Abstract
Study Objectives:
Sleep disordered breathing (SDB) of the obstructive type causes hemodynamic consequences, leading to an increased cerebrovascular risk. The severity of SDB at which detrimental circulatory consequences appear is matter of controversy. Aim of the present study is the investigation of cerebral hemodynamics in patients with SDB of variable severity using near-infrared spectroscopy (NIRS).
Design:
N/A.
Setting:
Sleep laboratory.
Patients or Participants:
Nineteen patients with SDB.
Interventions:
N/A.
Measurements and Results:
Patients underwent nocturnal videopolysomnography (VPSG) coupled with cerebral NIRS. NIRS data were averaged for each patient, and a new method (integral) was applied to quantify cerebral hemodynamic alterations. Nocturnal VPSG disclosed various severities of SDB: snoring (7 patients, apnea-hypopnea index [AHI] = 2 ± 2/h, range: 0.5–4.5); mild SDB (7 patients, AHI = 14 ± 8/h, range: 6.3–28.6); and severe obstructive sleep apnea syndrome (5 patients, AHI = 79 ± 20/h, range: 39.6–92.9). Relative changes of NIRS parameters were significantly larger during obstructive apneas (compared with hypopneas; mean deoxygenated hemoglobin [HHb] change of 0.72 ± 0.23 and 0.13 ± 0.08 μmol/L per sec, p value = 0.048) and in patients with severe SDB (as compared with patients with mild SDB and simple snorers; mean HHb change of 0.84 ± 0.24, 0.02 ± 0.09, and 0.2 ± 0.08 μmol/L per sec, respectively, p value = 0.020). In this group, NIRS and concomitant changes in peripheral oxygen saturation correlated.
Conclusions:
The results of this study suggest that acute cerebral hemodynamic consequences of SDB lead to a failure of autoregulatory mechanisms with brain hypoxia only in the presence of frequent apneas (AHI > 30) and obstructive events.
Citation:
Pizza F; Biallas M; Wolf M; Werth E; Bassetti CL. Nocturnal cerebral hemodynamics in snorers and in patients with obstructive sleep apnea: a near-infrared spectroscopy study. SLEEP 2010;33(2):205–210.
Keywords: Sleep disordered breathing, cerebrovascular circulation, hemodynamics, near-infrared spectroscopy
SLEEP DISORDERED BREATHING (SDB) OF THE OBSTRUCTIVE TYPE IS A CONTINUUM OF SLEEP-RELATED BREATHING ALTERATIONS CONSISTING OF recurrent upper airway obstructions with consequent intermittent hypoxia and sleep fragmentation, leading to several daytime symptoms, such as reduction in alertness, excessive daytime sleepiness, and cognitive functions, that finally constitute the obstructive sleep apnea syndrome (OSAS).1
Hemodynamic consequences of obstructive SDB include acute and chronic effects. Acutely, the recurrent cycling of apnea and recovery generates intermittent large negative intrathoracic pressures during obstructive apneas (associated with increased myocardial oxygen consumption and changes in ventricular preload and afterload), followed by rises in arterial blood pressure at the termination of the apnea, with parallel repetitive bradycardia and tachycardia episodes during sleep.2 Chronically, the impact of obstructive SDB on the circulatory system is severe and includes the development of systemic hypertension as well as the promotion of inflammation and atherosclerosis.3,4 Several studies have already proven a strong link between SDB and cardiovascular events (including stroke) and have suggested an increase in cardiovascular morbidity and mortality independent of associated cardiovascular risk factors.5–8
