Abstract
Only large increases in adult cessation will rapidly reduce population smoking prevalence. Evidence-based smoking-cessation treatments and treatment policies exist but are underutilized. More needs to be done to coordinate the widespread, efficient dissemination and implementation of effective treatments and policies. This paper is the first in a series of three to demonstrate the impact of an integrated, comprehensive systems approach to cessation treatment and policy. This paper provides an analytic framework and selected literature review that guide the two subsequent computer simulation modeling papers to show how critical leverage points may have an impact on reductions in smoking prevalence. Evidence is reviewed from the U.S. Public Health Service 2008 clinical practice guideline and other sources regarding the impact of five cessation treatment policies on quit attempts, use of evidence-based treatment, and quit rates. Cessation treatment policies would: (1) expand cessation treatment coverage and provider reimbursement; (2) mandate adequate funding for the use and promotion of evidence-based state-sponsored telephone quitlines; (3) support healthcare systems changes to prompt, guide, and incentivize tobacco treatment; (4) support and promote evidence-based treatment via the Internet; and (5) improve individually tailored, stepped-care approaches and the long-term effectiveness of evidence-based treatments. This series of papers provides an analytic framework to inform heuristic simulation models in order to take a new look at ways to markedly increase population smoking cessation by implementing a defined set of treatments and treatment-related policies with the potential to improve motivation to quit, evidence-based treatment use, and long-term effectiveness.
Introduction
The greatest declines in smoking-caused death in the U.S. over the next 30 years will come from increasing adult cessation.1 While about 70% of U.S. smokers want to quit2 and almost 45% make serious quit attempts annually,3 fewer than 10% quit successfully.4,5 Behavioral and pharmacologic treatments generally double unassisted quit rates across a range of populations,6 hold enormous potential to increase cessation nationwide, and are among the most cost effective of all prevention programs.7,8 However, evidence-based cessation treatments currently are used by only a small fraction of U.S. smokers who try to quit.9,10 Treatment use is particularly limited among smokers with the highest smoking prevalence, including those with comorbid psychiatric and substance abuse problems, and lower levels of income and education, thereby contributing even more strongly to poor outcomes and to disparities in disease burden and mortality.11,12
National panels have focused on the need to expand treatment use by aligning cessation treatments and the policies that support their use and delivery among all levels of medicine and public health. The 2007 NIH State-of-the-Science Conference13 and the National Tobacco Cessation Collaborative Consumer Demand Roundtable14 highlighted the need to maximize the reach, use, and population impact of treatments. The 2008 IOM report Ending the Tobacco Problem: A Blueprint for the Nation15 called for a coordinated, comprehensive, national strategy to dramatically increase the number of smokers who quit each year and concluded that “systems integration is arguably the single most critical missing ingredient needed to maximize the as yet unrealized potential to significantly increase population cessation rates.”16
It should be noted for this series of papers that the “systems integration” concept referred to herein is much broader than the usual call for the integration of cessation services into the healthcare delivery system. Systems integration involves multilevel integration of at least three overlapping domains: (1) better consumer awareness of, access to, and use of the full range of evidence-based cessation interventions; (2) improved reach to smokers at the individual, group, neighborhood, organizational, community, state, and national levels, and across different modes of delivery; and (3) better alignment of cessation treatment and policy across cessation episodes to support smokers through multiple quit attempts and to ensure sustained maintenance of cessation (for details, see the 2008 IOM report16).
This paper and the two that follow17,18 take a fresh look at ways to markedly increase smoking cessation at the population level by modeling the implementation of a defined set of policies to improve the reach, use, and impact of smoking-cessation treatments. The paper begins with an analytic framework to map the impact of cessation treatments and policies on the core components of the population quit rate: (1) quit attempts; (2) treatment use; and (3) long-term treatment effectiveness. Next the evidence is selectively reviewed regarding the impact of each of the cessation treatments on each element of the population quit rate. Finally, five treatment-related policies were reviewed, that if implemented in a coordinated fashion could increase reach, access, use, and long-term effectiveness (i.e., reduce relapse rates) of treatment, and ultimately accelerate reductions in the population prevalence of smoking. Three of the policies have a strong evidence base: (1) expanded cessation treatment coverage and provider reimbursement; (2) adequate funding for the use and promotion of evidence-based, state-sponsored telephone quitlines; and (3) incentives for the adoption of healthcare system supports proven to increase the delivery of evidence-based brief provider interventions. Two promising approaches are considered that could play a role in enhancing the effectiveness of evidence-based treatments: (4) promoting effective Internet-based cessation programs, and (5) providing a more coordinated national treatment strategy (i.e., systems integration, referred to above and in the recent IOM Report16) that includes tailoring of treatment, stepped-care approaches, and more comprehensive care management and continuity of care.16 A more speculative examination is made of the potential synergies and interactions that are likely to occur when these policies are implemented in tandem. In areas where the evidence is less robust or nonexistent, this series of papers identifies gaps in our knowledge base that will need to be addressed.
Using the framework and findings in this paper, the second paper17 in this series models the impact of individual and combined cessation treatment policies on population quit rates. The third paper18 uses the SimSmoke model19,20 to expand the analyses in the second paper to include the effects of three public health policies: tax increases, clean indoor air laws, and health communication interventions such as antismoking media campaigns. Together the series describes how multiple cessation-related policies can be combined to create a comprehensive population cessation strategy (i.e., systems integration16), making use of simulation modeling to paint a vision of “plausible futures” with respect to impact on quit rates and national prevalence. The models serve as heuristic guideposts for policymakers, stakeholders, and healthcare, public health and other agencies, identifying promising policy “levers” to promote adult cessation.
Analytic Framework for Modeling the Population Impact of Interventions
The primary outcome for evaluating the impact of cessation is the adult population quit rate, defined as the proportion of the U.S. smoking population that, on an annual basis, quits smoking and maintains abstinence for 6 months.21–23 Figure 1 depicts the framework of annual population quit rates as a function of three components: (1) the proportion of all current smokers who make a serious quit attempt each year; (2) the proportion of serious quitters who make use of one or more evidence-based cessation treatments; and (3) the long-term effectiveness of those treatments. The framework focuses on annual rates of quitting because it is the standard time-frame for retrospective self-reports on quit attempts employed in national surveys, and because the generally accepted standard measure of sustained abstinence is 6–12 months.24 In national surveys, a serious quit attempt is generally defined as an intentional effort to quit smoking for 24 hours or longer. As described below, quit rates are higher among individuals that utilize evidence-based treatment than among those who quit without evidence-based treatments.6
Figure 1. Impact of treatment-related policies on various components of the population.
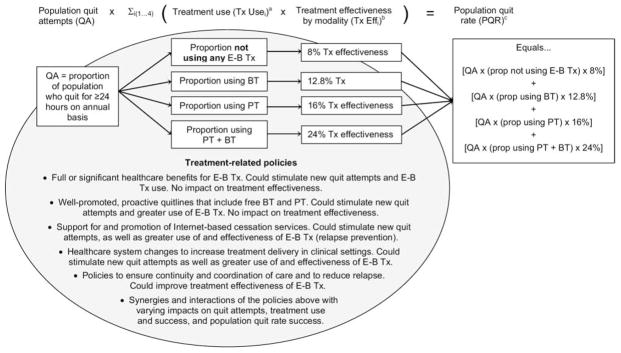
aTx Use is the proportion of smokers using each category of treatment in their quit attempt.
bTx Eff is the percentage of those using a given treatment modality that can be expected to have quit successfully at 12 months.
cPQR is the proportion of the population that is expected to have successfully quit at the end of 1 year, computed from the proportion of smokers making a quit attempt and the expected effectiveness rate for each form of treatment used.
