Abstract
Background:
Longer-term pharmacologic studies for insomnia in older individuals are sparse.
Objective:
To evaluate the efficacy and safety of 12 weeks of nightly eszopiclone in elderly outpatients with insomnia.
Methods:
Participants (65–85 years) met DSM-IV-TR criteria for insomnia with total sleep times (TST) ≤ 6 h, and wake time after sleep onset (WASO) ≥ 45 min. Participants were randomized to 12 weeks of eszopiclone 2 mg (n = 194) or placebo (n = 194), followed by a 2-week single-blind placebo run-out. Subject-reported measures of sleep (sTST, sleep latency [sSL], sWASO) and daytime function (alertness, concentration, well-being, ability to function) were assessed. AEs were monitored.
Results:
Subjects treated with 2 mg eszopiclone slept longer at night on average and at every individual time point compared to baseline than placebo subjects, as measured by TST over the 12-week double-blind period (P < 0.0001). Mean sTST over the double-blind period for eszopiclone-treated subjects was 360.08 min compared to 297.86 min at baseline, a mean change of 63.24 min. Over the double-blind period, eszopiclone-treated subjects also experienced a significantly greater improvement in sSL compared to placebo, with a mean decrease of 24.62 min versus a mean decrease of 19.92 min, respectively (P = 0.0014). Eszopiclone subjects also experienced a significantly greater decrease in WASO (mean decrease of 36.4 min) compared to placebo subjects (decrease of 14.8 min) (P < 0.0001). Post-discontinuation, sleep parameters were statistically improved versus baseline for eszopiclone (P-values ≤ 0.01), indicating no rebound. The most common AEs (≥ 5%) were headache (eszopiclone 13.9%, placebo 12.4%), unpleasant taste (12.4%, 1.5%), and nasopharyngitis (5.7%, 6.2%).
Conclusion:
In this Phase IV trial of older adults with insomnia, eszopiclone significantly improved patient-reported sleep and daytime function relative to placebo. Improvements occurred within the first week and were maintained for 3 months, with no evidence of rebound insomnia following discontinuation. The 12 weeks of treatment were well tolerated.
Clinical Trial Information:
A Long-Term Safety and Efficacy Study of Eszopiclone in Elderly Subjects With Primary Chronic Insomnia; Registration #NCT00386334; URL - http://www.clinicaltrials.gov/ct2/show/NCT00386334?term=eszopiclone&rank=24
Citation:
Ancoli-Israel S; Krystal AD; McCall WV; Schaefer K; Wilson A; Claus R; Rubens R; Roth T. A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. SLEEP 2010;33(2):225-234.
Keywords: Eszopiclone, primary insomnia, elderly, sleep, next day function
ESTIMATES OF THE PREVALENCE OF INSOMNIA IN ADULTS RANGE FROM 10% TO 40% DEPENDING UPON THE DEFINITION OF INSOMNIA USED AND THE population under investigation.1,2 The prevalence of sleep disturbances among individuals over the age of 65 is higher than in younger adults, with estimates ranging from 44% to 69%.3–7
Chronic sleep disturbances in older adults are associated with poorer health outcomes,8,9 cognitive and functional impairments,8,10,11 decrements in quality of life,12 increased nursing home placements,13 and increased risk of falls.14–16 In addition, 3 objective studies of sleep found that low sleep efficiency and insufficient sleep are associated with greater risk of mortality in older individuals.17–19 Although it is yet unknown if these relationships are casual or perhaps even bidirectional, it is clear that poor sleep is associated with poorer functional outcome.
The use of sedative-hypnotics is more prevalent in older adults than in their younger counterparts.20 Despite concerns about tolerance, dependence, and residual drug effects, older adults tend to use these medications for longer periods of time.21 The duration of treatment of nearly all published placebo-controlled trials that assess the efficacy and tolerability of sedative-hypnotics or cognitive behavioral therapy in older adults ranges from 2 to 6 weeks.22
Eszopiclone is approved for the treatment of primary insomnia including chronic use where required. It is a single-isomer, non-benzodiazepine, cyclopyrrolone agent that interacts with α 1-, 2-, 3-, and 5- GABA-A receptor subtypes. In two 2-week trials in older adults with primary insomnia, eszopiclone 2 mg improved both objective23 and subjective23,24 measures of sleep onset, sleep maintenance, and sleep quality, as well as some measures of next-day function. The current study extended the treatment period to 12 weeks of administration of eszopiclone 2 mg in older adults.
METHODS
This was a randomized, double-blind, placebo-controlled trial conducted at 82 private practice clinics and clinical research sites in the United States from October 2006 to February 2008. The protocol and informed consent form were reviewed and approved by the institutional review boards of participating institutions. The trial complied with the principles of the Declaration of Helsinki (1989) and standards of good clinical practices, and all subjects gave written, signed, informed consent prior to participation.
Patients
Eligible men and women between 65 and 85 years of age (inclusive) met DSM-IV-TR criteria for primary insomnia. Qualified participants also reported that during the month prior to screening, their total sleep times (TST) were ≤ 6 h per night for ≥ 3 nights per week, and their wake time after sleep onset (WASO) was ≥ 45 min per night for ≥ 3 nights per week. Eligible participants were required to go to bed between 21:00 and 00:00. All had Mini-Mental State Examination scores ≥ 28. Eligible participants were allowed a primary DSM-IV-TR Axis I Clinical Disorder diagnosis (or any other psychiatric diagnosis) within 6 months of screening as long as the condition was under good symptomatic control. Consistent with this, the following SSRIs were permitted as concomitant medications during the study provided participants were on a stable dose for 60 days prior to screening and remained on the same dose throughout the trial: fluoxetine (2 subjects in the eszopiclone group), sertraline (1 subject in the placebo group) paroxetine (2 subjects in the eszopiclone group), citalopram (1 subject in the placebo group), and escitalopram oxalate (1 subject in the placebo group and 3 subjects in the eszopiclone group).
Individuals with another primary or secondary sleep disorder (e.g., sleep apnea, restless legs syndrome, periodic leg movement disorder) or a known or suspected acute medical or psychiatric condition that could have affected sleep (e.g., chronic pain, benign prostatic hypertrophy) were excluded. Overnight PSG was not used to screen patients for sleep disorders. Participants with any unstable medical abnormality or chronic disease, clinically significant electrocardiographic abnormalities at screening, or any condition that could have interfered with drug metabolism (e.g., malabsorption, gastrointestinal disorder) were excluded. Any participants in a previous zopiclone or eszopiclone clinical trial, or who were previously treated with zopiclone or eszopiclone, and individuals with a history of drug or alcohol abuse or dependence within 6 months of screening were excluded. Substances affecting sleep (e.g., hypnotics, sedatives, anxiolytics, herbal supplements, antihistamines) were not permitted.