A pathophysiologic link between nocturnal recurrent hypoxemia and brain damage has been established by metabolic studies disclosing subtle, but irreversible, white matter and cortical metabolic impairment in patients with OSAS, even in subjects free from associated vascular risk factors.9–11 Transcranial doppler (TCD) studies assessing cerebral blood flow velocity (CBFV) of the middle cerebral artery during sleep have shown significant alterations in patients with OSAS.12 The common findings are an overall reduction of CBFV during sleep in patients with OSAS and the occurrence of CBFV swings associated with the respiratory events (with most of the authors reporting a transient compensatory increase in CBFV followed by a decrease to baseline or lower levels during obstructive apneas).13–16
Cerebral near-infrared spectroscopy (NIRS) noninvasively monitors the concentration of oxygenated and deoxygenated haemoglobin (O2Hb, HHb), thus allowing the measurement of tissue oxygen saturation (StO2) and total hemoglobin (tHb) concentration, which is considered an equivalent of blood volume in NIRS studies, in the tissue volume illuminated by near-infrared light. Four studies have applied NIRS during sleep in a total of 43 patients with SDB (data from 8 presented twice in 2 studies).17–20 Three studies showed a consistent decrease of O2Hb and StO2 together with an increase of HHb and tHb during obstructive apneas in 24 patients with OSAS. The cerebral hemodynamic changes correlated with the duration of the apnea and were associated with concomitant alterations of the cytochrome oxidase redox state.17–19 The fourth study suggested lower mean indexes of brain tissue oxygenation during sleep in 19 patients with SDB (even if independently influenced by age).20 These NIRS studies share an important limitation because they all assessed almost exclusively obstructive apneas of patients with severe SDB, without further investigating the complex disease spectrum.
In addition to having methodologic limitations, these studies did not address the question regarding a possible threshold for the impairment of cerebral circulatory autoregulation. The main aim of the current study, therefore, was to assess the impact of different types of respiratory events on cerebral hemodynamics in patients with SDB of variable severity.
METHODS
Nineteen patients (18 men, 1 woman) referred to the sleep laboratory of the Neurology Department of the University Hospital Zurich for habitual nocturnal snoring and daytime sleepiness were assessed. Clinical examination included standardized scales for the assessment of respiratory symptoms and of subjective daytime sleepiness, i.e., the Epworth Sleepiness Scale (ESS).21 Full-night videopolysomnography (VPSG) was coupled with cerebral NIRS recording performed with the OxiplexTS (ISS, Champaign, IL). The VPSG was performed and scored according to international criteria for clinical purposes.22 The study was approved by the local ethics board, and patients signed a written informed consent.
Patients
The 18 men and 1 woman (mean age 54 ± 14 years) had a mean body mass index (BMI) of 29 kg/m2 (± 5, range 21-39 kg/m2). They all had symptoms supporting the clinical diagnosis of SDB, and the mean ESS was 11 (± 5, range 3-19; 12 patients with an ESS score > 11; normal value ≤ 11).21 The nocturnal VPSG disclosed a wide spectrum of SDB, with a mean apnea-hypopnea index (AHI) of 24 events per hour (± 30, range 0-93), a mean oxygen desaturation index (ODI) of 21 events per hour (± 27, range 0-78), a mean oxygen saturation of 93% (± 4%, range 80%#97%), and a minimum oxygen saturation of 84% (± 10%, range 59%–93%). Five patients had an AHI greater than 30 per hour (severe OSAS), 7 patients had an AHI between 5 and 30 per hour (mild SDB), and 7 subjects had simple snoring (an AHI < 5/h).1,23 Clinical and polysomnographic details of our patients are shown in Table 1.
Table 1.