BT, behavioral treatment; E-B Tx, evidence-based treatment; PT, pharmacologic treatment; Tx, treatment
Evidence-Based Cessation Treatments
The 2008 U.S. Public Health Service clinical practice guideline6 (hereafter referred to as “the 2008 Guideline”) recommends behavioral and pharmacologic cessation treatments as outlined in Table 1. The behavioral treatments include counseling, social support, problem solving, and cessation skills training offered in face-to-face, individual, or group formats, or via proactive telephone quitlines. Pharmacologic treatments include seven FDA-approved, first-line medications (i.e., bupropion SR, varenicline, and nicotine gum, inhaler, lozenges, nasal spray, and patches) and two second-line medications (clonidine and nortriptyline). Combined pharmacologic treatments (e.g., nicotine patch and gum) and combined behavioral and pharmacologic treatments are recommended as more effective than either alone.
Table 1.
Treating tobacco use and dependence: updated 2008 U.S. Public Health Service Clinical Practice Guidelines
| Evidence-based treatments | OR compared to control/comparison groupsa |
|---|---|
| Behavioral treatments | |
| Provider advice and brief counseling (3–10 minutes) | 1.6 |
| Face-to-face counseling (group, individual) | 1.3–1.7 |
| Proactive telephone counseling | 1.6 |
| Multiple format counseling combinations | 1.9–2.5 |
| Pharmacologic treatments | |
| First-line FDA-approved medications: nicotine gum, lozenge, patch, spray and inhaler, bupropion, varenicline | 1.5–3.1 |
| Second-line medications: nortriptyline, clonidine | 1.8–2.1 |
| Specific combinations of first- and second-line medications | 2.2–3.6 |
| Combination behavioral and pharmacologic treatments | |
| Specific combinations of the two treatment types compared to either alone | 1.3–1.7 |
Note: Further research recommended: individually tailored and stepped-care interventions; computerized E-health and Internet interventions; relapse prevention interventions; culturally tailored treatments for racial/ethnic minority populations.
In some cases, multiple treatments are subsumed under one heading. In such cases, the range of ORs are shown. ORs were obtained from Fiore et al., 2008.4
Using the analytic framework presented above, the evidence is reviewed for four mutually exclusive categories of treatment: (1) no formal or no effective evidence-based treatment (NoEBT); (2) one or more effective forms of evidence-based behavioral treatment without pharmacologic treatment; (3) one or more forms of evidence-based pharmacologic treatment without behavioral treatment; and (4) one or more forms of evidence-based behavioral treatment combined with one or more forms of evidence-based pharmacologic treatment.
As summarized in Table 1, behavioral treatments increase the odds of quitting 1.3 to 2.5 times, and pharmacologic treatments increase the odds of quitting 1.5 to 3.6 times compared to NoEBT.6 Treatments that combine one or more behavioral treatments with one or more pharmacologic treatments increase the odds of quitting 1.3 to 2 times over either pharmacologic treatment or behavioral treatment alone.6 Similar results occur when quitlines are expanded to provide free medication. While some states observed 50% increases in quit rates after including free NRT as part of the quitline service,25,26 most states found quit rates doubled25,27–31 (e.g., 6-month quit rates of 8%–12% for quitlines without pharmacotherapy compared to 15%–23% with free or discount pharmacotherapy). It is estimated that compared to NoEBT, quit rates are increased by 100% when pharmacologic treatment is used, by 60% when behavioral treatment is used, and by 200% when pharmacologic treatment and behavioral treatment are used. Given the range and the CIs from prior reviews, these estimates are bounded at 50% above and 50% below those levels.
The 2008 Guideline recommends that clinicians implement the full 5A’s intervention (i.e., Ask, Advise, Assess, Assist, Arrange) with all patients seen in primary care and other healthcare settings.6 In this paper and the two papers that follow, brief counseling (3–10 minutes, OR 1.6) is used as a relatively conservative estimate of impact (Table 1). Nearly all of the existing studies on physician interventions focus on the interventions’ impact on overall abstinence rates and do not distinguish their specific impact on quit attempts, treatment use, or treatment effectiveness. However, one study found that delivery of a brief intervention was associated with a 60% greater chance of the smoker making a quit attempt.32 Based on the above, it is estimated that brief interventions by clinicians increase the population quit rate by 60%, through a 60% increase in quit attempts (range, 40%–100%).
Evidence-Based Cessation Treatment Policies
This review of cessation treatment policy research is selective in extracting how current and future treatment policy levers could be used and integrated to increase quit attempts, cessation treatment use, long-term treatment effectiveness, and ultimately the population quit rate. It is designed to provide a range of plausible parameter estimates that are used in the two modeling papers that follow. Three public health policies and healthcare systems changes are reviewed that are intended to improve tobacco-cessation treatment reach, access, delivery, use, and long-term effectiveness (Figure 1). Reviews were based primarily on those of the CDC Community Preventive Services guideline33 and the 2008 Guideline.6 The 2008 Guideline provides evidence that healthcare policy and delivery systems changes (e.g., including treatment as a covered benefit, implementing a tobacco-user identification/reminder system, and provider training) significantly increase the likelihood that smokers will receive effective cessation treatments and achieve long-term quitting success. Several new studies estimate the impact of policies regarding quitlines that deliver free counseling and medication.
Cessation Treatment Coverage and Provider Reimbursement
There is strong evidence that policies that reduce smokers’ out-of-pocket treatment costs and reimburse their providers for cessation services increase treatment use and successful long-term quitting. The 2008 Guideline6 recommends that all insurers provide tobacco-cessation benefits that include: (1) payment for evidence-based counseling and medications (both prescription and over-the-counter); (2) coverage of at least four counseling sessions of at least 30 minutes each delivered via quitlines, face-to-face group or individual counseling; (3) coverage of treatments for at least two quit attempts per year; and (4) minimization of co-pays or deductibles.
Most smokers do not have benefits that meet these standards either through their health plans or through employer-sponsored insurance programs (e.g., Medicaid, Medicare, state and federal benefit plans). For instance, a 2006 study34 reported that only 24% of employers offered full or partial coverage for tobacco-use treatment. In 2007, the National Business Group on Health reported that only 2% of companies provide comprehensive smokingcessation benefits for employees.35 While virtually all of the best-selling managed care HMO packages offered full coverage for at least one recommended behavioral treatment or pharmacologic treatment, only a small percentage covered the full range of evidence-based behavioral treatments and pharmacologic treatments.36 Medicaid tobacco-cessation treatment benefits vary widely by state, with only one state providing coverage for all 2008 Guideline–recommended treatments.37
The power of expanded cessation benefits to increase quit attempts, treatment use, and long-term quitting is also often blunted by the lack of awareness by smokers (and their providers) of their benefits. For instance, it has been found38 that only 27.4% of well-educated, insured smokers were aware of their benefits in 2002; not surprisingly, cessation treatment use was markedly higher among those who were aware of their benefits (39.6% vs 3.5%). Similar results were reported39 among smokers unaware of a new health plan cessation drug benefit and in a follow-up,40 increased awareness of a health plan was found among 1930 smokers (39% in enhanced awareness vs 22% in standard care), but this increase did not translate into greater pharmacologic treatment use or higher cessation rates.
In two states with comprehensive Medicaid coverage of cessation treatments, only 36% of covered smokers and 60% of their physicians knew about these benefits.41 Thus, a policy to create or expand cessation treatment coverage and provider reimbursement must also include explicit, proactive communication steps to inform and educate beneficiaries of the availability of these treatment options, using direct to consumer marketing or other local and mass media tools.
