Abstract
Problem
Infection during pregnancy represents a significant cause of mobility and mortality. While viruses pose a major threat, little is known about their effect on early pregnancy, or the mechanisms involved. The objective of this study was to characterize the trophoblast response following exposure to viral ssRNA.
Method of study
First trimester trophoblast cells were treated with or without viral ssRNA. Cytokine production was measured by multiplex analysis and ELISA. Apoptosis was determined using Hoescht staining, cell viability, and caspase activity assays.
Results
Treatment of trophoblasts with viral ssRNA increased their secretion of IL-8, IL-6 and IFNβ. However, the ssRNA also induced trophoblast apoptosis. To test whether the viral ssRNA-induced inflammatory response was responsible for this induction of apoptosis, conditioned media (CM) from trophoblasts was added to a fresh culture of cells. The CM from viral ssRNA-treated induced higher levels of trophoblast apoptosis than the control CM. Moreover, recombinant IFNβ induced trophoblast apoptosis.
Conclusion
We demonstrate that viral ssRNA induces a pro-inflammatory and type I interferon response in the trophoblast, and that this inflammatory process may indirectly induce trophoblast apoptosis. These results provide a novel mechanism by which certain viral infections might compromise placental integrity and function, and therefore, pregnancy outcome.
Keywords: Caspase, Infection, Pregnancy, ssRNA, Toll-like receptor, Virus
INTRODUCTION
An intrauterine infection can significantly influence pregnancy outcome. Bacterial and viral infections can gain access to the maternal-fetal interface by one of three major routes: via the maternal circulation; by ascending into the uterus from the lower reproductive tract; or by descending into the uterus from the peritoneal cavity 1,2. There is now a strong clinical association between bacterial infections and preterm labor, particularly in those delivering before 30 weeks of gestation 1,3,4. Other complications, such as preeclampsia and intrauterine growth restriction (IUGR), may also have an underlying infectious element 5–8. While less is known about the impact viral infections have on pregnancy outcome, their potential role in pregnancy-related complications have been realized more recently, from clinical studies. In addition to the potential for vertical transmission, resulting in fetal infection and developmental complications, viral infections during pregnancy, such as Cytomegalovirus (CMV), Parvovirus B12, Herpes virus, Adeno-associated virus, influenza, and rubella virus, have all been associated with preterm delivery, spontaneous miscarriage, still birth, and preeclampsia 8–16.
The way in which infections can negatively impact pregnancy is thought to involve innate immune responses towards the microorganism, which inadvertently leads to excessive inflammation or apoptosis at the maternal-fetal interface 17,18. Studies focusing on the mechanisms involved have implicated innate immune pattern recognition receptors (PRRs), such as the Toll-like receptors (TLRs), as playing a key role in infection-related pregnancy complications. Indeed, gestational tissues such as the placenta, the decidua, and the fetal membranes, can recognize and respond to microbes at the maternal-fetal interface through PRRs (reviewed in 19). Moreover, animal models have demonstrated a role for TLRs in mediating inflammation-associated pregnancy complications triggered by bacteria or bacterial components 20–23. More recently, in vivo studies have suggested that TLRs may also be involved in preterm delivery and pregnancy failure induced by viral components 22,24,25. Nonetheless, little is still known about the effects of viral infections on early human pregnancy, or the mechanisms involved.
We previously described the function of TLR3 in human first trimester trophoblast cells in response to Poly(I:C), a synthetic analogue of viral dsRNA. Activation of TLR3 induces a rapid, potent, and highly specific pro-inflammatory and anti-viral response in first trimester trophoblast cells 24,26,27. These studies suggest that the trophoblast are able to recognize and specifically respond to viral dsRNA in a highly regulated fashion, and this may be important for the control of certain viral infections at the maternal-fetal interface. Since nothing is known about TLR8 function in early pregnancy, the objective of this study was to characterize the response of first trimester trophoblast cells following exposure to the TLR8 agonist, viral ssRNA.
Materials and Methods
Patient samples
First trimester placentas (8 – 12 weeks) were obtained from elective terminations of normal pregnancies performed at Yale-New Haven Hospital. All patients signed consent forms and the use of patient samples was approved under Yale University’s Human Investigations Committees.
Reagents and antibodies
The TLR8 agonist, viral ssRNA40/LyoVec (ssRNA) was purchased from Invivogen (San Diego, CA). The mouse anti-XIAP mAb (#610716) and the mouse anti-Bax mAb (#610982) were obtained from BD Transduction Labs (San Diego, CA), and the rabbit-anti-Bid polyclonal antibody (#2002) was purchased from Cell Signaling Technology (Danvers, MA). The mouse mAb for ®-actin was purchased from Sigma. Specific signals were detected using either a peroxidase-conjugated horse anti-mouse, or a peroxidase-conjugated goat anti-rabbit secondary antibody (Vector Laboratories). Human recombinant IFNβ was purchased from PBL Interferon Source (Piscataway, NJ).
Culture of cell lines
All cells were maintained at 37°C/5% CO2. Two first trimester trophoblast cell lines were used in these studies. The human trophoblast cell line, 3A, which was transformed by SV40 28,29. The human trophoblast cell line, HTR8 (referred to from hereon as H8) which was also transformed by SV40 30 was a kind gift from Dr Charles Graham (Queens University, Kingston, ON, Canada). The monocytic cell line, THP-1, was a gift from Dr Paul Guyre (Dartmouth Medical School, Lebanon, NH). All cell lines were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT), 10mM Hepes, 0.1mM MEM non-essential amino acids, 1mM sodium pyruvate, 100nm penicillin/streptomycin (Gibco).
RT-PCR
Total RNA was isolated from first trimester placenta, the trophoblast cells lines and THP-1 cells using the Rneasy kit from Qiagen (Valencia, CA). Reverse transcription was performed on 5⎧g of total RNA using the First Strand cDNA Synthesis kit from Amersham Biosciences (Buckinghamshire, UK) according to the manufacturer’s directions. The primers used for amplification of human TLR8 have been described previously 31 and have the following sequences:5'-CAGAATAGCAGGCGTAACACATCA-3', 5'AATGTCACAGGTGCATTCAAAGGG-3'. 35 cycles of PCR were performed at 94° for 30 seconds, 55°C for 30 seconds and 72°C for 1 minute. The size of the product was 581bp.