Procedures
After signing the informed consent, physical examinations, clinical laboratory assessments, and sleep, medical, and psychiatric histories were obtained at screening. Eligible participants were then provided with single-blind placebo and instructed to take the study medication nightly at bedtime for one week. Participants were also given electronic hand-held sleep/wake diaries, instructed in their use, and told to complete them twice each day. Daytime functioning parameters were recorded in the evening between 20:00 and 23:45; nighttime (sleep parameters) data were recorded in the morning between 06:00 and 10:00. Five to 9 days later, participants returned to the clinic for reassessment. Participants who took ≥ 4 doses of single-blind placebo and had TST values ≤ 6 h on ≥ 4 nights, with no night ≥ 7 h, were eligible to continue in the trial.
All continuing participants were given an additional week of single-blind placebo and instructed to take the study medication nightly at bedtime during this run-in period to establish baseline sleep. Five to 9 days later, study site personnel utilized an internet based randomization system to randomly assign patients to the study treatment. All randomized participants were dispensed double-blind study medication, and returned to the clinic at approximately 3-week intervals (21 ± 3 days) for interim clinic visits.
After 12 weeks of nightly dosing, participants entered a 4-week follow-up period to assess potential discontinuation effects. The first 2-week interval was a run-out period in which eszopiclone 2 mg was discontinued and all participants received single-blind placebo. The next 2-week interval was a no-drug period in which all study medications were withdrawn.
A subset of participants from selected sites were provided with an actigraph wrist monitor that was worn from the start of the single-blind placebo run-in period to the end of the follow-up period. The results of these actigraphy data will be the subject of a separate report.
No assessment of the success of blinding was used in this study.
Study Endpoints
The primary efficacy endpoint was the change from baseline in subject-reported TST averaged over the 12-week double-blind study period. The key secondary efficacy endpoints were the change from baseline in subject-reported SL and subject-reported WASO, both averaged over the 12-week double-blind study period. Efficacy was assessed from patient-reported data collected each morning and evening using electronic sleep/wake diaries. Efficacy variables assessed from the morning diary included sleep latency (sSL), total sleep time (sTST), wake time after sleep onset (sWASO), number of awakenings, quality of sleep, and depth of sleep. Variables assessed from the evening diary included ratings of daytime alertness, ability to function, ability to concentrate, and sense of physical well-being. These daytime function variables were assessed using an 11-point Likert scale ranging from 0 to 10, with 0 representing the least desirable outcome (e.g., “very sleepy,” “poor sleep quality”) and 10 representing the best outcome (e.g., “not sleepy at all,” “excellent sleep quality”). The number of naps taken and the length of naps were also recorded in the evening diaries. For these endpoints, averages were calculated based on the averages of the diary data for the relevant period.
Severity and impact of insomnia symptoms were assessed with an expanded version of the Insomnia Severity Index25 (ISI) which was completed at baseline (Week 0), at each 3-week interval during double-blind dosing (Weeks 3, 6, 9, and 12), and following treatment discontinuation during the follow-up period (Weeks 14 and 16). Quality of life was assessed with the 36-Item Short-Form Health Survey26 (SF-36) at baseline and at Weeks 6, 12, and 16. The Sheehan Disability Scale27 (SDS) was completed electronically at baseline and at Weeks 6, 12, 14, and 16. The Benzodiazepine Withdrawal Symptom Questionnaire28 (BWSQ) was completed at Weeks 12 and 14 to assess withdrawal post-discontinuation. Each of these assessments was completed via electronic data capture during in-clinic visits. Safety was assessed through adverse event (AE) monitoring, vital signs, and clinical laboratory tests. Specifically, AE monitoring was conducted at all study visits except screening, vital sign measurements were collected at all visits, and clinical laboratory assessments were collected at screening, the interim visit, end of double blind or the unscheduled early termination visit.
Statistical Methods
Assuming a standard deviation of 78 minutes, 300 participants (150 per treatment group) would provide 90% power to detect a treatment group difference of 30 min in sTST at the 0.05 significance level using a 2-tailed test. This sample size also provided at least 90% power to detect a 25% difference (i.e., an absolute difference of 0.288 on the log-transformed scale) between the treatment groups for sSL and sWASO, assuming standard deviations of 0.72 and 0.76, respectively, on the log-transformed scale. Approximately 430 participants were needed for randomization to ensure that 300 participants complete 12 weeks of treatment, assuming an attrition rate of 30%.
Continuous baseline and demographic variables were analyzed using an ANOVA model with fixed effects for treatment and site type. Categorical baseline and demographic variables were analyzed using the Cochran-Mantel Haenzel general association test, controlling for site type. All efficacy and safety analyses were conducted on the intent-to-treat (ITT) population, which consisted of randomized participants who received at least one dose of study medication. The efficacy analyses were based on the last-observation-carried-forward (LOCF) method, unless otherwise noted. Napping endpoints (number of naps/week and total nap time/week) were analyzed in ITT participants who reported at least one nap during the baseline period. Discontinuation effects were analyzed in participants who completed the double-blind treatment period and took at least one dose of single-blind placebo during the follow-up period.
The primary analysis compared the change from baseline in sTST averaged over the double-blind period between the eszopiclone and placebo groups using an analysis of covariance (ANCOVA) model with treatment and site type (actigraphy vs. non-actigraphy sites) as fixed effects and baseline as the covariate. The key secondary endpoints were the change from baseline in sSL and sWASO averaged over the double-blind period. These endpoints were analyzed using the same ANCOVA model as for the primary analysis, but were based on log-transformed data and used the respective parameter’s baseline (the average of the values during the week prior to randomization) sTST as a covariate. Changes from baseline in sTST, sSL, and sWASO were also evaluated weekly for the first 3 weeks, at 3-week intervals during the remainder of the double-blind treatment period (Week 6, Week 9, Week 12), and for each 2-week interval during the follow-up period (Week 14, Week 16) using the same methodology and model as for the primary analysis. All other sleep efficacy variables (e.g., number of awakenings, depth of sleep, number of naps), as well as ISI scores, SF-36 scales, and SDS scores were analyzed in a similar manner.
A sequential (step-down) approach to hypothesis testing was employed to control for multiple comparisons. Only if the primary analysis of sTST was significant at the 2-sided 5% significance level, would P-values of 0.05 or less be considered statistically significant for the treatment group comparisons of sSL. Next, if the analyses of sTST and sSL were statistically significant, then P-values of 0.05 or less were also considered to be statistically significant for the treatment group comparisons of sWASO.
Rebound insomnia was assessed for sTST, sSL, and sWASO. The change from baseline to each diary assessment during the single-blind placebo and no-drug periods was calculated. A Wilcoxon signed-rank test was performed for each treatment group to assess whether the distribution of changes from baseline was centered at zero, and between group comparisons were also performed using the same method as for the primary analysis. In addition, the difference between the treatment groups for the change in BWSQ scores from Week 12 to Week 14 was analyzed using an ANCOVA model with fixed effects for treatment and site type, and Week 12 scores as the covariate. Wilcoxon signed-rank tests were performed to compare Week 14 scores to Week 12 scores within each treatment group. LOCF methodology was not used to assess discontinuation effects.
