Abstract
Anterior decompression and fusion is an established procedure in surgical treatment for multilevel cervical spondylotic myelopathy (MCSM). However, contiguous corpectomies and fusion (CCF) often induce postoperative complications such as nonunion, graft subsidence, and loss of lordotic alignment. As an alternative, noncontiguous corpectomies or one-level corpectomy plus adjacent-level discectomy with retention of an intervening body has been developed recently. In this study, we prospectively compared noncontiguous anterior decompression and fusion (NADF) and CCF for MCSM in terms of surgical invasiveness, clinical and radiographic outcomes, and complications. From January 2005 to June 2007, 105 patients with MCSM were randomized to NADF group (n = 55) and CCF group (n = 50), and followed up for average 31.5 months (range 24–48 months). Average operative time and blood loss decreased significantly in the NADF group as compared with those in the CCF group (p < 0.05 and <0.001, respectively). For VAS, within 3 months postoperatively, there was no significant difference between the two groups. But at 6 months after surgery and final follow-up, VAS improved significantly in NADF group than that in CCF group (p < 0.05). No significant difference of JOA score was observed between the two groups at every collection time. In NADF group, all 55 cases obtained fusion at 1 year after operation (average 5.1 months). In CCF group, 48 cases achieved fusion 1 year postoperatively, but the other 2 cases were performed posterior stabilization and achieved fusion 6 months later. The differences of cervical lordosis between two groups were insignificant at the same follow-up time. But the loss of lordosis and height of fusion segments in 6 months postoperatively and final follow-up were significantly more in CFF group than in NADF group (p < 0.001). Complications were similar in both groups. But in CCF group three cases needed reoperation, one case with extradural hematoma was immediately re-operated after anterior decompression and two cases mentioned above were performed posterior stabilization at 1 year postoperatively. In conclusion, in the patients with MCSM, without developmental stenosis and continuous or combined ossification of posterior longitudinal ligaments, NADF and CCF showed an identical effect of decompression. In terms of surgical time, blood loss, VAS, fusion rate and cervical alignment, NADF was superior compared with CCF.
Keywords: Anterior, Noncontiguous decompression and fusion, Cervical spondylotic myelopathy, Multilevel, Prospective study
Introduction
The optimal surgical management of multilevel cervical spondylotic myelopathy (MCSM) remains controversial. Anterior decompression and fusion has been widely adopted and accepted as an effective and safe procedure with satisfactory results for MCSM [14–16, 22, 26]. Discectomy and corpectomy are the main procedures to anterior decompression of the cervical spine, including the resection of disc material and posterior osteophytes impinging on the spinal cord at or immediately adjacent to the level of the disc space [5, 11, 15, 16, 18]. In cases where three or more levels are affected, the techniques most frequently employed are contiguous corpectomies and fusion (CCF). However, the incidence of complications result from this surgical modality such as graft displacement, nonunion and hardware-related complications, increased as more levels were decompressed [4, 10, 19, 21, 31]. As an alternative, noncontiguous corpectomies or one-level corpectomy plus adjacent-level discectomy with retention of an intervening body, we defined it as noncontiguous anterior decompression and fusion (NADF) (Fig. 1), has been developed recently [2, 17]. But to our knowledge there was no report to compare these two different anterior procedures of cervical surgeries in prospective case study.
Fig. 1.
In multilevel cervical spondylotic myelopathy with three discs involved that should be decompressed (a), one-level corpectomy plus adjacent-level discectomy with retention of an intervening body can be performed (b). If four level discs need to be decompressed (c), two noncontiguous copectomies can be done with reservation of the middle vertebral body (d)
Therefore, the purpose of this study was to compare NADF and CCF in a prospective and randomized manner with focus on surgical invasiveness, clinical and radiographic outcomes, and complications.
Materials and methods
In the period of January 2005 to June 2007, 110 patients with MCSM were admitted in our units. The inclusion criterion was that the patient had myelopathy in physical examination and the spinal cord compression was seen in MRI at three or four disc levels. Five-level cases were excluded for the small population. The exclusion criteria were the developmental stenosis, continuous or combined ossification of the posterior longitudinal ligament and previous history of cervical spine surgery.
