Abstract
The antioxidant function of 2-Cys peroxiredoxin (Prx) involves the oxidation of its conserved peroxidatic cysteine to sulphenic acid that is recycled by a reductor agent. In conditions of oxidative stress, the peroxidatic cysteine can be overoxidized to sulphinic acid inactivating the Prx. An enzyme recently discovered, named sulfiredoxin (Srx), reduces the sulphinic 2-Cys Prx (Prx-SO2H). To explore the physiological functions of Srx in plants we have cloned, expressed and purified to homogeneity a Srx from Arabidopsis thaliana (AtSrx), as well as five variants by site-directed mutagenesis on amino acids involved in its activity. The activity of sulfiredoxin, determined by a new method, is dependent on the concentration of the sulphinic form of Prx and the conserved Srx is capable of regenerating the functionality of both pea and Arabidopsis Prx-SO2H. Molecular modelling of AtSrx and the facts that the R28Q variant shows a partial inactivation, that the activity of the E76A variant is equivalent to that of the native enzyme and that the double mutation R28Q/E76A abolishes the enzymatic activity suggests that the pair His100-Glu76 may be involved in the activation of C72 in the absence of R28. The knock-out mutant plants without Srx or 2-Cys Prx exhibited phenotypical differences under growth conditions of 16 h light, probably due to the signalling role of the sulphinic form of Prx. These mutants showed more susceptibility to oxidative stress than wild-type plants. This work presents the first systematic biochemical characterization of the Srx/Prx system from plants and contributes to a better understanding of its physiological function.
Keywords: Antioxidant defence, Arabidopsis, hydrogen peroxide, inorganic phosphate, peroxiredoxin, sulfiredoxin
Introduction
High levels of reactive oxygen species (ROS) such as peroxynitrite (ONOO–), superoxide ion () and hydrogen peroxide (H2O2) have been described as compounds capable of modifying protein, lipids, and DNA (Finkel and Holbrook, 2000). By contrast, low levels of H2O2 can function as a second messenger signal in cell proliferation, differentiation, and migration (Rhee, 2006; Sundaresan et al., 1995). The deregulation of these signalling processes leads to oxidative stress and disease states, including diabetes, cancer, and ageing (Finkel and Holbrook, 2000; Klaunig and Kamendulis, 2004). The ubiquitous peroxiredoxin (Prx) family, and specifically the 2-Cys Prxs, have been recognized as peroxide sensors that can be inactivated through hyperoxidation (Kang et al., 2005). Their catalytic cycle consists of two events: (i) the nucleophilic attack of the peroxide by the conserved peroxidatic cysteine that is oxidized to sulphenic acid (Cys-SPOH), and (ii) the resolution by attack of a free thiol to release water and form a disulphide. At high concentrations of H2O2, the peroxidatic cysteine can be overoxidized to the sulphinic acid form (Cys-SPO2H) inactivating the enzyme and acting itself as a signal (Vivancos et al., 2005).
In this context, the retroreduction of Prxs is essential to restore the peroxidase activity and the regulation of signalling events. However, the oxidation of the sulphenic acid (Cys-SPOH) to sulphinic acid in Prxs (Cys-SPO2H) was thought to be an irreversible step (Yang et al., 2002) until Woo et al. (2003a) reported that the sulphinic form produced during the exposure of cells to high levels of H2O2 was reduced to the catalytically active thiol form (Cys-SPH) and that an enzyme might be involved in the reduction. These results were further confirmed by the studies on the retroreduction of different mammalian 2-Cys Prxs by Chevallet et al. (2003). However, the identification of the proposed enzyme was carried out by Biteau et al. (2003), who found in yeast that H2O2 induced the overexpression of a new protein that they called sulfiredoxin (Srx) and that the deletion of the gene that encodes it reduced the tolerance to H2O2.
Srx is an antioxidant enzyme present in eukaryotes that contains a C-terminal cysteine residue conserved in all family members (Jönsson and Lowther, 2007). Interestingly, Srx is not apparent in prokaryotes; it is thought that this is due to the role of Srx in the restoration of over-oxidized 2-Cys Prx, whose counterparts in prokaryotes are not sensitive to oxidative inactivation (Wood et al., 2003). Unlike the extensive studies in yeast (Biteau et al., 2003; Vivancos et al., 2005) and mammals (Chang et al., 2004; Woo et al., 2005; Jeong et al., 2006) there are only two references that provide molecular evidence for the presence of functional homologues in higher plants (Liu et al., 2006; Rey et al., 2007). Plant Srxs contain all the conserved residues necessary for the binding of ATP and catalysis and a putative chloroplast targeting peptide. The single AtSrx gene in Arabidopsis encodes a 14 kDa polypeptide and knock-out plants in this protein increase the levels of sulphinic form of At-2-Cys Prx under stress. Although these two works deal with the importance of this antioxidant enzyme to maintain redox balance in chloroplasts, they do not provide a systematic biochemical characterization by a kinetic analysis of a plant Srx.
The involvement of the Prx/Srx system in growth factor signalling mediated by receptor tyrosine kinases has recently been reported in mammalian (Choi et al., 2005; Lei et al., 2008; Wei et al., 2008) and yeast (Vivancos et al., 2005) cells. Choi et al. (2005) have demonstrated that human Prx II is a negative regulator of platelet-derived growth factor (PDGF) and Prx II deficiency results in increased cell proliferation. In plants, the genome from Arabidopsis encodes receptor-like kinases (RLK) genes (Chae et al., 2009) and, recently (Smet et al., 2009), RLK signalling pathways have been identified as regulators in cell specification in plants. In this context, studies by Liu et al. (2006) have reported that the knock-out line of AtSrx was more susceptible to oxidative stress elicited by paraquat than WT plants, whereas Rey et al. (2007) have observed that this mutant line exhibits less oxidative damage than WT under photo-oxidative treatment.
From a mechanistical point of view, two schemes have been proposed to explain the mechanism of action of the Srx and both involve an exogenous thiol reductant, ATP, Mg2+, and a conserved Cys. According to the first proposed mechanism (Fig. 1), one oxygen atom on the sulphinic moiety of the oxidized Prx functions as a nucleophile and attacks the γ-phosphate of ATP at the Srx to yield a sulphinic acid phosphoryl ester intermediate that is resolved by the nucleophilic attack of the Cys from the Srx (Biteau et al., 2003; Jönsson et al., 2008a). In the second proposed mechanism, the Cys from the Srx attacks the γ-phosphate of ATP resulting in the formation of a thiophosphate intermediate that reacts with the sulphinic moiety of the Prx to yield sulphinic phosphoryl which is resolved by the external reductant (Jeong et al., 2006). Thus, Srx acts as a phosphotransferase and a thioltransferase in the first hypothesis, while its role is limited to the transfer of the phosphate to the Prx in the second one. Although both mechanisms are plausible there exists some experimental evidence that supports the former (Jönsson et al., 2008b).
Fig. 1.
Catalytic cycle of 2-Cys Prx, overoxidation by peroxide, and proposed reaction mechanism of retroreduction by Srx. On the left part of the figure the peroxide oxidizes the N-terminal cysteine (the peroxidatic cysteine, Cys-SPH) of one subunit to sulphenic acid (Cys-SPOH) (1), which reacts with the C-terminal cysteine (the resolving cysteine, Cys-SRH) of the other subunit to form an intermolecular disulphide that is reduced. However, at high concentrations of H2O2 the peroxidatic cysteine can be overoxidized to sulphinic acid (Cys-SPO2H) (2) inactivating the enzyme. According to the most accepted mechanism of retroreduction the sulphinic acid is phosphorylated, through a reversible step, by a direct attack on the γ-phosphate of ATP in the presence of Srx (3). The phosphoryl ester () converted into a thiosulphynate (Prx-SO-S-Srx) and inorganic phosphate (Pi) is released (4). A reducing agent (R-SH) (Trx, GSH or DTT) reduces this complex to release Prx-Cys-SPOH and Srx-S-S-R (5) that is subsequently reduced to Srx-SH (6).