A post hoc analysis examined the shift in distributions of the ISI categories from Week 12 to Week 14 within each treatment group using the Stuart-Maxwell test.
RESULTS
Participants
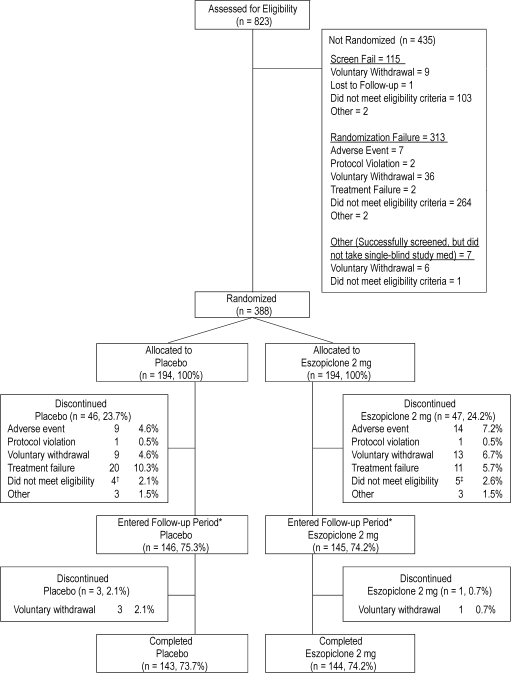
As shown in Figure 1, 823 participants were assessed for eligibility and 388 were randomized (194 in each treatment group) and received at least one dose of double-blind study medication. Approximately 74% of participants in both treatment groups completed the study.
Figure 1.
Study flow and patient disposition
*Thirty-six subjects dropped out of the study during the single-blind placebo run-in phase. The other 9 subjects were screen failures.
†3 subjects did not meet the criteria of TST ≤ 6 hours on 4 or more nights; 1 subject was non-compliant with visit schedules due to vacation.
‡1 subject did not meet the criteria of TST ≤ 6 hours on 4 or more nights; 1 subject took excluded psychiatric medications for an undisclosed psychiatric condition; 1 subject started working nights; 1 subject was enrolled in 2 investigational studies simultaneously; 1 subject had a positive urine drug screen for barbiturates.
There were no significant differences between treatment groups on any demographic or baseline sleep parameter (Table 1). Participants in both the eszopiclone (mean ISI total score of 16.1) and placebo group (mean ISI total score of 16.3) had moderate insomnia at baseline. Percent compliance during the double-blind period (the number of tablets taken divided by the number of days on-study multiplied by 100) was high in both groups (97.6% and 97.3% in the placebo and eszopiclone groups, respectively). The percentages of participants with clinically important comorbid conditions and the percentages of participants who used concomitant medications during the trial were also similar in both treatment groups (Table 2). Treatment groups were balanced for the category of psychoanaleptics, all but 2 of which were allowed by the protocol (one patient taking donepezil was discontinued; another patient taking trazodone was discontinued). Although psycholeptics were excluded, an equal percentage of patients in each treatment group used these during the study. Eight of these patients completed the study (4 of whom used the disallowed medication during the run-out period), and 6 were discontinued from the study. Slightly more patients in the placebo group used analgesics during the study compared with the eszopiclone group (26.8% vs 19.1%), though these differences were not statistically significant (p = 0.09). Additionally, in over 80% of the patients in each group the analgesics used were aspirin or acetaminophen.
Table 1.
Demographic characteristics and baseline sleep and assessment scores (ITT Population)
| Treatment |
||
|---|---|---|
| Eszopiclone 2 mg | Placebo | |
| Characteristic | (N = 194) | (N = 194) |
| Age (Years) | N = 194 | N = 194 |
| Mean (SD) | 71.6 (5.0) | 72.4 (5.2) |
| Gender | ||
| Male | 70 (36.1%) | 75 (38.7%) |
| Female | 124 (63.9%) | 119 (61.3%) |
| Racea | ||
| White | 178 (91.8%) | 181 (93.3%) |
| Black or African American | 8 (4.1%) | 9 (4.6%) |
| Asian | 4 (2.1%) | 2 (1.0%) |
| American Indian, Alaska Native | 1 (0.5%) | 0 |
| Native Hawaiian, Other Pacific Islander | 1 (0.5%) | 0 |
| Other | 2 (1.0%) | 2 (1.0%) |
| Weight, Overall (kg) | N = 193 | N = 194 |
| Mean (SD) | 76.98 (16.72) | 75.44 (17.07) |
| BMI, Overall(kg/m2) | N = 193 | N = 194 |
| Mean (SD) | 28.25 (4.90) | 27.28 (4.98) |
| Sleep Parameter | ||
| TST (minutes) | N = 191 | N = 192 |
| Mean (SD) | 297.86 (56.14) | 294.03 (63.30) |
| SL (minutes)b | N = 191 | N = 192 |
| Mean (SD) | 75.68 (56.63) | 82.17 (74.08) |
| WASO (minutes)b | N = 191 | N = 190 |
| Mean (SD) | 92.66 (58.11) | 90.86 (51.77) |
Abbreviations: BMI, body mass index; ITT, intent-to-treat; SD, standard deviation; Min/Max, minimum/maximum.
Race categories are mutually exclusive.
SL and WASO were log-transformed prior to analysis.
Notes: Percentages are based on the number of subjects in the ITT Population. Baseline age and height are based on data collected at Screening. Baseline weight is defined as the last available measurement taken at or before the first dose of double-blind medication. Baseline BMI is calculated using these height and weight measurements
Table 2.
Important comorbid conditions and concomitant medications
| Comorbid conditions by system organ class† | Placebo n (%)* | Eszopiclone 2 mg n (%)* |
|---|---|---|
| Musculoskeletal | 126 (64.9) | 125 (64.4) |
| Endocrine and metabolic | 118 (60.8) | 123 (63.4) |
| Cardiovascular | 117 (60.3) | 118 (60.8) |
| Gastrointestinal | 88 (45.4) | 89 (45.9) |
| Genitourinary | 45 (23.2) | 51 (26.3) |
| Neurologic | 34 (17.5) | 47 (24.2) |
| Psychiatric/Psychological | 18 (9.3) | 17 (8.8) |
| Concomitant medications by ATC Level 2‡ | ||
| Lipid modifying agents | 87 (44.8) | 89 (45.9) |
| Antithrombotic agents | 66 (34.0) | 62 (32.0) |
| Anti-inflammatory – antirheumatic products | 61 (31.4) | 58 (29.9) |
| Agents acting on the renin-angiotensin system | 59 (30.4) | 59 (30.4) |
| Analgesics | 52 (26.8) | 37 (19.1) |
| Mineral supplements | 45 (23.2) | 42 (21.6) |
| Psychoanaleptics§ | 5 (2.6) | 7 (3.6) |
| Psycholeptics∥ | 7 (3.6) | 7 (3.6) |
Percentage are based upon the number of ITT participants in each treatment group.