Each case of these 110 patients was given a serial number according to the consecutive sequence of hospitalization, and assigned to NADF group or CCF group randomly by computer according to the serial number. In the follow-up period, 5 patients were lost within 6 months postoperatively, and 105 patients included in this study. Among these 105 patients, there were 63 males and 42 females with average age 60.2 years (38–78 years). Written consent to participate in this study was obtained from all patients. Data were collected prospectively by independent observers using standardized data collection forms.
Surgical technique
All patients underwent a right anterior cervical approach. The surgical procedure of NADF group included a corpectomy plus a discectomy with retention of an intervening body in three levels involved (Fig. 2), and noncontiguous two-level corpectomies with retention of an intervening body in four levels involved (Fig. 3). Extensive decompression was performed including removal of the osteophytes, herniated nucleus pulposus and posterior longitudinal ligament to expose the dura throughout the length of the corpectomy and discectomy. PEEK cage (SCIENT’X Company, France) or titanium mesh (Synthes Inc, Switzerland) filled with the bone dust of the resected vertebra was placed in the anterior trench as well as instrumented an anterior cervical plate (Zephir, Medtronic Inc, USA; Vectra, Synthes Inc, Switzerland). The surgery was performed with fluoroscopic control.
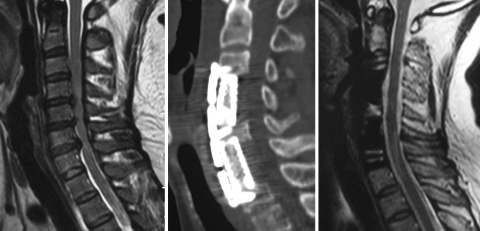
Fig. 2.
Preoperative sagittal T2-weighted image showed that the spinal cord compressed by C4/5,5/6,6/7 disc. In the level of C4/5 and C5/6, spinal cord compressed more severely than in C6/7 (1). Corpectomy of C5 and discectomy of C6/7 were performed with C6 body preserved (2 and 3)
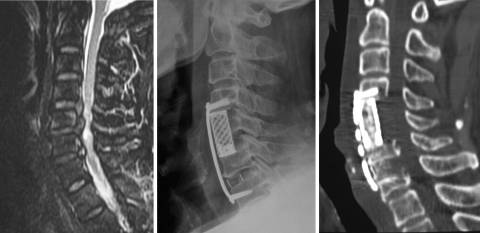
Fig. 3.
Preoperative sagittal T2-weighted image showed that the spinal cord compressed by four levels from C3/4 to C6/7 (1). Postoperative sagittal CT construction showed that corpectomies of C4 and C6 were performed and C5 body was reserved intact (2). Postoperative sagittal T2-weighted image showed that the spinal cord decompressed completely (3)
In the CCF group, two-level contiguous corpectomies were done in the three levels involved and three-level contiguous corpectomies were done in the four levels involved. A long titanium mesh filled with the bone dust of the resected vertebra was placed in the anterior trench. And anterior cervical plate was instrumented.
After surgery all patients were allowed to sit up the first postoperative day and walk on the second postoperative day with the cervical collar. The collars were applied within the first 3 months postoperatively.
Outcome measures
The visual analog scale (VAS; 0 mm = no pain, 100 mm = worst imaginable pain) was used to rate their neck pain preoperatively and at 1 day, 1 week, 4 weeks, 3 months, 6 months after surgery and final follow-up. Japanese Orthopedic Association (JOA) score was recorded for cervical myelopathy before and after surgery. Radiographs were taken in neutral laterally and extension–flexion positions at 1 day, 1, 3, 6, 9, 12 months and final follow-up to evaluate the sagittal alignment of cervical spine and the bony fusion after surgery. The C2–C7 tangent angle was measured as cervical lordosis. The height of the fusion segments was measured at the midportion of the adjacent upper and lower endplates. Loss of cervical lordosis and height of fusion segments was recorded at 1, 3, 6, 9, 12 months postoperatively and final follow-up when compared with that at day 1 after surgery. If it was difficult to decide whether bony fusion was obtained from radiograph, two-dimensional CT was taken additionally. Radiograph fusion criteria included the presence of bony trabeculation and absence of bony lucency at the strut/vertebral endplate interface, while two-dimensional CT studies additionally demonstrated ingrowth of bone into the central canal of the bony strut emanating from the endplate [7]. Radiograph and two-dimensional CT studies were independently evaluated by two spine surgeons and one radiologist. When there was a difference between the results of the two spine surgeons, the decision was made by the radiologist.