Studies of the impact of insurance coverage on cessation are reviewed by Hopkins et al.,42 Kaper et al.,43 and the 2008 Guideline.6 These reviews examined the impact of policies with similar coverage grouped together and did not distinguish their heterogeneity with regard to which medications were covered, whether behavior therapy was a required part of the pharmacotherapy regimen, or the promotion efforts of each program. The current review is limited to studies that examine full coverage. The study by Boyle et al.39 provides a lower-bound estimate of the impact of cessation benefit expansion because the new medication coverage they evaluated required physician involvement (a barrier to easy access) and because information about treatment coverage was not well communicated to eligible smokers. In contrast, an HMO in California introduced a well-publicized benefit that made NRT available free to smokers, and pharmacologic treatment use approximately doubled (OR=2.3) with a 10.2 percentage point increase.44 In another well-publicized benefit expansion with few restrictions on the type of treatment available to smokers,45 pharmacologic treatment and behavioral treatment use both increased by 4 percentage points. Results from the above studies and reviews are summarized in Table 2.
Table 2.
The effect of policies providing treatment coverage on quitting behaviors
| Treatment category | pp Δ (OR) | |||
|---|---|---|---|---|
| Between-group difference in treatment usea | Between-group difference in quit attemptsa | Between-group difference in quit ratea | ||
| Review studies | ||||
| Hopkins (2001)42 | PT+BT | 7.0 (not given) | NR | 7.8 (not given) |
| Kaper (2005)43 | PT | 0.12 (2.9) | 0.05 (1.3) | 0.02 (1.5) |
| BT | 0.02* (2.5*) | NR | NR | |
| Fiore (2008)6 | Any treatment | 9.3 (2.3) | 5.7 (1.3) | 3.8 (1.6) |
| Empirical studies | ||||
| Kaper (2005)45 | PT | 2.7 (4.0) | NR | NR |
| BT | 4.0 (5.1) | NR | NR | |
| PT+BT | 6.7 (2.9) | 2.6 (1.2) | 2.7 (2.3) | |
| Schauffler (2001)44 | PT | 10.2 (2.3) | 6.7 (1.4) | 4.7 (1.6) |
| Boyle (2002)39 | PT | 4.6 for Zyban* −1.9 for NRT* (not given) | 2.5* (not given) | 0.8* (not given) |
Note: For all studies in the table, changes in the effect of a treatment coverage policy are measured relative to a control group. In the present study, PT referred to NRT only.
Quit attempts were measured as 7-day point prevalence abstinence except for Kaper et al.,43 which combined studies using both 7-day point prevalence abstinence and continuous abstinence measures; and Hopkins et al.42 and Fiore et al.,6 where specific measures of abstinence were not stated.
Not significant
BT, behavior therapy; NR, not reported; pp Δ, percentage point change; PT, pharmacotherapy of any variety, usually NRT+bupropion; PT+BT, combined pharmacotherapy and behavior therapy
As reviewed above, the effects of full insurance coverage and information and outreach to make beneficiaries aware of their benefits on treatment utilization is based on the percentage of all smokers using treatment rather than just those smokers who make a quit attempt. The 4%–12% increases in pharmacologic treatment use in response to greater treatment coverage translates into an increase of 10%–30% in pharmacologic treatment use among those making a quit attempt, assuming that 40% of smokers attempt to quit each year. Large variations in projected effects on pharmacologic treatment use are due to differences in the control groups. For example, Schauffler et al.44 observed control group rates of pharmacologic treatment use of 17% compared to rates of 2% observed by Kaper et al.45 The 10%–30% increases in pharmacologic treatment use with coverage expansions translate into 100%44 to 450%45 increases relative to controls.
Changes in behavioral treatment use in response to changes in treatment coverage have been measured with less precision, with increases of 2%–4% of smokers using one or more behavioral treatments or about 5%–10% of those making a quit attempt following coverage expansions. These translate to increases in behavior therapy utilization ranging from 50% to 500%, in percentage terms relative to controls. No studies were found of coverage reductions or rollbacks.
Existing coverage studies often do not distinguish the effects of coverage expansions on the individual behavioral treatments or pharmacologic treatments that make up combined behavioral treatment and pharmacologic treatment treatment programs. It was estimated that well-publicized full coverage produces relative increases of 60% for pharmacologic treatment use alone, 100% for behavioral treatment use alone, and 125% for use of both treatments simultaneously, with a range of 50% below to 50% above each of these values. Levy and Friend21,22 obtained similar estimates.
In addition to increasing treatment use, the effects of providing full treatment coverage will depend on whether those who are induced to use treatment would not have made a quit attempt if the policy were not in effect. The current review indicates that quit attempts increase by 3–7 percentage points, which translates to about 50% of the new users of treatments who would not have otherwise made a quit attempt.
These reviewed studies report quit rates of all smokers in intervention and control groups but do not specifically consider treatment effectiveness among treatment users in the intervention group relative to the control. Policies that expand treatment coverage may yield long-term treatment effectiveness rates that are lower than those seen in clinical trials as less-motivated smokers may attempt to quit in response to the policy.21,22 Comparing the change in quit rates (ORs 2–3) and in treatment usage rates (ORs 1.5–2.3) relative to treatment use of 2–17 percentage points, it appears that providing reimbursement yields effectiveness rates as large as clinical studies.
Funding for the Use and Promotion of Evidence-Based State-Sponsored Telephone Quitlines
In 2004, the establishment of a national network of tobacco quitlines (1-800-QUIT-NOW) greatly expanded smokers’ access to evidence-based behavioral and pharmacologic treatment. State quitlines now have the potential to reduce access barriers to counseling and medication and to enhance long-term quit rates by better coordinating and tailoring proven counseling and pharmacologic treatments over time. However, financial support for state quitlines and their promotion is limited and uncertain, resulting in their use by only 1%–2% of U.S. smokers.10,46,47 The largest ever federal tobacco tax increase of 62 cents per pack that was implemented on April 1, 2009, resulted in as much as a three- to four-fold increase in quitline call volume during that time.48,49 This illustrates that there is upside potential to increase cessation beyond the usage rates of 1%–2%.
The type and duration of counseling provided by telephone quitlines vary across states, from single counseling sessions to multisession counseling that includes proactive follow-up calls to smokers who have made an initial contact.46,47 In 2006, most quitlines offered multilingual counseling and counseling protocols tailored to special populations such as teens and pregnant smokers. The extent to which pharmacotherapy is supplied free to callers also varies. In 2006, quitlines in 24 states (46%) provided free medications or medication vouchers to eligible adult callers: 24 states offered nicotine patches, 23 states offered nicotine gum, 20 states offered nicotine lozenges, 18 states offered free bupropion, and some had begun to provide discounted varenicline when it became available in August 2006.47 This variability in services could be reduced by implementing a policy that requires access to free or low-cost medications in all states.
Finally, there is wide variability in the degree to which state quitline services are advertised and promoted. Utilization of telephone quitline services is generally low, although current capacity could accommodate more callers. Only about 1% of U.S. smokers call a quitline each year.10,46 It has been estimated that even with current staffing levels, existing quitlines could accommodate as much as a tenfold increase in quitline calls (personal communication, Tim McAfee, Free & Clear, Inc., January 2009). Utilization of quitlines depends heavily on promotion efforts,50 which are often carefully titrated so that call volumes do not overwhelm existing quitline staffing and funding levels. Thus, national and state policies focused on telephone quitlines should not only address the types of services provided, but also ensure adequate marketing and promotion.
