Abstract
The study of sex differences has produced major insights into the organization of animal phenotypes and the regulatory mechanisms generating phenotypic variation from similar genetic templates. Teleost fishes display the greatest diversity of sexual expression among vertebrate animals. This diversity appears to arise from diversity in the timing of sex determination and less functional interdependence among the components of sexuality relative to tetrapod vertebrates. Teleost model systems therefore provide powerful models for understanding gonadal and non-gonadal influences on behavioral and physiological variation. This review addresses socially controlled sex change and alternate male phenotypes in fishes. These sexual patterns are informative natural experiments that illustrate how variation in conserved neuroendocrine pathways can give rise to a wide range of reproductive adaptations. Key regulatory factors underlying sex change and alternative male phenotypes that have been identified to date include steroid hormones and the neuropeptides GnRH and arginine vasotocin, but genomic approaches are now implicating a diversity of other influences as well.
Keywords: hermaphroditism, protogyny, protandry, alternative male phenotypes, hypothalamus, arginine vasotocin, GnRH, estrogen, androgen
1. Introduction
Nobel laureate August Krogh opened his Zoophysiological laboratory at the University of Copenhagen 100 years ago. In 1929, he wrote that “For such a large number of problems there will be some animal of choice or a few such animals on which it can be most conveniently studied” [94]. This approach of defining important questions and then carefully selecting the animal model in which to study them has been termed the ‘Krogh Principle’ and led to great advances in both physiology and behavior through the 20th century. Two of the central challenges facing modern biology are understanding the bases of variation in behavioral and physiological phenotypes and elucidating the mechanisms by which environmental inputs produce phenotypic variation. The study of sex determination and differentiation has been especially informative in this regard [see also 20,21,36,83,112,113,122,124,130,155]. This review focuses on socially controlled sex change and alternative male phenotypes, two closely related topics where fishes provide excellent models. Fishes exhibit enormous diversity in sexual expression including all the major forms of sex determination known from vertebrate animals: parthenogenesis, temperature dependent sex determination, both protandrous and protogynous sex change, and simultaneous hermaphroditism [22,25,107]. In addition to the variety in sex determination patterns, fishes also often display considerable intrasexual variation in the form of discrete alternate male phenotypes within species. Fish models have been particularly important in understanding the ecology, endocrine physiology, and neurobiology of intrasexual dimorphism.
The lability in sex determination and intrasexual variation in fishes may be linked to developmental, physiological, neurobiological mechanisms that allow significant environmental influence on the process of sexual differentiation. Three features of fish development and physiology are likely to be important here. First, even in fishes with strict genotypic determination of sex, sex determination appears generally very malleable prior to sexual maturation relative to tetrapod vertebrates. This lability of sex determination is routinely exploited to create monosex stocks through steroid hormone treatments in aquaculture [25]. Second, as explored in detail by Francis [41], fishes and tetrapods exhibit a generally reversed polarity of development with respect to the gonads and brain. While the gonads develop early in ontogeny in tetrapods and are important in organizing the brain, the reverse is true in the majority of fishes. For many marine species and especially coral reef fishes, juveniles are essentially ‘miniature adults’ living independently and exposed to a full range of environmental cues including social interactions before the gonads develop and sex is determined. The reversed developmental polarity (relative to tetrapods) creates the developmental opportunity for sex determination and differentiation to be shaped adaptively in response to environmental influences. Finally, at least some groups of fishes appear to show a reduced dependence on the gonads for organizing sexual behavior that may facilitate both sex change and the development of novel morph types such as female-mimic males.
This review addresses our understanding of socially controlled sex change and alternate male phenotype expression in fishes from the neuroendocrine and neurobiological perspectives with an emphasis on behavior. Section 2 discusses socially controlled sex change, primarily in coral reef fishes. Section 3 examines alternative male phenotype expression in gonochoristic species (non-sex changers) with examples chosen to highlight the strengths of particular groups for the study of this phenomenon. The final section synthesizes information from sections 2 and 3 in returning to a discussion of how features of teleost physiology and development may contribute to the sexual diversity seen in this vertebrate group.
2. Socially Controlled Sex Change
The Indo-Pacific cleaner wrasse (Labroides dimidiatus) has fascinated biologists and non-biologists alike with its habit of gaining nutrition through setting up ‘cleaning stations’ on reefs where larger fish visit and allow the cleaners to remove parasites. In 1972, Ross Robertson showed cleaner wrasses were equally remarkable sexually when he induced socially controlled sex change in the largest females of polygynous groups by removing dominant males [137]. Sex change had already been recognized as a valuable ‘natural experiment’ in reproductive biology [45], but his was the first demonstration of social control of the phenomenon and in the animal's natural habitat. This paper and a contribution by Fishelson [34] on the fairy basslet (Pseudanthias squamipinnis) stimulated a great deal of interest in socially-controlled sex change from both behavioral ecologists and neuroendocrinologists.
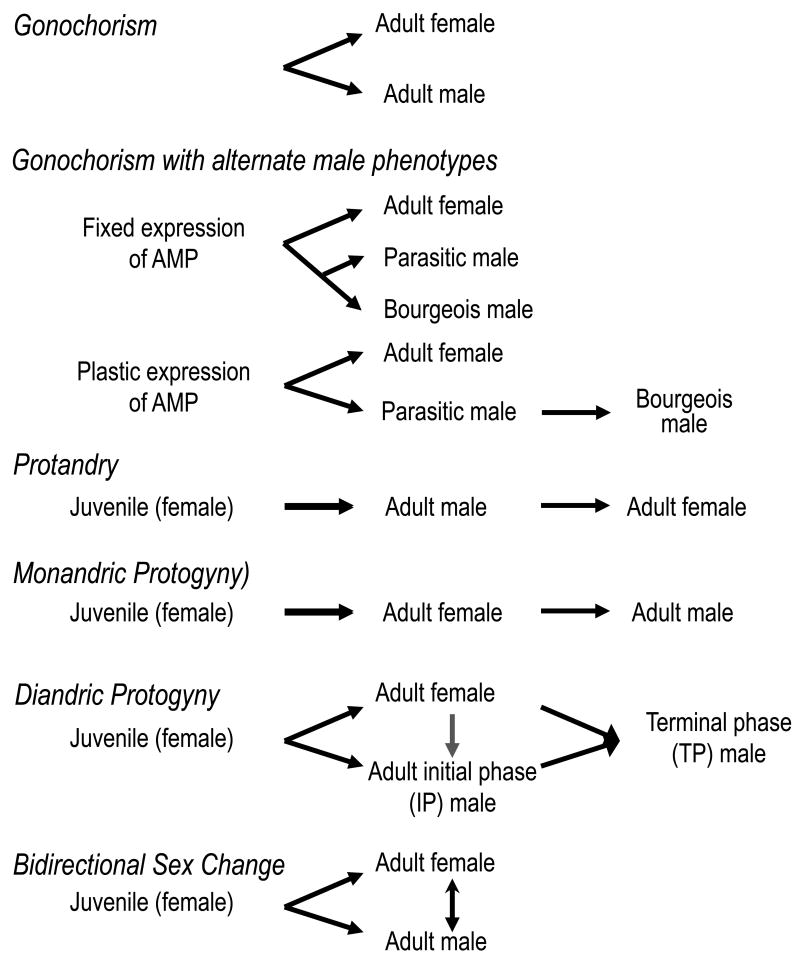
The range of sexual patterns in coral reef and warm temperate reef fishes includes male-to-female sex change (protandry), female-to-male functional sex change (protogyny), bidirectional sex change, and simultaneous hermaphroditism (Figure 1, [22]). Protogyny can be either monandric, where all males are sex changed females, or diandric, where individuals may mature as either males or females (the ‘initial phase’) and either can become a ‘terminal phase’ male. Sex change is observed in seven families across 27 orders of fishes and many species on coral reefs [22], but most of the research has focused on five families that the discussion here will be restricted to (wrasses – Labridae, parrotfishes – Scaridae, gobies – Gobiidae, damselfishes – Pomacentridae, groupers and basslets – Serranidae).
Figure 1.
Diversity of sexual patterns in fishes for both gonochoristic and hermaphroditic species. ‘AMP’ refers to alternate male phenotypes.
2.1 Steroid Hormones and Socially Controlled Sex Change
Gonadal steroid hormones are key regulators of sexual differentiation and reproduction across vertebrate animals. This is also true for sex change in fishes, but with some interesting variations in the key sites of steroidogenesis and especially the role of estrogen signaling. The first studies of the physiology of sex change used wrasses and hormone manipulations to determine whether protogynous sex change could be induced through administering androgens. Working with the bluehead wrasse, Thalassoma bifasciatum, Stoll [157] found that androgen implants in females could induce the blue coloration characteristic of terminal phase males. A role for androgens has been supported and extended in more recent studies using both correlational and manipulative approaches as described below.
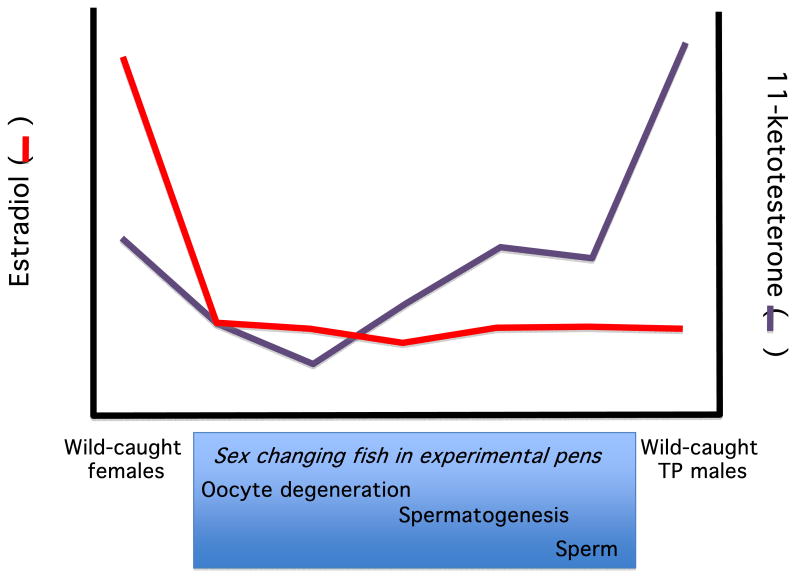
The most comprehensive studies of steroid correlates of socially-controlled sex change in a protogynous species have been in the Hawaiian saddleback wrasse, Thalassoma duperrey [116]. Sex change in T. duperrey can be induced by housing large females with smaller female conspecifics and inhibited by also housing them with a larger individual, which can be either a larger female or a terminal phase male [114,140]. These housing conditions will be referred to below as ‘socially permissive’ and ‘socially inhibitory’ respectively. Nakamura and colleagues compared females and terminal phase males caught and sampled directly from reefs on the island of Oahu (‘natural’ females and terminal phase males) to females sampled at different points during sex change induced by housing with smaller females in floating pens (‘experimental females’, [116]). Plasma levels of estradiol 17β (E2) in experimental females in pens were significantly lower than E2 levels in natural females from the earliest stages of sex change and comparable to the very low levels found in natural terminal phase males (Figure 2). Conversely, 11-ketotestosterone (11KT) levels were relatively low in natural females and elevated in natural terminal phase males. 11KT levels remained low in experimental females during sex change in pens. Interestingly with respect to patterns in tetrapods, testosterone (T) levels were not different between natural females and terminal phase males and did not exhibit significant variation across the sex change process. The lack of variation in T may be due to its being primarily a prohormone in many fishes, serving as a biochemical precursor for E2 and 11KT synthesis.
Figure 2.
Steroid hormone levels in plasma of the saddleback wrasse (Thalassoma duperrey) shown for natural females and TP males and in females induced to undergo sex change in experimental pens (Redrawn from Nakamura et al., 2005). Stages in blue box indicate progress in sex change process when samples were taken.
Gonadal steroid hormone patterns in saddleback wrasses are paralleled by differences in gonadal ultrastructure and steroid hormone synthetic capacity. Females exhibited greater gonadal E2 synthetic capacity than advanced sex changers in vitro when stimulated by salmon gonadotropin, while terminal phase males showed greater 11KT synthesis than females in vitro with advanced sex changers being intermediate [116]. A follow up study compared terminal phase males with female-mimic initial phase males [72]. Initial phase males are externally indistinguishable from females except for the genital papilla. This external similarity is likely important for success in ‘sneaker’ mating tactics (a topic I return to in section 3). Initial phase male saddleback wrasses show both lower plasma levels and lower production of 11KT by the testes in vitro than terminal phase males.
Gonadal steroid hormone patterns described for other sex changing species are generally consistent with those for saddleback wrasses. Stoplight parrotfish (Sparisoma viride) exhibit a similar pattern of sexual phenotype differentiation characterized by diandric protogyny with colorful and territorial terminal phase males and initial phase males that are very similar to females in external morphology. As with saddleback wrasses, terminal phase male stoplight parrotfish exhibit higher 11KT levels than females or initial phase males while females exhibit the highest E2 levels [16,17]. Cardwell and Liley were also able to sample fish undergoing natural female-to-male sex change and found elevations in plasma 11KT and decreases in E2 in these transitional individuals. Initial phase males undergoing only color change during development into terminal phase males showed increases in 11KT consistent with a role in development of the terminal phase male phenotype. The pattern of higher E2 levels in females and higher 11KT in males is also seen in gobies such as the blackeye goby Coryphopterus nicholsi [95] and a variety of grouper species [1,7,68,102,103].