Follow-up data were collected by an independent observer.
Statistical analysis
SPSS statistical program (version 10.0) was used for the statistical analysis. The results are given as mean and SD or range. The t test was used to perform statistical comparisons. p values < 0.05 were considered statistically significant.
Results
All the 105 patients were followed up for 24–48 months (mean 31.5 months). Of the 105 patients, 55 were in the NADF group and 50 were in the CFF group. There were no significant differences between two groups with regard to patient gender, age and levels involved (Table 1). The average operative time was 140 min in the NADF group and 168 min in the CCF group, the difference between two groups was significant (p < 0.05). The average blood loss during surgery was 269 ml in NADF group, significantly lower than CCF group with 379 ml (p < 0.001) (Table 1).
Table 1.
Patient demographics
| NADF | CFF | p | |
|---|---|---|---|
| Total patients | 55 | 50 | NS |
| Male/female | 33/22 | 30/20 | NS |
| Mean age (years) | 59.7 | 60.8 | NS |
| No. of levels | |||
| 3 | 45 | 42 | NS |
| 4 | 10 | 8 | NS |
| Operative time (min) | 140.2 ± 27.1 | 168.3 ± 31.7 | <0.05 |
| Blood loss (ml) | 269.1 ± 97.2 | 378.6 ± 111.4 | <0.001 |
Values are mean ± SD
NS not significant
VAS for neck pain
The mean VAS scores for each data collection time are depicted in Table 2. There was no significant difference between the two groups before surgery. And within 3 months after surgery, there was no significant difference in either group. But at 6 months postoperatively and final follow-up, VAS in NADF group was 8.2 and 9.5, which were significantly lower than in CCF group (p < 0.05), 13.3 and 14.3, respectively. There were no patients with a daily demand of NSAIDs for axial neck pain in both groups at final follow-up. But five patients (10%) in CCF group need an occasional medication of NSAIDs at final follow-up. The radiographic studies of these five cases showed no significant difference compared with other 45 patients in fusion rate, loss of lordotic angle and height of fusion segments.
Table 2.
VAS of the patients
| NADF | CCF | p | |
|---|---|---|---|
| Pre-op. | 50.1 ± 13.7 | 49.3 ± 13.3 | NS |
| Post-op. | |||
| 1 day | 45.2 ± 12.7 | 44.8 ± 13.3 | NS |
| 1 week | 29.8 ± 10.3 | 31.2 ± 9.6 | NS |
| 1 month | 13.6 ± 8.2 | 15.7 ± 8.1 | NS |
| 3 months | 9.3 ± 6.4 | 12.6 ± 7.5 | NS |
| 6 months | 8.2 ± 5.9 | 13.3 ± 7.1 | <0.05 |
| Final follow-up | 9.5 ± 5.8 | 14.3 ± 8.1 | <0.05 |
Values are mean ± SD
NS not significant
Neurological function
In NADF group, 44 patients reported subjective neurological improvement postoperatively, 11 patients demonstrated unchanged neurological status. In CCF group, 41 cases indicated neurological improvement postoperatively and 9 patients remained unchanged. The difference between both groups was not statistically significant. JOA score was 9.1 in the NADF group and 8.8 in the CCF group before surgery, 13.2 and 13.4 at 6 months after surgery, and 14.0 and 14.1 at final follow-up. There was no significant difference between the two groups. There were two patients, including one with three levels involvement and one with four levels involvement, who had deterioration of the JOA score of 1 point at final follow-up compared with 6 months postoperatively in CCF group. Radiograph studies showed that fusion was obtained in both the patients. Loss of lordotic angle and height of fusion segments was 4.2° and 4.8°, 3.5 and 4.1 mm, respectively. Both MRI showed no compressed spinal cord. No further management was performed in these two patients.