Six months after introducing a free nicotine replacement therapy to eligible adult callers, smoker utilization of quitline services was 2% in Minnesota27 and 3% in New York State.51,52 In Maine, the percentage of smokers who used the service was initially 3%31 and later increased to 6% as taxes increased.53 Based on the available evidence, it was estimated that quitlines that offer no-cost pharmacotherapy attract 4% of all smokers each year (range, 2%–6%) and 10% of those making a quit attempt (range, 5%–15%). Based on limited evidence from quitlines in California54 and New York29,30 and the evidence on the effect of treatment coverage policies, it was estimated that 50% of quitline callers (range, 25%–75%) are those who would not have otherwise made a quit attempt without the quitline. In should be noted that in some studies (e.g., the New York study), the introduction of free NRT often coincides with the introduction of a tobacco excise tax increase or new smokefree air laws so that estimates of NRT use may be inflated. These are important methodologic issues that are addressed in the third paper18 in this series.
Healthcare System Changes to Prompt, Guide, and Incentivize Tobacco Treatment
In this section, studies were reviewed that have evaluated changes in healthcare systems and policies to improve the consistent delivery of evidence-based brief healthcare provider interventions recommended as the “5A’s” intervention. As evidence is lacking on the specific effect when all components of the 5A’s are delivered, studies were reviewed of brief clinician interventions lasting 3–10 minutes, and consider opportunities for improved follow-up. Whereas many of the estimates in the previous sections were based on population-level data, the estimates presented in this section are based largely on the results of clinical trials.
The 2008 Guideline6 recommends that all clinicians implement each step of the 5A’s treatment model—Ask, Advise, Assess, Assist, and Arrange. However, despite the proven effectiveness of physician counseling for tobacco cessation, physicians still fail to assess and treat tobacco use consistently and effectively.6 For instance, it has been found55 that, while identification of smokers by physicians increased from 65% to 68%, rates of physician counseling declined from 22% in 1994–1996 to 20% in 2001–2003. In both time periods, the level of recommendations for smoking cessation was low (<2% of smokers’ visits).55 Overall, 32% of patient charts did not have information about tobacco use, and 81% of smokers did not receive assistance.56 Data from smokers are consistent with these statistics. To gauge patient recall of 5A’s counseling, results from adult members of nine nonprofit HMOs indicated inadequate delivery of the full 5A’s intervention: while 90% of smokers were asked about smoking, 71% were advised to quit; 56% were assessed for their willingness to quit; only 49% received assistance interventions; and less than 10% received any follow-up.57
Healthcare providers can be supported and encouraged to provide tobacco dependence treatment through policies and healthcare system changes that prompt, guide, and incentivize tobacco treatment. Evidence-based cessation policy and system changes include the use of tobacco intervention reminder systems, clinician training, and routine treatment performance measurement and feedback.58,59 Past clinical practice guide-lines60,61 were critical to the development of a National Committee for Quality Assurance Healthcare Effectiveness Data and Information Set (HEDIS) using patient reporting to track the delivery of the Ask, Advise, and Assist components of the 5A’s intervention in managed care settings.62,63 The HEDIS measure has not been incorporated in major national healthcare quality improvement and pay-for-performance metrics. The 2008 Guideline6 suggests that incentive and reward programs combined with feedback on provider performance may ultimately prove the best approach for improving physician intervention. Evidence from other types of health services confirm the importance of incentive and reward programs,64 but the few studies on the use of these approaches to encourage physician counseling for cessation have yielded mixed results.65,66 However, as the evidence base grows, existing healthcare performance standards and policies could be expanded to include evidence-based recommendations for cessation treatment–related performance measurement, feedback systems, and financial incentives to providers.58,59
The 2008 Guideline6 notes the need for all of these healthcare systems and policy change strategies, but high-quality studies were available only for estimating the impact of training programs and reminder systems. Training clinicians to provide cessation treatment increases the prevalence of physician counseling from 36.2% to 64.7% (OR 3.2), and increases quit rates among those counseled from 6.4% to 12.0% (OR 2.0).6 Combining a reminder system with training increases the percentage of providers assessing tobacco use from 58.8% to 75.2% (OR 2.1) and increases the proportion of smokers who set a quit date from 11.4% to 41.4% (OR 5.5). Hopkins et al.42 found that reminder systems alone increased the percentage of providers who deliver advice to quit by 13 percentage points, while reminder systems combined with clinician education program increased the number of patients advised to quit by 20% and the number of patients who successfully quit by 4.7%. Patient education combined with provider reminders and provider education increased the rate of advice to quit by 22% while also increasing the percentage who successfully quit by 5.7%.42 Given these data, it was estimated that provider training and reminders to providers to query their patients’ smoking status would lead to an additional 20% of smoking patients who receive brief intervention (range, 10%–30%).
Promising Cessation Treatment Policies
In this section, the impact was estimated of two policies with potential to improve the use and effectiveness of evidence-based cessation treatments: (1) policies to support and promote high-quality, evidence-based treatment via the Internet, and (2) policies to improve the long-term effectiveness of evidence-based treatments. Both approaches have been identified as promising by the 2008 Guideline6 and are now the subject of considerable research. Therefore, these were added as part of a future-oriented vision and assessment of the ways that cessation treatment policies could contribute to progress toward Healthy People 2010 and 2020 goals for adult smoking prevalence. Although the level of evidence available for more established policy approaches is lacking for these two policy categories, it is constructive to determine what the overall impact would be if such interventions were implemented.
Policies to Support and Promote Evidence-Based Computerized Treatment via the Internet
Over the past decade, the Internet has been widely adopted and represents a viable modality for the delivery of behavioral and pharmacologic treatments for tobacco dependence.67 Evidence-based Internet cessation treatments hold enormous potential to boost quit attempts, treatment use, and long-term quitting success. Millions of smokers look for cessation assistance online each year, and there is growing evidence for the erosion of the digital divide.68,69 The Internet is the only cessation treatment modality currently able to provide 24/7/365 cessation information, counseling, and sustained support to promote cessation and prevent relapse.70 In addition, treatment content can be readily and inexpensively targeted and tailored to address the quitting needs of specific sociodemographic populations and individual quitters. Recognizing these unique advantages of the Internet, by 2006 over two thirds of U.S. quitlines had begun to use the Internet to provide supplementary services.47
The field of Internet-based cessation is still relatively new, with most studies reporting on only the feasibility and usability of online programs. Several recent randomized trials of individually tailored, web-based smoking cessation programs have reported long-term quit rates of 7%–26%,71–73 and a recent review of computer-mediated and web-based cessation programs for adult tobacco users found that seven of 15 studies reported significantly improved outcomes over control conditions.74 The 2008 Guideline noted that such studies are promising but have not yet produced sufficient, high-quality evidence to be included as a recommended treatment strategy.
Indeed, the majority of quit-smoking websites available to consumers are not evidence-based and have not been tested in rigorous research trials.75,76 The result is a confusing and often misleading landscape for smokers seeking cessation assistance online. Development of a rating system for evidence-based Internet treatments similar to the system used by Consumer Reports could help millions of smokers that are motivated to search for cessation assistance online to locate effective interventions and avoid unproven sites. In addition, policies that encourage or incentivize the use of Internet interventions as an adjunct to other forms of evidence-based treatment covered by insurance could represent a cost-efficient strategy to provide sustained support to promote abstinence and prevent relapse. Currently, there is insufficient evidence to estimate the impact of web-based interventions on population quit rates. It was tentatively estimated that improved web-based treatment is used by 2.5% of smokers that attempt to quit (with a range of 1.5% to 3.5%), of which 1% would be encouraged to use pharmacologic treatment and 1.25% would be smokers attempting to quit for the first time. Effectiveness of high quality, evidence-based websites is estimated to be 60%, the same as for behavioral treatment.