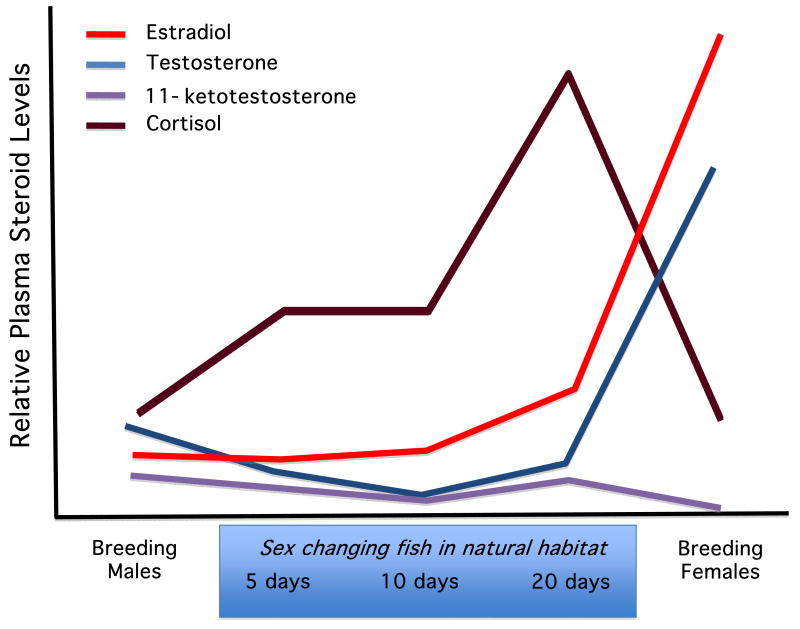
The associations between sex and circulating steroid levels in protandrous and bidirectionally changing species are somewhat less consistent than those for protogynous species, particularly with respect to 11KT. In agreement with the findings described for protogynous species and despite the reversed direction of sex change, the protandrous anemonefish Amphiprion melanopus displays higher levels of 11KT in males and higher levels of E2 in females (Figure 3; [46,47]). Testosterone levels are also higher in female A. melanopus, but this is likely explained by T's role as a biosynthetic precursor to E2. Gobies able to exhibit serial, bi-directional sex change are especially interesting in this context as they maintain both ovarian and testicular tissue in the adult gonad with only one type being active at a time [e.g., 87]. Consistent with other sex changing species, higher levels of E2 are observed when gobies are functioning as females [96,105]. By contrast, Lorenzi and coworkers [105] examined excreted 11KT in the bluebanded goby Lythrypnus dalli and found no difference between individuals functioning as males versus females, a finding consistent with the lack of differences across functional sexes in whole-body 11KT levels in the bidirectionally sex changing Gobiodon erythrospilus [98, both L. dalli and G. erythrospilus are very small bodied, precluding plasma measurements]. The lack of sex differences in 11KT levels may be related to their ability of serial, bidirectional sex change and/or their relative lack of external sexual dimorphism. Recall that initial phase male saddleback wrasses and stoplight parrotfish also lack male secondary sexual characters and have low 11KT levels, a feature generally consistent with other examples of teleost alternative male phenotypes and gonochoristic teleosts that do not display pronounced sexual dimorphism [10,11]. This relation between 11KT levels and the display of secondary sexual characters was also strongly supported in studies of the wrasse Pseudolabrus sieboldi, where shifts from the female-typical to terminal phase-male typical coloration of the anal fin were closely correlated to serum levels of 11KT [but not E2, 119].
Figure 3.
Steroid hormone levels in plasma of the anemonefish Amphiprion melanopus for breeding males and females captured from unmanipulated social groups as well as males undergoing protandrous sex change induced by removing dominant females from social groups. Relative levels of different hormones are depicted to approximately the same scale except for cortisol, where levels were substantially higher. (Redrawn from Godwin and Thomas, 1993).
There are relatively few in-depth studies of androgen signaling other than those described for saddleback wrasses above. Information is particularly lacking in terms of steroid receptor expression. In the single published study, Kim and colleagues [78] cloned androgen and estrogen receptors from the threespot wrasse Halichoeres trimaculatus. Fishes have been reported to express two distinct androgen receptors [154,162], but it was not clear which AR was cloned in this H. trimaculatus study due to incomplete sequence information. Three distinct ERs are known from teleost fishes [66] and the ER cloned from H. trimaculatus was the ERα type. Using RT-PCR approaches, these investigators found no differences in expression of either receptor mRNA in gonadal tissue across the female, initial phase male, and terminal phase male phenotypes. Similarly, ERα mRNA did not differ in brain samples. However, AR mRNA abundances were significantly elevated in terminal phase males compared to both females and initial phase males. The precise neuroanatomical locations and site of sexual phenotype differences in AR mRNA expression are not known from the RT-PCR approach employed (ERα mRNA differences at the level of discrete nuclei also cannot be excluded). Characterizing receptor differences at finer neuroanatomical resolution should be particularly interesting in future studies.
The results of manipulative studies are generally consistent with a causal role for androgens in protogynous sex change. However, several issues are important when considering such studies including the social conditions experienced by treated animals, effects of different types of androgens, whether effects were mediated by the androgen administered or following conversion of the androgen administered to another steroid, and the precise anatomical site(s) and nature of androgen effects.
Kramer and colleagues [91] failed to show an effect of T implants on the occurrence of gonadal sex change in female bluehead wrasses despite observing the complete development of terminal phase male coloration within 18 days (roughly comparable to the rate of color change with natural sex change in bluehead wrasses, pers. observation). The lack of effectiveness of T may be because it is a biosynthetic precursor for other steroid hormones including estradiol. By contrast, studies in wrasses, parrotfishes and gobies have shown that non-aromatizable androgens effectively induce gonadal sex change [16,61,95,131].
While androgens can clearly influence the occurrence of sex change, estrogenic activity appears likely to be the critical regulator in most examples of socially controlled sex change. Estrogenic control is suggested by several lines of evidence involving measures and correlates of estrogen synthetic capacity as well as estrogen manipulations. Higher E2 levels in females of many sex changing species were discussed above. The following section discusses evidence for alterations in estrogen synthetic capacity and effects of estrogen manipulations on sex change.
As in tetrapod vertebrates, estrogens in teleosts are synthesized from androgens through the actions of a cytochrome P450 enzyme termed aromatase. Fishes have two aromatase enzymes derived from distinct genes: i) gonadal aromatase, primarily expressed in the gonads (also referred to as p450AromA or cyp19A1), and ii) ‘brain aromatase’ (also referred to as p450AromB or cyp19A2) [15,40]. Teleosts can exhibit very high aromatase levels in brain tissue relative to those seen in tetrapods and evidence for brain aromatase activity regulating socially controlled sex change is accumulating. The gobies that exhibit bidirectional sex change are particularly interesting models here. The bluebanded goby Lythrypnus dalli is native to rocky reefs off the coast of California and exhibits bidirectional functional sex change through shifts in allocation between ovarian and testicular tissue in the gonad. Bluebanded gobies typically live in groups consisting of a behaviorally dominant male and several females in nature. Removal of the male from a social group leads to rapid gonadal, morphological, and behavioral changes in the largest female as she undergoes protogynous sex change [138,156]. The behavioral changes include increases in a dominance-related displacement behavior that are correlated with rapid declines in aromatase activity in the brain [8]. Interestingly, gonadal aromatase activity also decreases as a female changes sex, but these decreases are slower than those in the brain and are not significantly correlated with behavioral changes. Aromatase immunoreactivity in another goby displaying bidirectional sex change, Trimma okinawae, was higher in ovarian tissue than testicular tissue, but neural expression was not examined [159].
Manipulation using implants and aromatase inhibition also support a critical regulatory role for estrogen signaling in sex change. Implants of the aromatase inhibitor fadrozole are as effective in inducing protogynous sex change as 11KT implants in blackeye gobies [95]. The West Pacific goby Gobiodon erythrospilus exhibits serial bidirectional sex changes, an ability hypothesized to be adaptive by allowing formation of breeding pairs with any two fish resident on a coral and preventing the need for dangerous travel across the reef in this very small-bodied species. Fadrozole implants induce protogynous sex change in female G. erythrospilus while E2 implants induce protandrous sex change in males [97]. Aromatase inhibitors are also effective in inducing protogynous sex change in groupers [102] and wrasses [69]. Importantly, induction of protogynous sex change in threespot wrasses by either aromatase inhibition or 11KT could be prevented by co-implanting the animals with E2, suggesting estrogen action is the critical inhibitory influence with androgens potentially having their effects by inhibiting aromatization (11KT does inhibit aromatization by the ovary of the Atlantic croaker, a gonochoristic fish species [12]). Lastly, very recent findings in mammals show that ERα (ESR1) interacts with the forkhead transcription factor Foxl2 in the adult ovary to actively suppress Sox9 expression [166]. This suppression of Sox9 prevents somatic cell reprogramming and testicular differentiation. These authors speculate a Foxl2-mediated mechanism may be important in teleost sex change.
How might estrogen synthesis through aromatase activity be regulated in sex changing species? Relatively little information is available, but findings from gonochoristic fishes and other vertebrates suggest some intriguing possibilities. The promoter region of brain aromatase genes in the bi-directionally sex-changing gobies G. histrio and T. okinawae have estrogen response elements (ERE), but an ERE was suggested only for gonadal aromatase in T. okinawae [44, 85,86]. Estrogen responsiveness mediated transcriptionally through an ERE could help explain E2 effects on the occurrence or prevention of sex change if exogenous estrogen maintained or induced aromatase expression in brain or gonad. Consistent with this estrogenic regulation of neural aromatase expression, female bluehead wrasses receiving E2 implants do show higher brain aromatase mRNA abundances than controls (Hunkin et al., in preparation). Kobayashi and colleagues [86] also described cAMP response elements (CRE) in the promoter regions of both forms of aromatase, consistent with results from zebrafish. By contrast, Gardner did not describe CREs for either aromatase in G. histrio, but did suggest a glucocorticoid response element (GRE) in the gonadal aromatase gene (although a GRE was not found in two other sex changers examined). The somewhat conflicting results from different studies are difficult to reconcile, but efforts to sequence the genome from a representative of every vertebrate genus (www.genome10K.org) could provide data allowing characterization of aromatase promoters across fish species within a few years.
Glucocorticoids have been postulated to be critical regulators of the sex change process [127] This model is based on the link between glucocorticoids and social status in many vertebrates, the frequent suppression of reproductive function in subordinate animals, and glucocorticoid regulation of steroid synthesis [127]. Contrary to the model's predictions, glucocorticoid administration did not prevent sex change in the protogynous sandperch Parapercis cylindrica under permissive social conditions [43]. However, glucocorticoid effects can be complex and more studies would be useful.
2.2 Neural Signaling Systems and Sex Change
Important regulatory events in socially controlled sex change must take place in the brain. While our understanding of how transduction of social cues takes place is still very incomplete, characterization of the role of several neural signaling systems is progressing. This information is reviewed below with a focus on monoamine neurotransmitters, the hypothalamo-pituitary-gonadal (HPG) axis, and the neuropeptides arginine vasotocin (AVT) and isotocin (IST).
Monoamine neurotransmitters are key regulators of a wide variety of neural functions and it is therefore unsurprising that they are implicated in behavioral and gonadal aspects of socially controlled sex change. Monoamine function is linked to social status in many species and monoamines also interact with neuropeptide systems regulating reproductive and behavioral functions [158]. Saddleback wrasses undergoing female-to-male sex change in outdoor floating pens showed monoamine changes in a variety of brain regions [100]. The pattern was complex and included a decrease in serotonergic activity in the raphe nucleus during the first week when sex changing females were establishing behavioral dominance. Follow up studies showed monoamine manipulations could override social control of the process [99]. Reducing serotonergic signaling or increasing noradrenergic signaling pharmacologically induced sex change even in the presence of a large male (socially inhibitory conditions) while increasing serotonergic and reducing noradrenergic signaling could prevent sex change even when females were the largest fish in a group (socially permissive conditions).
The correlation between behavioral dominance and reductions in serotonergic activity in the raphe nucleus for sex-changing female saddleback wrasses is consistent with behavioral effects of fluoxetine, a selective serotonin reuptake inhibitor (trade name: ‘Prozac’) in the congeneric bluehead wrasse [126]. Both chronic and acute fluoxetine treatment, in the lab and field respectively, decreased aggression of terminal phase males towards a smaller terminal phase male intruder. The chronic fluoxetine treatment may have decreased aggressive behavior through augmentation of serotonergic signaling, but the treatment also reduced AVT mRNA abundances in the preoptic area (POA) and AVT is implicated in male-typical behavior in this species [149]. It is noteworthy that some SSRIs can also significantly alter neurosteroid synthesis. A study in mice using stereoisomers of fluoxetine showed that neurosteroidogenic rather than serotonergic effects best accounted for the decreased aggressive behavior of treated animals [129].