Medical histories coded using MedDRA v9.1.
Concomitant medications coded using the WHO Drug Dictionary (version 1stQ2006), Anatomical Therapeutic Chemical (ATC) classification.
Drugs that produce an arousing effect upon the patient (e.g., anti-depressants, anti-dementia agents, psychostimulants).
Drugs that produce a calming effect upon the patient (e.g., antipsychotics, anxiolytics, hypnotics, sedatives)
Sleep Efficacy
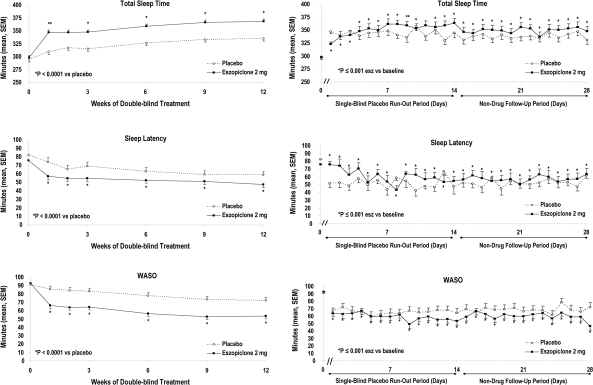
The primary endpoint of sTST and the key secondary endpoints of sSL and sWASO all significantly improved from baseline to treatment averaged over the 12 weeks (all P-values ≤ 0.001) in the eszopiclone group compared to placebo. As shown in Figure 2, these improvements were evident from the first week of dosing, and the effect was maintained at all subsequent time points during the 12-week treatment period (all P-values ≤ 0.02).
Figure 2.
Subject-reported sleep efficacy during 12 weeks of treatment and after discontinuation
Mean (SEM) sSL, sWASO, and sTST at each assessment time point during 12 weeks of double-blind treatment, and on each day after treatment discontinuation. Values represented at each week of the double-blind treatment period are for the ITT population (N = 194 in the eszopiclone group; N = 194 in the placebo group). Values represented on each day after treatment discontinuation are for those participants who completed double-blind treatment and received at least one dose of single-blind placebo (N = 145 in the eszopiclone group; N = 146 in the placebo group). Time 0 represents the baseline value at the start of the double-blind treatment period.
Other secondary endpoints were also significantly improved in the eszopiclone group compared to the placebo groups, with the reported number of awakenings per night reduced (all P-values ≤ 0.01), and quality (all P-values < 0.001) and depth of sleep (all P-values ≤ 0.001) improved at all assessed time points as well as for the 12-week average.
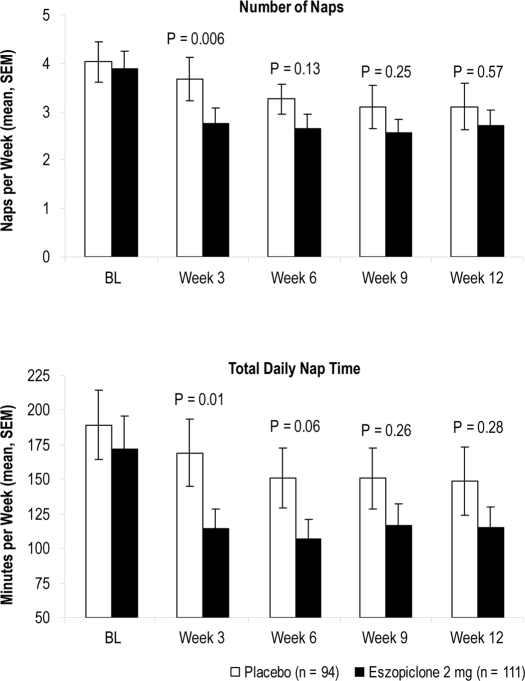
As shown in Figure 3, there were no significant differences in napping between the eszopiclone group (n = 94) and the placebo group (n = 111) during the baseline period for subjects who napped at least once during baseline. A significantly greater decrease in naps per week was noted over the first three weeks of treatment with eszopiclone (1.2 naps per week decrease) versus placebo (0.4 naps per week; P = 0.006), but not at Week 6, 9, or 12. Similar results were obtained for total nap time per week (Figure 5).
Figure 3.
Number of reported naps/week and total daily nap time/week
The mean (SEM) number of naps per week (top) and mean (SEM) total daily naptime per week (bottom) at each assessment time point. P-values are based on ANCOVA vs. placebo. BL, baseline.
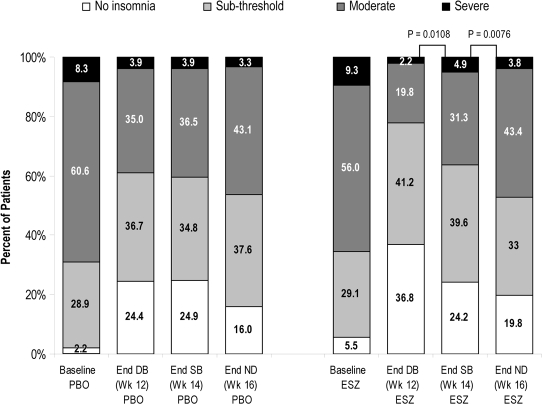
Figure 5.
Distribution of ISI total scores
Percentage of participants with ISI total scores categorized as no insomnia, sub-threshold insomnia, moderate insomnia, and severe insomnia at baseline, at the end of 12 weeks of treatment (Week 12), the end of the single-blind placebo period (Week 14), and the end of the no-drug period (Week 16). PBO, placebo; ESZ, eszopiclone 2 mg; End DB, end of double-blind period; End SB, end of single-blind placebo period; End ND, end of no-drug period. The shift in distribution of scores from Week 12 to Week 14 and Week 14 to Week 16 were analyzed using the Stuart-Maxwell test.
Insomnia Severity Index
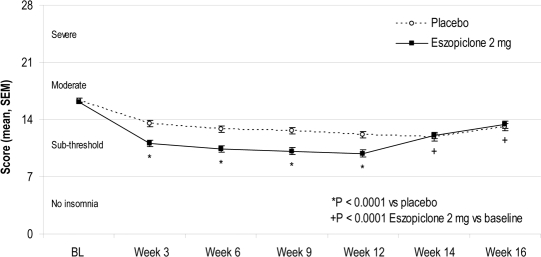
The eszopiclone-treated participants had significantly greater improvements from baseline in ISI total scores than placebo-treated participants at all time points (all P-values < 0.001, Figure 4) and for the double-blind average (P < 0.001). The percentage of participants with ISI total scores categorized as “no insomnia” and “sub-threshold insomnia” was greater in the eszopiclone group during double-blind treatment (78.0% at Week 12) than in the placebo group (61.1%; P < 0.05). There were commensurate decreases in the percentage with “moderate” and “severe” insomnia (Figure 5).
