Abstract
Obesity is one of the most prevalent chronic diseases globally, especially in the United States. While the United States gained an early lead in unnecessary weight gain, most other countries are quickly closing the gap. The latest U.S. National Health and Nutrition Examination Survey (NHANES, http://www.cdc.gov/nchs/nhanes.htm) documents that about one third of adults in the United States are now overweight [a body-mass index (BMI) between 25 and 30 kg/m2] and another one third (61 million) are considered obese (BMI > 30 kg/m2). Being obese is a strong risk factor for cardiovascular diseases, type 2 diabetes, osteoporosis, some cancers, and depression. The economic impact of this condition is staggering: in 2008, more than 147 billion dollars were spent just in the United States for medical costs related to obesity. Time lost from work and spending on weight loss costs even more.
Obesity is influenced to some extent by genetic and environmental factors. However, two centuries of research has repeatedly confirmed that the basic principle of energy balance (Figure 1) governs the accumulation of fat. Obesity results from years of slightly higher energy intake from food than energy expended (EE). The path to obesity leads from a positive energy balance of approximately 100–125 kcal per day to between 2 and 6 kg of weight gain per year (Figure 2;[1]). In other words, you can become overweight by eating just one or two cookies or drinking a can of sugar soda a day. This very slight energy imbalance means that measuring energy intake and expenditure are highly demanding, generally requiring high resolution over long periods. At present, we lack sufficiently accurate and reasonable means to measure ad libitum food intake in humans. Fortunately, continuing technological advances have permitted increasingly accurate measurement of energy expenditure in the laboratory and of freely living individuals.
Fig. 1.
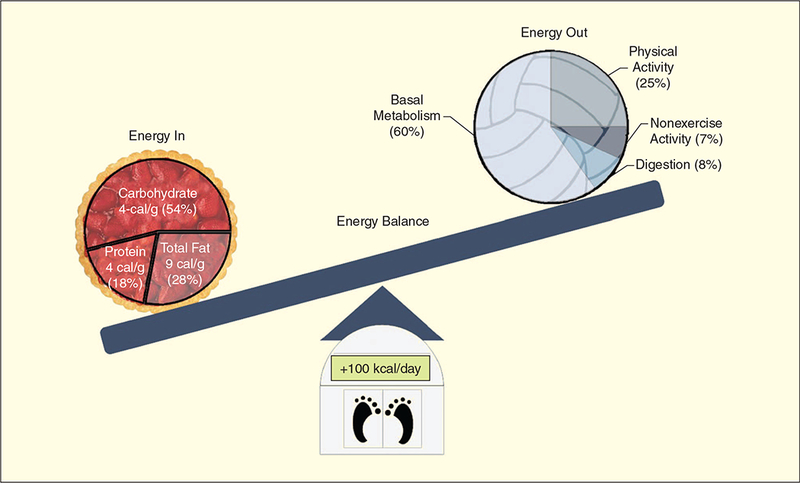
Major components of energy balance: expenditure, intake, and weight gain.
Fig. 2.
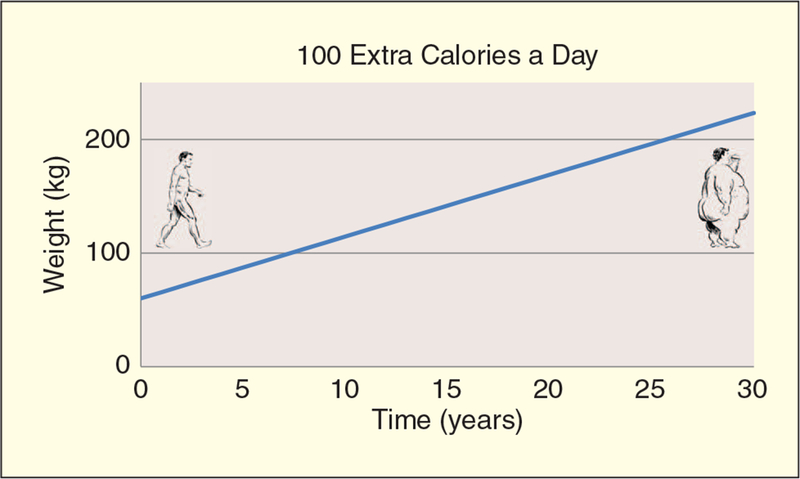
Long-term body weight increase from small energy imbalance. (Illustrations copyright Carol Lay.)