Neuropeptides are key regulators of reproductive function and behavior in fishes as in tetrapod vertebrates. Studies in sex changing fishes have focused primarily on gonadotropin-releasing hormone (GnRH) neurons and AVT because of their strong links to reproductive function and behavior in a variety of systems [36]. Studies of GnRH and AVT in sex changing fishes have been largely separate, but intriguing new findings from both mammalian models and some gonochoristic fish species suggest potential links between behavioral and gonadal sex change involving the GnRH and AVT systems. Teleost fishes have multiple GnRH neuronal populations, but the hypothalamic GnRH neurons are the key regulators of the hypothalamo-pituitary-gonadal axis and reproductive function [36]. This has made GnRH a logical focus for studies of the neuroendocrine basis of sex change. Findings to date do support a key role for hypothalamic GnRH neurons, although a detailed mechanistic model for how a change in their function could lead to a change of sex is still lacking.
Bluehead wrasse terminal phase males exhibit greater numbers of immunoreactive GnRH neurons in the POA of the hypothalamus than females or initial phase males [60] and GnRH neuron numbers are increased in females and initial phase males receiving an 11KT implant [which also induces sex change, 61]. Males of the monandric protogynous Ballan wrasse (Labrus berggylta) also show more GnRH neurons in the POA than do females, but no differences were found in the ventral telencephalon or the terminal nerve area populations [28]. Anemonefish, as noted above, display protandrous sex change. Interestingly though, the dimorphism in POA GnRH neurons in the anemonefish Amphiprion melanopus is similar to that of the wrasses studied in that males have greater numbers than females [27].
As with androgen and estrogen manipulations, experiments designed to mimic or produce changes in GnRH signaling can induce partial to complete sex change. In a series of studies, Kramer and colleagues demonstrated different degrees of sex change in female bluehead wrasses stimulated by treatment with human chorionic gonadotropin (hCG), a synthetic form of GnRH co-administered with a dopamine receptor antagonist, or neuropeptide Y [90,92,93]. There is some concern with these experiments since females were housed singly rather than in an inhibitory social environment with a larger fish as in some of the monoamine experiments with saddleback wrasses discussed above. However, the findings are generally consistent with gonadotropic signaling being an important regulator of sex change and are supported by a study showing hCG stimulation of protogynous sex change in the wrasse Coris julis [132]. More recent findings also implicate gonadotropic signalling and suggest potential mechanisms. Diurnal patterns of expression of various gonadotropin subunit mRNAs in the pituitary of the wrasse Pseudolabrus sieboldi were similar between males and females for the common α subunit, but strikingly different for the FSH and LH specific β subunits [120]. Perhaps the most interesting finding comes from the serially bidirectional sex changing goby Trimma okinawae. This species has a gonad with both ovarian and testicular tissue, but only one tissue is active at a time and this can be experimentally controlled through social manipulations. Kobayashi and colleagues found that gonadotropin receptor (FSHR and LHR) expression was very strongly biased to that portion of the gonad that was currently active and producing functional gametes (i.e., ovarian portion for females and testicular portion for males, [87]). Socially induced sex change produced significant shifts in gonadotropin receptor mRNA expression within 12 to 24 hours of the initiation of change and in the predicted directions. Finally, incubation with hCG and measurement of steroid production confirmed that mRNA changes were correlated with the predicted changes in gonadotropin receptor function. The mechanisms underlying the shift in gonadotropin receptor expression remain unknown and it is also unknown whether similar shifts occur in species without a clear morphological separation of ovarian and testicular precursors, but this example does suggest changes in target tissue responsiveness are likely to be important mediators of the sex change process.
Gonadal sex change can be relatively rapid with some species transitioning from functional female to functional male in as few as eight to ten days [e.g.,168]. However, behavioral sex change is often much faster, occurring sometimes within minutes to hours [137,168]. The rapidity of behavioral change and its temporal precedence over gonadal change calls into question a role for the gonads and gonadal products in regulating the process. In at least the bluehead wrasse, behavioral change does not depend on the gonads, occurring even in ovariectomized females made socially dominant on small patch reefs [48]. These ovariectomized, socially dominant females develop the full suite of terminal phase male-typical territorial and courtship behaviors, but do not develop terminal phase male coloration, presumably because they sufficient levels of 11KT [implanting 11KT into ovariectomized females does induce terminal phase male coloration, 148].
What neural mechanisms regulate behavioral sex change? Attention has focused largely on AVT and, to a lesser extent, IST because of patterns across vertebrate animals linking these neuropeptides and their homologues in tetrapods to important sociosexual behaviors [5,23,56]. AVT neurons in teleosts are primarily localized in the POA with anterior parvocellular and more caudally distributed large neurons in magnocellular and gigantocellular populations. The parvocellular and magnocellular cell groups are likely to be homologous to the supraoptic and paraventricular arginine vasopressin neuron populations of mammals respectively (see [149] for a discussion of homologies). The magnocellular neurons are complex, with projections to both the pituitary gland and other brain regions including the thalamus and telencephalon in at least the rainbow trout [143]. There is also a more caudal group of small AVT neurons in the tuberal hypothalamus [55,59, and Godwin, unpublished in situ hybridization results for the bluehead wrasse], but very little is known about this cell population.
The first report of a difference in AVT neuronal populations was in the goby Trimma okinawae [62]. A later study in the bluehead wrasse showed higher levels of AVT mRNA in the POA of terminal phase males than females and rapid increases in AVT mRNA with experimentally induced sex change [49]. Since behavioral and gonadal change are ‘confounded’ in an experimental sense during normal sex change, Semsar and Godwin compared AVT expression in four experimental groups: females undergoing sex change with or without gonads and females not changing sex that were either ovariectomized or not. Social status influenced AVT mRNA levels in the POA while gonadal status did not [147 and Figure 4]. In companion studies, neither castration of terminal phase males nor 11KT implants in ovariectomized females affected AVT mRNA abundances in bluehead wrasses [147]. Manipulative approaches are also consistent with a key role for AVT in behavioral control and sex change in this species. AVT injections can induce territorial behavior and increase courtship in non-territorial terminal phase males while injections of an AVP V1 receptor antagonist reduce both territorial aggression and courtship behavior in territorial terminal phase males [146]. The importance of AVT for the development of territorial status is also supported by experiments where an AVP V1 receptor antagonist blocked the development of territorial behavior in non-territorial terminal phase males and behavioral sex change in females made socially dominant through terminal phase male removals [148].
Figure 4.
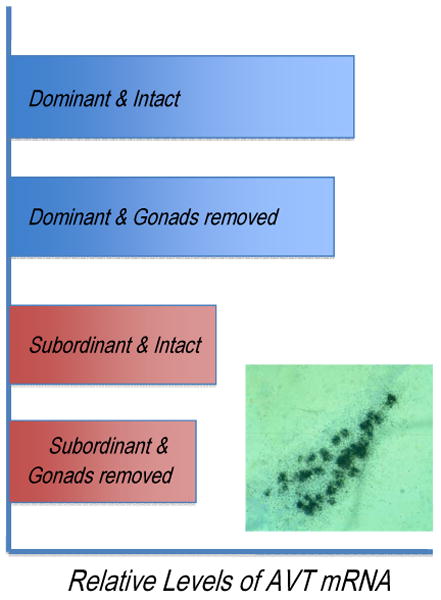
Social status influences AVT mRNA levels in the preoptic area of the hypothalamus in bluehead wrasses while gonadal status does not. Dominant individuals were the largest in their social groups while subordinants were not. Intact animals underwent ‘sham’ operations to control for the surgical procedure. Inset shows AVT mRNA in the preoptic area visualized by the technique of in situ hybridization. Figure depicts relative AVT mRNA levels in this comparison from Semsar and Godwin (2003).
Very little information is available regarding a potential role for isotocin in sex changing fishes. The single published study found greater numbers of isotocin-immunoreactive neurons in females and early-stage protogynous sex changers than in late stage sex change or males in the bluebanded goby Lythrypnus dalli [9].
What neural mechanisms influence AVT expression in bluehead wrasses and other sex changing species? Based on effects of the SSRI fluoxetine on AVT mRNA abundances described above, both serotonergic and neurosteroidogenic influences are possible [149]. Serotonergic effects on AVT neurons could be reminiscent of serotonin-induced reductions in AVP release in the anterior hypothalamus of golden hamsters [32]. We have also found both aromatase-immunoreactive glia and tyrosine-hydroxylase immunoreactive neurons in very close proximity to AVT neurons in the POA of bluehead wrasses [108]. Expression of estrogen receptor α, βa, and βb mRNAs is found throughout the POA in another perciform teleost [66,67]. Expression of Erβb is especially strong in some large magnocelular neurons that may also express AVT, which would be generally consistent with ERβ expression in mammalian AVP neurons [73,110].
Could interactions between the AVT, IST, and GnRH systems link behavioral and gonadal sex change? Too little information is available to assess this possibility presently, but findings from other teleosts and mammals suggest it is worth exploring. AVT and IST neurons in trout receive GnRH innervation and their electrical activity can be modulated with GnRH and a GnRH antagonist [142]. Isotocin and GnRH neurons have a broadly overlapping distribution in the POA of the dwarf gourami (Colisa lalia) and some parvocellular neurons in this region were immunopositive for both IST and GnRH [106]. Conversely, AVT could activate GnRH neurons. While certainly speculative, this possibility is suggested by vasopressin stimulating a LH surge in ovariectomized rats with suprachiasmatic nucleus lesions [125], contacts between vasopressin and GnRH neurons in the monkey supraoptic nucleus [163], and a recent report that vasopressin neurons in the suprachiasmatic nucleus of mice innervate kisspeptin neurons [76]. Kisspeptin neurons have recently emerged as key regulators of GnRH neuron activity across vertebrate animals including fishes [118,121], exhibit sexual dimorphisms and steroid sensitivity in at least one teleost [the medaka, 77], and have been linked to puberty in fishes [33,109]. Studies of kisspeptin involvement in socially controlled sex change have the potential to be very informative.
3. Alternative Male Phenotypes
As noted in the introduction, understanding the neural and physiological bases of behavioral variation is a central challenge for biology. Sex differences have provided very productive study systems for understanding this variation because individuals are ‘pre-classified’ into comparison groups that often show striking differences in behavior and physiology (but see [21] for a discussion of limitations to this approach). The sex changing species that are the focus of section 2 above are interesting because they allow study of the transition between the two sexes. However, there are two general challenges faced by this approach. The first is that comparisons are confounded because the groups also differ in genotype with respect to sex chromosomes and gonadal sex [112,113]. The genetic and gonadal sex confounds have been approached in several ways. These approaches include exploiting within sex variation generated by differences in the embryonic environment with phenomena such as intrauterine position effects in rodents and incubation temperature influences on adult phenotypes in reptiles [141,144]. Another approach is to focus on the effects of disruptions in key endocrine signaling systems mediating sexual differentiation. Examples include testicular feminization in mice and various disorders of sexual development associated with androgen signaling in humans including androgen insensitivity syndrome, 5α-reductase deficiency, and congenital adrenal hyperplasia [19,74]. The second general challenge is essentially one of repeatability because sex differences typically provide a comparison between only two groups. Species that display discrete, within-sex alternate mating phenotypes provide valuable opportunities to explore the bases of behavioral and physiologica variation because they provide options that help address both of these challenges.
Morphologically discrete alternate mating phenotypes are seen in all vertebrate groups except mammals, but occur most frequently and are best studied in squamate reptiles and fishes [113,160,161]. The best studied reptile in this respect is the tree lizard Urosaurus ornatus in which both fixed and plastic variation are observed across alternate male types and a series of studies has explored the mechanistic bases of the morphological, neuroendocrine, and behavioral variation among male types [82,113]. Fishes have received somewhat more attention with information now available from a number of species representing phylogenetically divergent groups and both hermaphroditic and gonochoristic sexual patterns. Examples of alternate male phenotypes in sex changing wrasses and parrotfishes were described in section 2 above. Well-studied examples of alternate male phenotypes from gonochoristic fishes include the bluegill sunfish (Lepomis macrochirus), Atlantic salmon (Salmo salar), and the plainfin midshipman (Porichthys notatus). An interesting feature of these model systems emerges in terms of the sexual phenotypes represented. As with wrasses and parrotfishes, each includes a large territorial male morph characterized often by exaggerated morphological secondary sexual characters, aggressive defense of a territory where breeding takes place, and often conspicuous courtship behaviors. The large territorial morphs have been termed ‘bourgeois’ males by Taborsky [160,161]. Females are usually smaller than males even in the gonochoristic species and do not display the morphological specializations exhibited by large males that are related to courtship and/or territorial defense (examples include coloration, fin extensions, and specializations for sound production). The alternate male mating phenotypes tend to represent an apparent decoupling and ‘resorting’ of components from the phenotypic characters seen in bourgeois males and females to produce a third, reproductively successful sexual phenotype (see also discussion in[124]). Alternate male phenotypes are fixed in some species with no switching later in ontogeny Other species show more plastic expression, with smaller males displaying a parasitic male phenotype then switching to a bourgeois male phenotype with growth and an increased ability to successfully compete for breeding opportunities (Figure 1).