Cell viability assay
The effects of viral ssRNA or conditioned media (CM) on trophoblast cell viability was determined using the CellTiter 96™ viability assay (Promega, Madison, WI), as previously described 32. First trimester trophoblast cells were plated in wells of a 96 well plate at 1 × 104 cells per well in growth media and cultured until 70% confluent. The media was then replaced with the reduced serum medium, Opti-MEM (Gibco) and cultured for another 4 hours prior to treatment. Following treatment, the CellTiter substrate, MTS tetrazolium, was added to all wells and following a 1–4 hour incubation at 37°C, optical densities were read at 490nm. All samples were assayed in triplicate and cell viability was presented as a percentage relative to the no treatment (NT) control.
Hoechst Staining
Apoptosis was monitored by Hoechst Staining as described previously 29. Following treatment with or without ssRNA, the trophoblast cells were incubated for 20 minutes with Hoechst 33342 dye (Molecular Probes) at 5⎧g/ml. The cells were then analyzed by fluorescent microscopy.
Caspase activity assay
The effects of viral ssRNA or recombinant IFN® on trophoblast caspase activity was determined using the Caspase-Glo™ assay (Promega, Madison, WI). Briefly, 10⎧g of whole cell lysates were incubated at room temperature in the dark for 1 hour with either the caspase-3, caspase-8, or caspase-9 substrate. Following incubation, luminescence was measured using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). The amount of luminescence detected as relative light units (RLU) was proportional to caspase activity. All samples were assayed in triplicate.
Western blot analysis
For analysis of intracellular apoptotic proteins, cells were lysed using 1% NP40 and 0.1% SDS in the presence of protease inhibitors (Roche, address). Protein concentrations were calculated by BCA assay (Pierce Biotechnology, Rockford, IL). Proteins were then diluted with gel loading buffer to 20⎧g and boiled for 5 minutes. Proteins were resolved under reducing conditions on 12% SDS-PAGE gels and then transferred onto PVDF paper (PerkinElmer, Boston, MA). Membranes were blocked at room temperature for 1 hour with 5% fat-free powdered milk (FFPM) in PBS/0.05% Tween-20 (PBS-T). Following three washes for 10 minutes each with PBS-T, membranes were incubated overnight at 4°C with primary antibody in PBS-T/1% FFPM. The anti-XIAP mAb was used at a 1:1000 dilution, the anti-Bax mAb at a 1:500 dilution and the anti-Bid polyclonal at a 1:2500 dilution. Following this incubation, membranes were washed three times as before and then incubated at room temperature for 1 hour with the appropriate secondary antibody conjugated to peroxidase at a 1:10,000 dilution in PBS-T/1% FFPM. Following three washes for 10 minutes each with PBS-T and three washes for 10 minutes each with distilled water, the peroxidase-conjugated antibody was detected by enhanced chemiluminescence (PerkinElmer). ®-actin was used as internal control, in addition to Ponceau Red, to validate the amount of protein loaded onto the gels.
Cytokine studies
Trophoblast cells were treated with or without viral ssRNA. Following a culture of 12 – 48 hours, the cell-free supernatants, also termed conditioned media (CM), were collected by centrifugation at 400g for 10 minutes and stored at −80°C until analysis was performed. The supernatants were evaluated by multiplex analysis using the BioPLex assay (BioRad) for the following cytokines/chemokines: IL-1®, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, G-CSF, GM-CSF, GRO-〈, IFN©, MCP-1, MIP-1〈, RANTES and TNF〈. Detection and analysis were performed using the Luminex 100 IS system (Upstate Biotechnology, Charlottesville, VA). The levels of IFN〈 and IFN® in the culture supernatants were measured using specific ELISAs (PBL Interferon Source).
Statistical analysis
Experiments were performed at least three times, and where appropriate, assayed in triplicate. Data are expressed as mean ± standard deviation (S.D.) of either representative or pooled experiments. Statistical significance (p< 0.05) was determined using either the one-way ANOVA with the Bonferroni correction for multiple comparisons, or the paired student’s t-test.
RESULTS
Viral ssRNA reduces first trimester trophoblast cell viability
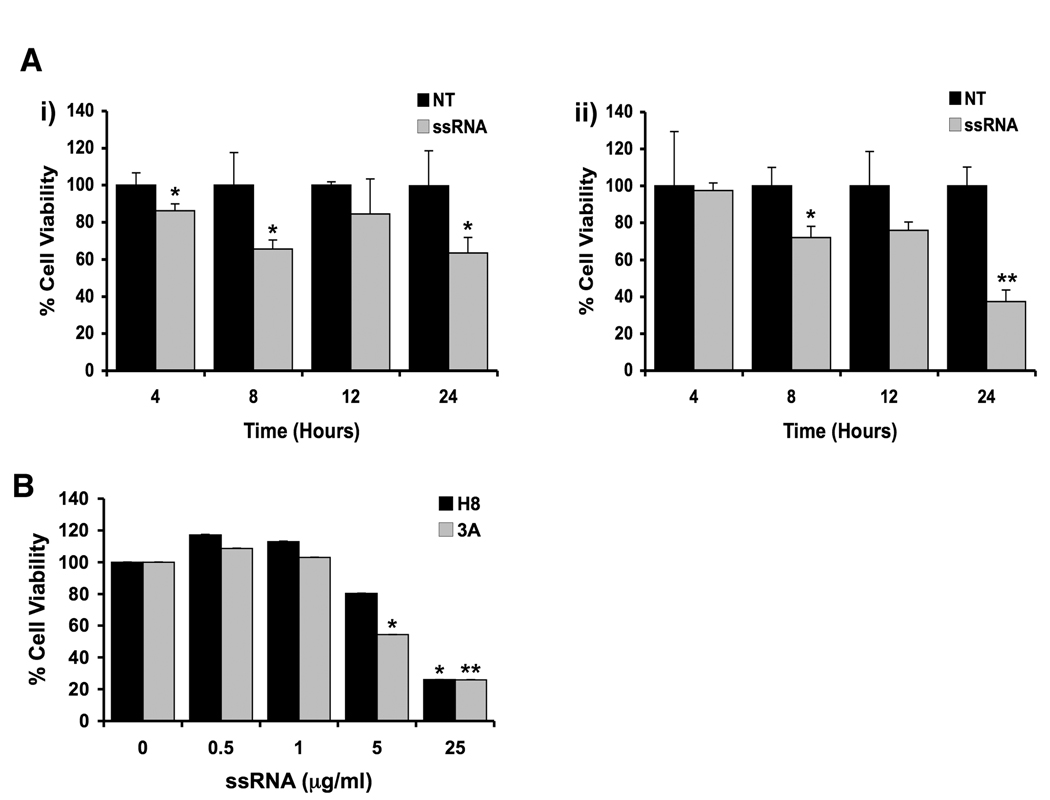
Our first objective was to determine the effect of viral ssRNA on the first trimester trophoblast cell lines, H8 and 3A. As shown in Figure 1, treatment of both trophoblast cell lines with viral ssRNA resulted in a significant time- (Figure 1A) and dose- (Figure 1B) dependent decrease in cell viability. Interestingly, after 24 hours of treatment with ssRNA at 25⎧g/ml, and 48 hours of treatment with ssRNA at 5⎧g/ml, the 3A cells were more sensitive to the ssRNA in terms of cell viability when compared to the H8 cells.