In this article, the first systematic biochemical and functional characterization of a plant Srx is reported. The heterologous expression and purification to homogeneity of Srx from Arabidopsis thaliana, as well as of five variants (R28Q, K40Q, C72S, E76A, and R28Q/E76A) are described, with the aim of understanding its action mechanism. To this end, a new method has been designed to determine the Srx activity based on the ATP hydrolysis. The response of AtSrx and At-2-Cys-Prx knock-out lines to oxidation stress has also been explored. The results provide additional insight into the function of Srx and the crucial role in plants that of the sulphinic form of Prx.
Materials and methods
Cloning, mutagenesis, and purification of recombinant AtSrx and mutants R28Q, K40Q, C72S, E76A, and R28Q/E76A
Total RNA was isolated from 2 g of leaves from Arabidopsis thaliana (ecotype Columbia) by the phenol/SDS method (Sambrook et al., 1989). The cDNA library was generated by RT-PCR using Superscript II reverse transcriptase (Invitrogen, Carlsbad, USA) and oligo dT20 as a primer. The full-length coding AtSrx sequence (309 pbs) which encodes the mature protein (GenBank accession number Q8GY89) was amplified by PCR. Forward and reverse primers were designed with NcoI and BamHI (Roche, Mannheim, Germany) restriction sites respectively (underlined): AtSrx-F (5′-CACCATGGACGGTTCGCCGCCGGTGAT-3′), AtSrx-R (5′-TAAGGATCCATCTTCTCTGAGGTACCAA-3′).
The PCR were performed at an annealing temperature of 55 °C and the DNA products were gel-purified, cloned into the pGEM-T vector (Promega, Madison, USA) and sequenced. The PCR cDNA encoding the predicted mature protein (amino acids 23–125), lacking chloroplast transit peptide, was digested with HindIII and BamHI, and cloned into pETM-11 expression vector (Novagen, Darmstadt, Germany). The R28Q, K40Q, C72S, and E76A mutations were introduced by PCR as described by Rouhier et al. (2002) using a mix of cloning and mutagenic primers (mutagenic bases marked in bold):
AtSrx-F (as above), AtSrx-R (as above), R28Q-F (5′-TTGGAGAAGATACGACAACCGTTGAT-3′), R28Q-R (5′-ATCAACGGTTGTCGTATCTTCTCCAA-3′); K40Q-F (5′-TCTTTCACTTGGTTCTGATCGTTGGA-3′), K40Q-R (5′-TCCAACGATCAGAACCAAGTGAAAGA-3′); C72S-F (5′-TATCTGTGACTTCCCGAGAACCCATA-3′), C72S-R (5′-TATGGGTTCTCGGGAAGTCACAGATA-3′); E76A-F (5′-TGTCACTAGAACGCGGCGCATCAG-3′), E76A-R (5′-CTGATGCGCCGCGTATCTGTGACT-3′).
PCR were performed with 35 cycles using a temperature profile of 30 s at 94 °C, 30 s at 65 °C and 60 s at 72 °C. The purified PCR products were digested with NcoI and BamHI and ligated into pGEM-T (Promega) at the corresponding restriction sites. The sequences of the recombinant plasmids were verified by sequencing. The PCR products of the mutants were digested with HindIII and BamHI for cloning into pETM-11 (Novagen, Darmstadt, Germany).
For the expression of His-tagged AtSrx and its variants, Escherichia coli strain BL21 (DE3) was transformed with the recombinant plasmids (AtSrx-pETM-11, R28Q-pETM-11, K40Q-pETM-11, C72S-pETM-11, E76A-pETM-11, and R28Q/E76A-pETM-11). Transformed cells were cultured at 37 °C in Luria–Bertani medium supplemented with kanamicin to 0.6 OD600 and then the overexpression was induced by 1 mM IPTG for 6 h. Cells were harvested by centrifugation and stored at –70 °C. Frozen cells were resuspended in 50 mM TRIS-HCl (pH 7.5), 500 mM NaCl and disrupted with French press. The crude extract was centrifuged and the supernatant was supplemented with 20 mM imidazole and DNAase (Roche) prior to loading onto a Ni2+ column equilibrated with 50 mM TRIS-HCl (pH 7.5), 500 mM NaCl, and 20 mM imidazole in a FPLC system. Proteins were eluted with a gradient of imidazole from 20 mM to 500 mM. Fractions with the protein were dialysed against 50 mM TRIS-HCl (pH 7.5) and loaded onto a Mono Q 5/50 column (GE Healthcare, Upssala, Sweden) and eluted with a linear gradient from 0 to 1 M NaCl. Fractions with Srx were dialysed against 50 mM TRIS-HCl (pH 7.5). The success of each step was monitored by SDS-PAGE.
Cloning and purification of recombinant 2-Cys Prxs
The cDNA coding for mature Arabidopsis 2-Cys Prx (At-2-Cys Prx) (GenBank accession number Y10478) was amplified from a cDNA library using primers containing 5’ extensions with BamHI (Roche) site (underlined) for the forward and reverse primers: At2CP-F (5′-GGATCCATGGCGTCTGTTGCTTCTTC-3′); At2CP-R (5′-GGATCCCTAAATAGCTGAGAAGTACTC-3′). The 798 pb digested product was cloned into pQE32 vector (Qiagen). The cDNA encoding At-2-Cys Prx was assessed by sequencing. Expression in BL21 cells was induced by adding 1 mM IPTG. The His-tagged 2-Cys Prx protein was purified by trapping onto Ni-nitriloacetic acid Sepharose and elution with 500 mM imidazole (Barranco-Medina et al., 2008).
Chloroplast 2-Cys Prx from Pisum sativum (Ps-2-Cys Prx) was cloned without His-tag, and expressed and purified as described by Bernier-Villamor et al. (2004).
Plant material
Arabidopsis thaliana (ecotype Columbia) plants were grown in greenhouse with a 16/8 h light/dark photoperiod, with 250 μE m−2 s−1, temperature regime of 22/18 °C (day/night) and a relative humidity of 55%.
Two mutant lines (SALK 149311 and SALK 015324) for At-2-Cys Prx B and AtSrx, corresponding to T-DNA insertions events, termed ΔPrx B and ΔSrx, respectively, were isolated to obtain homozygous mutants using leaf genomic DNA extracted with the DNeasy Plant Mini Kit (Qiagen). Plants homozygous for the T-DNA insertion were identified from the T3 population and confirmed in the T4 generation using genomic PCR according to published methods (http://signal.salk.edu/).
The identification of the mutant lines was carried out by PCR to confirm the presence of transcript of At-2-Cys Prx A, At-2-Cys Prx B, and AtSrx (see Supplementary Fig. S1A at JXB online), using the following primers: 2-Cys Prx A-F (5′-CAAAGCCCAGGCCGATGATC-3′), 2-Cys Prx A-R (5′-TCATTGTCTCATCAACGCTTCG-3′); 2-Cys Prx B-F (5′-CATCCTCTTCTCTCTGTTCCG-3′), 2-Cys Prx B-R (5′-TCTCATTGTCTCATCAACACTTC-3′); AtSrx-F (as above), AtSrx-R (as above).
The presence of the T-DNA insert fragment into each knock-out line was assessed with the primer T-DNA Left border a1 (Lba1) (5′-TGGTTCACGTAGTGGGCCATCG-3′) with 2-Cys Prx B-R (as above) or AtSrx-R (as above) (see Supplementary Fig. S1B at JXB online).
The selection of the mutants and the study of the response of At-2-Cys Prx B and AtSrx knock-out lines to oxidative stress in plates were carried out on MS plates supplemented with kanamicin. In order to compare the oxidation stress response of mutants and wild-type (WT) plants, 5-week-old seedlings were sprayed with a solution containing 20 mM H2O2 and 0.5% Tween 20.
Isolation of chloroplasts
Young leaves (10 g.) from Arabidopsis thaliana (Col.) were homogenized in an ice-cold buffer containing 330 mM sorbitol, 50 mM MES-KOH (pH 6.5), 2 mM ascorbate, and 5 mM MgCl2. Chloroplasts were purified on a Percoll gradient (König et al., 2002) and resuspended in sorbitol buffer. The amount of chloroplastic protein was determined by the Bradford (1976) assay and the approximate number of chloroplast was estimated with a Neubauer camera.