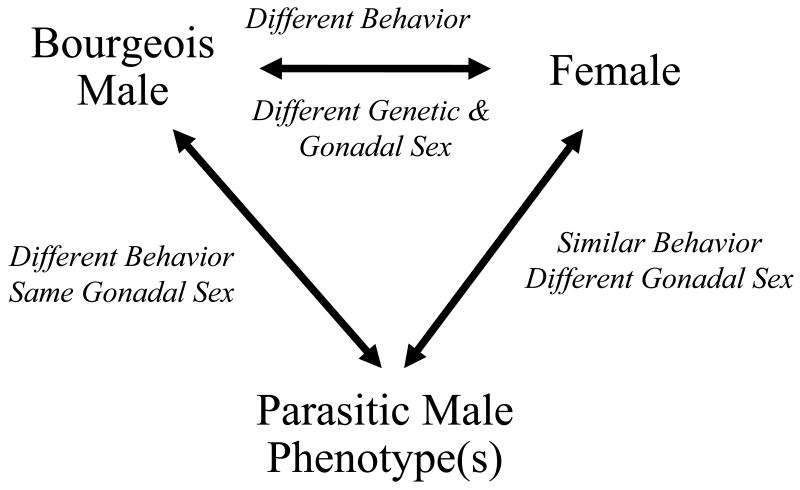
Species with alternate male phenotypes are useful in providing three (or more) discrete comparison groups that can be used to address questions about behavioral and physiological variation (Figure 5). As noted by Moore and colleagues [112,113], alternate male phenotypes allow the comparison of discrete, ‘pre-classified’ groups of males that display what is often pronounced dimorphism in morphology and/or behavior without the confound of differing gonadal sex. Equally valuable for some questions is the ability to ‘hold behavior constant’ and vary gonadal sex by comparing both females and alternative male morphs to the bourgeois males. In this section, I first review alternative male phenotype expression and the behavioral, morphological, and neuroendocrine correlates of this phenotypic variation for sunfishes, salmonids, and the plainfin midshipman with brief treatment of blennies and cichlids as well. Each group is characterized by particular features or developing resources that are useful for exploring the physiological bases of traits showing inter- and intrasexual dimorphism. The summaries will necessarily be brief, but serve as a basis for discussing general patterns that emerge and the implications of these patterns for the physiological and evolutionary origins of alternate male phenotypes. It has recently been argued that alternative phenotype adaptations are relatively well understood from a theoretical standpoint, but that more information on their mechanistic bases is very much needed [139]. Teleosts provide excellent model systems for exploring such mechanisms. They also allow comparisons across phylogenetically divergent groups as the salmonid fishes and perciforms such as sunfishes and wrasses likely diverged in the Jurassic period [104]. Such comparisons provide an unusually good opportunity to look for common principles underlying this sort of behavioral variation without the confound of recent common ancestry.
Figure 5.
Comparisons possible across sexual phenotypes in species with AMPs. Notes beside arrows indicate factors that are being varied or held ‘constant’ in each comparison.
Section 3.1 – The Sunfishes
Four species of sunfishes, all in the genus Lepomis, are known to display alternate male phenotypes, but the bluegill sunfish (L. macrochirus) has received the most attention [117]. Bluegill sunfish inhabit inland bodies of fresh water ranging from Canada into the southern United States. Key advantages of this species are that their habitats are accessible for field study, their behavior is easily observed, their behavioral ecology is well studied, and they have been the subjects of seminal early studies exploring the behavioral and reproductive correlates of alternate male phenotype expression [65, reviewed in 117]. Four sexual phenotypes are exhibited: females, large parental males (the bourgeois males in this system), satellite males, and sneaker males (Figure 6). Parental males are large, mature relatively late at large body size, exhibit display coloration, and defend nest sites that females visit to deposit eggs. Sneaker males gain fertilizations by darting into nests during spawning between parental males and females while satellite males mimic females in order to join spawning pairs and release sperm. Sneaker males develop into satellite males with growth, but this developmental trajectory is distinct from that leading to the large parental males.
Figure 6.
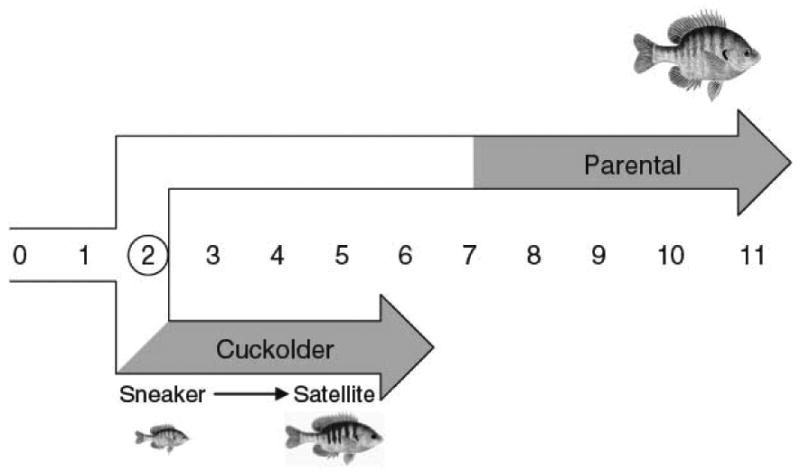
Ontogeny of alternative male phenotypes in the bluegill sunfish, Lepomis macrochirus. Numbers reflect age in years and gray fill in lines indicates approximate timing of sexual maturity (figure is from Neff and Knapp, 2009 with permission).
Neuroendocrinological studies of alternate male phenotypes in sunfishes have focused on bluegills and information is still somewhat limited, but the differences between morph types suggest this will be a very interesting model system going forward [see full review in 117]. Sneaker and satellite males have smaller testes than parental males, but their investment in gonads is greater when adjusted for body mass. There are also sperm differences with those of sneaker and satellite males showing greater energy reserves and greater swimming speed. As in the wrasses and parrotfish, the bourgeois parental males in bluegill sunfish show the highest circulating levels of 11KT, likely associated with the development of display coloration. Findings have been mixed for circulating T levels. Knapp and Neff [84] found higher levels of T in sneaker and satellite males than in parental males while an earlier study of the same population did not [80]. Most interestingly, Knapp and Neff found that sneaker and satellite males showed cortisol levels that were significantly elevated relative to both parental males and females. Estradiol levels also appeared to be elevated in sneaker and satellite males relative to nesting parental males on the day of spawning. Given the greater aromatase activity in the brains of female gobies and the non-territorial morph of the plainfin midshipman (see below), it is possible differences in circulating estrogen could be the result of neural synthesis differences.
Section 3.2 – The Salmonids (Salmon and Trout)
Several salmonid fishes also display alternative male phenotypes that have been the subjects of considerable research from the behavioral and life history perspectives [reviewed in 35]. These species provide a very good opportunity to explore the neuroendocrine bases of male phenotype variation because their physiology and genetics are well studied due to their ecological and economic importance. Many salmonid species have an anadromous life history where spawning and early development take place in freshwater, followed by downstream migration and maturation in the ocean before returning to freshwater as large fish to spawn. These migratory males pursue a bourgeois strategy, obtaining fertilizations through aggressive defense of nest sites on the spawning grounds. The alternate male phenotypes are referred to as ‘jacks’ or ‘parrs’. These parasitic males forgo migration, mature earlier and at much smaller size than the downstream migrant males, and display sneaking behavior to obtain fertilizations. As with sunfishes, these are fixed strategies in salmonids as sneaker males cannot develop into bourgeois males.
The noteworthy features of salmonids for understanding the neuroendocrinology of alternative male phenotypes are the extensive body of research on salmonid reproductive ecology and physiology and, more recently, the development of genomic resources for the group. The success of using genomic approaches to explore phenotype differences in social insects (e.g., [169]) suggests this is likely to be a similarly fruitful approach in salmonids and other groups of fishes. Working with brown trout (Salmo trutta), Kolm and colleagues [89] found that sneaker males had larger brains relative to their body size than migratory bourgeois males, although the cerebellum was relatively larger in the migratory morph. This brain difference is consistent with other studies demonstrating plasticity in salmonid brains [79,101]. As with the GnRH differences described between male phenotypes in bluehead wrasses in section 2, the HPG axis is important in differentiating parr from immature migratory males in Atlantic salmon with the maturing parr showing greater LHβ and FSHβ subunit mRNA levels in the pituitary, greater LH and FSH receptor mRNA in the testes, and greater testis size and 11KT levels. However, also consistent with the general observation of higher 11KT in mature bourgeois males, 11KT levels in precociously mature parr are relatively low compared with migrant bourgeois males returning to the spawning grounds [111]. These endocrine findings have been extended using salmonid cDNA microarrays in Atlantic salmon (Salmo salar). A pair of studies has shown differences in the expression of a number of key neuroendocrine genes at the developmental point when males are either maturing into non-migratory sneaker males or remaining immature and initiating the migration to the ocean [3,4]. The differences found include some that might be expected to be regulated such as gonadotropin subunits, prolactin, proopiomelanocortin (POMC), somatolactin, and somatotropin. However, a variety of additional transcripts that would not have been predicted also showed significant covariation with the neuronendocrine genes. The authors suggest coordinated suites of gene expression controlled by one or few master regulatory genes govern critical life history transitions.
Section 3.3 – The Plainfin Midshipman (Porichthys notatus)
The best-studied and understood example of alternative male phenotypes from a mechanistic standpoint is the Plainfin Midshipman, Porichthys notatus. An elegant series of studies have examined physiological, neuroendocrine, and neurophysiological differences between male morphs in P. notatus ranging from steroid hormone signaling to the activity and response properties of the relevant neural circuitry for key advertisement and communication behaviors (see [64] and citations below). The plainfin midshipman is a marine species native to the west coast of North America. During the summer breeding season, large ‘type I’ males court females through advertisement calls emitted from nest sites that they aggressively defend in the intertidal and shallow subtidal zones. Females are attracted to nests and deposit eggs the parental type I male then provides care for. Type II males are smaller, do not defend nest sites or court females, and attempt to obtain fertilizations through sneaking tactics similar to those employed by sneaker sunfish males. Type I males are the bourgeois males while type II males are the parasitic males in Taborsky's categorization. Females and type II males produce agonistic ‘grunt’ vocalizations, but not the courtship vocalizations used by type I males. Key advantages of P. notatus for exploring behavioral variation lie in the precisely quantifiable and reproducible nature of its acoustic communication signals and especially the suitability of its vocal-motor system for testing mechanistic hypotheses in manipulable neurophysiological preparations.
Patterns of plasma androgens in the plainfin midshipman are similar to other species discussed above in that the bourgeois male morph exhibits higher levels of 11KT. Indeed, observations from this species stimulated an important review linking 11KT primarily to display characters such as advertisement calls in fishes [11]. These results have been extended in recent years with characterizations of plasma steroid patterns both across the nesting cycle and across breeding and non-breeding seasons [81,152], demonstrations of seasonally varying auditory sensitivity in females that are under estrogenic control [150-153], expression of aromatase in glial cells (rather than neurons) that is widespread across both vocal and non-vocal brain regions [37-40,145], and recently a demonstration of rapid actions of the three major classes of steroid hormones on vocalization behavior [133,134]. Most directly relevant to this review are the findings of strong divergence in aromatase expression and activity, rapid steroid actions, and neuropeptide effects across sexual phenotypes.
Aromatase activity differs across sexual phenotypes in the midshipman [145]. Levels are comparable in the telencephalon, but higher for females in the diencephalon. Interestingly with respect to dimorphic vocal behavior, the greatest differences in aromatase activity are seen in the hindbrain where the sonic motor nucleus, a key vocal control region, is located. Females and type II males exhibit hindbrain aromatase activity levels 300-500% greater than those of type I males and these differences persist after castration in males. As predicted from these activity findings, both aromatase mRNA and protein were widely expressed in the brain including in the sonic motor nucleus of the hindbrain and POA, critical regions for vocalization and male-typical reproductive behaviors respectively [37]. Immunocytochemical colocalization studies showed brain aromatase was predominantly in glia in the midshipman, a surprising result relative to patterns in tetrapods. Comparing brain aromatase mRNA expression across sexual phenotypes in the breeding and non-breeding seasons showed a complex pattern of changes, but one generally consistent with the differences in aromatase enzymatic activity [38]. Circulating steroid hormone changes may contribute to aromatase expression changes as both T and E2 implants administered to ovariectomized females increase aromatase mRNA levels in the sonic motor nucleus and POA [39] and at least ERα is expressed in both regions [40].
Rapid steroid hormone actions also appear to be important in P. notatus and show fascinating variation across the sexual phenotypes. Elucidation of the vocal circuit in the plainfin midshipman has allowed experimental tests of various hormonal effects in intact and semi-intact preparations through monitoring of ‘fictive vocalizations’ – neuronal discharges of the pacemaker circuit to the sonic muscles that faithfully mirror vocal behavior at the whole animal level. Using this approach in type I males, Remage-Healey and Bass [133] showed that cortisol, 11KT, and E2 (but not T) could modulate vocal patterning within five minutes and that these effects could be blocked with specific and appropriate antagonists. Extending the investigation to females and type II males revealed fascinating between- and within-sex dimorphisms in rapid steroid effects [134]. While 11KT rather than T modified vocal patterning in type I males, the converse was true in type II males and females where T rapidly and dramatically increased call burst duration. Cortisol effects also differed between phenotypes, but decreased burst duration in type II males and females as opposed to increasing it in type I males. In contrast to androgen and cortisol effects, E2 effects were similar across the three phenotypes.