Historical Perspective
Measurement of human metabolism has enjoyed a lengthy history. In 1614, Santorio Sanctorius conducted one of the first controlled experiments in human metabolism by weighing himself before and after a number of his typical daily activities. He found that the majority of the food he consumed was lost through insensible perspiration [2]. Later that century, chemist and natural philosopher Robert Boyle and his colleagues studied combustion and metabolism by removing gases from containers, thereby extinguishing lit candles and killing birds. An astonishing 100 years later, Antoine Lavoisier (1743–1794) and his contemporaries finally identified oxygen and studied its role in animal respiration. They measured oxygen consumption volumetrically in humans and animals at different states (resting, postmeal, and exercise) by inventing the concept of indirect calorimetry. In the 1800s, EE measurement was being propelled to the forefront of scientific inquiry because of its implications in the rapidly developing fields of organic chemistry and biochemistry. During this period, Carl von Voit, Max Rubner, and their colleagues extended calorimetry studies and technology to a new level. Advances by the U.S. investigators such as Wilbur O. Atwater, Francis G. Benedict, and Graham Lusk soon followed.
The understanding of metabolic rates, heat exchange, and body size was greatly enhanced in the area of clinical nutrition in the late 1800s and early 1900s. At this point, normal basal metabolic rates (BMRs) could be predicted with reasonable accuracy using easily obtained measurements such as age, weight, and height. The only clinical need for in-depth measurement of individual BMR was to evaluate diseases of the thyroid. By 1950, however, this use was replaced by blood tests for thyroid hormone and its metabolites, which caused studies of whole-body EE in humans to decline. In the 1970s, the field of calorimetry was rejuvenated by advances in total parenteral nutrition (TPN) or intravenous feeding, which required accurate assessment of daily energy requirements. This was soon followed by the first notice of an epidemic of obesity in the 1980s and highlighted the need to quantify differences between energy intake and expenditure.
Calorimetry
All EE eventually ends up as heat; measuring heat lost is generally called calorimetry. Physiologic calorimetry can be either direct or indirect. Direct calorimetry specifically measures the rate at which a person dissipates heat. Although some direct calorimeter setups have been shown to be very accurate [3]–[6], in general, these systems are very expensive and difficult to operate. In addition, they are relatively slow compared with physiologic changes in metabolism (typical response time is 10–30 min) [3], [5]–[7].
Alternatively, indirect calorimetry relies on the metabolic conversion of food energy with oxygen to create carbon dioxide. EE can be calculated from the volume of oxygen consumed (VO2) and carbon dioxide produced (VCO2) with standard equation, [7], [8], such as,
| (1) |
Indirect calorimetry also has been shown to be very accurate (0.5–2.0% error), and for certain systems, response times below 30 s are possible [3]. Additionally, measuring human gas exchange is far less complex than directly quantifying heat loss. As a result, indirect calorimetry is widely viewed as the method of choice in humans and laboratory animals. The relative efficiency of indirect calorimetry has spawned several techniques to capture respired gases to compute VO2, VCO2, and EE. Each of these methods has specific advantages and limitations based on the aspects of human metabolism, which they were designed to capture.
Indirect Calorimeters
Metabolic Carts
Metabolic carts measure EE using a face-mask, mouthpiece, or a domed hood to collect gases entering and leaving the lungs (Figure 3). The quantity of exhaled gas is measured, and samples are pumped to a stationary gas-analyzer system. When calibrated and positioned correctly on the subject, a metabolic cart is accurate and can provide fast response times because the dead space is relatively small compared with minute ventilation rate. Still, a person tethered to the system has limited mobility. The metabolic cart is also uncomfortable and makes communication and eating almost impossible. Consequently, metabolic carts are generally restricted to periods from 20 min to at most 6 h.