Histochemical detection of H2O2 and superoxide radicals () in leaves
The histochemical detection of H2O2 was performed using endogenous POX-dependent in situ histochemical staining, in which whole leaves were vacuum-infiltrated with 0.1 mg ml−1 3, 3′-diaminobenzidine (DAB) in 50 mM TRIS-acetate buffer (pH 5.0) and incubated at 25 °C, in the dark, for 24 h (Hernández et al., 2001). Leaves were rinsed in 80% (v/v) ethanol for 10 min at 70 °C, mounted in lactic acid:phenol:water (1:1:1, by vol.), and photographed directly.
Fluorescence measurements
Modulated chlorophyll fluorescence emission from the upper surface of dark-adapted leaf discs was measured at midday using a PAM-2000 modulated Walz (Effeltrich, Germany) fluorometer. After dark adaptation of whole plants for 30 min, Fo and Fm measurements were performed from 6–8 leaves per plant. The initial level (Fo) of chlorophyll fluorescence was excited by a dim red light modulated at 600 Hz and determined with far-red light after a 2 s illumination, while the maximal level (Fm) was induced by an 800 ms pulse of intense white light. The efficiency of excitation energy capture by PSII, corresponding to the probability that an absorbed photon reaches the PSII reaction centres, was calculated as (Fm–Fo)/Fm=Fv/Fm.
Obtaining the sulphinic form of 2-Cys Prx
The sulphinic form of Prx (2-Cys Prx-SO2H) was generated in 50 mM TRIS-HCl pH 7.5 by a first incubation with 10 mM DTT for 30 min at 37 °C and then 2 mM H2O2 was added to the reaction mixture and the sample was incubated for an additional 30 min at 37 °C. The excess of DTT and H2O2 was removed by dialysis in a PD-10 column (GE Healthcare, Upssala, Sweden) equilibrated with 25 mM TRIS-HCl pH 7.5. The sulphinic form of Prx was checked by immuno-blot analysis after treatment with AMS (Biteau et al., 2003) and by identification with specific antibodies (Woo et al., 2003b).
In vitro Srx activity assays
The determination of the Srx activity by the spectrophotometric quantification of inorganic phosphate (Pi) is based on the method described by Chen et al. (1956). Reaction mixtures containing 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2, and various concentrations of ATP (0.25 or 1 mM), 2-Cys Prx (reduced and overoxidized) (15–30 μM) and Srx (1–5 μM), with or without GSH (10 mM), were incubated at 30 °C. At the indicated times (from 0 min to 120 min), aliquots of 100 μl were withdrawn and added to 350 μl of the colour reagent (two volumes of 10% (w/v) trichloroacetic acid/four volumes of 0.4% (w/v) ammonium molybdate in 3% (v/v) sulphuric acid/one volume of fresh L-ascorbic acid at 66.7 mg ml−1). After exactly 3 min of incubation at room temperature, 50 μl of 1.3 M tri-sodium citrate (Merck, Darmstadt, Germany) were added (Baginski et al., 1975; Gawronski and Benson, 2004) and the reaction was allowed to equilibrate for 45 min at 37 °C before reading the absorbance at 820 nm. For each determination a standard curve was obtained with potassium di-hydrogen phosphate (0–100 μM).
Srx activity was also measured by the spectrophotometric quantification of ADP by coupling to the oxidation of NADH in the presence of phosphoenolpyruvate, pyruvate kinase and lactate dehydrogenase (Kornberg and Pricer, 1951). Aliquots of the reaction mixtures prepared as described above were withdrawn at the indicated times and added to a mixture containing 50 mM TRIS-HCl (pH 7.5), 10 mM MgCl2, 10 mM KCl, 0.4 mM NADH, 10 U of pyruvate kinase, and 10 U of lactate dehydrogenase, with or without (blank cuvette) 2.5 mM phosphoenol pyruvate, and the absorbance at 340 nm was measured.
The ATP/ADP ratio during Srx-dependent retroreduction of Prx-SO2H was monitored by chromatrography in a Mono Q 5/50 GL column as described by Jönsson et al. (2005).
Kinetic parameters
KM and kcat of Srx from Arabidopsis and pea were determined by spectrophotometric quantification of the inorganic phosphate (Pi) released at 5 min and 10 min (to check a lineal increase) of reaction using saturating concentrations of purified Srx (30 μM), for its substrates: sulphinic form of 2-Cys Prx (Prx-SO2H) using 1 mM ATP, and for ATP using 40 μM Prx-SO2H. All determinations were made under the same conditions of reaction: 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2, from 0–40 μM of Prx-SO2H, and from 0 to 500 μM of ATP, incubated at 30 °C in the absence of reductant.
Peroxidase activity
Peroxide-dependent peroxidase activity of the recombinant 2-Cys Prxs were measured as described by Bernier-Villamor et al. (2004).
Polyacrylamide gel electrophoresis and Western blot analysis
Denaturing SDS-PAGE was performed as described by Laemmli (1970) with acrylamide concentrations of 6% (stacking gel) and 12.5% (resolving gel). Gels were stained with Coomassie Brilliant Blue R-250. For Western blot analysis, the proteins were transferred onto a nitrocellulose membrane (Amersham Bioscience, Germany) by electroblotting. Immunoreaction was carried out with rabbit serum against mature 2-Cys Prx, Srx or hyperoxidized Prx, diluted 1/5000, 1/1500, or 1/2000, respectively, in PBST containing 5% dried milk. Horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma, St Louis, USA) was used as the secondary antibody. The antigen was detected by chemiluminescence using a Western Lighting chemiluminscence reagent plus (Perkin-Elmer, Boston, USA) following the manufacturer's protocol.
Antibodies against 2-Cys Prx were obtained as described by Bernier-Villamor et al. (2004), while antibodies against the Srx peptide (CHRYEAHQKLGLPTI) were generated by Abyntek (Vizcaya, Spain) and antibodies against hyperoxidized Prx were purchased from LabFrontier (Seoul, Korea).
Molecular modelling of the AtSrx and pKa predictions
Modelling was approached by homology modelling at the Geno3D server (Combet et al., 2002) using as template the human Srx PDB entries 1xw3 and 3cyi that share about 39% identity, the psi-blast E-values (Altschul et al., 1997) being 9e−6 (9e−21 to 1e−19 when only structures in the PDB sharing less than 95% pairwise identity are considered). Both models comprise residues 21 to 102 (80% coverage) although that generated using 3cyi as template led to better parameters than that from 1xw3. Thus, the Errat overall quality factors (Colovos and Yeates, 1993) were 100 versus 91.9, the percentage of residues with an averaged 3D-1D score (Lüthy et al., 1992) better than 0.2 was 91.8% versus 98.8%.
Predictions of pKa were carried out at PROPKA server. It implements a method based on empirical relationships that uses as input 3D coordinates. The reported overall accuracy on the basis of the comparison of 314 experimental pKa values with those estimated by PROPKA is ±0.89 pH units. For the particular case of Cys, PROPKA predictions are withing 1 pH unit of experimental values in 83% of the cases (Li et al., 2005).
Results and discussion
Isolation of recombinant AtSrx and production of sulphinic form of Prx
A 375 pb cDNA was isolated from Arabidopsis thaliana RNA by RT-PCR and primers designed from the sequence of the putative AtSrx. The cDNA sequence encoded a 125 amino acids peptide with a molecular mass of 13 914 Da and a sequence-derived isoelectric point of 10.76, bearing a chloroplast transit peptide in the N-terminus and the signature sequence FG/SCHRY present in plant Srxs (Liu et al., 2006; Rey et al., 2007). A second PCR amplification with suitable primers led to the cDNA of the mature protein consisting of 103 amino acids with predicted molecular weight and isoelectric point of 11 546 and 9.86 Da, respectively. This cDNA was subcloned into pETM-11 expression vector and overexpressed in E. coli BL21 (DE3) to yield the recombinant N-terminal 6× His-tagged fusion mature Srx (AtSrx). The SDS-PAGE analysis showed that the His-tag approach led to a partially pure sample and additional Mono Q chromatography was needed to isolate the recombinant AtSrx (Fig. 2A). The Srx identity of the isolated recombinant protein was confirmed by Western blotting with antibodies against a Srx peptide and revealed a faint band at a molecular weight corresponding to a dimer of Srx (Fig. 2B).
Fig. 2.