Policies to Improve Individually Tailored, Stepped-Care Approaches and the Long-Term Effectiveness of Evidence-Based Treatments
Improving treatment efficacy requires reducing the very high rate of relapse through development of more effective and efficient interventions, improvement of continuity of care, and delivery of repeated treatments geared toward re-cycling smokers who relapse. Reducing relapse is a powerful yet largely unexplored lever in the pathway toward boosting population-level cessation rates.77,78 Smokers try multiple times to quit and make as many as 5–8 quit attempts before they successfully maintain cessation.79
Coordinated treatment models to reduce post-treatment relapse and improve long-term treatment effectiveness might include those that ensure continuity of care over time, that help to coordinate multiple types and modalities of treatment using tailoring or stepped-care algorithms, and that offer timely or sustained follow-up tailored to the unique needs of smokers who relapse. Each smoker should receive care based on routine follow-up assessment and triage into a level and type of treatment that is appropriately matched to their prior history, personal characteristics, and abilities. This kind of treatment matching should include a combination of tailoring or targeting specific treatment elements as well as a stepped-care approach that takes into account the intensity, duration, frequency, and cost of the treatment (IOM report16; Abrams et al.80). Such interventions may be aided by the increasing use of electronic medical records, web-based cessation programs, and computer-aided follow-up to guide ongoing tailoring and stepped-care approaches as part of a comprehensive system of care management. Delivery of appropriate follow-up and re-cycling measures could also be included as HEDIS metrics. The evidence base for these intuitively appealing but more complex integrated “systems approaches to comprehensive care management”16 is lacking, but there are promising emerging trends in support of the general concept.
The 2008 Guideline6 identifies individually tailored and stepped-care interventions as promising approaches. An example of such an approach is a study81 that evaluated a re-cycling intervention among U.S. veterans who had been issued prescriptions for pharmacologic treatment and who reported still smoking 6 months post-treatment. Almost two thirds of relapsed smokers were interested in quitting again (re-cycling) within 30 days. An evaluation was made82 of a standard 12-week treatment compared to an extended multicomponent intervention that combined, coordinated, and individually tailored multiple behavioral and pharmacologic treatments delivered over a 12-month period using face-to-face and telephone quitline formats, and stepped care. The multi-component intervention yielded a 50% 1-year quit rate compared to 18% in standard treatment. Several studies provide promising results for tailored interventions83–87 as well as culturally tailored support geared to the needs of specific subgroups of smokers.88,89 A meta-analysis showed that smokers who were provided with individually tailored self-help materials were more likely to succeed in their quit attempt than those who were provided nontailored materials (OR=1.42).90 There is promising yet insufficiently robust evidence to estimate the effects of such programs. Thus it was estimated that integrative systems approaches that support tailored, extended treatment can double the effectiveness of evidence-based treatments, with sensitivity analysis conducted at 50% and 150% increases.
Interacting Effects of Policies Implemented in Tandem
When implemented simultaneously, several of the policies described above can exhibit synergistic effects, creating benefits that are greater than the sum of their parts. For example, it is easy to imagine a greater impact of brief clinician interventions when implemented in the context of comprehensive coverage for evidence-based treatments, adequate funding of quitlines, sustained support via the Internet, or care management systems for extended follow-up. Similarly, with the removal of financial, informational, and convenience barriers as a result of direct-to-consumer marketing and well-publicized treatment coverage policies, clinicians can be expected to more consistently encourage treatment use. Indeed, this effect has been observed with the spread of the new Ask–Advise–Refer-to-Quitline version of the 5A primary care intervention model.90
Synergies might also be expected through improved treatment effectiveness brought about by enhanced follow-up based on better triage, matching treatments to individual needs, and the use of stepped-care approaches. Such improvements could be implemented not only in traditional healthcare delivery settings, but also via worksites or community health centers. In addition, web-based programs can widen access to and the use of behavioral treatments and pharmacologic treatments, and also promote higher long-term quit rates given their continuous availability. Mass media campaigns can also increase impact when combined with other policies and interventions (IOM report15,16), but media approaches were not included in this paper as it is difficult to estimate their additive and interactive effect.
Offsetting the synergies described above, other interactions may have overlapping or duplicative effects that could weaken the effects of individual policies. For example, when policies supporting and promoting quitlines that offer free NRT are implemented in conjunction with policies that expanded treatment coverage for all behavioral treatments and pharmacologic treatments, the combined impact on smokers may be less than the sum of their individual effects, as both reduce smokers’ out-of-pocket costs for behavioral treatment and pharmacologic treatment. Some smokers may prefer the relative anonymity and convenience of a website or a telephone quit-line while others may prefer face-to-face contact. The value of implementing policies in a coordinated fashion is that combined policies give smokers the option of selecting the treatments best suited to their needs. The precise quantitative nature of the interactions between and among treatments was considered in the second paper of this series.
Conclusion
An analytic framework for understanding the population impacts of a defined set of tobacco-cessation treatments and related policies is introduced. Recognizing that population cessation is determined by (1) the number of smokers who make a serious quit attempt; (2) the proportion of serious quitters who make use of evidence-based treatments; and (3) the long-term effectiveness of those treatments, this paper selectively reviewed the evidence for the independent effects of treatments and related policies on each of these three components. The second paper in this series17 uses the estimates derived in this review to model the effects of combined treatment-related policies on quit rates. The third paper18 widens the lens to project the progress that could be made toward reaching Healthy People 2010 and 2020 goals by aligning the adoption of these treatment-related polices with broader tobacco control policies known to have powerful independent effects on quit attempts (i.e., tobacco tax increases, clean air laws, and health communication interventions such as antismoking media campaigns).
The policies focused on included evidence-based policies governing expansions in treatment coverage and in quitline services, which have reduced financial and other barriers to treatment use, along with healthcare system changes and policies found to improve the delivery of brief counseling by healthcare providers.91 To date, none of these policies have been fully implemented. Few, if any, other activities could produce greater health and economic benefits to our nation as rapidly and as cost-efficiently as fully implementing smoking-cessation treatments and policies. Moreover, few other approaches can reduce population health disparities in the same powerful way as smoking cessation, especially among low-income Americans and those with limited formal education at greatest risk for disparities in tobacco-related disease.11,12 Also considered were the potential effects of promising cessation treatments and policies that would leverage the unique potential of the Internet, promote integrated systems of care management, and provide sustained support to reduce relapse propensity among smokers, as well as policies to increase the long-term treatment effectiveness of existing interventions.
Several limitations of this paper are noted. Most of the studies reviewed reported the impact of policies on treatment use or quit rates, without specifically distinguishing their effects on quit attempts or long-term treatment effectiveness. It was also necessary to extrapolate beyond the robust evidence base about the likely impacts of some policy levers, such as Internet-based programs; tailored, stepped care; media campaigns, and comprehensive systems of care management. There were studies that were not covered in this selective review or in the two papers that follow. For the most part, omitted studies were not deemed sufficiently relevant, representative, replicable, or robust enough to determine parameters for the models. The parameters were also set at a level of granularity that used the most robust and relevant data available with appropriate sensitivity bounds. For example, some studies with insufficient evidence included multilevel interaction effects on aggregate units such as combined media-, community-, neighborhood-, worksite-, home-, and school-level interactions. Other studies not covered note possible differences in outcomes due to factors such as comorbidity of psychiatric or substance abuse disorders, low literacy, and low SES, and suggest that culturally tailored interventions may improve lackluster outcomes for interventions with culturally diverse subgroups.92–94
This paper presents one among many possible approaches to developing a framework to inform simulation modeling. All frameworks and simulations are ultimately heuristic and are simplifications of the complexity of systems interactions in the “real world.”95–97 New study designs and data collection methods are needed to inform the current gaps in knowledge in order to develop more fine-grained algorithms and more complex frameworks to improve the utility of future simulations. The limitations noted in this paper illustrate the need to advance the field with increasingly more sophisticated “comprehensive systems integrated”16 approaches to intervention and policy to maximize impact on reducing population prevalence of smoking.