Clinical and polysomnographic features of the population
| Sex | Age, y | BMI, kg/m2 | ESS | AHI, no./h | ODI, no./h | Mean SaO2% | Min SaO2% |
|---|---|---|---|---|---|---|---|
| M | 56 | 33.1 | 5 | 4.5 | 3.2 | 94.7 | 88 |
| M | 47 | 31.9 | 18 | 2.1 | 4.4 | 94.3 | 85 |
| F | 83 | 34.7 | 12 | 4.3 | 4.3 | 93.3 | 89 |
| M | 58 | 24.5 | 8 | 0.2 | 0.2 | 95.5 | 92 |
| M | 26 | 24.1 | 8 | 0.5 | 1 | 96.4 | 92 |
| M | 58 | 21.1 | 14 | 1.8 | 1.8 | 94.9 | 91 |
| M | 57 | 27.4 | 11 | 1.7 | 1.7 | 95.8 | 91 |
| M | 51 | 39.4 | 13 | 13.8 | 15.8 | 92.3 | 85 |
| M | 55 | 29.1 | 14 | 11.2 | 7.9 | 95.7 | 83 |
| M | 70 | 27.8 | 16 | 28.6 | 12.4 | 95.5 | 87 |
| M | 52 | 26.6 | 18 | 10.4 | 1.1 | 97.3 | 93 |
| M | 52 | 27.5 | 10 | 6.3 | 7.6 | 95.9 | 90 |
| M | 46 | 24.3 | 14 | 8.5 | 4.9 | 96.5 | 89 |
| M | 66 | 25.3 | 6 | 20.3 | 17.3 | 93.7 | 85 |
| M | 52 | 33.1 | 15 | 72.2 | 76.2 | 91.3 | 68 |
| M | 39 | 33.9 | 12 | 92.9 | 77.6 | 84.4 | 64 |
| M | 60 | 26.4 | 20 | 80.2 | 56.1 | 93.7 | 87 |
| M | 75 | 37.7 | 12 | 64.6 | 68.3 | 79.8 | 59 |
| M | 61 | 31.9 | 3 | 39.6 | 37.7 | 94.6 | 79 |
Abbreviations: BMI refers to body mass index; ESS, Epworth Sleepiness Scale score; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; SaO2, peripheral oxygen saturation; M, male; F, female.
Objective Testing
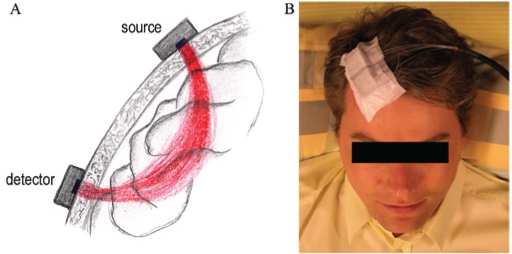
The OxiplexTS is based on a frequency-domain technology, which provides absolute values of StO2, O2Hb, HHb and tHb.24 In short, the instrument operates at 2 wavelengths, 690 nm and 830 nm, corresponding to high absorption of HHb and O2Hb, respectively. The light generated by 16 laser diodes (8 per wavelength) is intensity modulated at a frequency of 110 MHz. The light from the instrument to the tissue and back to the instrument is guided through optical fibers. The light is collected in 2 photomultiplier tube detectors and demodulated and its mean intensity, modulation amplitude and modulation phase, which correspond with the time the light needs to travel through the tissue, are determined and stored in a computer. These parameters are measured at different distances, i.e., a multidistance approach. This allows fully quantifying the light-scattering and absorption properties of the tissue. From the absorption, StO2, O2Hb, HHb, and tHb concentrations are determined. In addition, the multidistance approach subtracts the superficial layers (skin and skull) of the tissue and thus yields values of the brain (Figure 1A).25 The average intensity of the light we use images a volume of the cortical brain equal to 1 to 2 cm3. In our recordings, the sampling frequency was set at 1 Hz. A single probe with 4 light sources and 1 detector was calibrated using a calibration block of known optical properties and subsequently attached to the right forehead via a medical adhesive and shielded from external light with a cotton bend before the start of the recording (Figure 1B). NIRS data were subsequently postprocessed by OxiTS® software (Version 3.1.10, ISS Inc., Champaign, IL) and imported in the Somnologica Studio® environment (Embla Inc., Broomfield, CO) for further analysis.
Figure 1.