Figure 4.
Insomnia Severity Index total scores with clinical categories
Mean (SEM) ISI total scores at each assessment time point. Clinical categories were based on the ISI total scores: 0–7 = no clinically meaningful insomnia; 8–14 = sub-threshold insomnia; 15–21 = moderate insomnia; 22–28 = severe insomnia. BL, baseline.
Daytime Function
Changes from baseline in self reports of daytime alertness, ability to function, ability to concentrate, and sense of physical well-being were significantly increased in the eszopiclone group when compared with placebo at all assessment times (all P-values ≤ 0.001) and for the double-blind average (all P-values < 0.001) (Table 3).
Table 3.
Next-day function
| Daytime alertness | Week 3 | Week 6 | Week 9 | Week 12 | DB Av |
|---|---|---|---|---|---|
| Placebo | 0.4 (0.9) | 0.6 (1.3) | 0.7 (1.5) | 0.8 (1.7) | 0.6 (1.3) |
| Eszopiclone | 0.8 (1.0) | 1.0 (1.3) | 1.1 (1.5) | 1.2 (1.6) | 1.0 (1.3) |
| P-value* | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Ability to concentrate | |||||
| Placebo | 0.3 (0.9) | 0.4 (1.3) | 0.6 (1.5) | 0.7 (1.7) | 0.5 (1.3) |
| Eszopiclone | 0.7 (1.0) | 0.9 (1.2) | 1.0 (1.5) | 1.1 (1.6) | 1.0 (1.3) |
| P-value* | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Ability to function | |||||
| Placebo | 0.4 (0.9) | 0.5 (1.3) | 0.6 (1.5) | 0.7 (1.7) | 0.5 (1.3) |
| Eszopiclone | 0.7 (1.0) | 0.9 (1.3) | 1.0 (1.5) | 1.1 (1.6) | 0.9 (1.3) |
| P-value* | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Physical well being | |||||
| Placebo | 0.4 (0.9) | 0.5 (1.3) | 0.6 (1.5) | 0.7 (1.7) | 0.5 (1.3) |
| Eszopiclone | 0.7 (1.0) | 0.8 (1.3) | 0.9 (1.5) | 1.0 (1.6) | 0.9 (1.3) |
| P-value* | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Eszopiclone 2 mg vs. placebo; Values are mean changes (SD) from baseline; DB Av = 12-week double-blind average
Change from baseline (Week 0) was summarized and compared between treatment groups using an ANCOVA model with fixed effects for treatment and site and a covariate of baseline score.
Participants treated with eszopiclone also had statistically significant improvements relative to placebo in the vitality scale of the SF-36 at Week 6 (P = 0.04) and Week 12 (0.008), and in the general health scale at Week 12 (P = 0.009). There were no statistically significant differences between the treatments on the other SF-36 individual scale scores (Table 4), or on the mental or physical component summary scores. On the SDS, there were statistically significant improvements observed in the eszopiclone group relative to placebo for the social life and family life/home responsibilities items (both P-values ≤ 0.03) at Week 6, but not at Week 12. There was no statistically significant difference between the treatments on the work/school item at either time point.
Table 4.
SF-36 individual scale scores
| Physical functioning | Placebo |
Eszopiclone 2 mg |
P-value | ||
|---|---|---|---|---|---|
| Baseline | 67.9 | (26.1) | 71.4 | (23.9) | |
| Week 6 | 70.6 | (26.8) | 73.0 | (24.4) | 0.72 |
| Week 12 | 71.3 | (25.8) | 73.1 | (23.0) | 0.99 |
| Role physical | |||||
| Baseline | 65.3 | (24.1) | 66.6 | (22.6) | |
| Week 6 | 71.7 | (23.6) | 72.9 | (23.3) | 0.47 |
| Week 12 | 70.9 | 22.6) | 73.3 | (23.2) | 0.23 |
| Bodily pain | |||||
| Baseline | 71.8 | (23.5) | 73.0 | (23.7) | |
| Week 6 | 71.8 | (22.9) | 73.3 | (22.5) | 0.37 |
| Week 12 | 69.8 | (23.6) | 73.0 | (21.3) | 0.15 |
| General health | |||||
| Baseline | 71.3 | (20.4) | 71.5 | (20.3) | |
| Week 6 | 70.3 | (19.2) | 72.0 | (18.4) | 0.07 |
| Week 12 | 68.5 | (20.1) | 71.6 | (18.9) | 0.009 |
| Vitality | |||||
| Baseline | 51.6 | (20.4) | 52.4 | (23.1) | |
| Week 6 | 54.7 | (20.5) | 57.5 | (21.5) | 0.04 |
| Week 12 | 55.1 | (20.3) | 58.9 | (21.2) | 0.008 |
| Social functioning | |||||
| Baseline | 77.8 | (22.7) | 76.2 | (22.3) | |
| Week 6 | 80.5 | (21.9) | 81.1 | (21.2) | 0.19 |
| Week 12 | 79.9 | (21.4) | 78.7 | (20.9) | 0.74 |
| Role emotional | |||||
| Baseline | 74.1 | (23.0) | 74.3 | (23.8) | |
| Week 6 | 77.0 | (22.1) | 78.5 | (22.1) | 0.30 |
| Week 12 | 77.0 | (22.0) | 79.1 | (21.5) | 0.20 |
| Mental health | |||||
| Baseline | 75.2 | (16.8) | 74.5 | (18.1) | |
| Week 6 | 74.9 | (18.1) | 75.7 | (18.1) | 0.15 |
| Week 12 | 73.9 | (18.4) | 75.5 | (16.4) | 0.07 |
Data presented are means (SD)
12-week double-blind averages (computed using the LOCF imputation approach) were analyzed using the same ANCOVA model as for the primary analysis.
Safety and Tolerability
Adverse Events: The overall incidence of AEs during double-blind treatment was 59.3% for eszopiclone and 50.5% for placebo. The most common (≥ 5%) AEs reported by participants in the eszopiclone group were headache (13.9%; 12.4% for placebo), unpleasant taste (12.4% and 1.5%, respectively; the only adverse event that was significantly different from placebo [P < 0.001], and nasopharyngitis (5.7% and 6.2%, respectively). Of the 24 participants who experienced unpleasant taste in the eszopiclone group, 18 reported this adverse event as mild in severity.