Figure 1. Viral ssRNA reduces first trimester trophoblast cell viability.
(A) The first trimester trophoblast cells, (i) H8 and (ii) 3A, were incubated with either no treatment (NT), or ssRNA (25⎧g/ml) for 4, 8, 12 and 24 hours. (B) The first trimester trophoblast cells, H8 and 3A, were incubated with viral ssRNA at 0, 0.5, 1, 5 or 25⎧g/ml for 48 hours. Following each time point, cell viability was determined using the CellTiter 96 assay. Barcharts show percentage cell viability relative to the untreated control. Treatment with ssRNA significantly reduced trophoblast cell viability in a dose and time dependent manner (*p <0.05, **p <0.001). Data are representative of at least three independent experiments.
Viral ssRNA induces trophoblast apoptosis
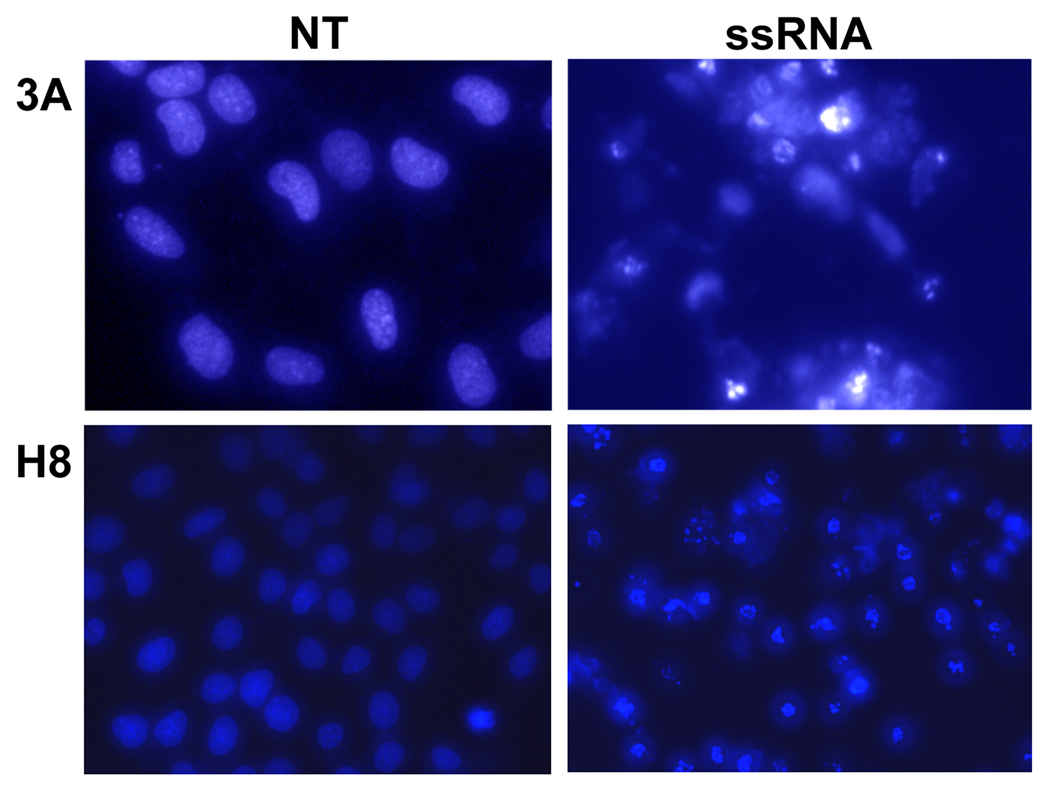
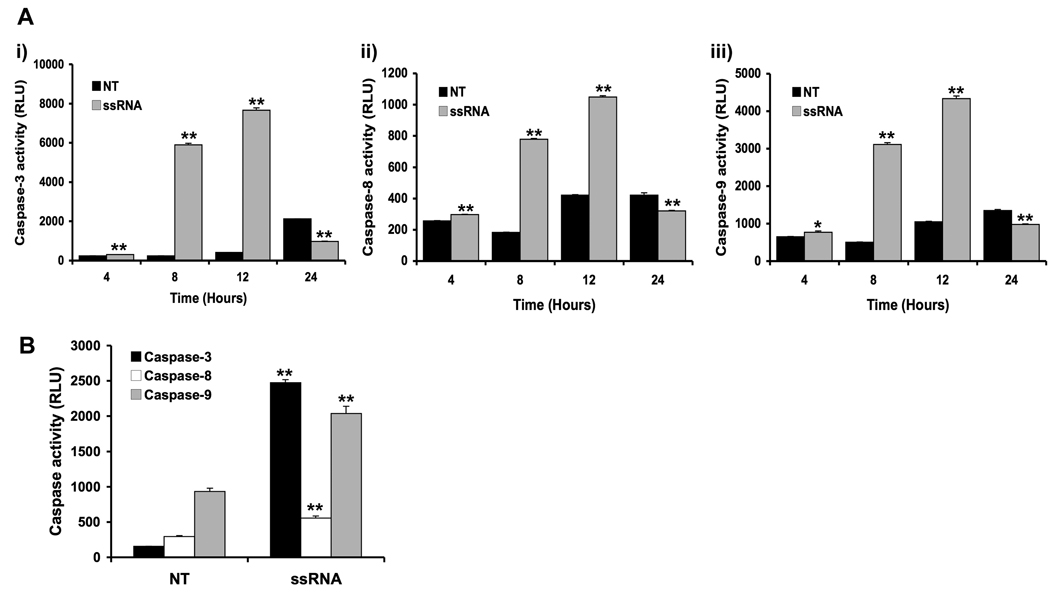
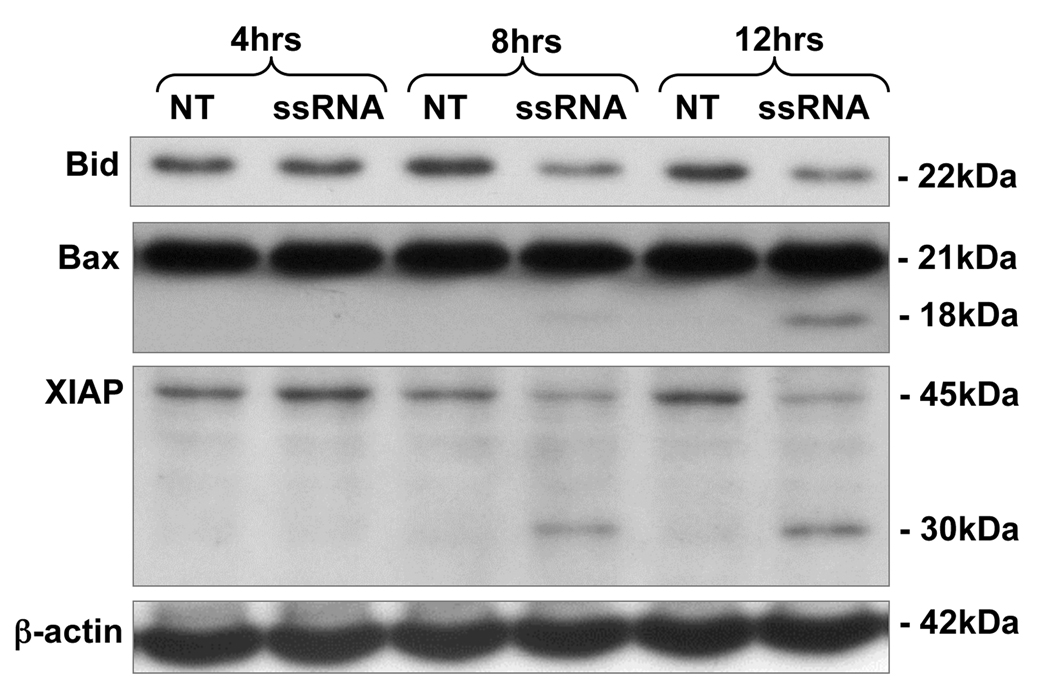
Our next objective was to determine whether the decrease in trophoblast cell viability induced by viral ssRNA was a result of the induction of apoptosis, rather than a cytotoxic effect. Hoechst staining revealed that treatment of the trophoblast cells with viral ssRNA resulted in DNA condensation, a change in morphology, and fragmented nuclei; all of which are indicative of apoptosis (Figure 2). To further confirm that the viral ssRNA was indeed triggering trophoblast apoptosis, cells were incubated with or without the ssRNA, after which protein was extracted from cell lysates, and caspase activation was determined using an activity assay. As shown in Figure 3, treatment of both trophoblast cell lines with viral ssRNA significantly increased trophoblast caspase-3, caspase-8, caspase-9 activity, with levels peaking at 12 hours post treatment. By 24 hours post-treatment, caspase activity in the ssRNA treated cells was significantly lower than the no treatment (NT) controls (Figure 3A). Using Western blot analysis, this pro-apoptotic effect of the viral ssRNA was shown to involve the mitochondrial pathway. Following treatment with ssRNA, we observed a decrease in total Bid expression (22kDa), and activation of Bax, evidenced by appearance of the 18kDa active cleavage product. Viral ssRNA-induced trophoblast apoptosis also involved inactivation of the X-linked inhibitor of apoptosis, XIAP, as evidenced by the appearance of the 30kDa inactive cleavage product and a reduction in the active 45Da form (Figure 4).