Fig. 3.
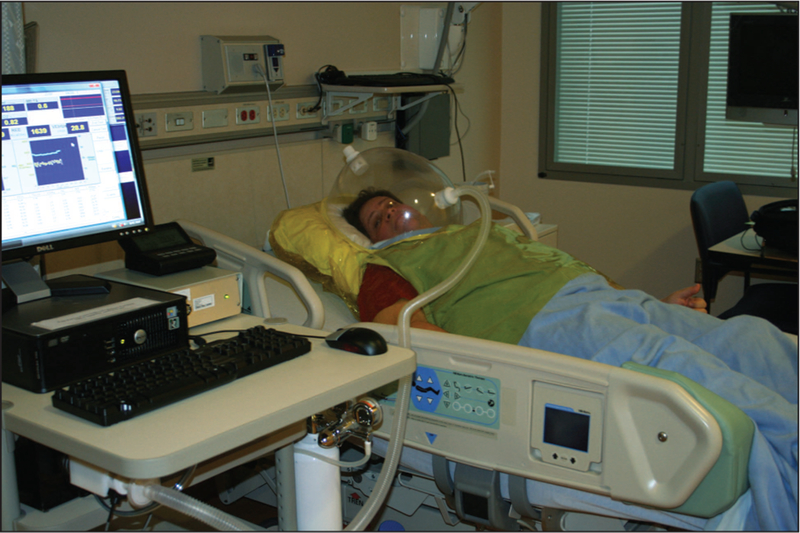
Metabolic cart with hood. (Photo courtesy of Dr. Kong Chen.)
Metabolic carts were introduced in the later half of the 20th century when electronic flow meters and process gas analyzers were perfected. Before that, exhaled gas was collected into a Douglas bag (or a small weather balloon to save money) or a bell respirometer. The period of measurement was limited by the number and volume of the containers available in the laboratory and the test subject’s respiration rate. Usually, only VO2 was measured by analyzing a sample of mixed gas chemically or in an analyzer while the contents of the container was forced out through a dry gas meter.
Portable Calorimeters
Portable calorimeters can achieve accuracy comparable to metabolic carts [9], [10]. However, they still require subjects to wear a mouthpiece and mask (Figure 4). A small gas analyzer and power supply (battery) are worn in a backpack. Or, exhaled gas is measured with a Fleisch meter, and periodic gas samples are collected in a series of vacuum containers of the same sort used for exposure monitoring. The portable configuration increases mobility and provides an environment that is closer to free living. However, the added weight of the analyzers makes the equipment more cumbersome and necessarily alters the desired measurement. The power required limits the recording time to approximately 4 h before recharging the battery.
Fig. 4.

COSMED portable calorimeter. (Photo courtesy of Dr. Kong Chen.)
Whole-Room Indirect Calorimeters
Whole-room indirect calorimeters also are called respiration or metabolic chambers. They are constructed as nearly air-tight rooms that capture all gases respired by a person inside. The room is ventilated with a carefully measured flow of air to keep CO2 concentration below a level that would affect metabolism. EE is calculated from the net flow rate of each gas species and changing composition of the air within the room (2). Room calorimeters offer the potential for robust measurement of a wide range of metabolic states. The larger the room the more comfortable the person inside will be, which interferes less with typical activity and permits longer studies (up to several days). As a result, whole-room calorimetry is the gold standard to measure total EE and its components.