The differences in responsiveness across sexual phenotypes found for steroid hormones are also seen with the key neuropeptides AVT and isotocin in the midshipman [54] (Goodson and Bass, 2000). Both neuropeptides innervate key vocal-acoustic areas of the midshipman brain with the vasotocinergic innervation being more restricted to these areas [55,57,58]. Goodson and Bass assessed responsiveness to exogenous AVT and isotocin across sexual phenotypes using fictive vocalizations as in the studies described above. Type I males and females showed exactly opposite patterns of responsiveness to these neuropeptides. Type I males responded to AVT with significant decreases in vocal motor output, but did not respond to isotocin. Females reduced output in response to isotocin while AVT was ineffective. Importantly, specific antagonists to AVT and isotocin also affected vocal motor output, but increased it in type I males and females respectively. Type II males provided the most striking results, showing patterns of responsiveness essentially identical to those of females rather than type I males. These responses to neuropeptides are very similar to those described above for rapid modulation of vocal motor output by T in this species and the lack of effects of AVT administration on the behavior of either females or female-mimic initial phase males in bluehead wrasses [148].
Section 3.4 –Blennies and Cichlids
Two other groups of fishes displaying pronounced intrasexual dimorphism that have been explored at the neuroendocrinological level are the blennies (Blenniidae) and cichlids (Cichlidae). The treatment here is briefer than that for the previous groups in this section because the patterns for both are similar in many respects to those described in section 2 for sex changing species and because excellent recent reviews are already available [24,31,122,124].
The neuroendocrinology of alternative mating phenotypes in blennies has been best studied in the peacock blenny Salaria pavo [122,124]. Peacock blennies are found in shoreline habitats in the eastern Atlantic Ocean. Nest sites are limited and occupied by older, larger males that differ from females in possessing a large head crest and anal glands on their anal fin. While large males compete for nest sites and provide parental care to eggs, it is the females that exhibit courtship in the populations that have studied (presumably because nest sites and nesting males are limiting). Young, small males cannot compete for nest sites and instead adopt a female-mimic strategy characterized by female coloration, lack of the head crest and anal glands, greater investment in gonads than nest holder males, female-like courtship, and low levels of both T and 11KT. Administering 11KT to sneaker males caused development of head crests and anal glands as well as inhibited female-like courtship behaviors, but did not induce nest holder behavior [123]. Conversely, castration of sneaker males induced increases in female-like courtship and coloration. The induction of female courtship and coloration could be blocked with implants of T together with the aromatase inhibitor fadrozole while E2+fadrozole implants had no effect [51]. Peacock blenny brain aromatase activity did not follow the pattern seen in the plainfin midshipman. While females had the highest activity in the brainstem, levels in sneaker males were significantly lower and not elevated relative to nesting males [52]. Particularly interesting is the finding that AVT expression in the POA is greater in females and sneaker males than in large nest holding males [63]. Consistent with this female-biased difference and the association of AVT and courtship in some other species, AVT injections induce female-like courtship and coloration in sneaker males [18] (but not male-typical behaviors).
The cichlid fishes have provided valuable model systems for a variety of fields due to their adaptability to aquarium conditions, complex patterns of social behavior, and the spectacular species diversity within the family. The cichlid ‘species flocks’ of the African rift lakes are perhaps the best example of a rapid adaptive radiation in vertebrates. The usefulness of cichlids as models is likely to continue to grow as genomic resources are developed [70,88,135]. These fishes should therefore continue to be excellent model systems for behavioral neuroendocrinology [130,136]. The best-studied example of intra- and intersexual dimorphism in reproductive function among the cichlids is Astatotilapia burtoni (formerly Haplochromis burtoni). This small-bodied species is native to Lake Tanganyika and rivers draining into the lake where territorial males defend breeding territories in shallow water [30]. Males compete for a limited number of territories and transitions between territorial and non-territorial status are frequent. Transition to territorial status involves rapid activation of the gonads, increases in plasma androgens, and development of display coloration and aggressive behavior (reviewed in [31]). The changes in the HPG axis are triggered by activation of forebrain GnRH neurons, which show increases in expression of the immediate early gene EGR-1 within a very short period after males perceive an opportunity to ascend to territorial status and begin displaying dominance related behaviors [13]. Shifts in social status are also accompanied by changes in the expression of AVT [59], somatostatin and somatostatin receptors [71, 165], and androgen and estrogen receptors [14]. Functional genomic resources have also now been developed for A. burtoni in the form of cDNA microarrays to allow global gene expression profiling. Initial efforts have provided confirmatory evidence of variation in signaling systems previously examined and intriguing new candidate genes and pathways that may be important for behavioral variation [136]. As with the salmon example in section 3.2 [3,4], most exciting is the ability to begin examining and comparing suites of coordinately regulated genes that shape the neural substrates of social dominance and reproductive adaptations both within and between species [130]. We return to the promise of contributions from genomics below.
4. Synthesis and Summary
From a mammalian perspective, sexual phenotypes are typically thought of as tightly integrated suites of characters whose morphology and response properties are largely organized during early development by gonadal steroid hormones [128, but see 2]. The gonadal steroid environment, in turn, depends on sex chromosome inheritance. Fishes deviate from the mammalian pattern both temporally, with key sex determination and differentiation events taking place across the lifespan in many species, and in decoupling and ‘resorting’ different components of sexuality to generate successful alternate reproductive phenotypes. This concluding section considers whether there are general principles in the neuroendocrine regulation of the plastic sex determination and differentiation in fishes, how patterns of regulation may contribute to and facilitate plasticity in sex determination and differentiation, and promising future directions.
4.1 – Regulatory Pathways and Sexual Plasticity in Fishes
Estrogens and androgens play key roles in regulating socially controlled sex change and intrasexual dimorphism in male fishes. However, in contrast to patterns in tetrapods, emerging information from several species suggests the precise nature of these mechanisms may be very different in fishes. Whereas estrogens play a key stimulatory role in inducing male-typical neuronal and behavioral phenotypes in many birds and mammals [6,164], the opposite appears true for many fishes. Examples include bluebanded gobies where brain aromatase activity declines rapidly at the onset of female-to-male sex change, the plainfin midshipman where hindbrain aromatase activity is much higher in females and female-mimic males, and the numerous studies showing inhibition of female-to-male sex change and/or male-typical behaviors through E2 administration. In contrast, androgens are reliably linked to the development of bourgeois male phenotypes in both sex changing and gonochoristic species, although the precise mechanisms and scope of these effects remains somewhat unclear. Brantley and colleagues [11] noted that the correlation between androgen levels and display of male secondary sexual characters was much stronger for 11KT than for T and more recent findings are consistent with this role for 11KT. These authors also noted a stronger linkage between elevated 11KT levels and morphological display characters than elevated 11KT levels and male-typical behaviors, a pattern also supported by more recent work. For example, gonads are not necessary for behavioral sex change in bluehead wrasses, but are necessary for development of terminal phase male coloration. Elevated 11KT levels may be necessary, but not sufficient for the display of bourgeois male behaviors in some other cases [123].
While it is clear that steroid hormones are important in some components of sex change and alternative male phenotype expression in fishes, it is less clear what the mechanisms and anatomical sites of their effects are. One mechanism is through direct effects of the hormones of interest such as the modulation of vocal behavior in the plainfin midshipman where a direct action is supported by the effectiveness of receptor antagonists. However, equally plausible in many cases are indirect effects where substrate utilization may indirectly reduce either androgenic or estrogenic signaling. High levels of aromatase in the sonic motor nucleus of female and type II male plainfin midshipman were suggested to potentially prevent masculinization by converting T to E2 [145]. Conversely, rapid decreases in brain aromatization at the onset of sex change in L. dalli may indirectly increase 11KT levels by increasing substrate (T) available to the enzymes 11β-hydroxysteroid dehydrogenase (11β-HSD) and 11β-hydroxylase [8]. Knapp [83] has also suggested the potential of stress to affect 11KT levels through substrate competition for the enzymes 11β-hydroxylase and 11β-HSD. These two enzymes are important for biosynthesis of both cortisol and 11KT and Knapp hypothesized that the elevations of cortisol in sneaker male sunfish could therefore interfere with 11KT synthesis. The anatomical and neuroanatomical sites of key steroid hormone effects in this context are also unclear. Both 11KT and aromatase inhibitor treatments can induce female-to-male sex change in a number of species, but treatments have been applied systemically rather than site-specifically. Given the more rapid declines in aromatase activity in brain than gonads at the onset of sex change in L. dalli, it is possible that the critical effects in androgen and aromatase inhibition experiments occur in the brain with gonadal change being a consequence of altered gonadotropin signaling (as in [87]). Site-specific manipulations in the brain would be very useful in this regard.
The neuropeptide hormones GnRH and AVT are both implicated in regulating sex change and alternative male phenotype expression, but a general explanatory framework applicable across species has not yet emerged. Challenges to developing such an interpretive framework include the diversity of reproductive patterns and behaviors represented, diversity in timing of reproduction with both year round and seasonal breeders having been studied, and in the methods employed to examine the neural systems. Results to date do appear generally consistent in linking bourgeois male phenotype expression to greater activation of the hypothalamic GnRH system. This association would be strengthened considerably with more demonstrations of GnRH system activation during transitions to dominant male status like that provided for A. burtoni [13]. Activation of the AVT system in some species is closely linked with the display of aggressive social dominance and active courtship (bluehead wrasses, Astatotilapia) while territorial, courting males in the plainfin midshipman show less well developed AVT systems and AVT can suppress aggressive fictive vocalizations in this morph [36,54]. The reasons for this disparity are not understood, but it is relevant that AVT/AVP systems in vertebrates are noted for variation in expression and function across species exhibiting different social structures [56].
4.2 – Developmental Polarity and Decoupling of the Components of Sexuality?
Intense competition for limited mating opportunities is hypothesized to be the key selective force favoring the evolution of both alternate male phenotypes and many examples of sex change [45,117,160,167]. Intrasexual competition provides the selective pressure favoring these adaptations, but the occurrence of natural selection also requires the developmental or physiological mechanisms necessary to effect an adaptation. Are there features of teleost reproductive development that facilitate these changes in patterns of sexual development? This section summarizes evidence that the timing and relative ‘coupling’ of different aspects of sexual determination and differentiation are likely to facilitate sexual lability in teleosts both physiologically and evolutionarily.
As noted in the introduction, Francis [41] proposed that the reversed polarity of brain-gonad development in teleosts relative to tetrapods creates the opportunity for individuals to use environmental information to adaptively direct sex determination and differentiation. Socially controlled sex change is perhaps the most spectacular example of this sexual lability, but there are also a number of examples of social environment cues directing initial sex determination in fishes [41,42]. The ability of an individual to use information about its social environment to guide differentiation into either a bourgeois or parasitic male morph during development could be strongly adaptive under conditions of intense mate competition. Do environmental cues contribute to the process of intrasexual differentiation prior to sexual maturity? No information is yet available to address this question directly. However, the social control of sex before maturation in some species [42,115] and of male phenotype differentiation after maturation in species like the bluehead wrasse suggest it is certainly plausible.
Do teleost fishes show a relative ‘decoupling’ of different components of sexual development relative to many tetrapods? In 1970, Alfred Jost [75] conceptualized sexuality as consisting of components including genetic sex, gonad sex, body sex, and brain sex. The different components of sexuality are typically tightly linked in mammals due to the cascading effects of chromosomally determined genetic sex leading to the differentiation of either ovaries or testes. The resultant differences in hormonal environment then organize body and brain sex (see [20] for a discussion of hormonal sex). By contrast, a relative decoupling of components of sexuality is suggested by several observations from both sex change and alternate male phenotypes in fishes.
Increases in 11KT levels, presumably mediated by increases in the activity of 11β-hydroxylase and 11β-hydroxysteroid dehydrogenase, lead to dramatic alterations in phenotype in species like the bluehead wrasse and stoplight parrotfish. Aromatase activity changes very rapidly in the brain in species like the bluebanded goby with the onset of sex change and differences in aromatase expression appear to contribute to within-sex dimorphism in the plainfin midshipman. Therefore, what appear to relatively minor alterations in endocrine physiology from a regulatory standpoint can have substantial consequences for development of the sexual phenotype. The variations in endocrine signaling and their effects discussed above for fishes are reminiscent of disorders in human and mouse sexual development where disruptions of 5α-reductase function or androgen receptor signaling lead to the development of female external phenotypes in genotypically XY individuals [19,74]. The difference in fishes is that the alternate sexual phenotypes are reproductively fully functional. Indeed, parasitic males of many species have larger testes than bourgeois males despite low to non-detectable levels of 11KT [11]. This decoupling of testicular function from elevated 11KT and elaborate 11KT-dependent secondary sexual characters may have important consequences both ontogenetically and evolutionarily. Ontogenetically, decoupling provides a physiological mechanism for altering expression of male display characters. In the absence of elevated 11KT, parasitic male phenotypes appear to generally follow a ‘default’ pathway that characterizes female development and results in a female-typical external phenotype (see [75] for similar arguments regarding mammals). Evolutionarily, such a decoupling could make the origin of alternate male phenotypes more likely if the loss or reduction of 11KT synthesis requires only a relatively minor genetic change. It is interesting in this context that elaborate secondary sexual characteristics in males appear to be lost more often than they are gained evolutionarily and a link to androgen metabolism has been suggested [29,170].