Light path of near-infrared (NIRS) light from the light source to the detector through the tissue (A) and positioning of the NIRS optode on the right forehead
Figure 1A shows the light path of NIRS light from a single light source through superficial layers (skin and skull) to the cerebral tissue and back to the light detector. The multidistance approach uses different light sources at specified distances from a common light detector to allow the subtraction of the influence of superficial tissue from NIRS recording. The light path is bent and reaches a maximum depth of about 2 cm beneath the center of the multidistance sensor, defining the region of sensitivity of NIRS that originates at the light source and extends to the detector.
Figure 1B shows an example of the position of the NIRS optode (i.e., a sensor with 4 light sources and 1 detector) on the right forehead. The NIRS optode is fixed to the head by a medical adhesive, before being shielded from external light with a cotton bend.
NIRS data associated with respiratory events (obstructive apneas and hypopneas) occurring in different sleep stages (non-rapid eye movement [NREM] and rapid eye movement [REM] sleep) have been exported together with SpO2 data within a time window of 40 seconds before and 80 seconds after the occurrence of a clear airflow alteration in the nasal-flow channel. Hypopnea events were included in the analysis if the reduction of nasal airflow was accompanied by a minimum of 4% of peripheral oxygen desaturation. Data associated with movement artifacts detectable on electroencephalographic channels were excluded from further analysis, as were data not related to sleep, as indicated by the VPSG.
Thereafter, we averaged NIRS (and peripheral oxygen saturation, SpO2) data within each subgroup (type of respiratory event occurring in specific sleep stage) for each patient. The averaged traces were normalized to value 0 (μmol/L for tHb, O2Hb, and HHb or percentage for StO2 and SpO2) at second 0 of the exported time window, i.e., the occurrence of the nasal-flow alteration in the VPSG. The SpO2 was plotted to detect when the SpO2 signal began decreasing and when it recovered, thus defining the length of the respiratory event. The SpO2 signal was integrated, calculating the area of the event included between the horizontal line passing through the first marker and the SpO2 average (Figure 2A). Averaged NIRS parameters were subsequently plotted together to detect when the first hemodynamic change (decrease of StO2 or O2Hb, increase of HHb) was clearly occurring close to time 0 seconds. Synchronous integrations on each NIRS parameter were applied from the start of the cerebral event, calculating areas having the length of the respiratory event, as defined on the SpO2 channel (Figure 2B). The final results of the integrals were adjusted for the duration of the respiratory event, leading to relative changes per time unit (second) of SpO2, StO2, tHb, O2Hb, and HHb.
Figure 2.
Averaged signals of (A) peripheral oxygen saturation (SpO2), and (B) oxygenated (O2Hb), deoxygenated (HHb), and total hemoglobin (tHb) concentrations during obstructive apneas in non-rapid eye movement (NREM) sleep of a patient with severe obstructive sleep apnea syndrome (OSAS).
Figure 2A shows the average of the SpO2 signal occurring during obstructive apnea in NREM sleep in a patient with severe OSAS. The arrow marks the start of the respiratory event; the grey color depicts the area of the integral calculation between the maxima SpO2 levels (respiratory-event duration).
Figure 2B shows the averages of O2Hb, HHb, and tHb concentration signals during obstructive apnea in NREM sleep of a patient with severe OSAS. The arrow marks the start of the integral calculation that is computed across the respiratory-event duration. After 5 seconds from the beginning of the obstructive apnea, the O2Hb decreases and the HHb increases abruptly (which corresponds with a severe tissue oxygen desaturation), whereas the tHb shows a concomitant transient increase. After 50 seconds from the beginning of the event, the O2Hb, HHb, and tHb concentrations are back to baseline values.