One of the advantages of simulation modeling as shown in the subsequent two papers17,18 in this series is that one can “push the envelope” beyond the current status quo of evidence to examine plausible future impacts. This heuristic “what if” simulation can point the way toward new research and practice initiatives. For example, what if an estimated level of improvement in maintenance of cessation (relapse prevention) turns out to be one of the most powerful policy levers to increase population cessation rates? This projected result encourages researchers, practitioners, and policymakers to focus on developing more effective interventions and policies to achieve that estimated level of intervention impact even if it is not currently supported by existing evidence.
In sum, this paper and the two simulation papers17,18 address the growing need for healthcare reform, illustrating the enormous benefits of this opportunity to capitalize on the smoking-cessation research and policy advances made over the past two decades by expanding adult cessation and policy. Failing to act now to implement a nationwide comprehensive smoking-cessation system of care is an extraordinary opportunity lost, with devastating consequences in terms of premature death, reduced quality of life, disease burden, preventable disparities in health and health care, and unsustainable healthcare costs.
Acknowledgments
This paper was conducted under the auspices of the national Consumer Demand Roundtable and was supported by funds provided by the Office of Behavioral and Social Sciences Research (OBSSR) at the NIH and the Robert Wood Johnson Foundation (RWJF). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the American Legacy Foundation, the University of Baltimore, OBSSR, or RWJF.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Levy DT, Cummings KM, Hyland A. A simulation of the effects of youth initiation policies overall cigarette use. Am J Public Health. 2000;90(8):1311–4. doi: 10.2105/ajph.90.8.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Cigarette smoking among adults—U.S. 2000. MMWR Morb Mortal Wkly Rep. 2002;51(29):642–5. [PubMed] [Google Scholar]
- 3.CDC. Cigarette smoking among adults—U.S. 2006. MMWR Morb Mortal Wkly Rep. 2007;56(44):1157–61. [PubMed] [Google Scholar]
- 4.CDC. Annual smoking-attributable mortality, years of potential life lost, and productivity losses—U.S. 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54(25):625–8. [PubMed] [Google Scholar]
- 5.CDC. Tobacco use among adults—U.S. 2005. MMWR Morb Mortal Wkly Rep. 2006;55(42):1145–8. [PubMed] [Google Scholar]
- 6.Fiore M, Jaén C, Baker T, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville MD: USDHHS, Public Health Service; 2008. [Google Scholar]
- 7.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31(1):52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 8.IOM. Priority areas for national action: transforming health care quality. Washington: National Academies Press; 2003. [PubMed] [Google Scholar]
- 9.Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med. 2005;28(1):119–22. doi: 10.1016/j.amepre.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the U. S Am J Prev Med. 2008;34(2):102–11. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Orleans CT. Increasing the demand for and use of effective smoking-cessation treatments reaping the full health benefits of tobacco-control science and policy gains—in our lifetime. Am J Prev Med. 2007;33(6S):S340–8. doi: 10.1016/j.amepre.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Gollust S, Schroeder S, Warner K. Helping smokers quit: understanding the barriers to utilization of smoking cessation services. Millbank Q. 2008;86(4):601–27. doi: 10.1111/j.1468-0009.2008.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backinger CL, O’Connell ME. Developing consensus on tobacco control and research. Am J Prev Med. 2007;33(6S):S311–3. doi: 10.1016/j.amepre.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 14.National Tobacco Cessation Collaborative. Innovations in building consumer demand for tobacco cessation products and services. Washington: Academy for Educational Development; 2007. [Google Scholar]
- 15.IOM. Ending the tobacco problem: a blueprint for the nation. Washington: The National Academies Press; 2007. [Google Scholar]
- 16.Abrams D. Comprehensive smoking cessation policy for all smokers: systems integration to save lives and money. In: Bonnie RJ, Stratton K, Wallace RB, editors. Ending the tobacco problem: a blueprint for the nation. Washington: The National Academies Press; 2007. [Google Scholar]
- 17.Levy DT, Mabry PL, Graham AL, Abrams DB, Orleans CT. Modeling the impact of smoking cessation treatment policies on quit rates. Am J Prev Med. 2010;38(3S):S364–S372. doi: 10.1016/j.amepre.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy DT, Mabry PL, Graham AL, Orleans CT, Abrams DB. Reaching Healthy People 2010 by 2013: a SimSmoke simulation. Am J Prev Med. 2010;38(3S):S373–S381. doi: 10.1016/j.amepre.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy DT, Nikolayev N, Mumford EA. The Healthy People 2010 Smoking Prevalence and Tobacco Control Objectives: results from the SimSmoke Tobacco Control Policy Simulation Model. Cancer Causes Control. 2005;16(4):359–71. doi: 10.1007/s10552-004-7841-4. [DOI] [PubMed] [Google Scholar]
- 20.Levy DT, Bauer JE, Lee HR. Simulation modeling and tobacco control: creating more robust public health policies. Am J Public Health. 2006;96(3):494–8. doi: 10.2105/AJPH.2005.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy DT, Friend K. A simulation model of policies directed at treating tobacco use and dependence. Med Decis Making. 2002;22(1):6–17. doi: 10.1177/0272989X0202200101. [DOI] [PubMed] [Google Scholar]
- 22.Levy DT, Friend K. Examining the effects of tobacco treatment policies on smoking rates and smoking-related deaths using the SimSmoke computer simulation model. Tob Control. 2002;11(1):47–54. doi: 10.1136/tc.11.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns D, Anderson C, Johnson M, et al. Population based smoking cessation: proceedings of a conference on what works to influence cessation in the general population. Smoking and Tobacco Control Monograph No. 12. Bethesda MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Nov, 2000. Cessation and cessation measures among daily adult smokers: national- and statespecific data. National Cancer Institute. NIH Pub. No. 00–4892. [Google Scholar]
- 24.Hughes JR, Benowitz N, Hatsukami D, Mermelstein RJ, Shiffman S. Clarification of SRNT workgroup guidelines for measures in clinical trials of smoking cessation therapies. Nicotine Tob Res. 2004;6(5):863–4. doi: 10.1080/1462220042000282564. [DOI] [PubMed] [Google Scholar]