AEs of interest in older adults and their incidence rates were: dizziness (4.1% and 1.5%, respectively), falls (1.0% and 0.5%, respectively), hallucinations (0.5% and 0%, respectively), memory impairment (1.0% and 0%, respectively), attention disturbance (0.5% and 0%, respectively), nervousness (1.5% and 0%, respectively), and anxiety (2.1% and 1.0%, respectively). There were 2 deaths in the eszopiclone group: a 69 year-old man who had been taking paroxetine committed suicide 12 days after completing the trial; and a 71-year-old female died as the result of arteriosclerotic heart disease. Both deaths were assessed by the investigator as unlikely to have been related to treatment. Other serious AEs in eszopiclone-treated participants were cholecystitis and rectocele, both assessed by the investigator as unrelated to treatment. Two placebo-treated participants experienced serious AEs: perforated appendix and pneumonia.
Discontinuation Effects
There was no evidence of rebound insomnia following eszopiclone discontinuation. Significant improvements in sSL, sWASO, and sTST relative to baseline were observed in the eszopiclone group (all P-values ≤ 0.01) throughout the 14-day single-blind run-out period and the 14-day no-drug period. (Figure 2; Supplemental Table—available online only at www.journalsleep.org)
ISI total scores in eszopiclone-treated participants were also significantly lower than baseline at the end of both the single-blind run-out and the no-drug follow-up periods (Figure 4; P-values < 0.001). In the eszopiclone group, the percentage of participants with ISI total scores categorized as “no insomnia” and “sub-threshold insomnia” declined at Weeks 14 and 16 relative to the percentage observed at Week 12 (Week 12 = 78.0%, Week 14 = 63.8%; Week 16 = 52.8%) but was higher than the percentage seen at baseline (34.6%). There were commensurate increases in the percentage of participants with “moderate” and “severe” insomnia. (Figure 5) The shifts in the distributions of scores in the eszopiclone group from Week 12 to Week 14 and Week 14 to Week 16 were significant (P < 0.01).
There were no statistically significant differences in eszopiclone-treated participants on BWSQ scores assessed at the end of treatment at Week 12 relative to those observed at Week 14 (mean [SD] change: −0.31 [3.34] vs. 0.23 [2.65] for placebo).
The rates of central nervous system and psychiatric AEs that occurred only during the follow-up period, or those that worsened in severity or frequency during the follow-up period, were similar in the eszopiclone and placebo groups (headache: 3.4% and 4.1%, respectively; dizziness: 2.1% and 1.4%; agitation: 0.7% and 0%; restlessness: 0.7% and 0%).
DISCUSSION
In this study of older adults with primary insomnia, 12 weeks of nightly eszopiclone 2 mg significantly improved subjective measures of sleep onset, maintenance, and duration, as well as increased ratings of sleep quality, depth of sleep, and daytime function. Improvements were observed within the first week of treatment and were maintained for the entire 12 weeks of dosing with no evidence of tolerance. There was also no evidence of rebound insomnia after discontinuation of eszopiclone following 12 weeks of dosing. These results extend the findings from the two 2-week trials of eszopiclone in this population.23,24 The lack of tolerance and rebound insomnia are particularly notable as this study is the longest placebo-controlled trial of insomnia pharmacotherapy conducted in older adults. These findings are consistent with findings in two separate 6-month double-blind placebo-controlled studies of eszopiclone 3 mg in younger adults with primary insomnia.29,30
Improvement was noted not only in measures of sleep but also in syndromal insomnia outcomes. When patients mention their sleep problems to their health care professionals, they usually complain of having insomnia, which they define as difficulty falling or staying asleep. A measure of perception of insomnia may be more clinically relevant therefore than a measure of sTST or sSL. The number of participants who shifted from rating themselves as having insomnia on the Insomnia Severity Index to having no insomnia at the end of 12 weeks was greater in the eszopiclone group than in the placebo group. These participants perceived that not only did their sleep improve, they no longer characterized themselves as having insomnia. These results are similar to those reported in younger adults with primary insomnia, where eszopiclone 3 mg reduced the Insomnia Severity Index to below clinically meaningful levels in 50% of the participants.30 In addition, in this study as in the others, mean ISI scores in the eszopiclone 2 mg group remained significantly lower than baseline after discontinuation of treatment, suggesting no evidence of rebound insomnia. The percentage of eszopiclone-treated participants who categorized their insomnia as “severe” increased from 2.2% at the end of treatment to 4.9% during the 2-week single-blind placebo run-out. Thus, the ISI may be a more sensitive measure of the impact of treatment discontinuation than time-based measures of sleep.
Previous studies have shown that older adults often nap during the day and that increased napping is associated with daytime sleepiness, depression, pain and nocturia31 as well as falls, fractures, functional impairment, and mortality.19,32–34 In this study, the number of naps and the duration of naps both significantly decreased at 3 weeks in the eszopiclone 2 mg group compared with the placebo group. This decrease in napping was maintained at a stable level over the entire study in the eszopiclone group. However, in the placebo group, napping also decreased. It is unclear why the number of naps continued to decrease in the placebo group when the amount of insomnia did not improve. It is possible that all subjects adopted some sleep hygiene measures just from being in an insomnia trial, but, as mentioned, it took the placebo group longer to stabilize. Sleep hygiene alone would not significantly improve nighttime sleep, but might influence napping behavior.
The 2005 NIH State-of-the-Science Conference on Insomnia35 called for studies to begin examining the effect of treatment of insomnia on daytime functioning, in particular, quality of life and performance. The results of this study showed that not only did treatment of participants’ insomnia with eszopiclone not impair function, a number of aspects of daytime functioning were significantly improved. This is especially important in older adults, who may be particularly vulnerable to experiencing daytime impairment with certain medications due to their slower drug elimination, and who may already be experiencing some cognitive impairment or decreased quality of life. Thus, pharmacologic studies that at a minimum show no decrement as a result of treatment and more importantly, show improvement, are particularly valuable.
In the current study, a comprehensive assessment of daytime function showed that subjective reports of daytime alertness, ability to function, ability to concentrate, and sense of physical well-being were all significantly improved in the eszopiclone group compared with placebo. Similar results have been reported in younger adults both after six months of double-blind treatment30 and after an additional six months of open-label treatment36 with eszopiclone 3 mg. These findings are of particular importance in older adults as problems with daytime functioning and concentration are often misinterpreted as cognitive impairment,37 but may actually be a function of poor sleep. When the impact of treatment on quality of life and disability was assessed using the SF-36 and the SDS, significant differences in the Vitality domain and the General Health domain of the SF-36 were significantly improved compared with placebo at the end of treatment, with differences of 3.1–3.8 units. No other SF-36 or SDS domains were significant at Week 12. This is in contrast to a second 6-month eszopiclone study in adults with primary insomnia, which demonstrated significant improvements in Physical functioning, Social Functioning, and Vitality, with slightly larger differences of 3.6–6.5 units.38 It is entirely possible that sleep has a differential effect on different aspects of quality of life and functioning in elderly patients with insomnia, with overall health and vitality being most affected by good sleep, though to a lesser degree than in younger adults.