A nonparametric univariate analysis of variance (Kruskall-Wallis test) was performed to assess significant differences between different types of respiratory events and different degrees of disease severity for the relative changes of SpO2 and cerebral NIRS parameters. Subsequently, relative changes of SpO2 were related to cerebral hemodynamic changes in the different subgroups based on type of respiratory event (obstructive apneas and hypopneas) and disease severity (snorers, mild SDB, severe OSAS) using Pearson and Spearman correlations. All the analyses have been performed using the software Matlab (Version 7.3.0.298, Mathworks Inc., Natick, MA) and SPSS (Version 15.01, SPSS Inc., Chicago, IL). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
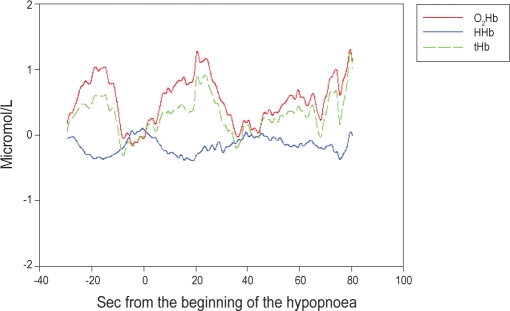
During hypopneas, a parallel change of tHb and O2Hb, with an initial decrease and subsequent recovery mirrored by opposite HHb changes, was the most common cerebral hemodynamic pattern (Figure 3). During obstructive apneas, cerebral hemodynamics disclosed a steep O2Hb decrease mirrored by an opposite HHb increase, which was associated with an opposite slight increase of tHb (Figure 2B).
Figure 3.
Averaged signals of oxygenated (O2Hb), deoxygenated (HHb), and total hemoglobin (tHb) concentrations during hypopneas in non-rapid eye movement (NREM) sleep of a patient with mild obstructive sleep apnea syndrome (OSAS). The O2Hb and tHb signals show parallel changes that are opposite to those of the HHb. After an initial decrease of O2Hb with a parallel increase of HHb (which corresponds to a tissue oxygen desaturation), a sudden increase of O2Hb and tHb with a decrease of HHb appears, consistent with an increase of local cerebral blood flow leading to a focal hyperoxygenation (i.e., a hemodynamic activation pattern).
Relative changes in NIRS parameters (and SpO2) differed significantly when comparing obstructive apneas and hypopneas: relative changes on SpO2, StO2, and HHb were higher during obstructive apneas than during hypopneas. The Kruskall-Wallis test confirmed a significant difference for SpO2 (p = 0.021, confidence interval [CI] = 0.017–0.024), StO2 (p = 0.011, CI = 0.009-0.014), and HHb (p = 0.048, CI = 0.042-0.053). SpO2 and NIRS parameters were significantly correlated (positively for StO2 and O2Hb, negatively for HHb and tHb) only during obstructive apneas (Tables 2 and 3).
Table 2.
Descriptive statistics of peripheral and cerebral hemodynamic changes associated with different types of respiratory events and disease severities
| Parameter | Hypopnea | Obstructive apnea | Snorers | Mild SDB | Severe OSAS |
|---|---|---|---|---|---|
| SpO2, % | −3.56 (0.43) | −7.18 (1.39) | −2.95 (0.39) | −2.55 (0.23) | −8.82 (1.39) |
| StO2, % | −0.57 (0.14) | −1.65 (0.45) | −0.77 (0.30) | −0.43 (0.08) | −1.76 (0.48) |
| tHb, μmol ·−1·s−1 | −0.08 (0.11) | 0.09 (0.07) | −0.04 (0.13) | −0.22 (0.14) | 0.22 (0.08) |
| O2Hb, μmol ·L−1·s−1 | −0.24 (0.09) | −0.63 (0.19) | −0.27 (0.14) | −0.27 (0.09) | −0.62 (0.22) |
| HHb, μmol ·>L−1·s−1 | 0.13 (0.08) | 0.72 (0.23) | 0.20 (0.08) | 0.02 (0.09) | 0.84 (0.24) |
Data are shown as mean (SEM) of the results of integrals for each subgroup. SDB refers to sleep disordered breathing; OSAS, obstructive sleep apnea syndrome; SpO2, peripheral oxygen saturation; StO2, cerebral oxygen saturation; O2Hb, oxygenated haemoglobin concentration; HHb deoxygenated hemoglobin concentration; tHb, total hemoglobin concentration.