Is decoupling of gonadal sex and brain sex more common in fishes? Again, several observations from species with sex change and alternate male phenotypes suggest this is the case. For sex changing fishes, these observations include very rapid behavioral changes that appear to greatly precede gonadal change in wrasses [137,168], behavioral sex change in the absence of gonads in bluehead wrasses [48], and declines in aromatization occurring more quickly in brain than the gonads in bluebanded gobies [8]. For alternate male phenotypes, female-mimic behavior in a number of species suggests female-like neural substrates. Neuroendocrine observations are consistent with this suggestion. Female and initial phase male bluehead wrasses have similar GnRH neuronal phenotypes and both are distinct from terminal phase males. Female and type II plainfin midshipman exhibit strong similarities in aromatase expression and vocalization responses to steroid and neuropeptide hormones that are distinct from those seen in type I males. The female-like levels of 11KT in parasitic males of many species also provide support as these endocrine differences likely originate with different patterns of hypothalamic neuroendocrine signaling. Lastly, gene expression profiling in the brain suggests greater similarity of socially subordinate male phenotypes to females than to dominant males in two cases. Comparing neural gene expression profiles using hierarchical cluster analysis in A. burtoni, Renn and colleagues [136] showed greater overall similarity between females and subordinate males than either phenotype showed with dominant males. A similar approach in Atlantic salmon also showed greater similarity between immature female and sneaker male neural gene expression profiles than either showed to the immature males that would migrate and later become large bourgeois males [3]. Quantitative RT-PCR based comparisons of proopiomelanocortin and prolactin in the same study revealed identical patterns across the salmon phenotypes at a more focused neuroendocrine level. Neither the studies in A. burtoni nor Atlantic salmon provide an ideal test of whether alternate male phenotypes have ‘female-like brains’ because one or two of the three phenotypes being compared in each is either reproductively suppressed or immature. However, both studies are generally consistent with this hypothesis. Also consistent are results of brain gene expression profiling for bluehead wrasses where we have found greater overall similarity between females and initial phase males than either phenotype shares with terminal phase males (Passador-Gurgel and Godwin, in preparation). More data are clearly needed to fully address the question and gathering these data will be greatly facilitated by recent advances in genomic technologies.
4.3 – Summary and Future Directions
The last decade has seen substantial progress in our understanding of sex change and sexual differentiation in fishes. Progress should accelerate as genomic resources become widely accessible for a variety of vertebrates. For example, efforts are underway to sequence the genome from a representative of every vertebrate genus (www.genome10K.org). The availability of extensive genomic data should provide the foundation to rigorously examine the regulation of key neuroendocrine pathways through promoter analysis as well as greatly facilitate the development of molecular tools for fish models. An intriguing possibility is the use of transcriptional profiles as response variables in testing the effects of potential regulators of sex differentiation responses. For example, do endocrine manipulations that induce sex change produce similar transcriptional changes to those that occur with socially stimulated sex change? If so, it would increase confidence that the experimental manipulations are relevant to the natural situation.
There are also unresolved questions that will require more traditional approaches. Prominent among these is the question of whether the differentiation of alternate male morphs in many species is genetically fixed or phenotypically plastic in expression. If plastic, the regulation of male morph development is likely to be through social cues in many species. Elucidating the neural pathways by which social cues are ‘transduced’ into neuroendocrine processes is a challenging, but key issue for the emerging field of ‘social neuroscience.’ Teleost systems provide attractive models because of their dramatic and often very rapid phenotypic responses to social environment. Understanding ransduction of social cues will also likely require much more detailed information about neural signaling systems, their structure and activity at a circuit level, and especially interactions between these systems in the brain.
Acknowledgments
The author wishes to thank Mary Beth Hawkins and two anonymous reviewers for constructive comments that improved the manuscript. The author's research is supported by 0416926 from the U.S. National Science Foundation. This is a contribution of the W.M. Keck Center for Behavioral Biology at North Carolina State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Alam MA, Bhandari RK, Kobayashi Y, Nakamura S, Soyano K, Nakamura M. Changes in androgen-producing cell size and circulating 11-ketotestosterone level during female-male sex change of honeycomb grouper Epinephelus merra. Molec Reprod Develop. 2006;73:206–214. doi: 10.1002/mrd.20393. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior - A reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 3.Aubin-Horth N, Landry CR, Letcher BH, Hofmann HA. Alternative life histories shape brain gene expression profiles in males of the same population. Proc Roy Soc B-Biol Sci. 2005;272(1573):1655–1662. doi: 10.1098/rspb.2005.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubin-Horth N, Letcher BH, Hofmann HA. Gene-expression signatures of Atlantic salmon's plastic life cycle. Gen Comp Endocrinol. 2009;163:278–284. doi: 10.1016/j.ygcen.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balment RJ, Lu W, Weybourne E, Warne JM. Arginine vasotocin a key hormone in fish physiology and behaviour: A review with insights from mammalian models. Gen Comp Endocrinol. 2006;147:9–16. doi: 10.1016/j.ygcen.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Balthazart J, Taziaux M, Holloway K, Ball GF, Cornil CA. Behavioral effects of brain-derived estrogens in birds. In: Vaudry H, Roubos EW, Coast GM, Vallarino M, editors. Trends Comp Endocrinol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annals New York Acad Sci. 2009;1163:31–48. doi: 10.1111/j.1749-6632.2008.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari RK, Komuro H, Nakamura S, Higa M, Nakamura M. Gonadal restructuring and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper (Epinephelus merra) Zool Sci. 2003;20:1399–1404. doi: 10.2108/zsj.20.1399. [DOI] [PubMed] [Google Scholar]
- 8.Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, lythrypnus dalli. Proc Royal Soc B-Biol Sci. 2005;272:2435–2440. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black MP, Reavis RH, Grober MS. Socially induced sex change regulates forebrain isotocin in Lythrypnus dalli. Neuroreport. 2004;15:185–189. doi: 10.1097/00001756-200401190-00036. [DOI] [PubMed] [Google Scholar]
- 10.Borg B. Androgens in teleost fishes. Comp Biochem Physiol C - Pharmacol Toxicol & Endocrinol. 1994;109:219–245. [Google Scholar]
- 11.Brantley RK, Wingfield JC, Bass AH. Sex steroid-levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm Behav. 1993;27:332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- 12.Braun AM, Thomas P. Androgens inhibit estradiol-17beta synthesis in Atlantic croaker (Micropogonias undulatus) ovaries by a nongenomic mechanism initiated at the cell surface. Biol Reprod. 2003;69:1642–1650. doi: 10.1095/biolreprod.103.015479. [DOI] [PubMed] [Google Scholar]
- 13.Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Horm Behav. 2007;51:164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callard GV, Tchoudakova AV, Kishida M, Wood E. Differential tissue distribution, developmental programming, estrogen regulation and promoter characteristics of cyp19 genes in teleost fish. J Steroid Biochem Molec Biol. 2001;79:305–314. doi: 10.1016/s0960-0760(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 16.Cardwell JR, Liley NR. Hormonal control of sex and color-change in the stoplight parrotfish, Sparisoma viride. Gen Comp Endocrinol. 1991;81:7–20. doi: 10.1016/0016-6480(91)90120-u. [DOI] [PubMed] [Google Scholar]
- 17.Cardwell JR, Liley NR. Androgen control of social status in males of a wild population of stoplight parrotfish, Sparisoma viride (Scaridae) Horm Behav. 1991;25:1–18. doi: 10.1016/0018-506x(91)90035-g. [DOI] [PubMed] [Google Scholar]
- 18.Carneiro LA, Oliveira RF, Canario AVM, Grober MS. The effect of arginine vasotocin on courtship behaviour in a blenniid fish with alternative reproductive tactics. Fish Physiol Biochem. 2003;28:241–243. [Google Scholar]
- 19.Couse JF, Korach KS. Exploring the role of sex steroids through studies of receptor deficient mice. J Molec Med. 1998;76:497–511. doi: 10.1007/s001090050244. [DOI] [PubMed] [Google Scholar]
- 20.Crews D. The organizational concept and vertebrates without sex-chromosomes. Brain Behav Evol. 1993;42:202–214. doi: 10.1159/000114155. [DOI] [PubMed] [Google Scholar]
- 21.Crews D. On the Organization of Individual Differences in Sexual Behavior. Am Zool. 1998;38:118–132. [Google Scholar]
- 22.de Mitcheson YS, Liu M. Functional hermaphroditism in teleosts. Fish and Fisheries. 2008;9:1–43. [Google Scholar]
- 23.de Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desjardins JK, Fernald RD. How do social dominance and social information influence reproduction and the brain? Integ Comp Biol. 2008;48:596–603. doi: 10.1093/icb/icn089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- 26.Elizur A. The KiSS1/GPR54 system in fish. Peptides. 2009;30:164–170. doi: 10.1016/j.peptides.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Elofsson UO, Winberg S, Francis RC. Number of preoptic GnRH-immunoreactive cells correlates with sexual phase in a protandrously hermaphroditic fish, the dusky anemonefish (Amphiprion melanopus) J Comp Physiol A – Sens Neural Behav Physiol. 1997;181:484–492. doi: 10.1007/s003590050132. [DOI] [PubMed] [Google Scholar]
- 28.Elofsson UO, Winberg S, Nilsson GE. Relationships between sex and the size and number of forebrain gonadotropin-releasing hormone-immunoreactive neurones in the Ballan wrasse (Labrus berggylta), a protogynous hermaphrodite. J Comp Neurol. 1999;410:158–170. [PubMed] [Google Scholar]
- 29.Emerson SB. Phylogenies and physiological processes – The evolution of sexual dimorphism in southeast Asian frogs. Syst Biol. 1996;45:278–289. [Google Scholar]
- 30.Fernald RD, Hirata NR. field-study of Haplochromis burtoni - quantitative behavioral observations. Anim Behav. 1977;25:964–975. [Google Scholar]
- 31.Fernald RD. Social regulation of the Brain: Status, sex, and size. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; New York: pp. 435–444. [Google Scholar]
- 32.Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filby AL, van Aerle R, Duitman JW, Tyler CR. The Kisspeptin/Gonadotropin-Releasing Hormone Pathway and Molecular Signaling of Puberty in Fish. Biol Reprod. 2008;78:278–289. doi: 10.1095/biolreprod.107.063420. [DOI] [PubMed] [Google Scholar]
- 34.Fishelson L. Protogynous sex reversal in the fish Anthias squamipinnis (Teleostei, Anthiidae) regulated by the presence or absence of a male fish. Nature. 1970;227:90–91. doi: 10.1038/227090b0. [DOI] [PubMed] [Google Scholar]
- 35.Fleming IA. Pattern and variability in the breeding system of Atlantic salmon (Salmo salar), with comparisons to other salmonids. Can J Fish Aq Sci. 1998;55:59–76. [Google Scholar]
- 36.Foran CM, Bass AH. Preoptic GnRH and AVT: Axes for sexual plasticity in teleost fish. Gen Comp Endocrinol. 1999;116:141–52. doi: 10.1006/gcen.1999.7357. [DOI] [PubMed] [Google Scholar]
- 37.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: Aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: Divergence across sex and vocal phenotypes. J Neurobiol. 2005;65:37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- 39.Forlano PM, Bass AH. Steroid regulation of brain aromatase expression in glia: Female preoptic and vocal motor nuclei. J Neurobiol. 2005;65:50–58. doi: 10.1002/neu.20178. [DOI] [PubMed] [Google Scholar]
- 40.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: New lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Francis RC. Sexual lability in teleosts - Developmental factors. Quart Rev Biol. 1992;67:1–18. [Google Scholar]