Purification of recombinant AtSrx and confirmation of the Srx identity. (A) SDS-PAGE and Coomassie Brilliant Blue R-250 staining. Lane 1, crude extract; lane 2, after His-tag column; lane 3, pure protein after Mono Q chromatography. (B) Western blot with a specific antibody against Srx.
Srx is involved in the restoration of the sulphinic form of 2-Cys peroxiredoxin (Prx-SO2H). Hence the production and overoxidation of Prx was a prerequisite to characterize the functionality of the AtSrx. Recombinant 2-Cys Prx from Arabidopsis thaliana was isolated and oxidized to the sulphinic form by a first incubation with DTT to reduce any disulphide bond blocking the cysteine residues of the active site and then by treatment with H2O2 to oxidize them to the sulphinic form. The formation of the sulphinic species was monitored by 4-acetamido-4′-maleimidylstilbene-2,2′-disulphonic acid (AMS) treatment (Biteau et al., 2003) which alkylates cysteines only in the free SH-form and increases the molecular weight in about 0.5 kDa (Fig. 3A) and also by Western blot using specific antibodies against the Prx-SO2H (Woo et al., 2003b) (Fig. 3B). Both assays confirmed the production of the overoxidized Prx (Prx-SO2H) and HPLC-mass spectrometry verified the sulphinic identity of the overoxidazed form and the absence of sulphonic forms.
Fig. 3.
Assessment of the production of overoxidized forms of At-2-Cys Prx. (A) Purified 2-Cys Prx was overoxidized to the Prx-SO2H form in the presence of H2O2, and then treated with AMS as described in the Materials and methods. After SDS-PAGE, reduced (2× AMS) and overoxidized (1× AMS) forms of 2-Cys Prx were visualized with Western blot with an antibody against 2-Cys Prx. The reduced form of 2-Cys Prx without AMS is also visualized as a control. (B) Western blot of reduced and oxidized forms of purified 2-Cys Prx with antibodies against hyperoxidized Prx (2-Cys Prx-SO2H) and 2-Cys Prx.
Determination of the sulfiredoxin activity
The activity of Srx may be determined by two different approaches that have been implemented in several methods not exempt of some drawbacks: (i) detection and quantification of the redox state of the Prx (Biteau et al., 2003; Chang et al., 2004) and (ii) determination of the hydrolysis of the ATP (Jeong et al., 2006; Roussel et al., 2008; Kim et al., 2009). The enzymatic activity of the recombinant AtSrx was determined by two variants of the methods based on the hydrolysis of ATP. One relies in the oxidation rate of NADH to report the ADP formation via coupling to the conversion of phosphoenolpyruvate into pyruvate and then into lactate by pyruvate kinase and lactate dehydrogenase, respectively. Both enzymes are commercial and results may be standardized, circumventing the strong variability of the enzymatic determination of the hydrolysis of ATP currently in use. The second method is based on the formation of a coloured phosphomolybdate complex between molybdate and the phosphate resulting from the hydrolysis of ATP. The determination of the enzymatic activity of the recombinant AtSrx by the two alternative methodologies is summarized in Table 1. Results demonstrate the feasibility of both approaches and show that the molar concentration of ADP after 2 h of reaction at different Srx:Prx-SO2H stoichiometries in the presence of 250 μM or 1 mM ATP corresponds to the concentration of phosphate.
Table 1.
Comparison between Srx activity measured by the determination of released phosphate and by the determination of released ADP
| AtSrx (μM) | Prx-SO2H (μM) | ATP (mM) | Pi (μM) | ADP (μM) | Significance |
| 5 | 15 | 0.25 | 35.9±4.3 | 42.1±2.5 | ≥0.99 |
| 5 | 30 | 0.25 | 80.4±11.5 | 78.4±2.1 | ≥0.99 |
| 2.5 | 15 | 0.25 | 24.4±3.1 | 26.4±3.4 | ≥0.99 |
| 5 | 15 | 1 | 63.2±5.0 | 77.5±5.3 | ≥0.98 |
| 5 | 30 | 1 | 96.6±7.6 | 97.6±4.5 | ≥0.99 |
| 2.5 | 15 | 1 | 44.3±4.4 | 59.6±3.1 | ≥0.98 |
Reaction mixtures containing 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2 and the indicated concentrations of AtSrx, Prx-SO2H, and ATP. After 2 h incubation at 30 °C, released phosphate and ADP were determined as indicated in the Materials and methods. Data are means ±SD of values from three independent experiments and the similarity between both methods were statistically analysed by a 2-tailed Student t test.
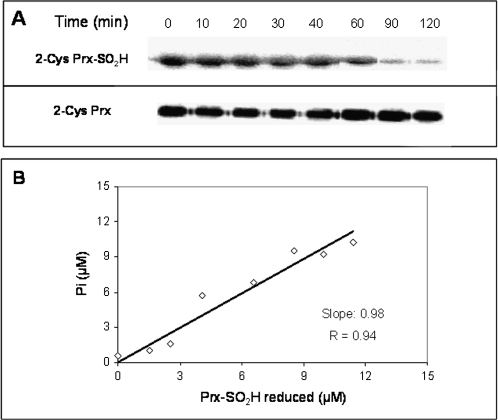
The simplicity of colorimetric assay is especially attractive and further evidences of its feasibility were obtained by comparison with the method based on the detection and quantification of the redox state of the Prx by Western blotting (Chang et al., 2004). To this end, the Srx activity of aliquots withdrawn at different times from a sample containing 5 μM AtSrx, 15 μM Prx-SO2H, 10 mM GSH, and 250 μM ATP was determined by quantification of both the sulphinic Prx with specific antibodies and the phosphate by the colorimetric assay. As shown in Fig. 4A, the intensity of the band detected by antibody against the hyperoxidized Prx decreased as the incubation time with AtSrx is longer whereas the intensity of the band detected with antibodies against 2-Cys Prx remained unchanged. The plot of the amount Prx-SO2H reduced by AtSrx estimated from the Western blot, versus the concentration of phosphate (Pi) resulting from the hydrolysis of ATP, reveals a linear relationship with R coefficient of 0.94 and an estimated slope of 0.98 (Fig. 4B) that validates the colorimetric assay for the determination of the sulfiredoxin activity.
Fig. 4.
Correlation between Srx activity measured by the determination of released phosphate and that using specific antibodies against hyperoxidized Prx. Reaction mixture containing 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2, 250 μM ATP, 10 mM GSH, 15 μM Prx-SO2H, and 5 μM Srx was incubated at 30 °C. (A) At the indicated times, aliquots were withdrawn and subjected to Western blot with specific antibodies against Prx-SO2H and 2-Cys Prx (control). (B) The concentration of Prx-SO2H reduced was determined from the corresponding Western blot band intensity (by analysed imaging) and plotted against the concentration of phosphate determined in the same samples.
Characterization of the sulfiredoxin from Arabidopsis
The biochemical characterization of mammalian sulfiredoxins by Jeong et al. (2006) revealed that the hydrolysis of ATP was dependent on the sulphinic form of peroxiredoxin (2-Cys Prx-SO2H) and that, in the presence of glutathione, it reached the concentration of the sulphinic Prx (Fig. 1, step 5). In the absence of the reductant (GSH, DTT, Trx, …), the hydrolysis of ATP progressed further and this experimental result was explained on the basis of a futile cycle of phosphorylation and dephosphorylation that consumes ATP continuously as a consequence of the formation of a sulphinic phosphoryl ester Prx intermediate (Fig. 1, steps 3 and 4) that is hydrolysed to regenerate the sulphinic acid group. The fact that the kinetic of the inorganic phosphate (Pi) formation is hyperbolically saturated led to the suggestion of an inactivation as a result of a disulphide formation between Srx and Prx.