Table 3.
Pearson and Spearman correlations between peripheral oxygen saturation changes and cerebral hemodynamic changes occurring in different types of respiratory events and various disease severities
| Hypopnea |
Obstructive apnea |
Snorers |
Mild SDB |
Severe OSAS |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson | Spearman | Pearson | Spearman | Pearson | Spearman | Pearson | Spearman | Pearson | Spearman | |
| StO2 | 0.23 | −0.01 | 0.79b | 0.59b | 0.01 | −0.09 | −0.29 | −0.27 | 0.78b | 0.74b |
| tHb | −0.13 | −0.20 | −0.62b | −0.61b | 0.03 | −0.01 | 0.30 | −0.04 | −0.51a | −0.41a |
| O2Hb | −0.03 | 0.08 | 0.79b | 0.44b | −0.10 | −0.01 | 0.25 | −0.11 | 0.75b | 0.63b |
| HHb | −0.19 | −0.13 | −0.89b | −0.68b | −0.08 | 0.04 | 0.35 | 0.25 | −0.86b | −0.75b |
Results are shown as correlation coefficients. SDB refers to sleep disordered breathing; OSAS, obstructive sleep apnea syndrome; StO2, cerebral oxygen saturation; tHb, total hemoglobin concentration; O2Hb, oxygenated hemoglobin concentration; HHb deoxygenated hemoglobin concentration.
p ≤ 0.05
p ≤ 0.001
Relative changes of peripheral and cerebral hemodynamic changes differed significantly when comparing patients with different disease severity (7 snorers, 7 with mild SDB, and 5 with severe OSAS), with values that were higher in patients with severe OSAS, as compared with those with mild SDB or who had simple snoring. The Kruskall-Wallis test confirmed significant differences for SpO2 (p < 0.0001, CI included between values < 0.0001), tHb (p = 0.003, CI = 0.002–0.005) and HHb (p = 0.020, CI = 0.014–0.021). SpO2 and NIRS parameters were significantly correlated (positively for StO2 and O2Hb, negatively for HHb and tHb) exclusively in the group of patients with severe OSAS (Tables 2 and 3).
DISCUSSION
This is the fifth study applying cerebral NIRS to nocturnal polysomnographic recordings of patients with SDB.17–20 For the first time, the full SDB spectrum was explored in terms of severity and types of respiratory events analyzed. In addition, a signal-analysis strategy improving the signal-to-noise ratio was used to quantify cerebral hemodynamic changes associated with respiratory events. Finally, overnight VPSG recordings were performed to assess the cerebral hemodynamics of SDB in treatment-naive patients.
The main results of the study are a qualitatively distinct behavior of cerebral hemodynamics in different types of respiratory events, with a modification of cerebral hemodynamics and SpO2 occurring selectively in patients with severe OSAS and during obstructive apneas. Although an increase of tHb (parallel to O2Hb) generally overcame brain oxygen desaturation leading to focal hyperoxygenation during hypopneas, during obstructive apneas, cerebral hypoxia mostly prevailed.