- 25.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16(1S):i53–59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinkelman D, Wilson SM, Willett J, Sweeney CT. Offering free NRT through a tobacco quitline: impact on utilisation and quit rates. Tob Control. 2007;16(1S):i42–6. doi: 10.1136/tc.2007.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An LC, Schillo BA, Kavanaugh AM, et al. Increased reach and effectiveness of a statewide tobacco quitline after the addition of access to free nicotine replacement therapy. Tob Control. 2006;15(4):286–93. doi: 10.1136/tc.2005.014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fellows JL, Bush T, McAfee T, Dickerson J. Cost effectiveness of the Oregon quitline “free patch initiative. Tob Control. 2007;16(1S):i47–52. doi: 10.1136/tc.2007.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metzger KB, Mostashari F, Kerker BD. Use of pharmacy data to evaluate smoking regulations’ impact on sales of nicotine replacement therapies in New York City. Am J Public Health. 2005;95(6):1050–5. doi: 10.2105/AJPH.2004.048025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller N, Frieden TR, Liu SY, et al. Effectiveness of a large-scale distribution programme of free nicotine patches: a prospective evaluation. Lancet. 2005;365(9474):1849–54. doi: 10.1016/S0140-6736(05)66615-9. [DOI] [PubMed] [Google Scholar]
- 31.Swartz SH, Cowan TM, Klayman JE, Welton MT, Leonard BA. Use and effectiveness of tobacco telephone counseling and nicotine therapy in Maine. Am J Prev Med. 2005;29(4):288–94. doi: 10.1016/j.amepre.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Gilpin EA, Pierce JP, Johnson M, Bal D. Physician advice to quit smoking: results from the 1990 California Tobacco Survey. J Gen Intern Med. 1993;8(10):549–53. doi: 10.1007/BF02599637. [DOI] [PubMed] [Google Scholar]
- 33.CDC. Increasing tobacco use cessation. Atlanta GA: CDC; 2009. Guide to Community Preventive Services. [Google Scholar]
- 34.Bondi MA, Harris JR, Atkins D, French ME, Umland B. Employer coverage of clinical preventive services in the U. S Am J Health Promot. 2006;20(3):214–22. doi: 10.4278/0890-1171-20.3.214. [DOI] [PubMed] [Google Scholar]
- 35.National Business Group on Health. Exploring employers’ understanding and perceptions of the business impact of smoking. Washington: 2007. http://www.businessgrouphealth.org/tobacco/surveys/index.cfm. [Google Scholar]
- 36.McPhillips-Tangum C, Rehm B, Carreon R, Erceg CM, Bocchino C. Addressing tobacco in managed care: results of the 2003 survey. Prev Chronic Dis. 2006;3(3):A87. [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. State Medicaid coverage for tobacco-dependence treatments—U.S. 2006. MMWR Morb Mortal Wkly Rep. 2008;57(5):117–22. [PubMed] [Google Scholar]
- 38.Burns ME, Rosenberg MA, Fiore MC. Use of a new comprehensive insurance benefit for smoking-cessation treatment. Prev Chronic Dis. 2005;2(4):A15. [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle RG, Solberg LI, Magnan S, Davidson G, Alesci NL. Does insurance coverage for drug therapy affect smoking cessation? Health Aff (Millwood) 2002;21(6):162–8. doi: 10.1377/hlthaff.21.6.162. [DOI] [PubMed] [Google Scholar]
- 40.Alesci NL, Boyle RG, Davidson G, Solberg LI, Magnan S. Does a health plan effort to increase smokers’ awareness of cessation medication coverage increase utilization and cessation? Am J Health Promot. 2004;18(5):366–9. doi: 10.4278/0890-1171-18.5.366. [DOI] [PubMed] [Google Scholar]
- 41.McMenamin SB, Halpin HA, Bellows NM. Knowledge of Medicaid coverage and effectiveness of smoking treatments. Am J Prev Med. 2006;31(5):369–74. doi: 10.1016/j.amepre.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001;20(2S):16–66. doi: 10.1016/s0749-3797(00)00297-x. [DOI] [PubMed] [Google Scholar]
- 43.Kaper J, Wagena EJ, Severens JL, Van Schayck CP. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database Syst Rev. 2005;(1):CD004305. doi: 10.1002/14651858.CD004305.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Schauffler HH, McMenamin S, Olson K, Boyce-Smoth G, Rideout JA, Kamil J. Variations in treatment benefits influence smoking cessation: results of a randomized controlled trial. Tob Control. 2001;10:175–80. doi: 10.1136/tc.10.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaper J, Wagena EJ, Willemsen MC, van Schayck CP. Reimbursement for smoking cessation treatment may double the abstinence rate: results of a randomized trial. Addiction. 2005;100(7):1012–20. doi: 10.1111/j.1360-0443.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 46.Cummins SE, Bailey L, Campbell S, Koon-Kirby C, Zhu SH. Tobacco cessation quitlines in North America: a descriptive study. Tob Control. 2007;16(1S):i9–15. doi: 10.1136/tc.2007.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.North American Quitline Consortium. North American quitlines: a profile of reach and services across the US and Canada—2006. Phoenix AZ: North American Quitline Consortium; 2008. [Google Scholar]
- 48.North American Quitline Consortium. US telephone quitlines struggle to serve all callers spurred to quit by federal tax hike. www.naquitline.org/resource/resmgr/worldnotobaccoday_naqcpressr.pdf.
- 49. [Accessed January 17, 2010]; http://www.usatoday.com/news/health/2009-04-02-smoking-hotlines_N.htm.
- 50.Farrelly MC, Hussin A, Bauer UE. Effectiveness and cost effectiveness of television, radio and print advertisements in promoting the New York smokers’ quitline. Tob Control. 2007;16(1S):i21–3. doi: 10.1136/tc.2007.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cummings KM, Fix B, Celestino P, Carlin-Menter S, O’Connor R, Hyland A. Reach, efficacy, and cost effectiveness of free nicotine medication giveaway programs. J Public Health Manag Pract. 2006;12(1):37–43. doi: 10.1097/00124784-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Cummings KM, Hyland A, Fix B, et al. Free nicotine patch giveaway program 12-month follow-up of participants. Am J Prev Med. 2006;31(2):181–4. doi: 10.1016/j.amepre.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 53.Woods SS, Haskins AE. Increasing reach of quitline services in a U.S. state with comprehensive tobacco treatment. Tob Control. 2007;16(1S):i33–6. doi: 10.1136/tc.2007.019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347(14):1087–93. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 55.Thorndike AN, Rigotti NA, Stafford RS, Singer DE. National patterns in the treatment of smokers by physicians. JAMA. 1998;279(8):604–8. doi: 10.1001/jama.279.8.604. [DOI] [PubMed] [Google Scholar]
- 56.Ferketich AK, Khan Y, Wewers ME. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001–2004 national ambulatory medical care survey (NAMCS) Prev Med. 2006;43(6):472–6. doi: 10.1016/j.ypmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Quinn VP, Stevens VJ, Hollis JF, et al. Tobacco-cessation services and patient satisfaction in nine nonprofit HMOs. Am J Prev Med. 2005;29(2):77–84. doi: 10.1016/j.amepre.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Casalino L, Gillies RR, Shortell SM, et al. External incentives, information technology, and organized processes to improve healthcare quality for patients with chronic diseases. JAMA. 2003;289(4):434–41. doi: 10.1001/jama.289.4.434. [DOI] [PubMed] [Google Scholar]
- 59.Keller PA, Fiore MC, Curry SJ, Orleans CT. Systems change to improve health and health care: lessons from addressing tobacco in managed care. Nicotine Tob Res. 2005;7(1S):S5–8. doi: 10.1080/14622200500077966. [DOI] [PubMed] [Google Scholar]
- 60.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. JAMA. 2000;283(24):3244–54. [PubMed] [Google Scholar]
- 61.Fiore M, Bailey W, Cohen S, et al. Smoking cessation: clinical practice guideline no. 18. Rockville MD: USDHHS; 1996. AHCPR Publication No. 96-0692. [Google Scholar]
- 62.Curry SJ, Keller PA, Orleans CT, Fiore MC. The role of health-care systems in increased tobacco cessation. Annu Rev Public Health. 2008;29:411–28. doi: 10.1146/annurev.publhealth.29.020907.090934. [DOI] [PubMed] [Google Scholar]