- 42.Francis RC, Barlow GW. Social-control of primary sex-differentiation in the midas cichlid. Proc Natl Acad Sci USA. 1993;90:10673–10675. doi: 10.1073/pnas.90.22.10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisch AJ, Walker SPW, McCormick ML, Solomon-Lane TK. Regulation of protogynous sex change by competition between corticosteroids and androgens: An experimental test using sandperch, Parapercis cylindrica. Horm Behav. 2007;52:540–545. doi: 10.1016/j.yhbeh.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Gardner L, Anderson T, Place AR, Dixon B, Elizur A. Sex change strategy and the aromatase genes. J Steroid Biochem Molec Biol. 2005;94:395–404. doi: 10.1016/j.jsbmb.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 45.Ghiselin MT. The evolution of hermaphroditism among animals. Quart Rev Biology. 1969;44:189–208. doi: 10.1086/406066. [DOI] [PubMed] [Google Scholar]
- 46.Godwin J. Behavioural aspects of protandrous sex change in the anemonefish, Amphiprion melanopus, and endocrine correlates. Anim Behav. 1994;48:551–567. [Google Scholar]
- 47.Godwin JR, Thomas P. Sex change and steroid profiles in the protandrous anemonefish Amphiprion melanopus (Pomacentridae, Teleostei) Gen Comp Endocrinol. 1993;91:144–157. doi: 10.1006/gcen.1993.1114. [DOI] [PubMed] [Google Scholar]
- 48.Godwin J, Crews D, Warner RR. Behavioural sex change in the absence of gonads in a coral reef fish. Proc Royal Soc London B-Biol Sci. 1996;263:1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- 49.Godwin J, Sawby R, Warner RR, Crews D, Grober MS. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav Evol. 2000;55:77–84. doi: 10.1159/000006643. [DOI] [PubMed] [Google Scholar]
- 50.Goncalves EJ, Almada VC, Oliveira RF, Santos AJ. Female mimicry as a mating tactic in males of the blenniid fish Salaria pavo. J Mar Biol Assoc UK. 1996;76:529–538. [Google Scholar]
- 51.Goncalves D, Alpedrinha J, Teles M, Oliveira RF. Endocrine control of sexual behavior in sneaker males of the peacock blenny Salaria pavo: Effects of castration, aromatase inhibition, testosterone and estradiol. Horm Behav. 2007;51:534–541. doi: 10.1016/j.yhbeh.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Goncalves D, Teles M, Alpedrinha J, Oliveira RF. Brain and gonadal aromatase activity and steroid hormone levels in female and polymorphic males of the peacock blenny Salaria pavo. Horm Behav. 2008;54:717–725. doi: 10.1016/j.yhbeh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- 55.Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J Comp Neurol. 2000;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res - Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 57.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 58.Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: Relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Roy Soc B-Biol Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grober MS, Bass AH. Neuronal correlates of sex/role change in labrid fishes: LHRH-like immunoreactivity. Brain Behav Evol. 1991;38:302–312. doi: 10.1159/000114396. [DOI] [PubMed] [Google Scholar]
- 61.Grober MS, Jackson IMD, Bass AH. Gonadal steroids affect LHRH preoptic cell number in sex/role changing fish. J Neurobiol. 1991;22:734–741. doi: 10.1002/neu.480220708. [DOI] [PubMed] [Google Scholar]
- 62.Grober MS, Sunobe T. Serial adult sex change involves rapid and reversible changes in forebrain neurochemistry. Neuroreport. 1996;7:2945–2949. doi: 10.1097/00001756-199611250-00029. [DOI] [PubMed] [Google Scholar]
- 63.Grober MS, George AA, Watkins KK, Carneiro LA, Oliveira RF. Forebrain AVT and courtship in a fish with male alternative reproductive tactics. Brain Res Bull. 2002;57:423–425. doi: 10.1016/s0361-9230(01)00704-3. [DOI] [PubMed] [Google Scholar]
- 64.Grober MS, Bass AH. Life History, Neuroendocrinology, and behavior in fish. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; New York: pp. 331–348. [Google Scholar]
- 65.Gross MR, Charnov EL. Alternative male life histories in bluegill sunfish. Proc Nat Acad Sci USA. 1980;77:6937–6940. doi: 10.1073/pnas.77.11.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawkins MB, Thornton JW, Crews D, Skipper JK, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc Nat Acad Sci USA. 2000;97:10751–10756. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawkins MB, Godwin J, Crews D, Thomas P. The distributions of the duplicate oestrogen receptors ER-beta a and ER-beta b in the forebrain of the Atlantic croaker (Micropogonias undulatus): Evidence for subfunctionalization after gene duplication. Proc Royal Soc – Biol Sci. 2005;272:633–641. doi: 10.1098/rspb.2004.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heppell SA, Sullivan CV. Identification of gender and reproductive maturity in the absence of gonads: muscle tissue levels of sex steroids and vitellogenin in gag (Mycteroperca microlepis) Can J Fish Aq Sci. 2000;57:148–159. [Google Scholar]
- 69.Higa M, Ogasawara K, Sakaguchi A, Nagahama Y, Nakamura M. Role of steroid hormones in sex change of protogynous wrasse. Fish Physiol Biochem. 2003;28:149–150. [Google Scholar]
- 70.Hofmann HA. Functional genomics of neural and behavioral plasticity. J Neurobiology. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- 71.Hofmann HA, Fernald RD. Social status controls somatostatin neuron size and growth. J Neurosci. 2000;20:4740–4744. doi: 10.1523/JNEUROSCI.20-12-04740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hourigan TF, Nakamura M, Nagahama Y, Yamauchi K, Grau EG. Histology, ultrastructure, and in vitro steroidogenesis of the testes of two male phenotypes of the protogynous fish, Thalassoma duperrey (Labridae) Gen Comp Endocrinol. 1991;83:193–217. doi: 10.1016/0016-6480(91)90023-y. [DOI] [PubMed] [Google Scholar]
- 73.Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinol. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 74.Imperato-McGinley J, Zhu YS. Gender and Behavior in subjects with genetic defects in male sexual differentiation. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 5. Academic Press; New York: pp. 303–345. [Google Scholar]
- 75.Jost A. Hormonal factors in sex differentiation of mammalian foetus. Phil Trans Royal Soc London B – Biol Sci. 1970;259:119–131. doi: 10.1098/rstb.1970.0052. [DOI] [PubMed] [Google Scholar]
- 76.Kallo I, Vida B, Deli L, Kalamatianos T, Hrabovszky E, Caraty A, Coen CW, Liposits Z. Evidence that kisspeptin neurons in the AVPV form part of a bisynaptic pathway connecting suprachiasmatic vasopressin neurons with preoptic GnRH cells. Program of the 38th Annual Meeting of the Society for Neuroscience; Washington, DC. 2008; Abstract 618.4. [Google Scholar]
- 77.Kanda S, Akazome Y, Matsunaga T, Yamamoto N, Yamada S, Tsukamura H, Maeda K, Oka Y. Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryzias latipes) Endocrinology. 2008;149:2467–2476. doi: 10.1210/en.2007-1503. [DOI] [PubMed] [Google Scholar]
- 78.Kim SJ, Ogasawara K, Park JG, Takemura A, Nakamura M. Sequence and expression of androgen receptor and estrogen receptor gene in the sex types of protogynous wrasse, Halichoeres trimaculatus. Gen Comp Endocrinol. 2002;127:165–173. doi: 10.1016/s0016-6480(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 79.Kihslinger RL, Lema SC, Nevitt GA. Environmental rearing conditions produce forebrain differences in wild Chinook salmon Oncorhynchus tshawytscha. Comp Biochem Physiol A Molec Integr Physiol. 2006;145:145–151. doi: 10.1016/j.cbpa.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 80.Kindler PM, Hilipp DP, Gross MR, Bahr JM. Serum 11-ketotestosterone and testosterone concentrations associated with reproduction in male bluegill (Lepomis macrochirus, Centrarchidae) Gen Comp Endocrinol. 1989;75:446–453. doi: 10.1016/0016-6480(89)90180-9. [DOI] [PubMed] [Google Scholar]
- 81.Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the plainfin midshipman fish (Porichthys notatus) Horm Behav. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- 82.Knapp R, Hews DK, Thompson CW, Ray LE, Moore MC. Environmental and endocrine correlates of tactic switching by nonterritorial male tree lizards (Urosaurus ornatus) Horm Behav. 2003;43:83–92. doi: 10.1016/s0018-506x(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 83.Knapp R. Endocrine mediation of vertebrate male alternative reproductive tactics: The next generation of studies. Integ Comp Biol. 2003;43:658–668. doi: 10.1093/icb/43.5.658. [DOI] [PubMed] [Google Scholar]
- 84.Knapp R, Neff BD. Steroid hormones in bluegill, a species with male alternative reproductive tactics including female mimicry. Biol Lett. 2007;3:628–631. doi: 10.1098/rsbl.2007.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kobayashi Y, Kobayashi T, Nakamura M, Sunobe T, Morrey CE, Suzuki N, Nagahama Y. Characterization of two types of cytochrome P450 aromatase in the serial-sex changing gobiid fish, Trimma okinawae. Zool Sci. 2004;21:417–425. doi: 10.2108/zsj.21.417. [DOI] [PubMed] [Google Scholar]
- 86.Kobayashi Y, Sunobe T, Kobayashi T, Nagahama Y, Nakamura M. Promoter analysis of two aromatase genes in the serial-sex changing gobiid fish, Trimma okinawae. Fish Physiol Biochem. 2005;31:123–127. doi: 10.1007/s10695-006-0013-6. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi Y, Nakamura M, Sunobe T, Usami T, Kobayashi T, Manabe H, Paul-Prasanth B, Suzuki N, Nagahama Y. Sex Change in the Gobiid Fish Is Mediated through Rapid Switching of Gonadotropin Receptors from Ovarian to Testicular Portion or Vice Versa. Endocrinology. 2009;150:1503–1511. doi: 10.1210/en.2008-0569. [DOI] [PubMed] [Google Scholar]
- 88.Kocher TD. Adaptive evolution and explosive speciation:The cichlid fish model Nature Rev. Genetics. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 89.Kolm N, Gonzalez-Voyer A, Brelin D, Winberg S. Evidence for small scale variation in the vertebrate brain: mating strategy and sex affect brain size and structure in wild brown trout (Salmo trutta) J Evol Biol. 2009;22:2524–2531. doi: 10.1111/j.1420-9101.2009.01875.x. [DOI] [PubMed] [Google Scholar]
- 90.Koulish S, Kramer CR. Human chorionic-gonadotropin (HCG) induces gonad reversal in a protogynous fish, the bluehead wrasse, Thalassoma bifasciatum (Teleostei, Labridae) J Exp Zool. 1989;252:156–168. doi: 10.1002/jez.1402520207. [DOI] [PubMed] [Google Scholar]
- 91.Kramer CR, Koulish S, Bertacchi PL. The effects of testosterone implants on ovarian morphology in the bluehead wrasse, Thalassoma bifasciatum (Bloch) (Teleostei: Labridae) J Fish Biol. 1988;32:397–407. [Google Scholar]
- 92.Kramer CR, Caddell MT, Bubenheimerlivolsi L. sGnRH-A[(D-Arg6,Pro9,net-)lhrh] in combination with domperidone induces gonad reversal in a protogynous fish, the bluehead wrasse, Thalassoma bifasciatum. J Fish Biol. 1993;42:185–195. [Google Scholar]
- 93.Kramer CR, Imbriano MA. Neuropeptide Y (NPY) induces gonad reversal in the protogynous bluehead wrasse, Thalassoma bifasciatum (Teleostei: Labridae) J Exp Zool. 1997;279:133–44. [PubMed] [Google Scholar]
- 94.Krogh A. The progress of physiology. Science. 1929;70:200–204. doi: 10.1126/science.70.1809.200. [DOI] [PubMed] [Google Scholar]
- 95.Kroon FJ, Liley NR. The role of steroid hormones in protogynous sex change in the blackeye goby, Coryphopterus nicholsii (Teleostei : Gobiidae) Gen Comp Endocrinol. 2000;118:273–283. doi: 10.1006/gcen.2000.7459. [DOI] [PubMed] [Google Scholar]
- 96.Kroon FJ, Munday PL, Pankhurst NW. Steroid hormone levels and bi-directional sex change in Gobiodon histrio. J Fish Biol. 2003;62:153–167. [Google Scholar]
- 97.Kroon FJ, Munday PL, Westcott DA, Hobbs JPA, Liley NR. Aromatase pathway mediates sex change in each direction. Proc Royal Soc B-Biol Sci. 2005;272:1399–1405. doi: 10.1098/rspb.2005.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kroon FJ, Munday PL, Westcott DA. Equivalent whole-body concentrations of 11-ketotestosterone in female and male coral goby Gobiodon erythrospilus, a bidirectional sex-changing fish. J Fish Biology. 2009;75:685–692. doi: 10.1111/j.1095-8649.2009.02274.x. [DOI] [PubMed] [Google Scholar]
- 99.Larson ET, Norris DO, Grau EG, Summers CH. Monoamines stimulate sex reversal in the saddleback wrasse. Gen Comp Endocrinol. 2003;130:289–298. doi: 10.1016/s0016-6480(02)00622-6. [DOI] [PubMed] [Google Scholar]
- 100.Larson ET, Norris DO, Summers CH. Monoaminergic changes associated with socially induced sex reversal in the saddleback wrasse. Neuroscience. 2003;119:251–263. doi: 10.1016/s0306-4522(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 101.Lema SC, Hodges MJ, Marchetti MP, Nevitt GA. Proliferation zones in the salmon telencephalon and evidence for environmental influence on proliferation rate. Comp Biochem Physiol – Molec Integr Physiol. 2005;141:327–335. doi: 10.1016/j.cbpb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Li GL, Liu XC, Zhang Y, Lin HR. Gonadal development, aromatase activity and P450 aromatase gene expression during sex inversion of protogynous red-spotted grouper Epinephelus akaara (Temminck and Schlegel) after implantation of the aromatase inhibitor, fadrozole. Aquacult Res. 2006;37:484–491. [Google Scholar]