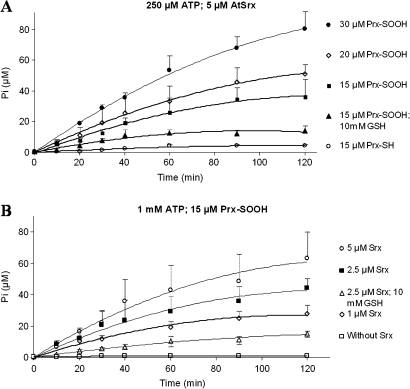
There is no similar study on plant Srxs, and AtSrx is a good model to test the generality of the above observations. The assay of activity using several pH (from 6.8 to 9.5), revealed that the optimal reduction of Prx-SO2H by AtSrx takes place at pH 7.8 (data not shown). In a first set of experiments at 250 μM ATP and 5 μM AtSrx, the influence of the Prx was analysed. As depicted in Fig. 5A, the release of phosphate was negligible when reduced 2-Cys Prx (Prx-SH) was assayed, confirming the requirement of the sulphinic moiety and ruling out a non-specific ATP hydrolysis. As its mammalian counterparts, in the presence of 10 mM GSH the concentration of released phosphate (13.9 μM) was that of the sulphinic Prx (15 μM) in the assay, being the extent of ATP hydrolysis in absence of reductant influenced by the concentration of the sulphinic Prx and larger than its concentration in the assay, in full agreement with the existence of the proposed phosphorylation/dephosphorylation futile cycle (Fig. 1, step 3).
Fig. 5.
Srx enzymatic activity determined by the quatification of the phosphate resulting from the hydrolysis of ATP. Reaction mixtures containing 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2, 250 μM or 1 mM ATP and various concentrations of 2-Cys Prx (reduced and overoxidized) and Srx were incubated at 30 °C. At the indicated times, aliquots of 100 μl were withdrawn and subjected to phosphate determination as described in the Materials and methods. (A) The reaction was carried out with 250 μM ATP, 5 μM Srx, and various concentrations of Prx-SO2H, namely, 30 μM (closed circles), 20 μM (open diamonds), 15 μM (open squares); 15 μM and 10 mM GSH (closed triangles); control with 15 μM Prx-SH (open circles). (B) The reaction was carried out with 1 mM ATP, 15 μM Prx-SO2H, and various concentrations of Srx, namely, 5 μM (open circles), 2.5 μM (closed squares), 1 μM (open diamonds); 2.5 μM and 10 mM GSH (open triangles); control without Srx (open squares). Data are means ±SD of values from three independent experiments.
In a second set of experiments at 1 mM ATP and 15 μM Prx-SO2H, the effect of the concentration of AtSrx was studied (Fig. 5B). As expected, in the presence of GSH the released Pi reached the concentration of sulphinic Prx in the assay (15.2 μM and 15 μM, respectively). On the other hand, the assay in the absence of the reductant supports the participation of the AtSrx in the futile cycle since at 15 μM Prx-SO2H, the hydrolysis of ATP at 5, 2.5, and 1 μM AtSrx yielded 63.2, 44.3, and 27.8 μM Pi, respectively. Moreover, a comparison of experiments at 15 μM Prx-SO2H and 5 μM AtSrx, and 250 μM ATP (Fig. 5A) or 1 mM ATP (Fig. 5B), reveals a release of Pi of 35.9 μM and 63.2 μM, respectively, suggesting that the yield of the futile cycle is also dependent on the concentration of ATP.
The kinetic parameters of AtSrx were determined by measuring the Pi released after 5 min of reaction. For the sulphinic form of At-2-Cys Prx (Prx-SO2H) using saturating concentrations of AtSrx (30 μM) and 1 mM ATP, the kinetic parameters were determined to be: KM=79 μM, kcat=0.66 min−1; the kinetic parameters for ATP using saturating concentrations of AtSrx (30 μM) and 40 μM sulphinic 2-Cys Prx were: KM=29 μM, kcat=0.025 min−1. These data are similar to those obtained from mammalian Srx (Jönsson and Lowther, 2007), suggesting that AtSrx, like mammalian Srx, is not a highly efficient enzyme despite our calculations revealing the low concentration of this enzyme in this organelle that was estimated as 2 ng of Srx in 1 μg of total chloroplastic protein (see Supplementary Fig. S2 at JXB online).
In order to evaluate the functionality of the regenerated Prx the peroxidase activity of retroreduced, overoxidized, and reduced Prxs specimens were analysed. Results showed a negligible activity for Prx-SO2H and that 3 h of retroreduction in the presence of Srx, GSH, and ATP led to a 96.8% restoration of the peroxidase activity compared with the reduced Prx (Fig. 6). On the other hand, there exists evidence of the capability of the human Srx to reduced mitochondrial peroxiredoxin III although the physiological mechanism of the mitochondrial translocation remains unclear. In fact it has been demonstrated that the overexpression of mitochondrion-targeted Srx promotes the restoration of Prx III and results in cellular resistance to apoptosis with enhanced elimination of mitochondrial H2O2 (Noh et al., 2009). In this context, the feasibility of AtSrx to restore the activity of other Prxs was put to test on the recombinant Pisum sativum 2-Cys Prx, lacking the His-tag and the sequence identity with that from Arabidopsis being 84.2%. Results revealed similar activity of the regenerated Prx regardless of the species and the presence of His-tag and the kinetic parameters are similar to those reported above for Prx-SO2H (KM=71 μM, kcat=0.65 min−1).
Fig. 6.
Recovery of the peroxidase activity of the sulphinic form of Prx. Reaction mixture containing 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2, 1 mM ATP, 10 mM GSH, 5 μM Prx-SO2H, and 1 μM Srx was incubated at 30 °C. At 1, 2, and 3 h aliquots were withdrawn and peroxidase activity was determined at the indicated times, as described in the Materials and methods. Peroxidase activity of recombinant reduced 2-Cys Prx (Prx-SH) and overoxidized Prx (Prx-SO2H) were also determined. Peroxidase activity without Prx was determined as a control.
Site-directed mutagenesis of AtSrx
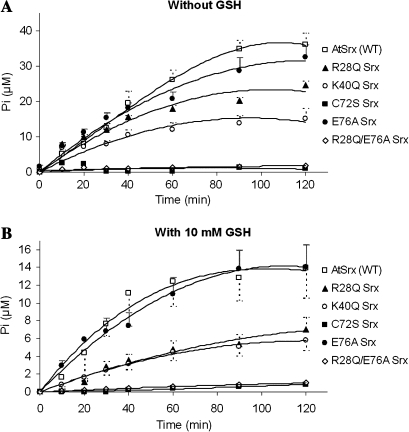
In order to gain additional insight into the features of AtSrx, site-directed mutagenesis was carried on, R28, K40, C72, and E76. Residues R28, K40, and C72 are conserved among Srx proteins from mammals, yeast, and plants and they have been demonstrated to play a critical role in the functionality of the enzyme (Jeong et al., 2006). Five mutants of AtSrx, namely R28Q, K40Q, C72S, E76A, and R28Q/E76A were generated and their Srx activity was assayed in the absence (Fig. 7A) and presence (Fig. 7B) of reductant (10 mM GSH) and no significant relative difference have been found between both conditions. The kinetic parameters (KM and kcat) and the catalytic efficiency as expressed by kcat/KM values for the assay in the absence of reductant are summarized in Table 2. The discrepancy observed for the lowest kcat/KM value reported for K40Q or R28Q, depending on the use of ATP or 2-Cys- Prx-SO2H as substrate, can be rationalized on the basis that both substrates interact in the catalysis, kcat/KM values being diminished for both substrates and both variants.
Fig. 7.
Activity of Srx variants. (A) Activity was measured as described in Fig. 5, in the presence of 250 μM ATP, 15 μM Prx-SOOH, and 5 μM Srx, namely, AtSrx WT (open squares); R28Q Srx (closed triangles); K40Q Srx (open circles); C72S Srx (closed squares); E76A Srx (closed triangles), and R28Q/E76A Srx (open diamonds). (B) Activity was measured with the same mix of reactions, adding 10 mM of GSH. Data are means ±SD of values from three independent experiments.
Table 2.
Kinetic parameters for AtSrx and its variants for Prx and ATP
| Enzyme | Substrate | kcat/KM (M−1 s−1) |
| AtSrx WT | 2-Cys Prx-SO2H | 1.39×102 |
| ATP | 0.14×102 | |
| R28Q AtSrx | 2-Cys Prx-SO2H | 0.27×102 |
| ATP | 0.03×102 | |
| K40Q AtSrx | 2-Cys Prx-SO2H | 0.47×102 |
| ATP | 0.02×102 | |
| E76A AtSrx | 2-Cys Prx-SO2H | 1.46×102 |
| ATP | 0.16×102 |
Data were determined by measuring Pi at 5 min in reactions containing 30 μM AtSrx, 1 mM Mg, 0–40 μM Prx-SO2H or 0–500 μM ATP in 50 mM TRIS-HCl (pH 7.5).