However, measuring human metabolism accurately using the metabolic chamber has several challenges. First, over the past 150 years, fewer than 30 institutions have been able to apply the resources and expertise needed to construct and operate accurate systems. So, the majority of researchers interested in nutrition and genetic determinants of obesity do not have access to the best measurement systems. Second, performance is still a difficult issue. Modern room calorimeters have an enclosed volume of 20,000–30,000 L. This is tremendously greater than the amount of O2 consumed by the person inside (0.2–0.4 L·min−1 for an average individual at rest). Room calorimeters are ventilated with a flow of fresh air between 20 and 100 L·min−1. This sets the time constant of large calorimeters from 150 to as much as 1,000 min, almost the entire duration of most studies. Equilibration to the subject’s gas exchange is even slower. Thus, although calorimeters can be easily modeled with the straightforward mass balance of (2), every analysis interval less than several hours is made by estimating the derivative of readings taken at the absolute resolution limit of the gas analyzers and flow meter.
| (2) |
Sources of variation and noise, such as change in ambient air concentrations of O2 and CO2, barometric pressure change, water vapor, and electromagnetic interference, degrade readings from the gas analyzers used to measure room O2 and CO2 composition and are amplified in the derivative. This interferes further with the response time and accuracy of the whole room system and makes it difficult to study rapid changes in EE caused by physical activities, pharmacology, or diet [11]. Consequently, aggressive data-acquisition techniques and software filter algorithms are needed to suppress noise. Moreover, there are different types of analyzers (e.g., paramagnetic and full-cell O2 analyzers) and various configurations (e.g., differential versus absolute sampling) to further complicate the methodology.
A variety of techniques have been developed over the past century and a half to deal with all the challenges of room calorimeters. Significant advances have been made in achieving response times close to the scale of separate human activities with automated data collection and faster instruments [11], [12]. There is no longer a practical limit to the resolution and duration at which data can be collected relative to human physiologic response. So, filter algorithms can be customized to the types of answers desired from information. So far, no one has compared the various approaches in a rigorous engineering evaluation. As a result, each calorimeter operates differently. Users disagree on what works, what does not, and how to assess performance. Another challenge we have yet to face is how to accommodate children from the age of ambulation to eight years old.
Free-Living Measurements
Measuring EE is very difficult outside of the laboratory. Challenges include limited monitoring duration, poor compliance, limited resolution of low energy activities, and lack of concurrency. Nearly every researcher needs information on different aspects of EE such as body posture, periods of relative inactivity, vigorous body movement, and extreme environments. Better devices are needed for nutrition research, clinical interventions, and self-monitoring. Studies are underway or are being planned to explore how contextual factors influence energy balance and related behaviors. These studies must be supported with technology to assess factors (mood, social environment, and location) relevant to variations in energy balance [food intake, weight, and physical activity (PA)] in individuals and in large populations.
The doubly labeled water (DLW) method uses stable isotopes of hydrogen and oxygen to tag total body water, carbonic acid (H2CO3), and expired CO2. If the relationship CO2 + H2O ⇔ H2CO3 is in equilibrium and distributed throughout the circulation, then the difference in the rate that the 2H and 18O tracers are eliminated represents the rate of expired CO2 [13]. To use the DLW method, a person drinks a small amount of 2H2 18O in water and provides occasional samples of urine or saliva over the next one to three weeks. They do not need to carry cumbersome equipment, which allows true free-living measurements for long periods. The major limitations to DLW are the high cost and scarcity of the isotopes and the poor availability of laboratories able to analyze the samples and that only a few values of total EE are provided across the entire measurement period. So, it is not feasible to use the DLW method to resolve EE patterns shorter than one day.
PA and heart rate (HR) are correlated closely with EE associated with physical activities (Figure 5). Portable PA monitors are engineered primarily to measure quantity and patterns of activity, focusing mainly on intensity, duration, and frequency [14]. Accelerometers are electronic devices that measure accelerations produced by whole body or limb movement. Westerterp reviewed laboratory validations of various accelerometers using indirect calorimetry in adults and determined Pearson correlations that ranged from r = 0.25 to 0.91 [15]. The variability was due to the use of different monitors, placement (hip, low back, or ankle), and the specific activities performed.
Fig. 5.