Figure 2. Viral ssRNA induces apoptosis in first trimester trophoblast cells.
The first trimester trophoblast cells, 3A (Mag. 40×) and H8 (Mag. 20×) were incubated for 24 hours with either no treatment (NT) or ssRNA (25⎧g/ml). Following incubation, the cells were stained with Hoechst 33342 dye (5⎧g/ml) and cells visualized by fluorescent microscopy. Note the increased numbers of condensed and fragmented nuclei in the ssRNA treated cells. Data are representative of at least three independent experiments.
Figure 3. Viral ssRNA activates the apoptotic caspase pathway in first trimester trophoblast cells.
(A) The first trimester trophoblast cells, 3A, were incubated with either no treatment (NT) or ssRNA (25⎧g/ml) for 4, 8, 12 or 24 hours, after which cell lysates were prepared and: (i) caspase-3; (ii) caspase-8; and (iii) caspase-9 activities were determined using the Caspase-Glo assay. (B) H8 cells were incubated with either no treatment (NT) or ssRNA (25⎧g/ml) for 12 hours, after which caspase-3, caspase-8 and caspase-9 activities were determined. Bar charts show caspase activity in relative light units (RLU). Viral ssRNA significantly increased trophoblast cell caspase-3, caspase-8 and capsase-9 activity, with levels peaking after 12 hours of treatment. *p<0.05; **p<0.001 relative to the NT control. Data are representative of at least three independent experiments.
Figure 4. Viral ssRNA alters apoptotic protein expression in first trimester trophoblast cells.
First trimester trophoblast cells (3A) were incubated with either no treatment (NT) or ssRNA (25⎧g/ml) for 4, 8 or 12 hours. After each time point, cell lysates were prepared and analyzed for Bid, Bax and XIAP expression levels by Western blot. ®-actin served as a loading control. Data are representative of three independent experiments.