Compared with actual NIRS literature on OSAS, this study confirmed the relationship between relative changes in peripheral saturation and cerebral NIRS parameters that have already been described during obstructive apneas.17–19 Our changes in cerebral hemodynamics were higher in amplitude in obstructive apneas and in patients with severe OSAS. Moreover, in these conditions, cerebral hemodynamic changes were also significantly correlated with changes in SpO2. Conversely, minor cerebral hemodynamic changes occurring during hypopneas, as well as in patients with mild SDB or snorers, were not correlated with changes in SpO2. The results of previous NIRS studies have suggested an insufficient ability of the brain to prevent hypoxia in patients with OSAS, but these studies examined data almost exclusively from patients with severe OSAS and those with obstructive apneas.17–19 When single mild cases (with AHI < 30/h) were included in the populations, they showed smaller changes in cerebral hemodynamics, with lower individual correlations between cerebral hemodynamics and apnea duration.17,18 Moreover, the single study that included hypopneas in the analysis did not compare cerebral hemodynamics among the different types of respiratory events.18
TCD studies performed during obstructive apneas in sleep have also shown changes in cerebral hemodynamics: an increase of CBFV followed by a secondary decrease (inconstantly leading to a transient CBFV undershoot) that was paralleled by electroencephalographic arousal or reduction of systemic blood pressure.13–15 A single TCD study has been conducted that included different types of respiratory events, showing a similar frequency of reductions in the CBFV signal amplitude during obstructive apneas and hypopneas, without providing further quantitative analysis of the CBFV alterations.16 Considering the composite of all information from TCD and NIRS studies of cerebral-hemodynamic changes associated with obstructive apneas, we summarize the data by saying that, during the cessation of breathing, a transient increase of cerebral CBFV (measured by means of TCD) is not entirely sufficient to prevent tissue hypoxia (StO2 measured by means of NIRS), thus supporting the hypothesis of a failure of autoregulatory mechanisms during SDB.
Our results suggest a complex threshold mechanism (inclusive of quality and frequency of breathing alterations during sleep) in the determination of cerebral hemodynamics during SDB. During hypopneas, the brain efficiently prevents severe hypoxia. We speculate that the cerebral hemodynamics associated with hypopnea could be a cortical activation pattern evoked by the incoming progressive cerebral hypoxia (i.e., an increase of tHb and O2Hb mirrored by a decrease of HHb) analogous to those changes found in functional NIRS studies.26–28 A failure of cerebral compensatory mechanisms appears only in patients with severe obstructive SDB. We speculate that the recurrence of obstructive apneas across the night above a specific frequency (in terms of AHI) could exhaust the cerebrovascular reserve. This could be mirrored by a daytime reduction in cerebrovascular reactivity to hypercapnia, which shows major impairment in the first hours after awakening.29,30
The present study has limitations. The NIRS technique has limited spatial resolution and measures a mixture of arterial and venous blood flow; therefore, we assumed that the hemodynamic changes measured in a restricted volume of superficial cerebral tissue could be an expression of global brain changes. The understanding of the relations between macroscopic (CBFV) and microscopic (StO2) hemodynamic changes associated with SDB would also benefit from a simultaneous TCD and NIRS recording.
Our data suggest that acute cerebral hemodynamic consequences of SDB are determined by the frequency and type of respiratory event and that the combination of the 2 can lead to a failure of cerebral circulatory mechanisms and eventually brain (tissue) hypoxia.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Massimiliano Siccoli, MD, and Philipp O. Valko, MD, for helpful assistance with NIRS recordings. The study was supported by Swiss National Science Foundation, Grant 320030_125069.
Footnotes
A commentary on this paper appears in this issue on page 146.
