease activity in the peritoneal fluid of patients with and without endometriosis. Fertil Steril. 2000;73:996–1000. doi: 10.1016/s0015-0282(00)00493-3. [DOI] [PubMed] [Google Scholar]
- Koprowski C, Ross RK, Mack WJ, Henderson BE, Bernstein L. Diet, body size and menarche in a multiethnic cohort. Br J Cancer. 1999;79:1907–1911. doi: 10.1038/sj.bjc.6690303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski C, Coates RJ, Bernstein L. Ability of young women to recall past body size and age at menarche. Obes Res. 2001;9:478–485. doi: 10.1038/oby.2001.62. [DOI] [PubMed] [Google Scholar]
- Koutsilieris M, Lavergne E, Lemay A. Association of protease activity against IGFBP-3 with peritoneal fluid mitogens: possible implications for the ectopic growth of endometrial cells in women with endometriosis. Anticancer Res. 1997;17:1239–1244. [PubMed] [Google Scholar]
- Koutsilieris M, Mastrogamvrakis G, Lembessis P, Sourla A, Miligos S, Michalas S. Increased insulin-like growth factor 1 activity can rescue KLE endometrial-like cells from apoptosis. Mol Med. 2001;7:20–26. [PMC free article] [PubMed] [Google Scholar]
- Koziel S, Jankowska EA. Effect of low versus normal birthweight on menarche in 14-year-old Polish girls. J Paediatr Child Health. 2002;38:268–271. doi: 10.1046/j.1440-1754.2002.00793.x. [DOI] [PubMed] [Google Scholar]
- Maclure M, Travis LB, Willett W, MacMahon B. A prospective cohort study of nutrient intake and age at menarche. Am J Clin Nutr. 1991;54:649–656. doi: 10.1093/ajcn/54.4.649. [DOI] [PubMed] [Google Scholar]
- MacMahon B, Trichopoulos D, Brown J, Andersen AP, Aoki K, Cole P, deWaard F, Kauraniemi T, Morgan RW, Purde M. Age at menarche, urine estrogens, and breast cancer risk. Int J Cancer. 1982;30:427–431. doi: 10.1002/ijc.2910300408. [DOI] [PubMed] [Google Scholar]
- Marsh E, Laufer MR. Endometriosis in premenarchal girls without an associated obstructive anomaly. Fertil Steril. 2005;83:758–760. doi: 10.1016/j.fertnstert.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Cakmak H, Fragouli YG, Goumenou AG, Mahutte NG, Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet. 2007;277:389–393. doi: 10.1007/s00404-007-0479-1. [DOI] [PubMed] [Google Scholar]
- Merzenich H, Boeing H, Wahrendorf J. Dietary fat and sports activity as determinants for age at menarche. Am J Epidemiol. 1993;138:217–224. doi: 10.1093/oxfordjournals.aje.a116850. [DOI] [PubMed] [Google Scholar]
- Meyer F, Moisan J, Marcoux D, Bouchard C. Dietary and physical determinants of menarche. Epidemiology. 1990;1:377–381. doi: 10.1097/00001648-199009000-00007. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004a;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004b;82:1501–1508. doi: 10.1016/j.fertnstert.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004c;104:965–974. doi: 10.1097/01.AOG.0000142714.54857.f8. [DOI] [PubMed] [Google Scholar]
- Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian country. Acta Obstet Gynecol Scand. 1997;76:559–562. doi: 10.3109/00016349709024584. [DOI] [PubMed] [Google Scholar]
- Moisan J, Meyer F, Gingras S. Diet and age at menarche. Cancer Causes Control. 1990a;1:149–154. doi: 10.1007/BF00053166. [DOI] [PubMed] [Google Scholar]
- Moisan J, Meyer F, Gingras S. A nested case–control study of the correlates of early menarche. Am J Epidemiol. 1990b;132:953–961. doi: 10.1093/oxfordjournals.aje.a115738. [DOI] [PubMed] [Google Scholar]
- Moisan J, Meyer F, Gingras S. Leisure physical activity and age at menarche. Med Sci Sports Exerc. 1991;23:1170–1175. [PubMed] [Google Scholar]
- Mueller WH. The changes with age of the anatomical distribution of fat. Soc Sci Med. 1982;16:191–196. doi: 10.1016/0277-9536(82)90022-3. [DOI] [PubMed] [Google Scholar]