As expected, mutation of C72S resulted in an abolition of the activity, confirming its critical role in the hydrolysis of ATP. Residues R28 and K40 correspond to R51 and K61 in human Srx (the residue numbers are lower by one in rat Srx) and their mutation to glutamine resulted in a partial reduction of the hydrolysis of ATP (Fig. 7). According to the X-ray structure of the human Srx (Jönsson et al., 2005) K61 interacts with one of the oxygen atoms of the α-phosphate of ADP and its mutation in rat Srx led to a weak ATPase binding and an ATPase activity of 10% of that of the Srx (Jeong et al., 2006). Thus ATPase activity of the K40Q AtSrx can be explained as a consequence of a diminished ATP binding capability.
Mutation of R28 was expected to result in the inactivation of the enzyme, as reported for rat Srx, since the structure of the human Srx shows that R51 is adjacent to C99 (C72 in AtSrx) and most likely functions to activate C99 to the thiolate form. However, the colorimetric assay showed that R28Q AtSrx retains a significant functionality (Fig. 7). This result was confirmed by the chromatographic determination of the ATP/ADP ratio during Srx-dependent retroreduction of Prx-SO2H since the retention time for ATP and ADP in a Mono Q 5/50 column is different enough even to monitor the ATPase activity (Fig. 8A). As shown in Fig. 8B, the amount of ADP produced by mutant C72S was negligible, which is in agreement with the date reported by Jönsson et al. (2005) using the same method and similar concentrations of Srx (25 μM), overoxidized Prx (25 μM), and ATP (0.5 mM). Variants R28Q and K40Q (Fig. 8B) showed activities comparable to those obtained by the phosphate determination (Fig. 7), R28Q being more active than K40Q and validating the results obtained by the colorimetric assay.
Fig. 8.
FPLC analysis of Srx activity. Reaction mixture containing 50 mM TRIS-HCl (pH 7.5), 1 mM MgCl2, 100 μM ATP, 15 μM Srx, and 30 μM Prx-SO2H (AtSrx and variants) were incubated at 30 °C. Aliquots of 200 μl were withdrawn at the indicated times and the ADP formed was separated from ATP in a Mono Q anion exchange column as indicated in the Materials and methods. (A) AtSrx activity with Prx-SO2H after the indicated times. (B) Variants Srx activity R28Q, K40Q, and C72S with Prx-SO2H after 3 h of reaction.
The above results suggest that the activation of C72 is either poorly relevant or resulting from the interaction with other residues besides R28. The former hypothesis does not seem realistic since this type of Arg–Cys interaction to activate the nucleophilicity of Cys has been described in other enzymes such as peroxiredoxins (Wood et al., 2003) and methionine sulphoxide reductases (Lowther et al., 2002). In fact the pKa predicted by PROPKA server (Li et al., 2005) for C99 from human Srx is 4.98. This anomalous pKa value for a cysteine (the standard value is 9.0) makes C72 especially reactive, being the hydrogen bonding with R51, S55 the main responsible for the enhanced nucleophilicity of C99 (Table 3).
Table 3.
pKa values as output by PROPKA for the catalytic Cys and the nearby His predicted from the structure of the human Srx and the models of AtSrx based on the PDB entries 3cyi (C99S human Srx) and 1xw3 (human Srx)
| Human Srx (1xw3) | ||||||||||||
| Residue | pKa | Locate | Desolvation effects |
Sidechain hydrogen bond | Backbone hydrogen bond | |||||||
| Massive |
Local |
|||||||||||
| Cys 99 | 4.98 | Surface | 0.00 | 363 | 0.00 | 0 | –1.46 | Ser | 55 | –0.73 | Val | 56 |
| –1.50 | Arg | 51 | –0.33 | Cys | 99 | |||||||
| His 100 | 6.29 | Surface | 0.00 | 278 | –0.21 | 3 | 0.00 | – | 0 | 0.00 | – | 0 |
| Model of the tertiary structure of AtSrx using 1xw3 as template | ||||||||||||
| Residue | pKa | Locate | Desolvation effects | Sidechain hydrogen bond | Backbone hydrogen bond | |||||||
| Massive | Local | |||||||||||
| Cys 72 | 6.46 | Surface | 0.00 | 358 | 0.07 | 1 | –1.60 | Arg | 28 | –0.36 | Thr | 33 |
| 0.00 | – | 0 | –0.62 | Cys | 72 | |||||||
| 0.00 | – | 0 | –0.03 | His | 73 | |||||||
| His 73 | 6.36 | Surface | 0.00 | 295 | –0.14 | 2 | 0.00 | – | 0 | 0.00 | – | 0 |
| Model of the tertiary structure of AtSrx using 3cyi as template | ||||||||||||
| Residue | pKa | Locate | Desolvation effects | Sidechain hydrogen bond | Backbone hydrogen bond | |||||||
| Massive | Local | |||||||||||
| Cys 72 | 4.29 | Surface | 0.00 | 337 | 0.14 | 2 | –1.60 | Thr | 33 | –0.81 | Thr | 33 |
| 0.00 | – | 0 | –0.78 | Cys | 72 | |||||||
| 0.00 | – | 0 | –1.67 | His | 73 | |||||||
| His 73 | 7.22 | Surface | 0.00 | 293 | 0.07 | 1 | 0.79 | Glu | 76 | 0.00 | – | 0 |
The contribution of the pKa shift is quantified in terms of three perturbations: desolvation, hydrogen bonding and charge-charge interactions.
To address the existence of other residues involved in the nucleophilic activation of C72 the tertiary structure of the AtSrx was modelled at the Geno3D server (Combet et al., 2002). The best two models were generated using as template the coordinates of the human Srx (PDB entry 1xw3) and the C99S mutant (PDB entry 3cyi). Both models comprised residues 21 to 102 and the fact that the alignment of the coordinates with Swiss-PDBviewer 4.0.1 (Guex and Peitsch, 1996) yielded an overall RMS deviation of 0.87 Å confirmed that they are equivalent. The prediction of the pKa value for C72 was 6.46 and 4.29 for the model based on the human Srx and C99S mutant, respectively. The analysis of the residues involved in the activation of C72 is summarized in Table 3 and it identifies R28 as being mainly responsible for the shift in the pKa for the model based on the Srx although the predicted value was far from that predicted for C99 from the human Srx. Unexpectedly, the model based on the C99S showed a completely different pattern, H73 and T33 being the two main contributors to the pKa shift that, however, yielded the pKa prediction closest to that for the human Srx. The inspection of both models revealed that the only difference was the position of the SH-group. In the model based on the coordinates of the C99S human Srx, the –SH group of the catalytic Cys is pointing to residue H100 that shows an anomalous predicted pKa value of 7.22 (standard value for histidine is 6.5) as a consequence of the interaction with E76 (Table 3).
The catalytic triad cysteine–histidine–glutamic acid predicted by the model is present in picornains 2A and 3C, cysteine peptidases from picornaviruses. Cysteine proteases are thought to attack the substrate by a thiolate-imidazolium ion par, the acidic component of the triad being essential for activity (Polgar, 2005). In order to assess the prediction, the double mutant R28Q/E76A was produced and the resulting protein was inactive in the absence and presence of reductor (Fig. 7), validating the prediction. The facts that, in mammals, the residue equivalent to E76 is A103 and that the mutation of R51 (equivalent to R28 in At-Srx) yields the inactivation of the enzyme encouraged us to generate the variant E76A to explore its role in catalysis. However, variant E76A shows the same activity as AtSrx WT (Fig. 7), suggesting that E76 is not crucial in the wild-type plant enzyme and that the pair histidine–glutamic acid may be involved in the activation of C72 in the absence of R28.