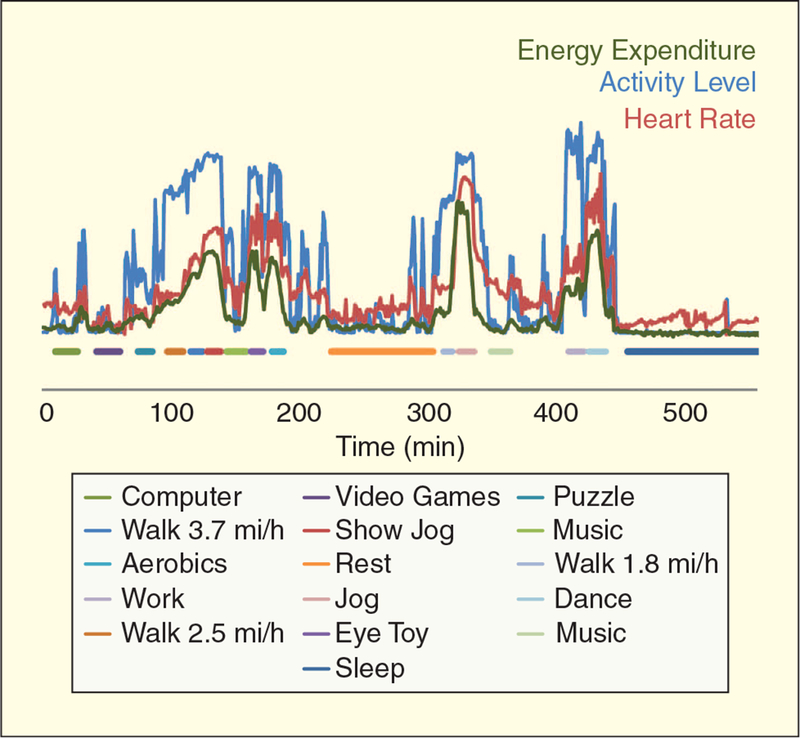
Details of energy expenditure, HR, and activity patterns in a room calorimeter. (Data courtesy of Dr. Nancy F. Butte, Baylor College of Medicine, Houston, Texas.)
Accelerometers provide an objective and reusable tool to assess PA, and they are increasingly relied upon as the criterion measure for new self-report surveys. Nevertheless, they have limited ability to assess cycling, stride length, locomotion on a gradient, or activities with limited torso movement. Current portable monitors generally have limited sensitivity (especially during the low-intensity activities that dominate the daily patterns for most overweight individuals), limited capacity to store data, power restrictions, or awkward wired connections between sensors and data logger units. Much longer and more detailed records of free-living behavior and conditions are badly needed.
With recent advances in sensor technologies, portable devices shrink in size, last longer, store more data, sense more information (e.g., multiaxial accelerometers), and are more sensitive. Thus, they should become more useful to determine free-living PA compared with devices from only a few years ago. Microelectromechanical systems (MEMS) accelerometers are the dominant class of motion transducers because of their size, affordability, low power consumption, multiple axes, programmable ranges, linearity, and fine sensitivity. A key advantage of MEMS accelerometers is that there is much less variation from one sensor to another.
HR monitoring remains the most common single-sensor physiologic monitor. Throughout the range of moderate through vigorous activity, HR increases linearly with the intensity of movement and the volume of oxygen consumed by the contracting skeletal muscle. Additionally, HR is well understood and defined with agreement for measurement, filtering, and storage.
Portable HR systems are comprised of a detector, usually attached to the chest, to sense skin surface cardiac depolarization potentials and a receiver/storage unit worn at the waist or wrist. The HR is represented by some proprietary radio-frequency encoding, but transmission occurs via standard schemes (Bluetooth, Zigbee, and ANT). Practical considerations in monitoring HR are that it remains susceptible to motion artifact, loss of electrical contact, and noise, generally from 60-Hz electrical interference generated by common household devices such as televisions and computers [16]. Newer units incorporate processing and a small amount of memory on board the detector module to create a store-and-forward arrangement that is less susceptible to interference with communication. Motion artifact and loss of contact are still addressed by using an electrolyte gel, increasing sensor surface area, and ensuring firm contact the sensor and skin.