Radiological outcomes
No hardware complications were noted in both the groups. In NADF group, all 55 cases obtained fusion at 1 year after operation with a mean period of 5.1 months, among which 52 cases (94.5%) achieved fusion within 6 months postoperatively. In CFF group, 18 cases (36%) did not achieve fusion at 6 months postoperatively. With a longer immobilization of neck brace 16 cases achieved fusion 1 year postoperatively. But the other two cases (4%) were performed posterior stabilization and fusion was noted 6 months later. The difference of fusion time between two groups was significant (p < 0.01).
The cervical alignment of neutral position in the two groups is summarized in Table 3. The differences of cervical lordosis between the two groups were insignificant at the same follow-up time. But the loss of lordosis and height of fusion segments in 6 months postoperatively and final follow-up were significantly more in CFF group than in NADF group (p < 0.001).
Table 3.
Radiographic outcomes of the patients
| NADF | CFF | p | |
|---|---|---|---|
| Lordosis of C2-7 (degrees) | |||
| Preoperative | 6.4 ± 8.6 | 6.7 ± 8.0 | NS |
| 1 day post-op. | 14.2 ± 7.2 | 13.1 ± 7.8 | NS |
| 6 months post-op. | 13.4 ± 6.6 | 11.2 ± 7.2 | NS |
| Final follow-up | 12.8 ± 6.3 | 9.1 ± 6.8 | NS |
| Loss of cervical lordosis (degrees) | |||
| 6 months after op. | 0.8 ± 0.9 | 2.0 ± 1.0 | <0.001 |
| Final follow-up | 1.4 ± 1.3 | 4.0 ± 1.4 | <0.001 |
| Loss of height of fusion segments (mm) | |||
| 6 months after op. | 0.8 ± 0.5 | 1.9 ± 0.7 | <0.001 |
| Final follow-up | 1.0 ± 0.6 | 3.1 ± 0.9 | <0.001 |
Values are mean ± SD
NS not significant
Surgical complications
Surgical complications were seen in 17 patients in this series, 8 in NADF group and 9 in CCF group (Table 4). There were no incidences of infections, esophageal or tracheal ruptures and death in this series. Seven patients complained of axial pain, which subsided gradually in 1–3 months without any treatment. One patient in CCF group had neurological deterioration 4 h after surgery, caused by extradural hematoma and recovered gradually when the hematoma was removed at once. Cerebral fluid leakage was noted in three cases, two in NADF group and one in CCF group. Two patients in CCF group, did not achieve fusion at 1 year postoperatively, were re-operated posteriorly to stabilize cervical spine and benefit for fusion 6 months later.
Table 4.
Surgical complications of the patients
| Complications | NADF | CCF |
|---|---|---|
| Hematoma | 0 | 1 |
| Cerebral fluid leakage | 2 | 1 |
| Re-operation posteriorly | 0 | 2 |
| Recurrent laryngeal nerve palsy | 1 | 1 |
| C5 paresis | 2 | 0 |
| Axial pain | 3 | 4 |
Transient recurrent laryngeal nerve palsy was observed in two patients without further management, one in NADF group and the other in CCF group. C5 paresis was seen in two cases postoperatively in NADF group, which subsided gradually within 6 months with conservative therapy.
Discussion
The primary goal of surgical treatment for MCSM remains the relief of neurological compression, stabilization of cervical spine and restoration of lordotic alignment. Posterior procedures, such as laminectomy and laminoplasty, associated with significant postoperative axial pain and high incidence of postoperative kyphosis, have driven surgeons away from posterior decompressive procedures as a treatment for MCSM [11, 13, 30, 33]. Anterior discectomy has good postoperative short-term stability and clinical results in single level disc herniation. But when used for MCSM, decompression of discectomy is limited and fusion rate is poor [12, 22, 23]. And, when anterior CCF are used in MCSM, the incidence of complications increased as more levels were decompressed [4, 10, 19, 21, 31]. Supplemental posterior stabilization usually should be done to increase postoperative stability after CCF to achieve solid fusion [1, 27]. Therefore, many modified methods for the anterior procedures of MCSM were developed in response to the limitations of previous techniques [3, 9, 25, 32]. Among these techniques, NADF had been found safe and efficient for anterior decompression in patients with MCSM, obviating the need for staged circumferential procedures [2, 17, 28]. So, this study was conducted to compare the clinical and radiographic outcome of patients with MCSM who underwent NADF and CCF in prospective case study.