Viral ssRNA upregulates trophoblast pro-inflammatory cytokine production and induces a type I interferon response
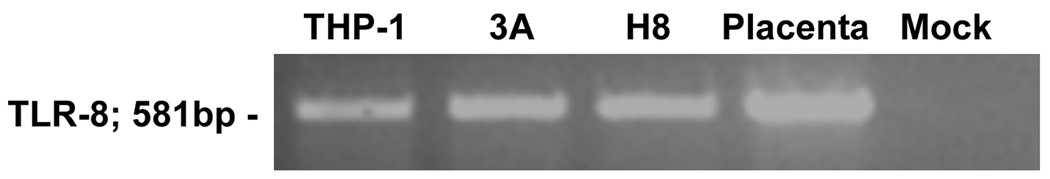
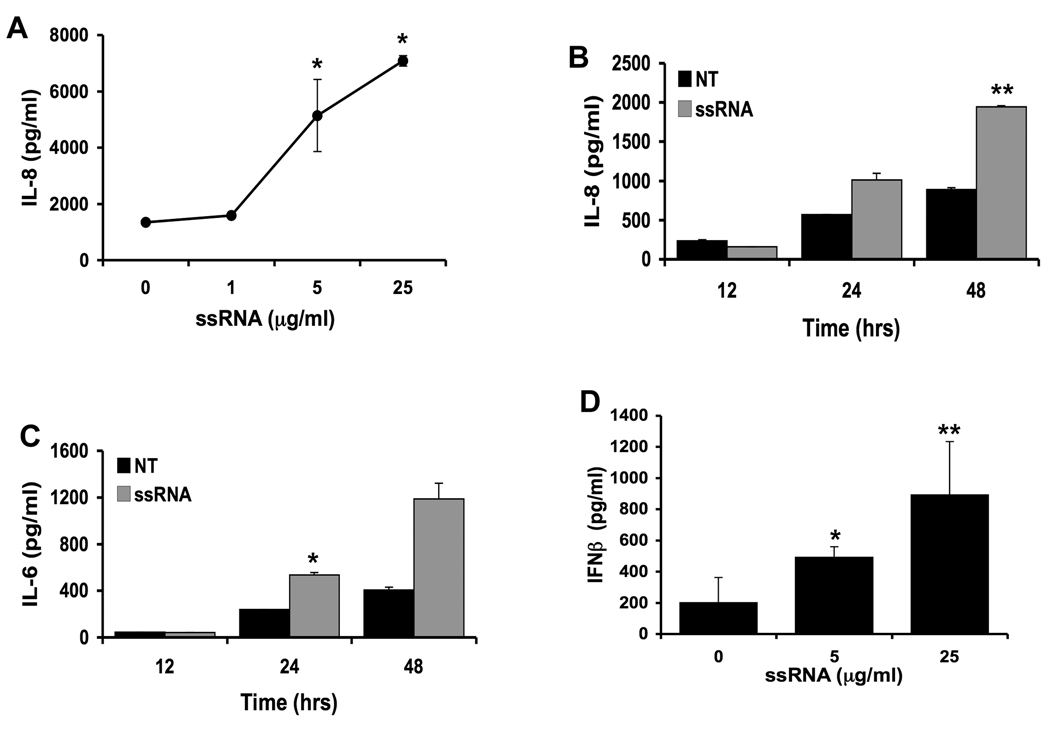
TLR8 has been shown to recognize viral ssRNA in immune cells 33. Upon binding to TLR8, viral ssRNA induces an inflammatory cytokine response 33. While term placental tissue and BeWo choriocarcinoma cells have been shown to express TLR8 mRNA 34–37, nothing is known about TLR8 expression in first trimester trophoblast cells. Therefore, we sought to determine the expression of TLR8 in first trimester placenta and first trimester trophoblast cells by RT-PCR. As shown in Figure 5, both the placental tissue, and the two first trimester trophoblast cell lines expressed TLR8 mRNA. Next the effects of viral ssRNA on trophoblast cytokine and chemokine production, as result of binding to TLR8, was determined. As shown in Figure 6, treatment of trophoblast cells with viral ssRNA induced a highly specific cytokine response, with only IL-8 and IL-6 production being increased in a dose and time-dependent manner (Figure 6 A, B &C). All other cytokines and chemokines tested by multiplex analysis were not significantly different from the no treatment (NT) control (data not shown).
Figure 5. First trimester trophoblast cells express Toll-like receptor 8.
TLR-8 mRNA expression was evaluated in first trimester trophoblast cells by RT-PCR. Panel shows TLR-8 expression (581bp) in the 3A and H8 trophoblast cells as well as 8-week placental tissue. The monocytic THP-1 cell line served as a positive control. No signal was detected in the mock control.
Figure 6. Viral ssRNA triggers an inflammatory cytokine response in trophoblast cells.
First trimester trophoblast cells, (H8), were incubated with either: (A) ssRNA at 0, 1, 5 or 25⎧g/ml for 48 hours; (B&C) no treatment (NT) or ssRNA (5⎧g/ml) for 12, 24 or 48 hours; or (D) ssRNA at 0, 5 or 25⎧g/ml for 48 hours. Cell-free supernatants were collected and analyzed for IL-8 and IL-6 secretion by multiplex analysis, and IFN® secretion by ELISA. *p<0.05; **p<0.001 relative to the untreated control. Data are representative of at least three independent experiments.
Having found that viral ssRNA was in fact triggering an inflammatory response, in addition to the pro-apoptotic response, we hypothesized that factors induced by TLR8 activation may trigger the apoptotic effect observed with ssRNA treatment. Since TLR8 is also known to trigger a type I interferon response in other cell types 33, and we previously reported that TLR3 activation by viral dsRNA triggers first trimester trophoblast cells to produce IFN® 26, supernatants were also evaluated for IFN〈 and IFN® by ELISA. While IFN〈 was undetectable in the supernatants (data not shown), treatment of trophoblast cells with viral ssRNA induced a strong IFN® response (Figure 6D). Interestingly, the levels of IFN® secreted by the trophoblast after TLR8 stimulation were at least 10-fold greater than the IFN® response induced by TLR3 activation 26.