While insomnia might be primary or comorbid in younger populations, in the elderly, insomnia is most often comorbid with medical or psychiatric conditions that generally require chronic treatment.37,39 Although cognitive behavioral therapy has been shown to be effective for a longer duration than sedative hypnotics,35 there may be times when comorbid chronic insomnia may also need chronic pharmacological treatment. Despite this need, few longer-term double-blind, placebo-controlled studies of sedative hypnotics have been undertaken in the older adult population, although no unanticipated adverse side effects were reported during 12 months of open-label treatment with zaleplon 5 and 10 mg.40 In this trial, which was the first double-blind, placebo-controlled study of at least 3-months duration in elderly subjects, the results suggest that longer-term treatment of insomnia with eszopiclone was effective, and that tolerance to the hypnotic effect did not develop. While this population of older insomnia patients included those with a variety of stable comorbid medical and psychiatric conditions, future studies are needed to examine the effect of treating insomnia in older patients with specific chronic comorbidities.
A second concern in older adults with chronic illness is the risk of certain AEs associated with the long-term use of hypnotics. In the current trial, the overall incidence of AEs was slightly higher in the eszopiclone group (59.3%) compared with the placebo group (50.5%), but the difference was accounted for by the presence of mostly mild reports of unpleasant taste in eszopiclone-treated participants (12.3% versus 1.5% in the placebo treatment group).
One limitation of this study was the lack of polysomnographic data. However, in clinical practice patients with insomnia do not receive overnight sleep recordings and physicians base the success of any given treatment on patient reports of improved sleep and well being. Therefore, the subjective improvements in this study are relevant for understanding the efficacy of eszopiclone in the treatment of insomnia in older patients.
In summary, 12 weeks of treatment with eszopiclone 2 mg was shown to be well tolerated, safe, and effective in older adults with insomnia. When compared with placebo, eszopiclone 2 mg significantly improved patient-reported measures of sleep onset, maintenance, and duration, as well as improved daytime function. Significant improvements in sleep relative to placebo occurred within one week and were maintained for three months of dosing, with no evidence of rebound insomnia following eszopiclone discontinuation. These findings provide evidence that longer-term pharmacotherapy of insomnia can be safe and effective for older adults who may need chronic insomnia treatment.
DISCLOSURE STATEMENT
Support for this study was provided by Sepracor Inc., Marlborough, MA. Dr. Ancoli-Israel has served as a consultant/advisory board member for Arena, Cephalon, Inc., Ferring Pharmaceuticals Inc., Orphagen Pharmaceuticals, Pfizer, Respironics, Sanofi-Aventis, Sepracor Inc., Schering-Plough, Somaxon, and Takeda Pharmaceuticals North America Inc. Additionally, Dr. Ancoli-Israel has received grants/contracts from Sepracor Inc., Takeda Pharmaceuticals North America, Inc, and Litebook, Inc. Dr. Krystal has received grant/research support from Sanofi-Aventis, Cephalon, GlaxoSmithKline, Merck, Neurocrine, Pfizer, Sepracor Inc., Samoxon, Takeda, Transcept, Respironics, Neurogen, Evotec, Astellas, and Neuronetics. Additionally, Dr. Krystal has served as a consultant for Actelion, Arena, Astellas, Axiom, AstraZeneca, BMS, Cephalon, Eli Lilly, GlaxoSmithKline, Jazz, Johnson and Johnson, King, Merck, Neurocrine, Neurogen, Novartis, Organon, Ortho-McNeil-Janssen, Pfizer, Respironics, Roche, Sanofi-Aventis, Sepracor Inc., Somaxon, Takeda, Transcept, Astellas, Research Triangle Institute, and Kingsdown Inc. Dr. McCall has received research support from Sanofi-Aventis, Sepracor Inc., and Mini-Mitter. He has also been a member of the speakers’ bureau and a scientific advisor for Sepracor Inc., Astra-Zeneca, and Sealy. Ms. Schaefer and Mr. Claus are both full-time employees of Sepracor Inc. At the time of the study, both Dr. Wilson and Dr. Rubens were also full-time employees of Sepracor Inc. Dr. Roth has received grants from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport. He has served as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, élan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Ceullular, Jazz, Johnson and Johnson, King, Lundbeck McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Additionally, Dr. Roth has served as a speaker for Cephalon, Sanofi, and Takeda.
Supplemental Table.
Assessment of rebound effect during 14-day single blind follow-up period and 14-day no drug period for subjective TST, SL, and WASO (Single Blind Follow-up Population)
| Days (change from baseline) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Single-Blind Follow-up Period | ||||||||||||||
| TST, minutes (SD) | ||||||||||||||
| Placebo | 51.05 | 42.98 | 52.97 | 42.50 | 49.36 | 51.48 | 53.56 | 45.88 | 37.98 | 61.65 | 47.17 | 58.44 | 34.62 | 51.37 |
| (83.17) | (85.01) | (79.37) | (99.89) | (83.67) | (100.59) | (74.92) | (90.64) | (92.84) | (84.65) | (92.87) | (82.43) | (94.32) | (85.63) | |
| Eszopiclone | 23.25 | 40.32 | 40.60 | 52.15 | 54.59 | 52.34 | 62.34 | 63.49 | 59.08 | 55.49 | 58.98 | 61.11 | 63.09 | 64.60 |
| 2 mg | (109.98) | (108.05) | (106.26) | (98.27) | (104.64) | (106.47) | (83.34) | (86.17) | (100.07) | (106.18) | (108.25) | (89.12) | (90.82) | (93.11) |
| SL, minutes (SD) | ||||||||||||||
| Placebo | −23.00 | −21.76 | −27.29 | −16.24 | −26.13 | −21.82 | −33.68 | −19.60 | −20.60 | −32.18 | −33.06 | −32.35 | −8.99 | −31.16 |
| (83.86) | (81.93) | (79.56) | (94.07) | (78.46) | (99.20) | (70.95) | (108.53 | (85.31) | (61.22) | (85.02) | (70.90) | (91.17) | (71.21) | |
| Eszopiclone | −1.75 | −4.04 | −15.27 | −7.39 | −25.29 | −14.37 | −24.78 | 37.27 | −14.74 | −15.28 | −21.64 | −21.52 | −26.40 | −21.73 |
| 2 mg | (110.08) | (104.23) | (91.74) | (93.63) | (88.67) | (103.15) | (67.82) | (59.32) | (94.35) | (101.40) | (84.06) | (87.08) | (68.18) | (97.91) |
| WASO, minutes (SD) | ||||||||||||||
| Placebo | −22.81 | −18.99 | −24.64 | −28.06 | −30.62 | −29.72 | −26.01 | −27.84 | −26.19 | −26.72 | −25.17 | −25.47 | −25.17 | −32.52 |
| (54.63) | (58.98) | (52.21) | (54.53) | (54.26) | (55.14) | (57.98) | (51.58) | (55.83) | (60.96) | (57.06) | (60.09) | (61.51) | (60.21 | |
| Eszopiclone | −29.08 | −28.48 | −29.03 | −29.09 | −35.44 | −32.67 | −34.01 | −31.98 | −42.82 | −38.05 | −35.11 | −42.06 | −38.63 | −38.49 |