In general, the sensors are problematic with respect to compliance. Most people find it uncomfortable to wear a sensor adhered or strapped tightly to their chest all day. In some individuals, mainly children who have more sensitive skin than do adults, contact dermatitis and chafing occur. This issue limits ubiquitous use of this technology, particularly when the outcome of interest is usual or habitual PA, which requires many days of monitoring. Recently, advances have been made in sensors woven into fabric that can be worn as functional clothing.
Despite these practical limitations, laboratory and field research that has examined the accuracy and precision of HR monitors currently available found good to excellent agreement between the most commonly used monitors and electrocardiogram (ECG) recordings [17]–[20]. In some situations, HR as a measure of PA, as opposed to motion-based PA sensors, may be exploited to better understand activity and health outcome relationships. For example, heart-rate monitoring has recently been used to examine the contribution of horseback riding to daily activity levels and functional fitness in wheel chair and ambulatory children with cerebral palsy [21]. Furthermore, because HR is a time domain parameter, the absolute amplitude of the ECG signal is not critical, as long as there is a sufficient signal-to-noise ratio. Therefore, in contrast to accelerometer-based activity monitoring studies, there is a greater comparability between heart-rate monitoring studies, even when different monitors and data modeling strategies are used.
One major limitation of using HR to estimate EE is that, while the two indices correlate well between individuals, the absolute relationship varies greatly. Resting HR and response to activity depend on age, fitness, autonomic status, presence of cardioactive medications, and a number of other factors. HR increases relative to the stress placed on the cardiorespiratory system; therefore, individuals participating in the same activity have different HRs based on their cardiovascular fitness differences. Other considerations include changes in HR that occur independent of PA, such as emotional or environmental stresses. Until recently, the individual relationship of HR and PA to EE had to be determined by indirect calorimetry in a laboratory across a range of activities and metabolic levels.
Consumer devices are available that monitor activity, global-positioning system (GPS) location, or HR fairly well. However, a few store data for longer than the equivalent of an exercise session or make that data available in raw form for thorough analysis. Totalizing monitors do not provide operators the ability to apply their own formulas to combine with other monitored data or in relations with body weight, fat-free mass, age, and other factors to improve assessment of EE.
Mobile phones are perhaps the most promising tool for free-living measurements and to study associated behaviors. Smart-phones contain quality accelerometers that were originally intended to determine orientation, but they are now used for many live action applications. They can also determine location, speed, and bearing very quickly with augmented GPS (a-GPS) and triangulation (e.g., the Skyhook service). Phones can receive local signals from separate sensors, such as HR or multiple accelerometer detectors placed about the body. Most powerful is the ability of smartphones to be the core of a body-area network (BAN) that links local and remote communications with rich user interactions and processing power. There is a great interest in using the ubiquitous and socially accepted (even among teenagers) cellular phone platform in support of diet and EE studies. Researchers in nutrition are working toward an integrated suite of technologies that support complete energy balance measurement (food diaries and photoimaging for intake, detailed EE, and weight gain).
The major difficulty in using the accelerometer, GPS receiver, and other hardware built into a smartphone is that the operating system and low-level software were not intended to acquire data for research. This creates a type of black box problem where sampling intervals, hardware (analog) filtering, sensor mounting, and phone dimension and mass must be carefully measured for each device. Once the black box transfer function is characterized, then raw data can be recovered from output data using an inverse function. Smartphones will probably be accepted quickly as essentially all dedicated PA monitors suffer the same limitation because they were never scrutinized closely during validation and are rarely calibrated. Part of the problem is that there isn’t a good calibration device that covers the range of physiologic center-of-mass measurement (± 3 g, 0.1–20 Hz).
Social Considerations
Approaches taken to measure EE are vastly different in a laboratory than free living (during normal, daily life). In the laboratory, indirect calorimetry is nearly ubiquitous to achieve the greatest accuracy, that is, if a calorimeter laboratory happens to be within a reasonable travel distance of the researcher and subject population. Most studies of free-living EE apply a unique combination of electronic monitors and aggregation of data. Unfortunately, comfort is almost always sacrificed for accuracy both inside and outside the laboratory. As in most real-world measurements, the greater the accuracy sought the more likely an investigation will alter the parameter being measured. And comfort is not the only issue, compliance to the requirements of a study decline rapidly if the techniques used are simply inconvenient or subject to peer or other social pressure.