Viral ssRNA-triggered inflammation induces first trimester trophoblast cell death
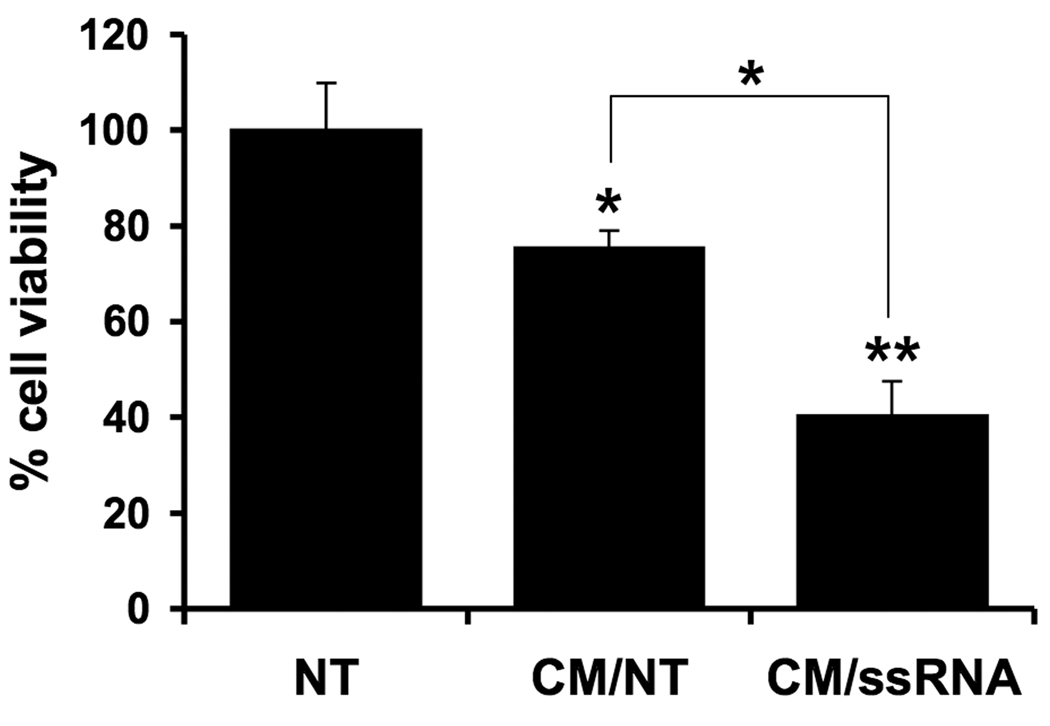
First trimester trophoblast cells are sensitive to TNF〈 and IFN©-induced apoptosis 38. While the type I interferons, IFN〈 and IFN® can induce apoptosis in neuronal cells and tumor cells 39,40, nothing is known about their effects on trophoblast survival. Since we found viral ssRNA to upregulate the trophoblast’s production of IFN®, we hypothesized that this type I interferon might be responsible for the ssRNA-induction of trophoblast cell death and apoptosis by acting back on the cells in an autocrine/paracrine manner. To test this, we took the supernatants from trophoblast cells treated with or without viral ssRNA (5⎧g/ml), now termed conditioned media (CM), and then exposed a new culture of trophoblast cells to this CM. The CM was diluted to 50% with media, thus the maximum residual ssRNA concentration was 2.5⎧g/ml, a concentration, that alone, does not induce cell death (Figure 2B). After 96 hours, trophoblast viability was evaluated. The CM from trophoblasts treated with the ssRNA (CM/ssRNA) caused a significantly greater reduction in trophoblast cell viability than the CM from untreated trophoblasts (CM/NT) (Figure 7).
Figure 7. Effect of viral-ssRNA induced trophoblast-derived factors on trophoblast cell viability.
First trimester trophoblast cells (H8) were treated with no treatment (NT) or ssRNA (5⎧g/ml) for 48 hours, after which the cell-free conditioned media (CM) was collected. A fresh culture of H8 cells were then incubated with either: no treatment (NT); 50% of CM from untreated trophoblast cells (CM/NT); or 50% of CM from trophoblast cells treated with ssRNA (CM/ssRNA). After 96 hours, cell viability was determined using the CellTiter™ assay. *p<0.05 and **p<0.001 relative to the NT control, unless otherwise indicated. Data are representative of at least three independent experiments.
IFN® induces first trimester trophoblast apoptosis
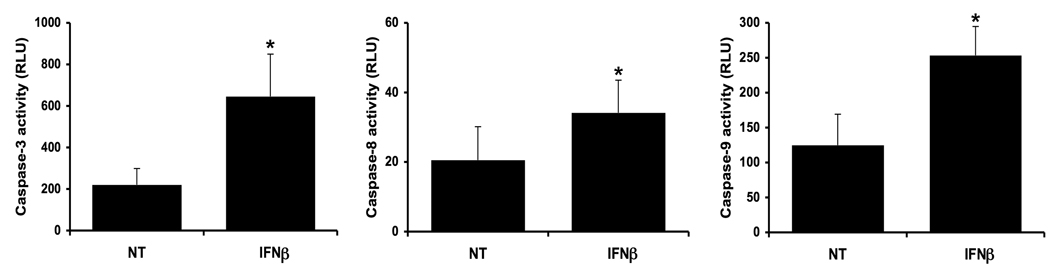
Having established that a soluble factor(s) produced by the trophoblast, following exposure to viral ssRNA, induced cell death, we questioned whether the ssRNA-induced apoptosis could be IFN®-mediated. To test this, we exposed first trimester trophoblast cells to recombinant human IFN® and assessed caspase activity. As shown in Figure 8, treatment of trophoblast with IFN® resulted in a significant increase in (i) caspase-3, (ii) caspase-8, and (iii) caspase-9 activity when compared to the no treatment (NT) control.
Figure 8. IFN® induces trophoblast apoptosis.
First trimester trophoblast cells (H8) were treated with no treatment (NT) or recombinant human IFN® (10,000U/ml) for 72 hours, after which (i) caspase-3; (ii) caspase-8; and (iii) caspase-9 activities were determined. *p<0.001 relative to the NT control. Data are from four independent experiments.
DISCUSSION
There is compelling evidence to suggest that viral infections may have detrimental effects on pregnancy, however, our knowledge of placental responses to viral products is still limited. In this study, we demonstrate for the first time, that viral ssRNA induces both inflammation and apoptosis in first trimester trophoblast cells, and that these outcomes are mechanistically connected. We have found that viral ssRNA triggers trophoblast cells to produce a highly restricted panel of chemokines, cytokines and type I interferons, which act back on the cells in an autocrine/paracrine manner to induce apoptosis and cell death. Our findings provide a novel mechanism by which certain viral infections might compromise placental integrity and function, and therefore, pregnancy outcome.