Response of ΔSrx and ΔPrx B plants to oxidative stress
The involvement of the Prx/Srx system in growth factor signalling mediated by receptor tyrosine kinases in mammalian (Choi et al., 2005; Kang et al., 2005; Lei et al., 2008; Wei et al., 2008) and yeast (Vivancos et al., 2005) and the existence of more than 600 receptor-like kinase homologes in the Arabidopsis thaliana genome whose role in signalling pathways has been recognized (Smet et al., 2009) prompted us to investigate the role of the Prx/Srx system in the growth under oxidative conditions. As shown in Fig. 9A, knock-out lines of Arabidopsis (ΔSrx and ΔPrx B) grown in a 16/8 h light/dark photoperiod exhibited phenotypic differences. Leaves were smaller in ΔSrx but larger in ΔPrx B compared with WT in 5-week-old plants. The average rosette diameter (cm) for n=10 plants were 7.87±0.91 (AtWT), 9.41±0.95 (ΔPrx B), and 6.21±0.57 (ΔSrx). These differences with respect to the WT were statistically significant by a 2-tailed Student t test at P >0.98. This is the first phenotype variation described for both lines of mutants in plants grown under a long cycle photoperiod. However, under a short cycle photoperiod (8/16 h light/dark) no phenotypic differences between both lines and WT were observed (data not shown), in full agreement with the bibliography (Liu et al., 2006; Rey et al., 2007). As expected, Western blot analysis of leaf extracts showed a small amount of 2-Cys Prx and of its sulphinic form in ΔPrx B (because 2-Cys Prx A is expressing) as well as the absence of Srx in ΔSrx (Fig. 9B). A closer analysis revealed a higher level of Prx-SO2H in ΔSrx than in WT and an inverse relationship between growth and level of Prx-SO2H. It is tempting to speculate that this inverse correlation may be related to a regulatory role of the sulphinic form of 2-Cys Prx B that may account for the phenotypical differences observed under a long photoperiod: the extended illumination may induce a moderate stress, increasing the amount of sulphinic Prx that yields a lower growth rate. Thus, ΔPrx B plants that produce a low amount of Prx-SO2H and ΔSrx that can not retroreduce it exhibited the largest and the smallest leaf size, respectively. This hypothesis demands additional studies to confirm by complementation lines whether the phenotypic differences are the result of the level of Prx-SO2H. Further work would open a wide field connecting Prx/Srx system and redox signalling.
Fig. 9.
Characterization of ΔPrx B and ΔSrx and its response to oxidative stress. (A) Phenotype of untreated plants at 5 weeks old under a long cycle photoperiod. (B) Western blot of levels of total Prx and overoxidized Prx (2-Cys Prx-SO2H) in 10 μg of total leaf protein and levels of Srx in 20 μg of total leaf protein from untreated plants and after 24 h or 48 h of 20 mM H2O2 treatment. (C) Fluorescence measurements (Fv/Fm) and staining with DAB of H2O2 and in leaves from untreated plants and treated for 24 h or 48 h with 20 mM H2O2. (D) Mutants grown on MS medium with 20 mM and 50 mM H2O2.
Plants treated with 20 mM H2O2 to induce an oxidative stress showed differences in the levels of the sulphinic form of 2-Cys Prx when compared with untreated plants (Fig. 9B). In ΔSrx, the absence of Srx yielded an increased level of Prx after 24 h and 48 h of treatment that has not been found in AtWT. Liu et al. (2006) reported an increase of Srx at the transcript level in WT plants under stress conditions. However, no changes in the level of AtSrx have been found in treated plants, probably due to the limited time of treatment. On the other hand, the fact that ΔPrx B plants yielded a lower level of Srx than the AtWT suggests that the sulphinic form of 2-Cys Prx in plants may regulate the expression of Srx as shown in yeast by Vivancos et al. (2005). To our knowledge, this is the first report of a lower level of the Srx protein in ΔPrx B compared with AtWT plants.
The effect of the manipulation of the Prx/Srx system on the photosynthetic efficiency (Fv/Fm) was also analysed. As shown in Fig. 9C, although Fv/Fm was not affected in untreated plants, mutants were more sensitive to H2O2 treatment and DAB detection revealed a larger accumulation of H2O2 and superoxide ion probably due to the difficulty of eliminating them. This result is in full agreement with those reported by Liu et al. (2006), who found that AtSrx knock-out mutants under paraquat treatment were more sensitive than WT and also had greater sensitivity of the Srx knock-down mammalian epidermal cells to hydrogen peroxide (H2O2) induced cell death (Wei et al., 2008). However, Rey et al. (2007) have reported that mutant lines lacking Srx increased stress tolerance and Baier et al. (2000) and Lamkemeyer et al. (2006) reported that plants with decreased levels of 2-Cys Prx and Prx Q, respectively, did not have an apparently different phenotype from WT at the plant level and that both lines had a decreased sensitivity to oxidants. Probably, these discrepancies may be related to the different growth conditions and oxidative treatments.
In order to gain additional insight into the response of ΔSrx and ΔPrx B to oxidative stress during germination, seedlings were grown in plates with MS medium and kanamicin containing H2O2. Results showed at ΔPrx B mutants were more sensitive than ΔSrx at low concentrations of H2O2 and that ΔSrx plants were more sensitive than AtWT at high concentration of H2O2 (Fig. 9D). These results reveal that, although both enzymes are not essential for viability of the plant, they protect the chloroplast with some specificity depending on the intensity of the oxidative stress, so the role of Srx is crucial in critical situations to switch on the antioxidant pathway to regenerate the oxidative damage (Vivancos et al., 2005).
Conclusions
To our knowledge, this is the first systematic biochemical and functional characterization of plant Srx by a new and reliable colorimetric method based on the detection of inorganic phosphate. Biochemical studies show that AtSrx behaves as its mammalian counterparts in terms of kinetic parameters and capability to retroreduce the activity of other Prxs. However, site-directed mutagenesis and molecular modelling have revealed that, whereas in mammals, mutation of R51 leads to the inactivation of the enzyme, the equivalent mutation in AtSrx only reduces the activity and only the additional mutation of E76 abolishes it. This unique feature of plant Srxs suggests that the pair histidine–glutamic acid may be involved in the activation of C72 in the absence of R28. Functional studies in Arabidopsis of ΔSrx and ΔPrx B knock-out lines grown under a long photoperiod led to the detection of a phenotype variation that could suggest a regulatory role of the growth process of the sulphinic form of 2-Cys Prx B. Induction of oxidative stress shows that, as in yeasts, 2-Cys Prx in plants may regulate the expression of Srx and that, although Srx and Prx are not essential for plant viability, they protect against oxidative stress. Further works are ongoing to investigate the signalling role of the sulphinic form of 2-Cys Prx in eukaryotic cells.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Identification of mutants ΔPrx and ΔSrx.
Supplementary Fig. S2. Determination of the Srx amount into the chloroplast isolated from Arabidopsis.
Supplementary Material
Acknowledgments
This work was supported by Dirección General de Investigación, Ministerio de Ciencia e Innovación (Project BFU2008-00745/BFI) and by Acción Integrada (Project HA2007-0077) between Spain and Germany. We would like to thank Joseph Ecker as the donor of the knock-out lines used in this work. We are grateful to the proteomic services of the University of Córdoba for the HPLC analysis and Dr M Barón's group for the help in measurements of Fv/Fm and DAB detection. FJLJ is indebted to Professor Santoyo for his support.