In this series, instead of using autografts of iliac crest or fibular bone, we prefer to reconstruct the spine with titanium mesh cages or PEEK cage packed with local corpectomy site, derived for all cases in two groups. This facilitates the grafting of autogenous local bone and obviates unexpected complications of harvesting bone block from the iliac crest or fibular bone [8]. However, TMC subsidence was a common phenomenon after anterior cervical corpectomy and fusion with TMC according to Chen’s study [4]. The level of corpectomy was a unique risk factor for severe subsidence which might have led to bad clinical results and subsidence-related complications, such as axial pain and recurrent symptoms [4]. Similarly in our study, loss of cervical lordosis and height of fusion segments in CCF group were more than in NADF group in 6 months postoperatively and final follow-up. TMC subsidence in CCF group was supposed to explain the decreased VAS in the follow-up period [4].
In surgical invasiveness, the significant difference between the two groups was in blood loss and operating time. Compared within CCF group, the reduced operating time in NADF group was considered due to absence of the procedure to perform an additional corpectomy. The vertebral body was reserved intact, resulting in less blood loss. However, whether the reserved vertebral body impaired the effect of decompression was one of the questions we wanted to investigate in this study.
Often, cervical stenosis of MCSM is confined to adjacent-level degenerated discs with the absence of bony riding behind the vertebral bodies. So, adequate decompression can often be achieved with multilevel discectomy or NADF technique [28, 32]. Corpectomy can effectively decompress the spinal cord in the level of removed vertebral body and immediately adjacent disc. In four levels of CSM, NADF can decompress the spinal cord completely. But if three levels are involved, corpectomy should be performed at the most severely involved levels [17]. And discectomy should be performed in the smaller herniated disc, removing the posterior osteophytes at or immediately adjacent to the level of the disc space. Although not a direct criterion to evaluate decompression, JOA score was used to assess the effectiveness of decompression in this study. The same JOA score improvement between the two groups indicated the effectiveness of decompression with NADF was as same as CCF.
Some authors deemed that incidence of pseudarthrosis increased in more graft–host interfaces where fusion has to occur, and advocated the use of one or more corpectomies as an alternative to multiple interbody grafts [6, 20]. But in our series, the fusion rate was not affected by greater number of graft-host interfaces. There were four graft–host interfaces in NADF group which seemed to accelerate fusion compared with two graft–host interfaces in CCF group. Moreover, long TMC graft in the CCF group may delay the fusion time and increase the incidence of pseudarthrosis [4]. To guarantee solid fusion, supplemental posterior stabilization was performed for two cases in CCF group 1 year after operation. Using in vitro mechanical testing, Singh [28] found that in the three levels involved NADF provided greater stability than the two-level continuous corpectomies. We presumed that more biomechanical stability in NADF group contributed to faster fusion and high fusion rate. But the biomechanical test for four levels involved should be done in the future.
The total complications of this series were seen in 17 cases (16.1%), similar in two groups. With regard to the severity of complications among these two groups, CCF group seemed to be more troublesome than NADF. Three cases (3/9) needed to be re-operated in CCF group. One case with extradural hematoma was immediately re-operated after anterior decompression and two cases with fusion-related complications were performed posterior stabilization at 1 year postoperatively. In NADF group, all eight cases with complications were subsided with conservative therapy. However, the incidence of complications was less than the other published series [24, 26, 29].
Optimal candidates for NADF are considered to be the patients with MCSM, with the pathology of CSM lying anteriorly mainly at disc space level. The patients with developmental spinal canal stenosis and continuous or combined ossification of posterior longitudinal ligament are contraindications for NADF technique. A potential limitation of the present study is the fact that CCF technique can decompress the contiguous or combined ossification of posterior longitudinal ligaments, which are contraindications for NADF technique. Thus the candidates for the present study were limited.
In conclusions, in the patients with MCSM, without developmental stenosis and continuous or combined ossification of posterior longitudinal ligaments, NADF and CCF showed an identical effect of decompression. In terms of surgical time, blood loss, VAS, fusion rate and cervical alignment, NADF was superior compared to CCF.