Context seeks to evaluate the physical and social environment, attitude, and perspective of a person in association with a measure of EE. In other words, context answers the questions of “what, where, and with whom” and sometimes it also links to “why.” Location is a key piece of context information that can be assessed objectively by a-GPS and other information. Or, it can be based on being present in monitored area or by proximity to a particular object (refrigerator, vending machine, and television) or person. Devices can already verify that the correct person is using exercise equipment or that a description of a workout session was provided on the same day as the workout. However, we cannot assess meaning unless the person being studied tells us that they are in a bad mood, with friends, at a preferred restaurant, or about to start running with their cross-country team instead of alone.
Compared with traditional motion and physiologic sensors, contextual assessments of EE offer a completely new perspective. Assessments of EE context provide rich information about individual behavior and the interaction of behavior with the environment. However, it also brings a new set of unique challenges that can only be addressed by having psychologists and sociologists work together with nutrition researchers, clinicians, and engineers. Context is proving to be the critically important additional element that allows us to foresee effective techniques for weight management that might actually have an impact on the epidemic of obesity.
Room calorimeters offer the potential for robust measurement of a wide range of metabolic states.
Biography
Robert Brychta received a B.S. degree in biology from Ursinus College, Collegeville, Pennsylvania, in 2000 and an M.Sc. and Ph.D. degrees in biomedical engineering from Vanderbilt University, Nashville, Tennessee, in 2003 and 2006, respectively. He is currently a postdoctoral fellow at the National Institute of Diabetes and Digestive and an intramural research training award recipient. He has been a Member of the IEEE since 2006 and is a member of the Tau Beta Pi and Phi Beta Kappa honor societies. His main research interests include advancing measurement techniques of human metabolism and physical activity, biomedical signal processing, biomedical pattern recognition, and autonomic influences on human metabolism.

Erica Wohlers received her B.S. degree in biomedical engineering from the University of Minnesota and B.S. degree in physics from the University of Wisconsin-La Crosse. She is currently a biomedical engineer at MEI Research, Ltd.

Jon Moon received his B.S. degree in mechanical engineering from Massachusetts Institute of Technology (MIT) and M.S. and Ph.D. degrees in biomedical engineering from the University of Texas. He is the president of MEI Research, Ltd., which provides technologies for human energy balance investigations.

Kong Chen received his B.S. degree in mechanical engineering from Tennessee Technological University, a Ph.D. degree in biomedical engineering and a special M.S. degree in clinical investigation from Vanderbilt University. He is currently a clinical investigator at the intramural research program of the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Clinical Endocrinology Branch in Bethesda, Maryland. His research interests include quantitative physiology including human energy metabolism, body composition, and physical activity.

References
- [1].Rosenbaum M, Leibel RL, and Hirsch J, “Obesity,” N. Engl. J. Med, 337, pp. 396–407, Aug. 1997. [DOI] [PubMed] [Google Scholar]
- [2].Eknoyan G, “Santorio Sanctorius (1561–1636)–Founding father of metabolic balance studies,” Amer. J. Nephrol, 19, pp. 226–233, 1999. [DOI] [PubMed] [Google Scholar]