| 2 mg | (63.05) | (82.99) | (62.85) | (72.07) | (62.02) | (59.48) | (55.99) | (69.77) | (59.91) | (64.98) | (67.520) | (62.79) | (63.44) | (55.19) |
| No Drug Period | ||||||||||||||
| TST, minutes (SD) | ||||||||||||||
| Placebo | 37.84 | 41.64 | 48.69 | 38.93 | 44.55 | 36.24 | 41.52 | 42.44 | 39.46 | 49.14 | 37.71 | 44.91 | 49.02 | 31.31 |
| (86.80) | (82.13) | (81.34) | (100.45) | (90.13) | (87.49) | (83.56) | (89.87) | (93.17) | (80.63) | (85.49) | (84.83) | (77.88) | (98.85) | |
| Eszopiclone | 48.88 | 44.33 | 54.60 | 51.81 | 52.51 | 47.08 | 54.69 | 54.46 | 39.45 | 53.20 | 53.31 | 58.42 | 59.41 | 56.30 |
| 2 mg | (89.19) | (88.92) | (88.99) | (90.44) | (93.76) | (89.89) | (76.01) | (83.63) | (102.67) | (82.87) | (96.65) | (92.25) | (89.70) | (89.33) |
| SL, minutes (SD) | ||||||||||||||
| Placebo | −24.26 | −24.57 | −34.33 | −21.07 | −30.33 | −19.81 | −27.34 | −28.04 | −25.98 | −20.01 | −23.31 | −23.29 | −25.54 | −7.69 |
| (70.02) | (68.86) | (66.61) | (96.79) | (64.52) | (84.26) | (72.99) | (68.01) | (82.99) | (70.00) | (65.06) | (73.23) | (53.33) | (87.18) | |
| Eszopiclone | −22.53 | −18.52 | −19.79 | −22.88 | −23.31 | −20.50 | −25.86 | −21.62 | −15.29 | −18.66 | −24.32 | −21.09 | −16.15 | −15.55 |
| 2 mg | (90.82) | (96.64) | (61.97) | (68.88) | (94.24) | (85.17) | (59.36) | (80.72) | (98.60) | (83.25) | (82.90) | (81.92) | (85.26) | (98.86) |
| WASO, minutes (SD) | ||||||||||||||
| Placebo | −24.00 | −24.57 | −21.46 | −24.63 | −22.13 | −21.30 | −23.55 | −23.50 | −23.72 | −28.59 | −12.76 | −21.42 | −25.33 | −22.31 |
| (53.73) | (59.36) | (59.93) | (61.76) | (62.71) | (62.39) | (59.97) | (62.71) | (61.67) | (49.84) | (58.36) | (59.04) | (68.47) | (65.40) | |
| Eszopiclone | −35.39 | −25.68 | −34.87 | −37.10 | −30.79 | −34.80 | −35.45 | −32.67 | −30.18 | −35.32 | −29.69 | −36.55 | −35.37 | −46.60 |
| 2 mg | (62.27) | (65.61) | (60.96) | (64.71) | (61.14) | (68.86) | (64.56) | (80.20) | (69.20) | (66.67) | (70.73) | (60.89) | (64.92) | (62.85) |
Placebo group, N=146; Eszopiclone 2-mg group, N=145
REFERENCES
- 1.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–353. [PubMed] [Google Scholar]
- 2.Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J Clin Psychiatry. 2005;66(Suppl 9):10–13. [PubMed] [Google Scholar]
- 3.Mellinger GD, Balter MD, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;2:225–32. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 4.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 5.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Sleep Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22:S366–372. [PubMed] [Google Scholar]
- 6.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–6. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 7.Paudel ML, Taylor BC, Diem SJ, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56:1228–35. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 9.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol: Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 11.Dam TT, Ewing S, Ancoli-Israel S, et al. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–1673. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byles JE, Mishra GD, Harris MA, Nair K. The problems of sleep for older women: changes in health outcomes. Age Ageing. 2003;32:123–4. doi: 10.1093/ageing/32.2.154. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S. Insomnia in the elderly: a review for the primary care practitioner. Sleep. 2000;23:S23–30. [PubMed] [Google Scholar]
- 14.Avidan AY, Fries BE, James ML, Szafara KL, Wright GT, Chervin RD. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53:955–96. doi: 10.1111/j.1532-5415.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- 15.Stone KL, Ancoli-Israel S, Blackwell T, et al. Poor sleep is associated with increased risk of falls in older women. Arch Intern Med. 2008;168:1768–75. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- 16.Stone KL, Ensrud KE, Ancoll-Israel -S. Sleep, insomnia and falls in elderly patients. Sleep Med. 2008;S1:S18–S22. doi: 10.1016/S1389-9457(08)70012-1. [DOI] [PubMed] [Google Scholar]
- 17.Dew MA, Hoch CC, Buysee DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 18.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96. [PMC free article] [PubMed] [Google Scholar]
- 19.Stone KL, Ewing SK, Ancoli-Israel S, et al. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57:604–11. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 21.Morgan K, Clarke D. Longitudinal trends in late-life insomnia: implications for prescribing. Age Ageing. 1997;26:179–84. doi: 10.1093/ageing/26.3.179. [DOI] [PubMed] [Google Scholar]
- 22.Krystal A. A compendium of placebo-controlled trials of the risks/benefits of pharmacologic treatments for insomnia: the empirical basis for clinical practice. Sleep Med Rev. 2009;13:265–274. doi: 10.1016/j.smrv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.McCall WV, Erman M, Krystal AD, et al. A polysomnography study of eszopiclone in elderly patients with insomnia. Curr Med Res Opin. 2006;22:1633–1642. doi: 10.1185/030079906X112741. [DOI] [PubMed] [Google Scholar]
- 24.Scharf M, Erman M, Rosenberg R, et al. A 2-week efficacy and safety study of eszopiclone in elderly patients with primary insomnia. Sleep. 2005;28:720–7. doi: 10.1093/sleep/28.6.720. [DOI] [PubMed] [Google Scholar]
- 25.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-Item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 27.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 28.Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 29.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 32.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman SE, Hall M, Boudreau R, et al. Association between nighttime sleep and napping in older adults. Sleep. 2008;31:733–40. doi: 10.1093/sleep/31.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stone KL, Ewing SK, Lui LY, et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1177–83. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 35.NIH State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults statement. J Clin Sleep Med. 2005;1:412–21. [PubMed] [Google Scholar]
- 36.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–95. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S, Cooke JR. Prevalence and co-morbidity of insomnia and impact on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 38.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: Effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foley DJ, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S, Walsh JK, Mangano RM, Fujimori M. Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Prim Care Companion J Clin Psychiatry. 1999;1:114–20. doi: 10.4088/pcc.v01n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]