Glossary
Abbreviations
- AMS
4-acetamido-4′-maleimidylstilbene-2,2′-disulphonic acid
- ADP
adenosine 5′-diphosphate
- ATP
adenosine 5′-triphosphate
- DAB
3,3′-diaminobenzidine
- DTT
dithiothreitol
- GSH
glutathione
- IPTG
isopropil-1-thio-β-D-galactoside
- NADH
nicotinamide adenine dinucleotide
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein data base search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginski ES, Epstein E, Zak B. Review of phosphate methodologies. Annals of Clinical and Laboratory Science. 1975;5:399–416. [PubMed] [Google Scholar]
- Baier M, Noctor G, Foyer CH, Dietz KJ. Antisense suppression of 2-Cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiology. 2000;124:823–832. doi: 10.1104/pp.124.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranco-Medina S, Kakorin S, Lázaro JJ, Dietz KJ. Thermodynamics of the dimmer-decamer transition of reduced human and plant 2-Cys peroxiredoxin. Biochemistry. 2008;47:7196–7204. doi: 10.1021/bi8002956. [DOI] [PubMed] [Google Scholar]
- Bernier-Villamor L, Navarro E, Sevilla F, Lázaro JJ. Cloning and characterization of a 2-Cys peroxiredoxin from Pisum sativum. Journal of Experimental Botany. 2004;55:2191–2199. doi: 10.1093/jxb/erh238. [DOI] [PubMed] [Google Scholar]
- Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine–sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chae L, Sudat S, Dudoit S, Zhu T, Luan S. Diverse transcriptional programs associated with environmental stress and hormones in the Arabidopsis receptor-like kinase gene family. Molecular Plant. 2009;2:84–107. doi: 10.1093/mp/ssn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS, Jeong W, Woo HA, Lee SM, Park S, Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulphinic acid in the active site to cysteine. Journal of Biological Chemistry. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- Chen PS, Jr., Toribara TY, Wagner H. Microdetermination in phosphorus. Analytical Chemistry. 1956;28:1756–1758. [Google Scholar]
- Chevallet M, Wagner E, Luche S, van Dorsselaer A, Leize-Wagner E, Rabilloud T. Regeneration of peroxiredoxins during recovery after oxidartive stress. Journal of Biological Chemistry. 2003;278:37146–37153. doi: 10.1074/jbc.M305161200. [DOI] [PubMed] [Google Scholar]
- Choi MH, Lee IK, Kim GW, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- Colovos C, Yeates TO. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Science. 1993;2:1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combet C, Jambon M, Deléage G, Geourjon C. Geno3D: automatic comparative molecular modelling of protein. Bioinformatics. 2002;18:213–214. doi: 10.1093/bioinformatics/18.1.213. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Gawronski JD, Benson DR. Microtiter assay for glutamine synthetase biosynthetic activity using inorganic phosphate detection. Analytical Biochemistry. 2004;327:114–118. doi: 10.1016/j.ab.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. Swiss-PDBViewer: a fast and easy-to-use PDB viewer for macintosh and PC. Protein Data Bank Quaterly Newsletter. 1996;77:7. [Google Scholar]
- Hernández JA, Ferrer MA, Jiménez A, Ros-Barceló A, Sevilla F. Antioxidant systems and /H2O2production in the apoplast of Pisum sativum L. leaves: its relation with NaCl-induced necrotic lesions in minor veins. Plant Physiology. 2001;127:817–831. doi: 10.1104/pp.010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Park SJ, Chang TS, Lee DY, Rhee GS. Molecular mechanism of the reduction of cysteine sulphinic acid of peroxiredoxin to cysteine sulphinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. Journal of Biological Chemistry. 2006;281:14400–14407. doi: 10.1074/jbc.M511082200. [DOI] [PubMed] [Google Scholar]
- Jönsson TJ, Lowther WT. The peroxiredoxin repair proteins. Subcellular Biochemistry. 2007;44:115–141. doi: 10.1007/978-1-4020-6051-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson TJ, Murray MS, Johnson LC, Lowther WT. Reduction of cysteine sulphinic acid in peroxiredoxin by sulfiredoxin proceeds directly through a sulphinic phosphoryl ester intermediate. Journal of Biological Chemistry. 2008a;283:23846–23851. doi: 10.1074/jbc.M803244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson TJ, Murray MS, Johnson LC, Poole LB, Lowther WT. Structural basis for the retroreduction of inactivated peroxiredoxins by human sulfiredoxin. Biochemistry. 2005;44:8634–8642. doi: 10.1021/bi050131i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson TJ, Tsang AW, Lowther WT, Furdui CM. Identification of intact protein thiosulphinate intermediate in the reduction of cysteine sulphinic acid in peroxiredoxin by human sulfiredoxin. Journal of Biological Chemistry. 2008b;283:22890–22894. doi: 10.1074/jbc.C800124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends in Molecular Medicine. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim H, Hong S, Rhee SG, Jeong W. A colorimetric assay for sulfiredoxin activity using inorganic phosphate measurement. Analytical Biochemistry. 2009;393:36–40. doi: 10.1016/j.ab.2009.06.030. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annual Review of Pharmacology and Toxicology. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- König J, Baier M, Horling F, Kahmann U, Harris G, Schürmann P, Dietz K-J. The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox-hierarchy of photosynthetic electron flux. Proceedings of the National Academy of Sciences, USA. 2002;99:5738–5743. doi: 10.1073/pnas.072644999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A, Pricer WE., Jr. Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. Journal of Biological Chemistry. 1951;193:481–495. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamkemeyer P, Laxa M, Collin V, et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. The Plant Journal. 2006;45:968–981. doi: 10.1111/j.1365-313X.2006.02665.x. [DOI] [PubMed] [Google Scholar]
- Lei K, Townsend DM, Tew KD. Protein cysteine sulphinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene. 2008;27:4877–4887. doi: 10.1038/onc.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Robertson AD, Jensen JH. Very fast empirical prediction and rationalization of protein pKa values. Proteins. 2005;61:704–721. doi: 10.1002/prot.20660. [DOI] [PubMed] [Google Scholar]
- Liu XP, Liu XY, Zhang J, Xia ZL, Liu X, Qin HJ, Wang DW. Molecular and functional characterization of sulfiredoxin homologs from higher plants. Cell Research. 2006;16:287–296. doi: 10.1038/sj.cr.7310036. [DOI] [PubMed] [Google Scholar]
- Lowther WT, Weissbach H, Etienne F, Brot N, Matthews BW. The mirrored methionine sulphoxide reductases of Neisseria gonorrhoeae pilB. Nature Structural Biology. 2002;9:348–352. doi: 10.1038/nsb783. [DOI] [PubMed] [Google Scholar]
- Lüthy R, Bowie JU, Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992;356:83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- Noh YH, Baek JY, Jeong W, Rhee SG, Chang TS. Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. Journal of Biological Chemistry. 2009;284:8470–8477. doi: 10.1074/jbc.M808981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar L. The catalytic triad of serine peptidases. Cellular and Molecular Life Sciences: CMLS. 2005;62:2161–2172. doi: 10.1007/s00018-005-5160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey P, Becuwe N, Barrault MB, Rumeau D, Havaux M, Biteau B, Toledano MB. The Arabidopsis thaliana sulfiredoxin is a plastidic cysteine-sulphinic acid reductase involved in the photooxidative stress response. The Plant Journal. 2007;49:505–514. doi: 10.1111/j.1365-313X.2006.02969.x. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signalling. H2O2, a necessary evil for cell signalling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rouhier N, Gelhaye E, Jacquot JP. Glutaredoxin-dependent peroxiredoxin from poplar. Protein–protein interaction and catalytic mechanism. Journal of Biological Chemistry. 2002;277:13609–13614. doi: 10.1074/jbc.M111489200. [DOI] [PubMed] [Google Scholar]
- Roussel X, Béchade G, Kriznik A, Van Dorsselaer A, Sanglier-Cianferani S, Branlant G, Rahuel-Clermont S. Evidence for the formation of a covalent thiosulphinate intermediate with peroxiredoxin in the catalytic mechanism of sulfiredoxin. Journal of Biological Chemistry. 2008;283:22371–22382. doi: 10.1074/jbc.M800493200. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Smet ID, Voss U, Jürgens G, Beeckman T. Receptor-like kinases shape the plant. Nature Cell Biology. 2009;11:1166–1173. doi: 10.1038/ncb1009-1166. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayté J, Toledano MB, Hidalgo E. A cysteine-sulphinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proceedings of the National Academy of Sciences, USA. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Jiang H, Matthews CP, Colburn NH. Sulfiredoxin is an AP-1 target gene that is required for transformation and shows elevated expression in human skin malignancies. Proceedings of the National Academy of Sciences, USA. 2008;105:19738–19743. doi: 10.1073/pnas.0810676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulphinic acid formation. Science. 2003a;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- Woo HA, Jeong W, Chang TS, Park KJ, Park SJ, Yang JS, Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. Journal of Biological Chemistry. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- Woo HA, Kang SW, Kim HK, Yang KS, Chae HZ, Rhee SG. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulphinic acid. Inmunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. Journal of Biological Chemistry. 2003b;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Schröder E, Harris JR, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemistry Science. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Yang KS, Kang WS, Woo AH, Hwang SH, Chae Z, Kim K, Rhee GS. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulphinic acid. Journal of Biological Chemistry. 2002;277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.