Viral infections pose a significant threat to both pregnancy outcome and fetal development. Many viruses that gain access to the maternal-fetal interface can infect the placenta 41, and fetal developmental problems can arise from a viral infection during pregnancy. Strong candidates for such fetal abnormalities include CMV, rubella virus and influenza 14,42. Furthermore, clinical studies have linked these, and other, viral infections to adverse pregnancy outcomes, such as spontaneous miscarriage and preterm delivery 8–16. These observations, coupled with recent animal studies 22,24,25, suggest that an innate immune response to a virus at the maternal-fetal interface may provide the basis for such obstetrical complications.
We previously reported the function of TLR3 in first trimester trophoblast cells. In response to viral dsRNA, first trimester trophoblast cells generate a strong pro-inflammatory cytokine/chemokine response, but also a robust anti-viral response, suggesting that the placenta may provide some level of host defense against invading viral infections 24,26,27. In this current study, we sought to examine the function of TLR8 in response to viral ssRNA in first trimester trophoblast cells. Similarly to TLR3 activation, we found that TLR8 stimulation by viral ssRNA triggered the trophoblast to generate a pro-inflammatory cytokine/chemokine response. However, unlike activation of the pattern recognition receptors: TLR3, TLR4, Nod1, or Nod2, which induce first trimester trophoblast cells to upregulate a range of cytokines and chemokines in response to their pathogenic agonists 24,26,27,29,43–45, viral ssRNA triggered a highly restricted panel of factors. The only cytokines/chemokines that we found to be upregulated following treatment with viral ssRNA were IL-6, IL-8, and the type I interferon, IFN®. In a recent study by Patni et al., treatment of term placental explants, with viral ssRNA, also led to elevated IL-6 and IL-8 production, but not TNF〈 or IL-10. However, the production of IFN® was not tested in this study 36. Nonetheless, these observations support our findings of ssRNA modulation of IL-6 and IL-8 in first trimester trophoblast cells. Moreover, studies using CMV have shown that viral infection of first trimester trophoblast cells also upregulate IL-6 and IL-8 46,47. Our observation that viral ssRNA also induces a strong IFN® response in the trophoblast is in keeping with the ability of the placenta to generate a specific anti-viral response. Studies from our lab, as well as others, have previously shown that viral products and viral infections trigger the trophoblast to produce type I interferons 26,48,49.
While we found that viral ssRNA triggered a strong inflammatory response in the trophoblast, in parallel to this, viral ssRNA triggered trophoblast cell death and apoptosis. This involved activation of the caspase pathway; activation of the pro-apoptotic proteins, Bid and Bax, which act on the mitochondria; and inactivation of the inhibitor of apoptosis, XIAP. This finding is supported by a recent study which showed that the ssRNA virus, H3N2 influenza, induces first trimester trophoblast apoptosis 50. Next, we questioned the mechanism involved and postulated that the ssRNA-induced cytokine response was acting back on the trophoblast in a paracrine/autocrine manner to induce apoptosis. Conditioned media from viral ssRNA-treated trophoblast cells induced higher levels of trophoblast cell death than the control conditioned media, suggesting that a soluble factor produced by the cells after TLR8 stimulation may be responsible. Since IL-8 and IL-6 are normally constitutively produced by first trimester trophoblast cells, and are commonly upregulated after microbial stimulation without inducing cell death 28,43,45, we focused on the possibility that the secreted IFN® was acting in a pro-apoptotic manner. Indeed, treatment of the trophoblast cells with recombinant IFN® induced trophoblast apoptosis, as evidenced by increased levels of caspase activity. While high levels of recombinant IFN® were required to induce this apoptotic response, exposure of trophoblast cells to viral ssRNA triggered a high level of IFN® production, at least 10-fold more than that induced by TLR3 activation 26. This may explain why TLR3 stimulation by dsRNA induces trophoblast inflammation, but not apoptosis. A study by Jung et al., showed that TLR4 activation of mouse microglial cells resulted in IFN® production, which in turn induced microglial apoptosis 51, and IFN® has been shown to induce apoptosis in various human tumor cell lines 52. Moreover, Chan et al., demonstrated that CMV can indirectly induce apoptosis in term trophoblast cells by activating TLR2 and inducing production of the pro-apoptotic cytokine, TNF〈 53. Therefore, our findings suggest that TLR8 activation by viral ssRNA indirectly induce first trimester trophoblast apoptosis via the production of IFN®, a mechanism that is very different from TLR2-mediated apoptosis in response to bacterial components 28,29.
In summary, we have demonstrated that viral ssRNA induces a pro-inflammatory cytokine/chemokine, and type I interferon response in the first trimester trophoblast. Furthermore, through the production of IFN®, this inflammatory process indirectly induces trophoblast apoptosis and cell death. These results provide a novel mechanism by which certain viral infections might compromise placental integrity and function. By triggering an intense pro-inflammatory and pro-apoptotic placental response to a viral infection at the maternal-fetal interface may trigger obstetrical complications like preterm delivery, spontaneous miscarriage, or preeclampsia.
ACKNOWLEDGMENTS
This study was in part supported by grants RO1HD049446 and P01HD054713 from the Eunice Kennedy Shriver, NICHD, NIH and 3N01 HD23342 from the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver, NICHD, NIH.