- [3].Levine JA, “Measurement of energy expenditure,” Public Health Nutr, 8, pp. 1123–1132, Oct. 2005. [DOI] [PubMed] [Google Scholar]
- [4].Snellen JW, “An improved estimation of mean body temperature using combined direct calorimetry and thermometry,” Eur. J. Appl. Physiol, 82, pp. 188–196, June 2000. [DOI] [PubMed] [Google Scholar]
- [5].Snellen JW, Chang KS, and Smith W, “Technical description and performance characteristics of a human whole-body calorimeter,” Med. Biol. Eng. Comput, 21, pp. 9–20, Jan. 1983. [DOI] [PubMed] [Google Scholar]
- [6].Tschegg E, Sigmund A, Veitl V, Schmid P, and Irsigler K, “An isothermic, gradient-free, whole-body calorimeter for long-term investigations of energy balance in man,” Metabolism, 28, pp. 764–770, July 1979. [DOI] [PubMed] [Google Scholar]
- [7].Webster JD, Welsh G, Pacy P, and Garrow JS, “Description of a human direct calorimeter, with a note on the energy cost of clerical work,” Br. J. Nutr, 55, pp. 1–6, Jan. 1986. [DOI] [PubMed] [Google Scholar]
- [8].Weir JB, “New methods for calculating metabolic rate with special reference to protein metabolism 1949,” Nutrition, 6, pp. 213–221, May-Jun 1990. [PubMed] [Google Scholar]
- [9].Duffield R, Dawson B, Pinnington HC, and Wong P, “Accuracy and reliability of a Cosmed K4b2 portable gas analysis system,” J. Sci. Med. Sport, 7, pp. 11–22, Mar. 2004. [DOI] [PubMed] [Google Scholar]
- [10].McLaughlin JE, King GA, Howley ET, Bassett DR Jr., and Ainsworth BE, “Validation of the COSMED K4b2 portable metabolic system,” Int. J. Sports Med, 22, pp. 280–284, May 2001. [DOI] [PubMed] [Google Scholar]
- [11].Sun M, Reed GW, and Hill JO, “Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure,” J. Appl. Physiol, 76, pp. 2686–2691, June 1994. [DOI] [PubMed] [Google Scholar]
- [12].Moon JK, Vohra FA, Valerio Jimenez OS, Puyau MR, and Butte NF, “Closed-loop control of carbon dioxide concentration and pressure improves response of room respiration calorimeters,” J. Nutr, 125, pp. 220–228, 1995. [DOI] [PubMed] [Google Scholar]
- [13].Schoeller DA and Taylor PB, “Precision of the doubly labelled water method using the two-point calculation,” Hum. Nutr. Clin. Nutr, 41, pp. 215–223, May 1987. [PubMed] [Google Scholar]
- [14].Chen KY and Bassett DR Jr., “The technology of accelerometry-based activity monitors: current and future,” Med. Sci. Sports Exerc, 37, pp. S490–S500, Nov. 2005. [DOI] [PubMed] [Google Scholar]
- [15].Westerterp KR, “Assessment of physical activity: A critical appraisal,” Eur. J. Appl. Physiol, 105, pp. 823–828, 2009. [DOI] [PubMed] [Google Scholar]
- [16].Janz KF, “Use of heart rate montiors to assess physical activity,” in Physical Activity Assessments for Health-Related Research, Welk G, Ed. Champaign, IL: Human Kinetics, 2002, pp. 143–161. [Google Scholar]
- [17].Bassett DR Jr., “Validity and reliability issues in objective monitoring of physical activity,” Res. Q. Exerc. Sport, 71, pp. S30–S36, June 2000. [PubMed] [Google Scholar]
- [18].Gamelin FX, Berthoin S, and Bosquet L, “Validity of the polar S810 heart rate monitor to measure R-R intervals at rest,” Med. Sci. Sports Exerc, 38, pp. 887–893, May 2006. [DOI] [PubMed] [Google Scholar]
- [19].Kingsley M, Lewis MJ, and Marson RE, “Comparison of Polar 810s and an ambulatory ECG system for RR interval measurement during progressive exercise,” Int. J. Sports Med, 26, pp. 39–44, Jan-Feb 2005. [DOI] [PubMed] [Google Scholar]
- [20].Treiber FA, Musante L, Hartdagan S, Davis H, Levy M, and Strong WB, “Validation of a heart rate monitor with children in laboratory and field settings,” Med. Sci. Sports Exerc, 21, pp. 338–342, June 1989. [PubMed] [Google Scholar]
- [21].Dirienzo LN, Dirienzo LT, and Baceski DA, “Heart rate response to therapeutic riding in children with cerebral palsy: An exploratory study,” Pediatr. Phys. Ther, 19, pp. 160–165, Summer2007. [DOI] [PubMed] [Google Scholar]