REFERENCES
- 1.Diagnostic and Coding Manual. 2nd ed. Westbrook, IL: American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders. [Google Scholar]
- 2.Somers VK, Javaheri S. Cardiovascular effects of sleep-related breathing disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier Inc; 2005. pp. 1180–91. [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti C, M Aldrich M, Chervin R, Quint D. Sleep apnea in the acute phase of TIA and stroke. Neurology. 1996;47:1167–1173. doi: 10.1212/wnl.47.5.1167. [DOI] [PubMed] [Google Scholar]
- 5.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke. Diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 9.Alchanatis M, Deligiorgis N, Zias N, et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J. 2004;24:980–986. doi: 10.1183/09031936.04.00127603. [DOI] [PubMed] [Google Scholar]
- 10.Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, Chen W. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001;71:334–339. doi: 10.1136/jnnp.71.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonon C, Vetrugno R, Lodi R, et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30:305–311. doi: 10.1093/sleep/30.3.305. [DOI] [PubMed] [Google Scholar]
- 12.Franklin KA. Cerebral hemodynamics in obstructive sleep apnea and Cheyne-Stokes respiration. Sleep Med Rev. 2002;6:429–441. doi: 10.1053/smrv.2001.0206. [DOI] [PubMed] [Google Scholar]
- 13.Siebler M, Nachtmann A. Cerebral hemodynamics in obstructive sleep apnea. Chest. 1993;103:1118–1119. doi: 10.1378/chest.103.4.1118. [DOI] [PubMed] [Google Scholar]
- 14.Blfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med. 1994;150:1587–1591. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- 15.Hajak G, Klingelhofer J, Schulz-Varszegi M, Sander D, Ruther E. Sleep apnea syndrome and cerebral hemodynamics. Chest. 1996;110:670–679. doi: 10.1378/chest.110.3.670. [DOI] [PubMed] [Google Scholar]
- 16.Netzer N, Werner P, Jochums I, Lehmann M, Strohl KP. Blood flow of the middle cerebral artery with sleep-disordered breathing: correlation with obstructive hypopneas. Stroke. 1998;29:87–93. doi: 10.1161/01.str.29.1.87. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa T, Terashima M, Kayukawa Y, Ohta T, Okada T. Changes in cerebral oxygenation and hemodynamics during obstructive sleep apneas. Chest. 1996;109:916–21. doi: 10.1378/chest.109.4.916. [DOI] [PubMed] [Google Scholar]
- 18.Valipour A, McGown AD, Makker H, O'Sullivan C, Spiro SG. Some factors affecting cerebral tissue saturation during obstructive sleep apnoea. Eur Respir J. 2002;20:444–50. doi: 10.1183/09031936.02.00265702. [DOI] [PubMed] [Google Scholar]
- 19.McGown AD, Makker H, Elwell C, Al Rawi PG, Valipour A, Spiro SG. Measurement of changes in cytochrome oxidase redox state during obstructive sleep apnea using near-infrared spectroscopy. Sleep. 2003;26:710–716. doi: 10.1093/sleep/26.6.710. [DOI] [PubMed] [Google Scholar]
- 20.Olopade CO, Mensah E, Gupta R, et al. Noninvasive determination of brain tissue oxygenation during sleep in obstructive sleep apnea: a near-infrared spectroscopic approach. Sleep. 2007;30:1747–1755. doi: 10.1093/sleep/30.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 23.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 24.Fantini S, Franceschini MA, Fishkin JB, Barbieri B, Gratton E. Quantitative determination of the absorption spectra of chromophores in strongly scattering media: a light-emitting-diode based technique. Appl Opt. 1994;33:5204–5213. doi: 10.1364/AO.33.005204. [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Wolf M, Toronov V, et al. Noninvasive determination of the optical properties of adult brain: near-infrared spectroscopy approach. J Biomed Opt. 2004;9:221–229. doi: 10.1117/1.1628242. [DOI] [PubMed] [Google Scholar]
- 26.Obrig H, Villringer A. Beyond the visible—imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- 27.Hoshi Y. Functional near-infrared spectroscopy: potential and limitations in neuroimaging studies. Int Rev Neurobiol. 2005;66:237–266. doi: 10.1016/S0074-7742(05)66008-4. [DOI] [PubMed] [Google Scholar]
- 28.Toronov V, Webb A, Choi JH, et al. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med Phys. 2001;28:521–527. doi: 10.1118/1.1354627. [DOI] [PubMed] [Google Scholar]
- 29.Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology. 1998;51:1051–1056. doi: 10.1212/wnl.51.4.1051. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AI, Christopher Winter W, Bliwise DL. Sleep fragmentation and morning cerebrovasomotor reactivity to hypercapnia. Am J Respir Crit Care Med. 1999;160:1244–1247. doi: 10.1164/ajrccm.160.4.9810111. [DOI] [PubMed] [Google Scholar]