REFERENCES
- 1.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194:630–637. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–174. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 4.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64:644–647. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- 5.Arechavaleta-Velasco F, Koi H, Strauss JF, 3rd, Parry S. Viral infection of the trophoblast: time to take a serious look at its role in abnormal implantation and placentation? J Reprod Immunol. 2002;55:113–121. doi: 10.1016/s0165-0378(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CD, Witter FR. Urogenital infection in preeclampsia. Int J Gynaecol Obstet. 1995;49:271–275. doi: 10.1016/0020-7292(95)02373-k. [DOI] [PubMed] [Google Scholar]
- 7.von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81:642–648. [PubMed] [Google Scholar]
- 8.von Dadelszen P, Magee LA, Krajden M, Alasaly K, Popovska V, Devarakonda RM, Money DM, Patrick DM, Brunham RC. Levels of antibodies against cytomegalovirus and Chlamydophila pneumoniae are increased in early onset pre-eclampsia. Bjog. 2003;110:725–730. [PubMed] [Google Scholar]
- 9.Arechavaleta-Velasco F, Gomez L, Ma Y, Zhao J, McGrath CM, Sammel MD, Nelson DB, Parry S. Adverse reproductive outcomes in urban women with adeno-associated virus-2 infections in early pregnancy. Hum Reprod. 2008;23:29–36. doi: 10.1093/humrep/dem360. [DOI] [PubMed] [Google Scholar]
- 10.Gibson CS, Goldwater PN, MacLennan AH, Haan EA, Priest K, Dekker GA. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population. Bjog. 2008;115:492–500. doi: 10.1111/j.1471-0528.2007.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen IP, Thorsen P, Jeune B, Moller BR, Vestergaard BF. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. Bjog. 2000;107:637–643. doi: 10.1111/j.1471-0528.2000.tb13306.x. [DOI] [PubMed] [Google Scholar]
- 12.Johansson S, Buchmayer S, Harlid S, Iliadou A, Sjoholm M, Grillner L, Norman M, Sparen P, Dillner J, Cnattingius S. Infection with Parvovirus B19 and Herpes viruses in early pregnancy and risk of second trimester miscarriage or very preterm birth. Reprod Toxicol. 2008;26:298–302. doi: 10.1016/j.reprotox.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Maeda T, Okuno T, Hayashi K, Miyamoto H, Utsunomiya A, Yamada Y, Mori T. Abortion in human herpesvirus 6 DNA-positive pregnant women. Pediatr Infect Dis J. 1997;16:1176–1177. doi: 10.1097/00006454-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen SA, Erickson JD, Reef SE, Ross DS. Teratology: from science to birth defects prevention. Birth Defects Res A Clin Mol Teratol. 2009;85:82–92. doi: 10.1002/bdra.20506. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spano LC, Lima Pereira FE, Gomes da Silva Basso N, Mercon-de-Vargas PR. Human cytomegalovirus infection and abortion: an immunohistochemical study. Med Sci Monit. 2002;8:BR230–BR235. [PubMed] [Google Scholar]
- 17.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 19.Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 20.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci. 2007;14:315–320. doi: 10.1177/1933719107302959. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 24.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Ren L, Wang W, Di J, Zeng S, Saito S. Effect of TLR3 and TLR7 activation in uterine NK cells from non-obese diabetic (NOD) mice. J Reprod Immunol. 2009;82:12–23. doi: 10.1016/j.jri.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Bole-Aldo P, Romero R, Wira CR, Mor G. Expression and secretion of anti-viral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I:C) Human Reproduction. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 27.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 28.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 30.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrahams VM, Straszewski SL, Kamsteeg M, Hanczaruk B, Schwartz PE, Rutherford TJ, Mor G. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 2003;63:5573–5581. [PubMed] [Google Scholar]
- 33.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 34.Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7 hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 35.Komine-Aizawa S, Majima H, Yoshida-Noro C, Hayakawa S. Stimuli through Toll-like receptor (TLR) 3 and 9 affect human chorionic gonadotropin (hCG) production in a choriocarcinoma cell line. J Obstet Gynaecol Res. 2008;34:144–151. doi: 10.1111/j.1447-0756.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- 36.Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1–9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;80:243–248. doi: 10.1095/biolreprod.108.069252. [DOI] [PubMed] [Google Scholar]
- 37.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 38.Aschkenazi S, Straszewski S, Verwer KM, Foellmer H, Rutherford T, Mor G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod. 2002;66:1853–1861. doi: 10.1095/biolreprod66.6.1853. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13:859–875. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- 41.Younes AS, Csire M, Kapusinszky B, Szomor K, Takacs M, Berencsi G. Heterogeneous Pathways of Maternal-fetal Transmission of Human Viruses (Review) Pathol Oncol Res. 2009 doi: 10.1007/s12253-009-9166-9. [DOI] [PubMed] [Google Scholar]
- 42.Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 44.Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, Chavez SL, Romero R, Mor G. Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 45.Mulla MJ, Yu AG, Cardenas I, Guller S, Panda B, Abrahams VM. Regulation of Nod1 and Nod2 in first trimester trophoblast cells. Am J Reprod Immunol. 2009;61:294–302. doi: 10.1111/j.1600-0897.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- 46.Halwachs-Baumann G, Weihrauch G, Gruber HJ, Desoye G, Sinzger C. hCMV induced IL-6 release in trophoblast and trophoblast like cells. J Clin Virol. 2006;37:91–97. doi: 10.1016/j.jcv.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Kovacs IJ, Hegedus K, Pal A, Pusztai R. Production of proinflammatory cytokines by syncytiotrophoblasts infected with human cytomegalovirus isolates. Placenta. 2007;28:620–623. doi: 10.1016/j.placenta.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Toth FD, Juhl C, Norskov-Lauritsen N, Mosborg Petersen P, Ebbesen P. Interferon production by cultured human trophoblast induced with double stranded polyribonucleotide. J Reprod Immunol. 1990;17:217–227. doi: 10.1016/0165-0378(90)90004-p. [DOI] [PubMed] [Google Scholar]
- 49.Toth FD, Norskov-Lauritsen N, Juhl CB, Aboagye-Mathiesen G, Ebbesen P. Interferon production by cultured human trophoblasts and choriocarcinoma cell lines induced by Sendai virus. J Gen Virol. 1990;71(Pt 12):3067–3069. doi: 10.1099/0022-1317-71-12-3067. [DOI] [PubMed] [Google Scholar]
- 50.Trinh QD, Izumi Y, Komine-Aizawa S, Shibata T, Shimotai Y, Kuroda K, Mizuguchi M, Ushijima H, Mor G, Hayakawa S. H3N2 influenza A virus replicates in immortalized human first trimester trophoblast cell lines and induces their rapid apoptosis. Am J Reprod Immunol. 2009;62:139–146. doi: 10.1111/j.1600-0897.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung DY, Lee H, Jung BY, Ock J, Lee MS, Lee WH, Suk K. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J Immunol. 2005;174:6467–6476. doi: 10.4049/jimmunol.174.10.6467. [DOI] [PubMed] [Google Scholar]
- 52.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 53.Chan G, Guilbert LJ. Ultraviolet-inactivated human cytomegalovirus induces placental syncytiotrophoblast apoptosis in a Toll-like receptor-2 and tumour necrosis factor-alpha dependent manner. J Pathol. 2006;210:111–120. doi: 10.1002/path.2025. [DOI] [PubMed] [Google Scholar]