Acknowledgments
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Contributor Information
Xiao-Feng Lian, Email: Xf909@tom.com.
Jian-Guang Xu, Phone: +86-21-64369181, Email: jianguangxu2004@yahoo.com.cn.
References
- 1.Acosta FL, Jr, Aryan HE, Chou D, Ames CP. Long-term biomechanical stability and clinical improvement after extended multilevel corpectomy and circumferential reconstruction of the cervical spine using titanium mesh cages. J Spinal Disord Tech. 2008;21(3):165–174. doi: 10.1097/BSD.0b013e3180654205. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi E, Smorgick Y, Rand N, et al. Anterior decompression combined with corpectomies and discectomies in the management of multilevel cervical myelopathy: a hybrid decompression and fixation technique. J Neurosurg Spine. 2005;3(3):205–209. doi: 10.3171/spi.2005.3.3.0205. [DOI] [PubMed] [Google Scholar]
- 3.Bruneau M, Cornelius JF, George B. Multilevel oblique corpectomies: surgical indications and technique. Neurosurgery. 2007;61(3 Suppl):106–112. doi: 10.1227/01.neu.0000289723.89588.72. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chen D, Guo Y, et al. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech. 2008;21(7):489–492. doi: 10.1097/BSD.0b013e318158de22. [DOI] [PubMed] [Google Scholar]
- 5.Ebersold MJ, Pare MC, Quast LM. Surgical treatment for cervical spondylitic myelopathy. J Neurosurg. 1995;82:745–751. doi: 10.3171/jns.1995.82.5.0745. [DOI] [PubMed] [Google Scholar]
- 6.Emery SE, Bohlman HH, Bolesta MJ, et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: two to seventeen year follow-up. J Bone Joint Surg Am. 1998;80:941–951. doi: 10.1302/0301-620X.80B6.9517. [DOI] [PubMed] [Google Scholar]
- 7.Epstein NE. Circumferential cervical surgery for ossification of the posterior longitudinal ligament: a multianalytic outcome study. Spine. 2004;29(12):1340–1345. doi: 10.1097/01.BRS.0000127195.35180.08. [DOI] [PubMed] [Google Scholar]
- 8.Feneyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16:561–564. doi: 10.1097/00007632-199110001-00022. [DOI] [PubMed] [Google Scholar]
- 9.George B, Gauthier N, Lot G. Multisegmental cervical spondylotic myelopathy and radiculopathy treated by multilevel oblique corpectomies without fusion. Neurosurgery. 1999;44:81–90. doi: 10.1097/00006123-199901000-00046. [DOI] [PubMed] [Google Scholar]
- 10.Hee HT, Majd ME, Holt RT, et al. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16:1–8. doi: 10.1097/00024720-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy and cervical laminoplasty for the surgical management of multiple level spondylotic myelopathy. Spine. 1988;13:774–780. doi: 10.1097/00007632-198807000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hilibrand AS, Fye MA, Emery SE, et al. Increased rate of arthrodesis with strut grafting after multilevel anterior cervical decompression. Spine. 2002;27:146–151. doi: 10.1097/00007632-200201150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi K, Bohlman HH. Multilevel cervical spondylosis. Laminoplasty versus anterior decompression. Spine. 1995;20(15):1732–1734. doi: 10.1097/00007632-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Hwang SL, Lee KS, Su YF, et al. Anterior corpectomy with iliac bone fusion or discectomy with interbody titanium cage fusion for multilevel cervical degenerated disc disease. J Spinal Disord Tech. 2007;20(8):565–570. doi: 10.1097/BSD.0b013e318036b463. [DOI] [PubMed] [Google Scholar]
- 15.Ikenaga M, Shikata J, Tanaka C. Anterior corpectomy and fusion with fibular strut grafts for multilevel cervical myelopathy. J Neurosurg Spine. 2005;3(2):79–85. doi: 10.3171/spi.2005.3.2.0079. [DOI] [PubMed] [Google Scholar]