- 103.Li GL, Liu XC, Lin HR. Seasonal changes of serum sex steroids concentration and aromatase activity of gonad and brain in red-spotted grouper (Epinephelus akaara) Anim Reprod Sci. 2007;99:156–166. doi: 10.1016/j.anireprosci.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 104.Long JA. The rise of fishes. 500 million years of evolution. Baltimore: The John Hopkins University Press; 1995. [Google Scholar]
- 105.Lorenzi V, Earley RL, Rodgers EW, Pepper DR, Grober MS. Diurnal patterns and sex differences in cortisol, 11-ketotestosterone, testosterone, and 17 beta-estradiol in the bluebanded goby (Lythrypnus dalli) Gen Comp Endocrinol. 2008;155:438–446. doi: 10.1016/j.ygcen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 106.Maejima K, Oka Y, Park MK, Kawashima S. Immunohistochemical double-labeling study of gonadotropin-releasing-hormone (GnRH)-immunoreactive cells and oxytocin-immunoreactive cells in the preoptic area of the dwarf gourami, Colisa lalia. Neuroscience Research. 1994;20:189–193. doi: 10.1016/0168-0102(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 107.Mank JE, Promislow DEL, Avise JC. Evolution of alternative sex-determining mechanisms in teleost fishes. Biol J Linnean Soc. 2006;87:83–93. [Google Scholar]
- 108.Marsh KE, Creutz LM, Hawkins MB, Godwin J. Aromatase immunoreactivity in the bluehead wrasse brain, Thalassoma bifasciatum: Immunolocalization and co-regionalization with arginine vasotocin and tyrosine hydroxylase. Brain Res. 2006;1126:91–101. doi: 10.1016/j.brainres.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinez-Chavez CC, Minghetti M, Migaud H. GPR54 and rGnRH I gene expression during the onset of puberty in Nile tilapia. Gen Comp Endocrinol. 2008;156:224–233. doi: 10.1016/j.ygcen.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 110.Masayoshi N, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res – Molec Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 111.Maugars G, Schmitz M. Expression of gonadotropin and gonadotropin receptor genes during early sexual maturation in male Atlantic salmon parr. Mol Reprod Dev. 2008;75:403–413. doi: 10.1002/mrd.20767. [DOI] [PubMed] [Google Scholar]
- 112.Moore MC. Application of organization activation theory to alternative male reproductive strategies - A review. Horm Behav. 1991;25:154–179. doi: 10.1016/0018-506x(91)90048-m. [DOI] [PubMed] [Google Scholar]
- 113.Moore MC, Hews DK, Knapp R. Hormonal control and evolution of alternative male phenotypes: Generalizations of models for sexual differentiation. Am Zool. 1998;38:133–151. [Google Scholar]
- 114.Morrey CE, Nagahama Y, Grau EG. Terminal phase males stimulate ovarian function and inhibit sex change in the protogynous wrasse Thalassoma duperrey. Zool Sci. 2002;19:103–109. doi: 10.2108/zsj.19.103. [DOI] [PubMed] [Google Scholar]
- 115.Munday PL, White JW, Warner RR. A social basis for the development of primary males in a sex-changing fish. Proc Roy Soc B-Biol Sci. 2006;273:2845–2851. doi: 10.1098/rspb.2006.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura M, Hourigan TF, Yamauchi K, Nagahama Y, Grau EG. Histological and ultrastructural evidence for the role of gonadal steroid hormones in sex change in the protogynous wrasse Thalassoma duperrey. Env Biol Fishes. 1989;24:117–136. [Google Scholar]
- 117.Neff BD, Knapp R. Alternative reproductive tactics in the Centrarchidae. In: Cooke SJ, Philipp DP, editors. Centrarchid fishes: diversity, biology and conservation. 2009. pp. 90–104. [Google Scholar]
- 118.Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocrine Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohta K, Hirano M, Mine T, Mizutani H, Yamaguchi A, Matsuyama M. Body color change and serum steroid hormone levels throughout the process of sex change in the adult wrasse, Pseudolabrus sieboldi. Mar Biol. 2008;153:843–852. [Google Scholar]
- 120.Ohta K, Mine T, Yamaguchi A, Matsuyama M. A Sexually Dimorphic Expression of Pituitary Glycoprotein Hormones in a Sex-Changing Fish (Pseudolabrus sieboldi) J Exp Zool – Part A Ecol Gen Physiol. 2008;309A:534–541. doi: 10.1002/jez.485. [DOI] [PubMed] [Google Scholar]
- 121.Oka Y. Three Types of Gonadotrophin-Releasing Hormone Neurones and Steroid-Sensitive Sexually Dimorphic Kisspeptin Neurones in Teleosts. J Neuroendocrinol. 2009;21:334–338. doi: 10.1111/j.1365-2826.2009.01850.x. [DOI] [PubMed] [Google Scholar]
- 122.Oliveira RF, Adelino L, Canario VM, Grober MS. Male Sexual Polymorphism, Alternative Reproductive Tactics, and Androgens in Combtooth Blennies (Pisces: Blenniidae) Horm Behav. 2001;40:266–275. doi: 10.1006/hbeh.2001.1683. [DOI] [PubMed] [Google Scholar]
- 123.Oliveira RF, Carneiro LA, Goncalves DM, Canario AVM, Grober MS. 11-ketotestosterone inhibits the alternative mating tactic in sneaker males of the peacock blenny, Salaria pavo. Brain Behav Evol. 2001;58:28–37. doi: 10.1159/000047259. [DOI] [PubMed] [Google Scholar]
- 124.Oliveira RF, Ros AFH, Goncalves DM. Intra-sexual variation in male reproduction in teleost fish: a comparative approach. Horm Behav. 2005;48:430–439. doi: 10.1016/j.yhbeh.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 125.Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93:659–666. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 126.Perreault HA, Semsar K, Godwin J. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol Behav. 2003;79:719–724. doi: 10.1016/s0031-9384(03)00211-7. [DOI] [PubMed] [Google Scholar]
- 127.Perry AN, Grober MS. A model for social control of sex change: Interactions of behavior, neuropeptides, glucocorticoids, and sex steroids. Horm Behav. 2003;43:31–38. doi: 10.1016/s0018-506x(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 128.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 129.Pinna G, Dong E, Matsumoto K, Costa E. A Guidotti In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Nat Acad USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pollen AA. HA Hofmann Beyond Neuroanatomy: Novel Approaches to Studying Brain Evolution. Brain Behav Evol. 2008;72:145–158. doi: 10.1159/000151474. [DOI] [PubMed] [Google Scholar]
- 131.Reinboth R. Spontaneous and hormone-induced sex-inversion in wrasses (Labridae) Publ Stazione Zool Napoli. 1975;39:550–573. [Google Scholar]
- 132.Reinboth R, Brusle-Sicard S. Histological and ultrastructural studies on the effects of hCG on sex inversion in the protogynous teleost Coris julis. J Fish Biol. 1997;51:738–749. [Google Scholar]
- 133.Remage-Healey L, Bass AH. Rapid, Hierarchical Modulation of Vocal Patterning by Steroid Hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Remage-Healey L, Bass AH. Plasticity in Brain Sexuality Is Revealed by the Rapid Actions of Steroid Hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Renn SCP, Aubin-Horth N, Hofmann HA. Biologically meaningful expression profiling across species using heterologous hybridization to a cDNA microarray. BMC Genomics. 2004;5:42. doi: 10.1186/1471-2164-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Renn SCP, Aubin-Horth N, Hofmann HA. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 2008;211:3041–3056. doi: 10.1242/jeb.018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robertson DR. Social Control of Sex Reversal in a Coral-Reef Fish. Science. 1972;177:1007–1009. doi: 10.1126/science.177.4053.1007. [DOI] [PubMed] [Google Scholar]
- 138.Rodgers EW, Drane S, Grober MS. Sex reversal in pairs of Lythrypnus dalli: Behavioral and morphological changes. Biol Bull. 2005;208:120–126. doi: 10.2307/3593120. [DOI] [PubMed] [Google Scholar]
- 139.Roff DA. Contributions of genomics to life-history theory. Nat Rev Genet. 2007;8:116–125. doi: 10.1038/nrg2040. [DOI] [PubMed] [Google Scholar]
- 140.Ross RM, Losey GS, Diamond M. Sex change in a coral-reef fish - dependence of stimulation and inhibition on relative size. Science. 1983;221:574–575. doi: 10.1126/science.221.4610.574. [DOI] [PubMed] [Google Scholar]
- 141.Ryan BC, Vandenbergh J. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 142.Saito D, Hasegawa Y, Urano A. Gonadotropin-releasing hormones modulate electrical activity of vasotocin and isotocin neurons in the brain of rainbow trout. Neurosci Lett. 2003;351:107–10. doi: 10.1016/j.neulet.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 143.Saito D, Komatsuda M, Urano A. Functional organization of preoptic vasotocin and isotocin neurons in the brain of rainbow trout: central and neurohypophysial projections of single neurons. Neuroscience. 2004;124:973–984. doi: 10.1016/j.neuroscience.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 144.Sakata JT, Crews D. Developmental sculpting of social phenotype and plasticity. Neurosci Biobehav Rev. 2004;28:95–112. doi: 10.1016/j.neubiorev.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 145.Schlinger BA, Greco C, Bass AH. Aromatase activity in hindbrain vocal control region of a teleost fish: divergence amoung males with alternative reproductive tactics. Proc Roy Soc B: Biol Sci. 1999;266:131–136. [Google Scholar]
- 146.Semsar K, Kandel FLM, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- 147.Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J Neurosci. 2003;23:4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm Behav. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 149.Semsar K, Perreault HA, Godwin J. Fluoxetine-treated male wrasses exhibit low AVT expression. Brain Res. 2004;1029:141–147. doi: 10.1016/j.brainres.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 150.Sisneros JA. Adaptive hearing in the vocal plainfin midshipman fish: Getting in tune for the breeding season and implications for acoustic communication. Integr Zool. 2009;4:33–42. doi: 10.1111/j.1749-4877.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 151.Sisneros JA. Steroid-dependent auditory plasticity for the enhancement of acoustic communication: Recent insights from a vocal teleost fish. Hearing Res. 2009;252:9–14. doi: 10.1016/j.heares.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 153.Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- 154.Sperry TS, Thomas P. Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities. Endocrinology. 1999;140:1602–1611. doi: 10.1210/endo.140.4.6631. [DOI] [PubMed] [Google Scholar]
- 155.St-Cyr S, Aubin-Horth N. Integrative and genomics approaches to uncover the mechanistic bases of fish behavior and its diversity. Comp Biochem Physiol Pt A. 2009;152:9–21. doi: 10.1016/j.cbpa.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 156.St Mary CM. Sex allocation in a simultaneous hermaphrodite, the blue banded goby (Lythrypnus dalli)—the effect of body size and behavioral gender and the consequences for reproduction. Behav Ecol. 1994;5:304–313. [Google Scholar]
- 157.Stoll LM. Hormonal control of the sexually dimorphic pigmentation of Thalassoma bifasciatum. Zoologica. 1955;40:25–131. [Google Scholar]
- 158.Summers CH, Winberg S. Interactions between the neural regulation of stress and aggression. J Exp Biology. 2006;209:4581–4589. doi: 10.1242/jeb.02565. [DOI] [PubMed] [Google Scholar]
- 159.Sunobe T, Nakamura M, Kobayashi Y, Kobayashi T, Nagahama Y. Aromatase immunoreactivity and the role of enzymes in steroid pathways for inducing sex change in the hermaphrodite gobiid fish Trimma okinawae. Comp Biochem Physiol A-Molec Integ Physiol. 2005;141:54–59. doi: 10.1016/j.cbpb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 160.Taborsky M. Sperm competition in fish: Bourgeois males and parasitic spawning. Trends Ecol Evol. 1998;13:222–227. doi: 10.1016/s0169-5347(97)01318-9. [DOI] [PubMed] [Google Scholar]
- 161.Taborsky M. The evolution of bourgeois, parasitic, and cooperative reproductive behaviors in fishes. J Hered. 2001;92:100–110. doi: 10.1093/jhered/92.2.100. [DOI] [PubMed] [Google Scholar]
- 162.Takeo J, Yamashita S. Two distinct isoforms of cDNA encoding rainbow trout androgen receptors. J Biol Chem. 1999;274:5674–5680. doi: 10.1074/jbc.274.9.5674. [DOI] [PubMed] [Google Scholar]
- 163.Thind KK, Boggan JE, Goldsmith PC. Interactions between vasopressin- and gonadotropin-releasing-hormone-containing neuroendocrine neurons in the monkey supraoptic nucleus. Neuroendocrinology. 1991;53:287–297. doi: 10.1159/000125731. [DOI] [PubMed] [Google Scholar]
- 164.Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Front Neuroendocrinology. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trainor BC, Hofmann HA. Somatostatin and somatostatin receptor gene expression in dominant and subordinate males of an African cichlid fish. Behav Brain Res. 2007;179:314–320. doi: 10.1016/j.bbr.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NH, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 167.Warner RR. Mating behavior and hermaphroditism in coral-reef fishes. Am Scientist. 1984;72:128–136. [Google Scholar]
- 168.Warner RR, Swearer SE. Social-control of sex change in the bluehead wrasse, Thalassoma bifasciatum (Pisces, Labridae) Biol Bull. 1991;181:199–204. doi: 10.2307/1542090. [DOI] [PubMed] [Google Scholar]
- 169.Whitfield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- 170.Wiens JJ. Widespread loss of sexually selected traits: How the peacock lost its spots. Trends Ecol Evol. 2001;16:517–523. [Google Scholar]