- 63.Orleans CT, Woolf SH, Rothemich SF, Marks JS, Isham GJ. The top priority: building a better system for tobacco-cessation counseling. Am J Prev Med. 2006;31(1):103–6. doi: 10.1016/j.amepre.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Kane RL, Johnson PE, Town RJ, Butler M. Economic incentives for preventive care. Evid Rep Technol Assess. 2004 Summ;(101):1–7. [PMC free article] [PubMed] [Google Scholar]
- 65.Andrews JO, Tingen MS, Waller JL, Harper RJ. Provider feedback improves adherence with AHCPR Smoking Cessation Guideline. Prev Med. 2001;33(5):415–21. doi: 10.1006/pmed.2001.0907. [DOI] [PubMed] [Google Scholar]
- 66.Roski J, Jeddeloh R, An L, et al. The impact of financial incentives and a patient registry on preventive care quality: increasing provider adherence to evidence-based smoking cessation practice guidelines. Prev Med. 2003;36(3):291–9. doi: 10.1016/s0091-7435(02)00052-x. [DOI] [PubMed] [Google Scholar]
- 67.Graham AL, Abrams DB. Reducing the cancer burden of lifestyle factors: opportunities and challenges of the Internet. J Med Internet Res. 2005;7(3):e26. doi: 10.2196/jmir.7.3.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox S. Health information online. Washington: Pew Internet and American Life Project; 2005. [Google Scholar]
- 69.Cobb NK, Graham AL. Characterizing Internet searchers of smoking cessation information. J Med Internet Res. 2006;8(3):e17. doi: 10.2196/jmir.8.3.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cobb NK, Graham AL, Bock BC, Papandonatos G, Abrams DB. Initial evaluation of a real-world Internet smoking cessation system. Nicotine Tob Res. 2005;7(2):207–16. doi: 10.1080/14622200500055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swartz LH, Noell JW, Schroeder SW, Ary DV. A randomised control study of a fully automated Internet-based smoking cessation programme. Tob Control. 2006;15(1):7–12. doi: 10.1136/tc.2003.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web-based computer-tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction. 2005;100(5):682–8. doi: 10.1111/j.1360-0443.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- 73.Muñoz RF, Lenert LL, Delucchi K, et al. Toward evidence-based Internet interventions: a Spanish/English website for international smoking cessation trials. Nicotine Tob Res. 2006;8(1):77–87. doi: 10.1080/14622200500431940. [DOI] [PubMed] [Google Scholar]
- 74.Walters ST, Wright JA, Shegog R. A review of computer and Internet-based interventions for smoking behavior. Addict Behav. 2006;31(2):264–77. doi: 10.1016/j.addbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Bock B, Graham A, Sciamanna C, et al. Smoking cessation treatment on the Internet: content, quality, and usability. Nicotine Tob Res. 2004;6(2):207–19. doi: 10.1080/14622200410001676332. [DOI] [PubMed] [Google Scholar]
- 76.Bock BC, Graham AL, Whiteley JA, Stoddard JL. A review of web-assisted tobacco interventions (WATIs) J Med Internet Res. 2008;10(5):e39. doi: 10.2196/jmir.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shiffman S. Reflections on smoking relapse research. Drug Alcohol Rev. 2006;25(1):15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- 78.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 79.Hughes JR. Four beliefs that may impede progress in the treatment of smoking. Tob Control. 1999;8(3):323–6. doi: 10.1136/tc.8.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Prochaska JO, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: a combined stepped-care and matching model. Annals of Behavioral Medicine. 1996;18(4):290–304. doi: 10.1007/BF02895291. [DOI] [PubMed] [Google Scholar]
- 81.Fu SS, Partin MR, Snyder A, et al. Promoting repeat tobacco dependence treatment: are relapsed smokers interested? Am J Manag Care. 2006;12(4):235–43. [PubMed] [Google Scholar]
- 82.Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161(11):2100–7. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 83.Strecher VJ, Marcus A, Bishop K, et al. A randomized controlled trial of multiple tailored messages for smoking cessation among callers to the cancer information service. J Health Commun. 2005;10(1S):S105–18. doi: 10.1080/10810730500263810. [DOI] [PubMed] [Google Scholar]
- 84.Strecher VJ, Shiffman S, West R. Moderators and mediators of a web-based computer-tailored smoking cessation program among nicotine patch users. Nicotine Tob Res. 2006;8(1S):S95–101. doi: 10.1080/14622200601039444. [DOI] [PubMed] [Google Scholar]
- 85.Shiffman S, Paty JA, Rohay J, DiMarino ME, Gitchell J. The efficacy of computer-tailored smoking cessation material as a supplement to nicotine patch therapy. Drug and Alcohol Dependence. 2001;64:35–46. doi: 10.1016/s0376-8716(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 86.Shiffman S, Paty JA, Rohay J, DiMarino ME, Gitchell J. The efficacy of computer-tailored smoking cessation material as a supplement to nicotine polacrilex gum therapy. Arch Intern Med. 2000;160(11):1675–81. doi: 10.1001/archinte.160.11.1675. [DOI] [PubMed] [Google Scholar]
- 87.Velicer WF, Prochaska JO, Redding CA. Tailored communications for smoking cessation: past successes and future directions. Drug Alcohol Rev. 2006;25(1):49–57. doi: 10.1080/09595230500459511. [DOI] [PubMed] [Google Scholar]
- 88.Barker DC, Orleans CT, Schauffler HH. Tobacco treatment services should be covered under Medicaid. Tob Control. 1998;7(1):92. doi: 10.1136/tc.7.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lipkus IM, Lyna PR, Rimer BK. Using tailored interventions to enhance smoking cessation among African Americans at a community health center. Nicotine Tob Res. 1999;1(1):77–85. doi: 10.1080/14622299050011181. [DOI] [PubMed] [Google Scholar]
- 90.Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev. 2005;(3):CD001118. doi: 10.1002/14651858.CD001118.pub2. [DOI] [PubMed] [Google Scholar]
- 91.Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, McAfee T. The feasibility of connecting physician offices to a state-level tobacco quitline. Am J Prev Med. 2006;30(1):31–7. doi: 10.1016/j.amepre.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 92.Lawrence D, Graber JE, Mills SL, Meissner HI, Warnecke R. Smoking cessation interventions in U.S. racial/ethnic minority populations: an assessment of the literature. Prev Med. 2003;36(2):204–16. doi: 10.1016/s0091-7435(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 93.Kreuter MW, McClure SM. The role of culture in health communication. Annu Rev Public Health. 2004;25:439–55. doi: 10.1146/annurev.publhealth.25.101802.123000. [DOI] [PubMed] [Google Scholar]
- 94.Nollen N, Ahluwalia JS, Mayo MS, et al. A randomized trial of targeted educational materials for smoking cessation in African Americans using transdermal nicotine. Health Educ Behav. 2007;34(6):911–27. doi: 10.1177/1090198106294652. [DOI] [PubMed] [Google Scholar]
- 95.Epstein J. Why Model?. Paper presented at: Second World Congress on Social Simulation; 2008; George Mason University; [Google Scholar]
- 96.Mabry PL, Olster DH, Morgan GD, Abrams DB. Interdisciplinarity and systems science to improve population health: a view from the NIH Office of Behavioral and Social Sciences Research. Am J Prev Med. 2008;35(S2):S211–24. doi: 10.1016/j.amepre.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sterman JD. Learning from evidence in a complex world. Am J Public Health. 2006;96(3):505–14. doi: 10.2105/AJPH.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]