- 16.Ikenaga M, Shikata J, Tanaka C. Long-term results over 10 years of anterior corpectomy and fusion for multilevel cervical myelopathy. Spine. 2006;31(14):1568–1575. doi: 10.1097/01.brs.0000221985.37468.0f. [DOI] [PubMed] [Google Scholar]
- 17.Koller H, Hempfing A, Ferraris L, et al. 4- and 5-level anterior fusions of the cervical spine: review of literature and clinical results. Eur Spine J. 2007;16(12):2055–2071. doi: 10.1007/s00586-007-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990–997. doi: 10.3171/jns.1997.86.6.0990. [DOI] [PubMed] [Google Scholar]
- 19.Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cages with anterior plating. Spine. 1999;24:1604–1610. doi: 10.1097/00007632-199908010-00016. [DOI] [PubMed] [Google Scholar]
- 20.McAfee PC, Bohlman HH, Ducker TB, et al. One stage anterior cervical decompression and posterior stabilization. J Bone Joint Surg Am. 1995;77:1791–1800. doi: 10.2106/00004623-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Nakase H, Park YS, Kimura H, et al. Complications and long-term follow-up results in titanium mesh cage reconstruction after cervical corpectomy. J Spinal Disord Tech. 2006;19(5):353–357. doi: 10.1097/01.bsd.0000210113.09521.aa. [DOI] [PubMed] [Google Scholar]
- 22.Nirala AP, Husain M, Vatsal DK. A retrospective study of multiple interbody grafting and long segment strut grafting following multilevel anterior cervical decompression. Br J Neurosurg. 2004;18(3):227–232. doi: 10.1080/02688690410001732643. [DOI] [PubMed] [Google Scholar]
- 23.Rao RD, Gourab K, David KS. Operative treatment of cervical spondylotic myelopathy. J Bone Joint Surg Am. 2006;88:1619–1640. doi: 10.2106/JBJS.F.00014. [DOI] [PubMed] [Google Scholar]
- 24.Riew KD, Sethi NS, Devney J, et al. Complications of buttress plate stabilization of cervical corpectomy. Spine. 1999;24:2404–2410. doi: 10.1097/00007632-199911150-00019. [DOI] [PubMed] [Google Scholar]
- 25.Rocchi G, Caroli E, Salvati M, Delfini R. Multilevel oblique corpectomy without fusion: our experience in 48 patients. Spine. 2005;30(17):1963–1969. doi: 10.1097/01.brs.0000176327.04725.1b. [DOI] [PubMed] [Google Scholar]
- 26.Saunders RL, Pikus HJ, Ball P. Four-level cervical corpectomy. Spine. 1998;23:2455–2461. doi: 10.1097/00007632-199811150-00022. [DOI] [PubMed] [Google Scholar]
- 27.Sevki K, Mehmet T, Ufuk T, et al. Results of surgical treatment for degenerative cervical myelopathy: anterior cervical corpectomy and stabilization. Spine. 2004;29(22):2493–2500. doi: 10.1097/01.brs.0000145412.93407.c3. [DOI] [PubMed] [Google Scholar]
- 28.Singh K, Vaccaro AR, Kim J, et al. Enhancement of stability following anterior cervical corporectomy: a biomechanical study. Spine. 2004;29:845–849. doi: 10.1097/00007632-200404150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Vaccaro AR, Falatyn SP, Scuderi GJ, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410–415. [PubMed] [Google Scholar]
- 30.Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long term follow-up study over 10 years. Spine. 2001;26:1443–1448. doi: 10.1097/00007632-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Wang JC, Hart RA, Emery SE, Bohlman HH. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine. 2003;28:1016–1021. doi: 10.1097/00007632-200305150-00011. [DOI] [PubMed] [Google Scholar]
- 32.Ying Z, Xinwei W, Jing Z, et al. Cervical corpectomy with preserved posterior vertebral wall for cervical spondylotic myelopathy: a randomized control clinical study. Spine. 2007;32(14):1482–1487. doi: 10.1097/BRS.0b013e318068b30a. [DOI] [PubMed] [Google Scholar]
- 33.Yonenobu K, Hosono N, Iwasaki M, et al. Laminoplasty versus subtotal corpectomy: a comparative study of results in multisegmental cervical spondylotic myelopathy. Spine. 1992;17:1281–1284. doi: 10.1097/00007632-199211000-00004. [DOI] [PubMed] [Google Scholar]