- Munoz KA, Ballard-Barbash R, Graubard B, Swanson CA, Schairer C, Kahle LL. Recall of body weight and body size estimation in women enrolled in the breast cancer detection and demonstration project (BCDDP) Int J Obes Relat Metab Disord. 1996;20:854–859. [PubMed] [Google Scholar]
- Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, Rand WM. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–679. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- Nagle CM, Bell TA, Purdie DM, Treloar SA, Olsen CM, Grover S, Green AC. Relative weight at ages 10 and 16 years and risk of endometriosis: a case–control analysis. Human Repro. 2009;24:1501–1506. doi: 10.1093/humrep/dep048. [DOI] [PubMed] [Google Scholar]
- Oral E, Arici A. Pathogenesis of endometriosis. Obstet Gynecol Clin North Am. 1997;24:219–233. doi: 10.1016/s0889-8545(05)70301-6. [DOI] [PubMed] [Google Scholar]
- Pardanani S, Barbieri RL. The gold standard for the surgical diagnosis of endometriosis: visual findings or biopsy results? J Gynecol Techn. 1998;4:121–124. [Google Scholar]
- Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Tworoger SS, Eliassen AH, Missmer SA, Holly JM, Pollak MN, Hankinson SE. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer. 2007;14:721.32. doi: 10.1677/ERC-06-0080. [DOI] [PubMed] [Google Scholar]
- Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case–control study. Ann Epidemiol. 1997;7:267–741. doi: 10.1016/s1047-2797(97)00017-3. [DOI] [PubMed] [Google Scholar]
- Stoll BA. Teenage obesity in relation to breast cancer risk. Int J Obes Relat Metab Disord. 1998a;22:1035–1040. doi: 10.1038/sj.ijo.0800769. [DOI] [PubMed] [Google Scholar]
- Stoll BA. Western diet, early puberty, and breast cancer risk. Breast Cancer Res Treat. 1998b;49:187–193. doi: 10.1023/a:1006003110909. [DOI] [PubMed] [Google Scholar]
- Stoll BA, Vatten LJ, Kvinnsland S. Does early physical maturity influence breast cancer risk? Acta Oncol. 1994;33:171–176. doi: 10.3109/02841869409098400. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety SS, Rowland LP, Sidman SW, Mathysee SW, editors. The Genetics of Neurological and Psychiatric Disorders. New York City: Raven Press; 1983. pp. 115–120. [PubMed] [Google Scholar]
- Tanahatoe S, Hompes PG, Lambalk CB. Accuracy of diagnostic laparoscopy in the infertility work-up before intrauterine insemination. Fertil Steril. 2003;79:361–366. doi: 10.1016/s0015-0282(02)04686-1. [DOI] [PubMed] [Google Scholar]
- Tehard B, van Liere MJ, Com Nougue C, Clavel-Chapelon F. Anthropometric measurements and body silhouette of women: validity and perception. J Am Diet Assoc. 2002;102:1779–1784. doi: 10.1016/s0002-8223(02)90381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Missmer SA, Baer H, Rich-Edwards J, Michels KB, Barbieri RL, Dowsett M, Hankinson SE. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:2494–2501. doi: 10.1158/1055-9965.EPI-06-0671. [DOI] [PubMed] [Google Scholar]
- Van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Insulin, androgen, and gonadotropin concentrations, body mass index, and waist to hip ratio in the first years after menarche in girls with regular menstrual cycles, irregular menstrual cycles, or oligomenorrhea. J Clin Endocrinol Metab. 2000;85:1394–1400. doi: 10.1210/jcem.85.4.6543. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Hauner H, Heinze E, Bockmann A, Benz R, Mayer H, Teller W. Body fat distribution and steroid hormone concentrations in obese adolescent girls before and after weight reduction. J Clin Endocrinol Metab. 1995;80:3469–3475. doi: 10.1210/jcem.80.12.8530585. [DOI] [PubMed] [Google Scholar]
- Witz CA. Current concepts in the pathogenesis of endometriosis. Clin Obstet Gynecol. 1999;42:566–585. doi: 10.1097/00003081-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Witz CA, Schenken RS. Pathogenesis. Semin Reprod Endocrinol. 1997;15:199–208. doi: 10.1055/s-2008-1068749. [DOI] [PubMed] [Google Scholar]